Abstract
Background
COVID-19 reduces lung functionality causing a decrease of blood oxygen levels (hypoxemia) often related to a decreased cellular oxygenation (hypoxia). Besides lung injury, other factors are implicated in the regulation of oxygen availability such as pH, partial arterial carbon dioxide tension (PaCO2), temperature, and erythrocytic 2,3-bisphosphoglycerate (2,3-BPG) levels, all factors affecting hemoglobin saturation curve. However, few data are currently available regarding the 2,3-BPG modulation in SARS-CoV-2 affected patients at the hospital admission.
Material and methods
Sixty-eight COVID-19 patients were enrolled at hospital admission. The lung involvement was quantified using chest-Computer Tomography (CT) analysed with automatic software (CALIPER). Haemoglobin concentrations, glycemia, and routine analysis were evaluated in the whole blood, while partial arterial oxygen tension (PaO2), PaCO2, pH, and HCO3− were assessed by arterial blood gas analysis. 2,3-BPG levels were assessed by specific immunoenzymatic assays in RBCs.
Results
A higher percentage of interstitial lung disease (ILD) and vascular pulmonary-related structure (VRS) volume on chest-CT quantified with CALIPER had been found in COVID-19 patients with a worse disease outcome (R = 0.4342; and R = 0.3641, respectively). Furthermore, patients with lower PaO2 showed an imbalanced acid-base equilibrium (pH, p = 0.0208; PaCO2, p = 0.0496) and a higher 2,3-BPG levels (p = 0.0221). The 2,3-BPG levels were also lower in patients with metabolic alkalosis (p = 0.0012 vs. no alkalosis; and p = 0.0383 vs. respiratory alkalosis).
Conclusions
Overall, the data reveal a different pattern of activation of blood oxygenation compensatory mechanisms reflecting a different course of the COVID-19 disease specifically focusing on 2,3-BPG modulation.
Keywords: 2,3- bisphosphoglycerate; Blood oxygenation; COVID-19; Chest computed tomography; Arterial blood gas (ABG)
Highlights
-
•
The lung involvement quantified by CALIPER software predicts the COVID-19 severity.
-
•
2,3 BPG are higher in COVID-19 patients that do not develop alkalosis.
-
•
Specific patterns of blood oxygenation parameters reflect different courses of the disease.
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the COVID-19 disease. Since its outbreak, the World Health Organization (WHO) estimates more than 770 million infected subjects and 6.85 million deaths [1]. The SARS-CoV-2 mainly affects the upper respiratory tract leading to the progression of pneumonia and acute respiratory distress syndrome (ARDS), nevertheless, several other extra-pulmonary manifestations have been widely reported [2]. Chest computed tomography (CT) is the gold standard imaging analysis to diagnose and monitor COVID-19 pneumonia and has been used to evidence the destruction of the lung parenchyma and the insurgence of fibrotic lesions [3]. Interestingly, the use of Computer-Aided Lung Informatics for Pathology Evaluation and Rating (CALIPER) software has been recently validated for CT-chest analysis, correlating a high percentage of interstitial lung disease (ILD) and vascular-related structure volume (VRS) with COVID-19 patient worse outcome [4,5].
The decrease of lung functionality is associated with the reduction of respiratory gas exchange, which causes a decrease of blood oxygen levels (hypoxemia) often related to a decreased cellular oxygenation (hypoxia) [6]. Besides lung injury, other factors are implicated in the regulation of oxygen availability and equilibrium between blood and tissues oxygen such as pH, partial arterial carbon dioxide tension (PaCO2), and temperature, but also intra-erythrocytic organic phosphates’ concentration, especially adenosine triphosphate (ATP) and 2,3-bisphosphoglycerate (2,3-BPG) [7], all factors affecting hemoglobin saturation curve.
At the early stage of hypoxemia, COVID-19 patients, activate compensatory mechanisms by increasing minute ventilation which leads to pH increase and respiratory alkalosis, which is an alteration of the acid-base homeostasis [8,9]. Acid–base disorders are classified as either respiratory or metabolic, depending on the PaCO2 and the bicarbonate ions (HCO3−) levels in physiological fluids [10]. The insurgence of respiratory alkalosis during SARS-CoV-2 infection has been widely related to the aggravation of patient clinical outcomes [[11], [12], [13], [14]].
The compensatory ventilatory response to hypoxemia leads to an excessive expulsion of CO2, a state called hypocapnia [15,16]. This condition can reduce respiratory stimuli, and, together with other factors such as fever, can cause an alteration of dyspnoea perception, a condition called ‘silent hypoxia’ [8,17,18]. Silent hypoxia can explain the worsening of hypoxemia in the presence of normal or even low carbon dioxide levels [8,17]. Another factor that regulates the respiratory stimuli is the PaO2 but not oxygen saturation (SaO2) [19]. Thus, mechanisms able to shift the hemoglobin saturation curve can affect oxygen availability without affecting the respiratory stimuli.
The 2,3-BPG is a metabolic intermediate of the Luebering-Rapoport glycolytic pathway, which occurs in the erythrocyte and regulates the oxygen release from hemoglobin to tissues. The 2,3-BPG preferentially binds deoxyhaemoglobin facilitating the oxygen release, thus being essential for the physiological adaptation to oxygen deprivation, such as during hypoxic states [20].
Hypoxia is one of the main pathological features that characterized COVID-19, and the modulation of PaO2 as well as of PaCO2 and pH during the infection progression have been widely investigated [21,22]. However, to the best of our knowledge, few data are currently available regarding the modulation of 2,3-BPG in SARS-CoV-2 affected patients at the hospital admission [23,24]. Thus, the present study aims to investigate possible changes on the oxygen homeostasis-related parameters (pH, PaCO2, haemoglobin and 2,3-BPG levels) in relation to lung involvement measured by CT, oxygenation, and acid-base states of SARS-CoV-2 positive patients at hospital admission. Overall, the results might help to improve our understanding of the COVID-19 infection hopefully improving diagnostic capabilities.
2. Materials and methods
2.1. COVID-19 patients: recruitment and inclusion criteria
The current study includes 68 COVID-19 positive patients with symptoms, all recruited from the “Emergency Medicine and Emergency Room Operating Unit” of Azienda Ospedaliera Universitaria Pisana (AOUP) and admitted at Emergency Department of the Pisa Hospital from May 2021 to September 2022. The SARS-CoV-2 positive infection was assessed by virus nucleic acid amplification through RT-PCR whereas pulmonary involvement was revealed by chest CT.
The study procedures were approved by the local Ethical Committee (Protocol number 19275, February 25, 2021) and the Great North West Area of Tuscany and were in accordance with the provisions of the Declaration of Helsinki. All participants gave written informed consent for the use of their clinical data and blood samples for research purposes.
2.2. CALIPER software for COVID-19 patients’ chest-CT analysis
The automatic software CALIPER, based on a texture analysis of chest-CTs, has been already approved for clinical trials and validated to assess the severity and progression of idiopathic pulmonary fibrosis (IPF) [4] as well as COVID-19 mortality [5]. CALIPER is a software developed by Mayo Clinic, whose licence is provided to “IMBIO” under the name LUNG TEXTURE ANALYSIS™. All the chest-CTs of recruited patients were anonymized and analysed with CALIPER software, by performing a segmentation of the right and left lungs followed by the texture analysis of interstitial lung abnormalities. The total interstitial lung abnormalities indicated as CALIPER ILD, represent the parenchymal involvement ratio considering ground glass and reticulation areas percentage of total lung volume, while the vascular-related structures, indicated by CALIPER VRS, define the ratio of total vessel volume divided by the total lung parenchymal volume. The chest-CT scan with severe motion artifacts or not technically adequate CALIPER CT segmentations were excluded.
2.3. Blood sampling and separation of erythrocytes from plasma
Routine and non-routine blood clinical analysis were performed at hospital admission for each patient. Specifically, haemoglobin (Hb) concentrations were evaluated in the whole blood, while PaO2, PaCO2, pH, and HCO3− were assessed by arterial blood gas analysis. Peripheral oxygen saturation was obtained with a commercial oximeter. The RBCs and plasma were separated at the “Unità Operativa Biobanche” of the AOUP by sequential centrifugations and stored at −80°, within 4 h from the sample's collection.
2.4. 2,3-BPG quantification in erythrocytes
The detection and quantification of 2,3-BPG was performed by a competitive enzyme-linked immunosorbent assay (ELISA) (COD.MBS288269-96, MyBioSource). The assay was performed on each patient's erythrocytes stored at −80°, within 3 months from the collection. Briefly, lyophilized 2,3-BPG was reconstituted, and serial dilutions were prepared to create the standard calibration line (standard concentrations: 2000-1000-500-250-125-62.5-31.2-0 nmol/mL). Therefore, standards, blank, and samples were added to each well of a 96-well plate precoated with antibody specific to target the 2,3-BPG antigen and the biotin-labelled target antigen (reagent A) was immediately added, and the plate was incubated for 1 h at 37 °C into a thermomixer (Eppendorf), under soft continuous shaking. Then, wells were washed to remove excess conjugate and unbound antigen; then, avidin conjugated to horseradish peroxidase (HRP) (reagent B) was added in each well and incubated for 45 min at 37 °C. Finally, the substrate reagent tetramethylbenzidine (TMB) was added and the reaction was stopped after 20 min of incubation at 37 °C by adding the acid solution. The absorbance was measured spectrophotometrically at a wavelength of 450 nm and the sample concentrations of 2,3-BPG were determined through interpolating the absorbances into the standard curve.
2.5. Statistical analysis
The statistical analysis and graphical presentations were performed by GraphPad Prism (version 8.0, Graphpad Software Inc., San Diego, California). The D'Agostino & Pearson test was carried out to assess the normal distribution of data. Since data did not assume a Gaussian distribution, the correlations between the selected parameters were performed with nonparametric Spearman correlation. The strength of each correlation was evaluated by the coefficient of correlation (r): r = 0.00–0.19 indicates a very weak correlation, r = 0.20–0.39 indicates a weak correlation, r = 0.40–0.59 indicates a moderate correlation, r = 0.60–0.79 indicates a strong correlation, and r = 0.80–1.00 indicates a very strong correlation [25]. A positive or negative coefficient of correlation suggests a direct or indirect correlation between the two considered parameters, respectively. For column analysis, Mann-Whitney test and unpaired t-test were used and a p-value <0.05 was considered statistically significant. All data were shown as mean ± SD.
3. Results
3.1. Demographic and clinical data
The demographic and clinical characteristics of the study cohort are reported in [Table 1]. Briefly, 68 COVID-19 patients were enrolled, composed by 25 (41.33%) women, with a mean age of 67 years (range 27–89). The percentage of the elderly patients (>67 years) was 57%. Among them, 30 (44.1%) were fully vaccinated (2 doses), and 6 (8.8 %) were partially vaccinated (1 dose). In addition, 18 (26.5 %) of them were ex-smokers and only 3 (4.4 %) patients were smokers. The most common comorbidities of the analysed cohort were hypertension (50.0 %), followed by cardiovascular risk (42.6 %), dyslipidaemia (22.1%), solid and blood tumours (17.2 % and 14.7 %, respectively) and COPD (16.2%). During the hospital admission, the most common symptoms were fever, cough, dyspnoea, and asthenia. Thus, 50 (73.5 %) patients received O2 therapy in the emergency room, while 59 (86.8 %) patients needed O2 therapy during hospitalization; among them, 15 (22.1%) underwent non-invasive ventilation (NIV) (i.e. bilevel or continuous positive airway pressure, BPAP or CPAP, respectively) or nasal canula (NC) high flux therapy. COVID-19 patients were mainly treated with oral or intravenous corticosteroid (hydrocortisone or dexamethasone), antiviral (remdesivir, tocilizumab, and baricitinib), and antithrombotic therapy.
Table 1.
Demographic and clinical data.
| Demographic data | Mean (%) |
|---|---|
| Age, yrs | 67 ± 16.48 |
| Sex (F) | 25 (36.8) |
| Clinical data | |
| Smokers | 3 (4.4) |
| Ex-smokers | 18 (26.5) |
| Vax-Complete cycle | 30 (44.1) |
| Vax-Incomplete cycle | 6 (8.8) |
| Hypertension | 34 (50.0) |
| CV pathology | 29 (42.6) |
| Asthma | 8 (11.8) |
| COPD | 11 (16.2) |
| Ictus | 4 (5.9) |
| Cerebrovascular pathology | 11 (16.2) |
| Dementia | 2 (2.9) |
| Obesity | 12 (17.2) |
| Dyslipidaemia | 15 (22.1) |
| Solid cancer | 12 (17.2) |
| Haematologic cancer | 10 (14.7) |
| Symptoms | |
| Fever | 41 (60.3) |
| Dyspnoea | 36 (52.9) |
| Cough | 25 (36.8) |
| Therapy | |
| O2in emergency room | 50 (73.5) |
| O2at Hospital | 59 (86.8) |
| NIV therapy (BPAP or CPAP) | 15 (22.1) |
| CN high flux | 15 (22.1) |
| Corticosteroids | 62 (91.2) |
| Antithrombotics | 53 (77.9) |
| Antiviral | 25 (36.8) |
| Outcome | |
| IUC/Sub-IUC | 18 (26.5) |
| Respiratory failure | 52 (76.5) |
| Facility discharge | 15 (22.1) |
| Respiratory supports at the discharge time | 10 (14.7) |
| Bacterial over-infection | 25 (36.8) |
| Thrombotic complications | 5 (7.4) |
Abbreviations: F:female;COPD:Chronic obstructive pulmonary disease;CV pathology:cardio-vascular pathology;NIV: non-invasive ventilation;BPAP:bilevel positive airway pressure;CPAP:Continuous Positive airway Pressure;CN:nasal canula;Corticosteroids used: dexamethasone, hydrocortisone, methylprednisone. The most used was dexamethasone; Antithrombotic therapy: New oral anticoagulants (NAO), Clexane, Coumadin, Arixtra; IUC/Sub-IUC, intensive unit care/sub-intensive unit care; The most used was Clexane. Data are presented as mean ± S.D or number (%, percentage).
Among the 68 COVID-19 patients, 18 (26.5 %) required Intensive Care Unit or sub–Intensive Care Unit (ICU) admission. Furthermore, 5 (7.4 %) patients had thrombotic complications, while 25 (36.8 %) had bacterial over-infections. At the time of discharge, 15 (22.1%) patients were transferred to assisted living facilities. Among all patients, 10 (14.7 %) required oxygen therapy at discharge. Only four patients died (5%).
3.2. Increased ILD and VRS CALIPER values correlate with longer hospitalization time
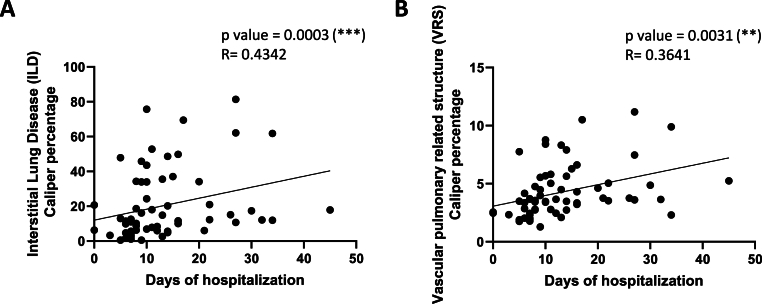
The possible association between ILD and VRS CALIPER with the disease severity were analysed in our patient cohort, assuming the hospitalization days as an index of the severity of COVID-19 pathology [Fig. 1]. The obtained results highlighted a direct correlation between ILD or VRS CALIPER and the hospitalization days ([Fig. 1A], p = 0.0003, R = 0.4342; [Fig. 1B], p = 0.0031, R = 0.3641), confirming these parameters as predictors of the disease severity.
Fig. 1.
CT parameters correlate with disease severity. Correlation between interstitial lung disease (ILD) (A) and vascular pulmonary related structure (VRS) (B) with days of hospitalization. The correlation between variables was determined by Spearman analysis, using GraphPad Prism 8. P and R values obtained for each correlation are reported in the respective panel.
3.3. Lower partial oxygen pressure at hospital admission was revealed in COVID-19 patients presenting pulmonary abnormalities and increased hospitalization time
The relationship between CALIPER ILD and VRS and the arterial partial oxygen pressure (PaO2) was then investigated. Indeed, COVID-19 infection has been related to the reduction of oxygen loading into the blood as a consequence of the gas exchange impairment [15,17]. As expected, CALIPER ILD and VRS values were inversely correlated with PaO2, however, only a weak correlation was obtained (p = 0.046, R = −0.2646 and p = 0.0449, R = −0.2640, respectively; data not shown).
To better investigate this relationship, the 68 patients were divided into two subgroups according to the median PaO2 value (67 mmHg). Most of the patients had a PaO2 value under the normal range indicating the insurgence of hypoxemia. As shown in [Table 2], patients belonging to the two subgroups had similar ages (p = 0.640) with a percentage of elderly patients of 53% and 62%, respectively. The two groups were sex-matched (p = 0.874); no significant differences were revealed considering the vaccination status (p = 0.775 for complete cycle and p = 0.296 for incomplete cycle) and most patients were no smokers. In the two subgroups, the patients showed no differences in symptomatic manifestation as fever (p = 0.882) and cough (p = 0.175), even if patients with lower PaO2 had higher dyspnoea (p = 0.038). Chronic obstructive pulmonary disease (COPD), asthma and obesity comorbidities presented the same percentages of incidence in the two subgroups (p = 0.958; p = 0.423; p = 0.171, respectively). Finally, patients with lower PaO2 received more oxygen treatments in the emergency room (p = 0.0072) and during hospitalization (O2, NIV therapy and CN high flux, p = 0.0013, p < 0.0001 and p = 0.0069, respectively).
Table 2.
Demographic and clinical data differences between the two patients’ subgroups based on PaO2 median value.
| Characteristics | PaO2 < 67 (n = 34) | PaO2 ≥ 67 (n = 34) | p-value |
|---|---|---|---|
| Demographic data | Mean (%) | Mean (%) | |
| Age, yrs | 66 ± 17.54 | 69 ± 1 5.50 | 0.640 |
| Sex (F) | 13 (38.2) | 12 (35.3) | 0.874 |
| Clinical data | |||
| Smokers | 2 (6.0) | 1 (3.0) | 0.502 |
| Ex-smokers | 9 (26.0) | 8 (24.0) | 0.559 |
| Vax-Complete cycle | 14 (41.2) | 16 (47.1) | 0.775 |
| Vax-Incomplete cycle | 2 (5.9) | 4 (11.8) | 0.296 |
| Hypertension | 18 (52.9) | 16 (47.1) | 0.714 |
| Asthma | 3 (8.8) | 5 (14.7) | 0.423 |
| COPD | 5 (14.7) | 5 (14.7) | 0.958 |
| Obesity | 8 (23.5) | 4 (11.8) | 0.171 |
| Symptoms | |||
| Fever | 20 (58.8) | 20 (58.8) | 0.882 |
| Dyspnoea | 22 (64.7) | 13 (38.2) | 0.038 |
| Cough | 10 (29.4) | 15 (44.1) | 0.175 |
| O2 therapy | |||
| O2in emergency room | 31 (91.2) | 19 (55.9) | 0.0072 |
| O2at Hospital | 34 (100.0) | 25 (74.5) | 0.0013 |
| NIV therapy (BPAP or CPAP) | 15 (44.1) | 0 (0.0) | < 0.0001 |
| CN high flux | 12 (35.3) | 3 (8.8) | 0.0069 |
| Outcome | |||
| IUC/Sub-IUC | 15 (44.1) | 3 (8.8) | 0,0010 |
| Respiratory failure | 32 (94.1) | 20 (58.8) | 0.0006 |
| Respiratory supports at the discharge time | 32 (94.1) | 24 (70.6) | 0.0015 |
Abbreviations:PaO2: arterial partial oxygen pressure; F female; COPD Chronic obstructive pulmonary disease; NIV, non-invasive ventilation; BPAP, bilevel positive airway pressure; CPAP, Continuous Positive airway Pressure; CN, nasal canula; IUC/Sub-IUC, intensive unit care/sub-intensive unit care. Data are presented as mean ± S.D or number (%, percentage). Differences between groups were evaluated by a non-parametric test (Mann-Withney test) and contingency test (Chi-sqaure test): ∗p < 0.05, ∗∗p < 0,01; ∗∗∗p < 0,001; ∗∗∗∗p < 0.0001.
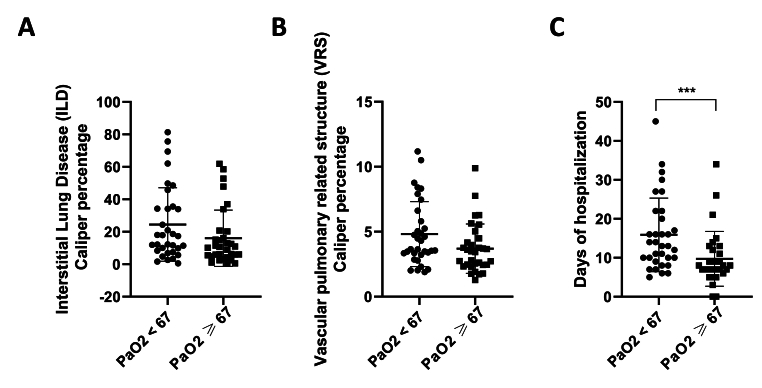
The CALIPER ILD and VRS values were assessed in the two groups [Fig. 2]. The obtained data showed a trend for an increase ([Fig. 2A], p = 0.0690; [Fig. 2B], p = 0.0695) of CALIPER ILD and VRS percentages in patients with lower PaO2. Furthermore, the hypoxemic patients had a longer hospitalization time ([Fig. 2C], p = 0.0009) in accordance with the higher values of ILD and VRS CALIPER. These results support in part the association existing between the extension of lung damage and the reduction of blood oxygenation; nevertheless, the aforementioned weak correlation between CALIPER ILD/VRS and the PaO2 suggests the involvement of other factors related to oxygen availability.
Fig. 2.
Lung involvement increased in patients with low PaO2 and correlated with a longer hospitalization period. Patients were subdivided into two groups according to the median PaO2 value. Caliper ILD and VRS percentages were obtained using CALIPER software for CT analysis and were expressed as percentages. ILD (A) and VRS (B) CALIPER parameters and days of hospitalization (C) in patients with lower and higher PaO2 than 67 mmHg, respectively. Data are reported as mean ± SD. Differences between groups were evaluated by an un-parametric test (Mann-Withney test): ∗∗∗ p < 0.001 vs. PaO2 ≥ 67. GraphPad Prism 8 was used to create the figure.
3.4. Lower partial oxygen pressure in COVID-19 patients was related to altered hemoglobin oxygenation parameters
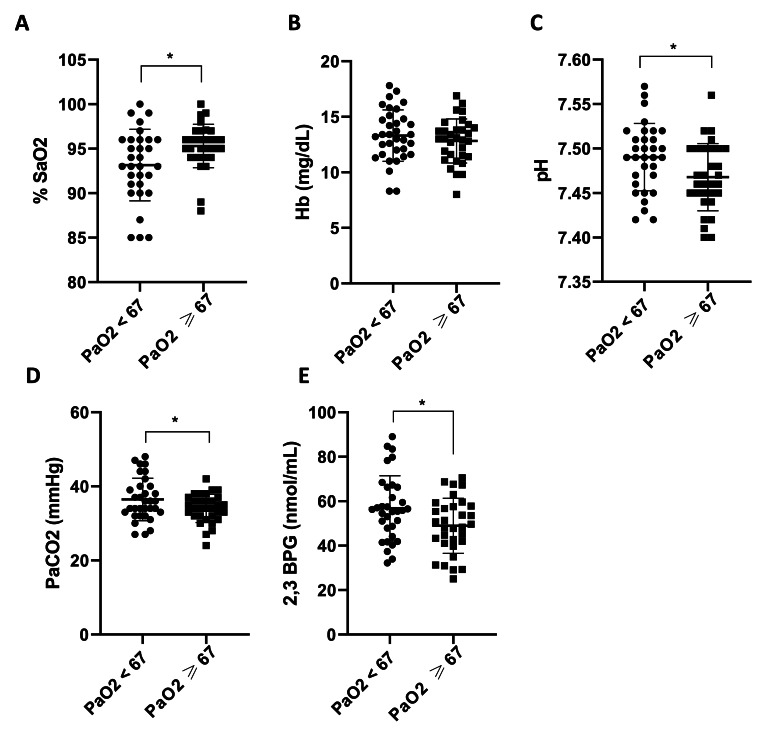
Besides the lung abnormalities, other factors can be implicated in the oxygen availability. Thus, the oxygen saturation percentage (%SaO2), the hemoglobin levels, and factors influencing the Hb-O2 dissociation (arterial blood gas analysis pH, PaCO2, and erythrocytes 2,3-BPG levels) were investigated in the two subgroups of patients [Fig. 3].
Fig. 3.
Several blood oxygenation parameters were altered in patients with lower PaO2. Oxygen saturation (SaO2) (A), haemoglobin (Hb) (B), pH (C), PaCO2 (D) and 2,3-BPG (E) levels in the two patient subgroups, accordingly to the PaO2 median value. The oxygen saturation was obtained with the oxymeter and the PaCO2 and pH by emo-gas analysis. 2,3-BPG levels were assessed by specific immunoenzymatic assays in RBCs of COVID-19 patients and reported here expressed as nmol/mL. Data are reported as mean ± SD. Differences between groups were evaluated by a non-parametric test (Mann-Withney test) and parametric test (Unpaired-t-test): ∗ p < 0.05 vs. PaO2 ≥ 67. GraphPad Prism 8 was used to create the figure.
As expected, the oxygen saturation (SaO2) was significantly decreased in COVID-19 patients with lower PaO2 (Fig. 3A, p = 0.0172), indicating decreased hemoglobin binding sites occupied by oxygen, although no differences in hemoglobin (Hb) concentrations were revealed (Fig. 3B, p = 0.364). In accordance with these data, arterial blood gas analysis revealed that pH and PaCO2 levels were increased in patients with lower PaO2 (Fig. 3C, p = 0.0208; Fig. 3D, p = 0.0496), suggesting the presence of acid-base imbalance. Finally, significantly higher 2,3-BPG levels were reported in patients with lower PaO2 (Fig. 3E, p = 0.0221). The 2,3-BPG mean value was 56.84 ± 14.61 nmol/mL for patients with lower PaO2 and 48.98 ± 12.38 nmol/mL for patients with higher PaO2.
3.5. SARS-CoV-2 infection leads to alkalemia and alkalosis onset
The herein presented data suggests a blood acid-base disequilibrium in COVID-19 patients. In fact, the majority of the patients presented high blood pH values, i.e. alkalosis in accordance with literature data [10]. To deeply investigate the effect of the alkalosis state on oxygen saturation (%SaO2) and the parameters influencing the O2 binding/release equilibrium in blood, the cohort of patients (68 patients) was divided into three groups based on the pH values, considering that the normal blood pH range is 7.35–7.45. Patients with pH values lower or equal to 7.45 were classified as “non-alkalosis”, while those presenting values higher than 7.45 were classified as “alkalosis”. Then, the patients included in “alkalosis” group were furthered classified based on PaCO2 blood concentrations. In particular, patients with PaCO2>35 mmHg were classified as “metabolic alkalosis” patients, while those with PaCO2<35 mmHg as “respiratory alkalosis” patients [26]. To confirm the metabolic alkalosis, the bicarbonate levels in the three groups were evaluated and a significant increase was found in the group of patients with metabolic alkalosis (25.6–40.3 mEq/L) compared to control and respiratory alkalosis ones. In the last group, the bicarbonate levels were normal or slightly lower than physiological values (21.5–27.8 mEq/L) (p < 0.0001, data not shown).
As shown in [Table 3], patients belonging to the three groups have similar ages (p = 0.778) with a percentage of elderly patients of 59%, 65%, and 52%, respectively. The three groups did not differ significantly in sex (p = 0.181). In relation to symptoms, no differences in the manifestation of dyspnoea (p = 0.250) fever (p = 0.861) and cough (p = 0.480) were found in the three groups. About electrolyte levels, all groups have the same sodium and creatine levels but different levels of potassium, which appears to be significantly lower in the group with metabolic alkalosis than in the group without acid-base alterations (p = 0.0404 (no alkalosis vs. metabolic alkalosis); 0.79 (no alkalosis vs. respiratory alkalosis); 0.13 (metabolic vs. respiratory alkalosis).
Table 3.
Demographic and clinical data in the three patient's groups, obtained considering the acid-base alterations.
| Characteristics | No alkalosis (n = 22) | Metabolic alkalosis (n = 17) | Respiratory alkalosis (n = 29) | p-value |
|---|---|---|---|---|
| Demographic data | Mean (%) | Mean (%) | Mean (%) | |
| Age, yrs | 68 ± 16.67 | 65 ± 19.91 | 68 ± 14.50 | 0.778 |
| Female | 6 (27.3) | 10 (58.8) | 9 (51.0) | 0.181 |
| Clinical data | ||||
| Smokers | 1 (4.5) | 2 (11.8) | 0 (0.0) | 0.0801 |
| Ex-smokers | 10 (45.5) | 3 (17.8) | 4 (13.8) | 0.0260 |
| Vax-Complete cycle | 14 (41.0) | 16 (47.0) | 0.482 | |
| Vax-Incomplete cycle | 2 (9.1) | 1 (5.9) | 3 (10.3) | 0.856 |
| Hypertension | 9 (40.9) | 8 (47.1) | 14 (48.3) | 0.612 |
| Asthma | 2 (9.1) | 1 (5.9) | 4 (13.8) | |
| COPD | 6 (27.3) | 2 (11.8) | 2 (6.9) | 0.0476 |
| Obesity | 5 (22.7) | 2 (11.8) | 5 (17.2) | 0.748 |
| Sodium (mg/dL) | 131.6 ± 28.21 | 137.5 ± 3.46 | 136,3 ± 3.79 | 0.424 |
| Potassium (mg/dL) | 4.2 ± 0.45 | 3.8 ± 0.51 | 4.0 ± 0.37 | 0.0360 |
| Creatinina (mg/dL) | 1.5 ± 1.29 | 0.9 ± 0.23 | 1.1 ± 1.01 | 0.204 |
| Symptoms | ||||
| Fever | 13 (59.1) | 8 (47.1) | 19 (65.5) | 0.861 |
| Dyspnoea | 10 (45.5) | 10 (58.8) | 16 (55.2) | 0.250 |
| Cough | 10 (45.5) | 6 (35.3) | 9 (31.0) | 0.480 |
| O2 therapy | ||||
| O2in emergency room | 16 (72.7) | 14 (63.6) | 19 (86.4) | 0.285 |
| O2at Hospital | 19 (86.4) | 14 (82.4) | 25 (86.2) | 0.650 |
| NIV therapy (BPAP or CPAP) | 4 (18.2) | 5 (29.4) | 5 (17.2) | 0.401 |
| CN high flux | 4 (18.2) | 4 (23.5) | 6 (20.7) | 0.647 |
| Outcome | ||||
| IUC/Sub-IUC | 4 (18.2) | 5 (29.4) | 8 (27.6) | 0.568 |
| Respiratory failure | 19 (86.4) | 12 (70.6) | 20 (69.0) | 0.238 |
| Respiratory supports at the discharge time | 13 (59.1) | 9 (40.9) | 16 (72.7) | 0.658 |
Abbreviations: F:female;COPD Chronic obstructive pulmonary disease;NIV:non-invasive ventilation;BPAP:bilevel positive airway pressure;CPAP:Continuous Positive airway Pressure;CN"nasal canula;IUC/Sub-IUC:intensive unit care/sub-intensive unit care. Sodium, potassium and creatinine levels were obtained by routine blood analysis. Data are presented as mean ± S.D or number (%, percentage). Differences between groups were evaluated by One-Way ANOVA non-parametric test (Kruskal-Wallis test): ∗ p < 0.05.
3.6. Oxygenation parameter change in relation to the acid-base alteration
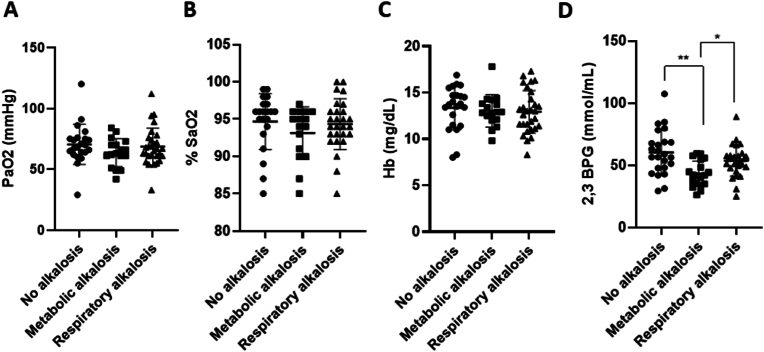
Firstly, the PaO2 and SaO2 trends in the three subgroups were evaluated showing no appreciable differences ([Fig. 4A], p = 0.420 (no alkalosis vs. metabolic alkalosis; 0.956 (no alkalosis vs. respiratory alkalosis); 0.649 (metabolic vs. respiratory alkalosis); [Fig. 4B], p = 0.480 (no alkalosis vs. metabolic alkalosis); 0.984 (no alkalosis vs. respiratory alkalosis); 0.638 (metabolic vs. respiratory alkalosis)). Also, no hemoglobin differences were reported, too ([Fig. 4C], p = 0.955 (no alkalosis vs. metabolic alkalosis); 0.867 (no alkalosis vs. respiratory alkalosis); 0.998 (metabolic vs. respiratory alkalosis)). Nevertheless, patients without alkalosis surprisingly showed significantly higher 2,3-BPG levels compared to patients with metabolic alkalosis; whereas comparable levels were found between the latter group compared to respiratory alkalosis patients ([Fig. 4D], p = 0.0012 (no alkalosis vs. metabolic alkalosis); p = 0.623 (no alkalosis vs. respiratory alkalosis). Interestingly, patients with metabolic alkalosis displayed a significant decrease of 2,3-BPG concentrations compared to those with respiratory alkalosis ([Fig. 4D], p = 0.0383 (metabolic vs. respiratory alkalosis)).
Fig. 4.
2,3-BPG levels were altered in the alkalosis state. PaO2 (A), %SaO2 (B), hemoglobin (C), and 2,3-BPG (D) levels in patients without acid-base imbalance and with metabolic and respiratory alkalosis. Patients were classified by pH and PaCO2 levels. The PaO2 was obtained by emo-gas analysis, while SaO2 by the oximeter. 2,3-BPG levels were assessed by specific immunoenzymatic assays in RBCs of COVID-19 patients and reported here expressed as nmol/mL. Data are reported as mean ± SD. Differences between groups were evaluated by One-Way ANOVA non-parametric test (Kruskal-Wallis test): ∗ p < 0.05; ∗∗p < 0.01 vs. metabolic alkalosis group. GraphPad Prism 8 was used to create the figure.
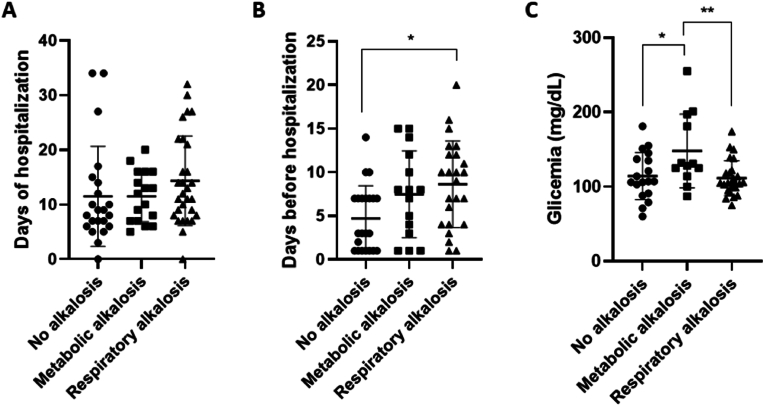
Furthermore, to investigate possible differences in lung involvement among the three groups, abnormalities of lung parenchyma and vasculature were evaluated. Of note, no significant changes were found in the three groups considering the CALIPER ILD and VRS, since they displayed comparable percentages (data not show; CALIPER ILD p = 0.975 (no alkalosis vs. metabolic alkalosis); p = 0.975 (no alkalosis vs. respiratory alkalosis), p > 0.99 (metabolic vs. respiratory alkalosis); CALIPER VRS p > 0.999 (no alkalosis vs. metabolic alkalosis); p > 0.999 (no alkalosis vs. respiratory alkalosis); p > 0.999 (metabolic vs. respiratory alkalosis)). Then, the hospitalization days after the diagnosis were considered depending on the alkalosis state. Results showed an increased trend of hospitalization time in patients with the alkalosis state with respect to the no alkalosis group ([Fig. 5A]; Days of hospitalization p > 0.999 (no alkalosis vs. metabolic alkalosis); p = 0.212 (no alkalosis vs. respiratory alkalosis); p > 0.999 (metabolic vs. respiratory alkalosis)). Of note, the patients showing a respiratory alkalosis state spent significantly more time at home before the hospitalization ([Fig. 5B], p = 0.205 (no alkalosis vs. metabolic alkalosis); p = 0.0159 (no alkalosis vs. respiratory alkalosis); p = > 0.999 (metabolic vs. respiratory alkalosis)).
Fig. 5.
Alkalosis state increased the time spent at home before hospitalization and was related to altered glycemia. Days of hospitalization (A), days spent at home before hospitalization (B) and glycemic levels (C) in patients without acid-base imbalance and with metabolic and respiratory alkalosis. Glycemia was measured by routine blood analysis. The data are showed mean ± SD. Differences between groups were evaluated by One-Way ANOVA non-parametric test (Kruskal-Wallis test): ∗ p < 0.05; ∗∗p < 0.01 vs. metabolic alkalosis group. GraphPad Prism 8 was used to create the figure.
The production of 2,3-BPG could be affected by several mechanisms such as pH and glycolysis rate [7], to shed light on the mechanisms regulating its synthesis in the different acid-base conditions, the glycemia was investigated [Fig. 5C]. Interestingly, the blood glucose levels were significantly higher in patients with metabolic alkalosis than those without alkalosis as well as compared to patients with respiratory alkalosis ([Fig. 5C], p = 0.0260 (no alkalosis vs. metabolic alkalosis); 0.992 (no alkalosis vs. respiratory alkalosis); 0.0089 (metabolic vs. respiratory alkalosis)).
4. Discussion
The study aimed to improve the knowledge about oxygen homeostasis in SARS-CoV-2 positive patients at hospital admission, investigating the parameters that affect the hemoglobin saturation curve (pH, PaCO2, and 2,3-BPG levels) in relation to patient blood oxygenation and acid-base state. The main results are: 1) a higher percentage of ILD and VRS volume, measured by the validated CALIPER software for chest-CT analysis in those COVID-19 patients who have a worse disease outcome represented by their higher number of hospitalization days; 2) patients with lower PaO2 showed an imbalanced acid-base equilibrium and a higher 2,3-BPG levels; 3) 2,3-BPG levels were lower in patients with metabolic alkalosis.
To the best of our knowledge, the results underlined, for the first time, an alteration of erythrocyte 2,3-BPG concentrations in COVID-19 patients, especially in relation to lower oxygen availability and acid-base imbalance.
Lungs are the main organ affected by SARS-CoV-2 infection [27] and chest-CT represents the gold standard method to evaluate the extent of pulmonary involvement in COVID-19 patients [28]. Despite its prognostic value, lung involvement assessment on CT is a qualitative analysis subjected to the inter-variability related to the operator [29]. Thus, CALIPER software has been developed to overcome this limit by quantifying parenchymal and vascular lung abnormalities [30]. Notably, CALIPER software has been recently validated for CT-chest analysis correlating ILD and VRS with an increasing trend in relation to the intensity of care. Furthermore, the increase of VRS parameters has been related to a higher mortality in COVID-19 patients [5]. In accordance with these data, herein, CALIPER ILD and VRS positively correlated to the hospitalization time that could be considered a parameter of COVID-19 severity. Since the beginning of the pandemic, patient death has been widely considered as a primary outcome to assess the COVID-19 severity, however, other outcomes have been proposed such as the hospitalization days [31]. Of note, the days of hospitalization could better describe the characteristics of the most recent pandemic waves, in which a decrease in mortality is evidenced and the severity of the disease passes through the clinical complexity of older or more fragile patients.
The SARS-CoV-2 infection leads to the alteration of alveolar structure, resulting in deep alveolar epithelial and capillary endothelial damage with alteration of vasoregulation, ventilation/perfusion (V/Q) mismatching, accelerated thrombogenesis, and edema [8,18]. These abnormalities drive a progressive reduction of blood oxygenation that represents the primary COVID-19 pathophysiologic feature, which characterizes all stages of the disease and mainly contributes to mortality in patients [32]. Although the reduction of oxygen levels is a direct consequence of lung involvement [2], our data showed only a weak correlation between the lung abnormalities, measured by CALIPER ILD and VRS percentages parameters, and PaO2 levels in the COVID-19 patients. This could be ascribed to a variable time between the symptom presentation and the hospital admission resulting in the observation of the pathophysiological phenomenon at different stages of the disease.
A progressive reduction of PaO2 is a common feature in early COVID-19 pneumonia, often in the absence of an appropriate shortness of breath [33]. On the other hand, PaO2 has been proposed as a prognosis marker of COVID-19 patients, and the percentage of oxygen saturation (%SaO2) less than 93% has been associated with hospitalized patients’ mortality [34]. In accordance, herein, patients with lower PaO2 values had a significant increase in hospitalization days, highlighting the hypoxemia role in contributing to worsening the COVID-19 outcome.
Actually, besides the direct lung injury involvement in SARS-CoV-2 infection, the blood oxygen levels and tissue oxygenation could be influenced by other factors, contributing to oxygen homeostasis dysregulation and hypoxemia/hypoxia development. Although the terms hypoxemia and hypoxia are often used interchangeably, they indicate two different abnormal oxygenation states. Indeed, hypoxemia is defined as a condition characterized by lower PaO2 than normal levels (80–100 mmHg) and it depends on the oxygen content of inspired gas. On the other hand, hypoxia is defined as the failure of oxygenation at the tissue level. Nevertheless, generally, the presence of hypoxemia suggests hypoxia, they may or may not occur together at the same level [35,36].
Besides PaO2, several other known parameters, such as PaCO2, pH, temperature, and other intra-erythrocytic factors, could affect oxygen availability, modifying the hemoglobin oxygen dissociation curve (ODC) [22]. Among intraerythrocytic factors, 2,3-BPG plays a crucial role [7], it binds hemoglobin in a 1:1 ratio, leading to a decrease of the oxygen affinity to hemoglobin, resulting in an ODC shift to the right and consequent increase of oxygen release at peripheral levels [37]. The synthesis and breakdown of 2,3-BPG is controlled by the phosphoglycerate cycle of Luebering–Rapoport; under physiological conditions, 2,3-BPG concentrations are controlled by a feedback loop involving the same 2,3-BPG concentration or rather by the phosphates and hydrogen ions concentration [7]. Indeed, the variation of plasma pH could influence the 2,3-BPG concentration. Moreover, a significant increase of erythrocyte 2,3 BPG concentration was related to the increase in erythrocytes phosphates concentration. This correlation may be related to the influence of RBCs glycolytic rate and or increased Pi shifting in GAPDH equilibrium with consequent increase of 1,3-BPG concentration [38,39]. Herein, despite similar hemoglobin levels, an increase of the 2,3-BPG levels has been observed for the first time in COVID-19 patients with lower PaO2, confirming the activation of compensatory mechanisms in order to guarantee the correct tissue oxygenation. Of note, few data have been reported in the literature regarding the changes of 2,3-BPG in COVID-19 patients. Thomas et al. measured an increase of 2,3-BPG concentrations in COVID-19 patients with respect to healthy subjects, however, only relative levels are reported [40]. Of note, it has been reported that the Rapoport-Luebering shunt decreases simultaneously with a decrease in 2,3-BPG concentration in RBCs in the elderly [41,42]. In our cohort of subjects, the distribution of elderly patients in the two groups did not differ significantly, however, a possible impact of the age in the modulation of the 2,3-BPG could not be excluded.
On the other hand, also extra-erythrocytic factors such as pH and PaCO2 levels can shift the ODC. The effect of hydrogen ions on oxygen affinity is called the “Bohr effect”. Specifically, an increase in hydrogen ions (lower pH) concentration leads to a right ODC shift. On the other hand, carbon dioxide can affect oxygen affinity in two ways: through the Bohr effect and the accumulation of carbamino compounds that are generated by chemical interactions, which in turn stabilizes the T hemoglobin state [43]. However, the majority of carbon dioxide enters red blood cells and is quickly converted to carbonic acid, which dissociates into bicarbonate and hydrogen ions, stabilizing the ODC right shift [43]. During the COVID-19 pandemic outbreak, several studies tried to investigate the alteration of ODC (reviewed in [22]). However, most of these studies lack the evaluation of all the main parameters affecting the ODC [44]. Only a few studies measured the complete ODCs, both using the automatic Hemox Analyzer, or using fixed values. Such as Ceruti et al. which set the 2,3-BPG concentration at a fixed value of 4.65 mmol/L [23]. Despite this limitation, they found a left shift of the ODC during the hospital stay, causing a significant decrease of p50 (the partial oxygen tension at which hemoglobin is saturated at 50%) during the first hospitalization day of critically ill COVID-19 [37]. Furthermore, Daniel et al. find a p50 rather higher (29.0 ± 2.3 mmHg) in 14 COVID-19 patients, but not significantly different from a control group of 11 patients with unknown diagnosis (28.5 ± 1.8 mmHg) [24]. Thus, to date, a deeper investigation of all the parameters affecting hemoglobin-oxygen affinity is pivotal.
In our cohort, lower PaO2 is associated with higher PaCO2 partial pressure and higher pH levels, suggesting a variation in oxygen affinity and an acid-base imbalance. Starting from these results, we investigated the onset of acid-base disorders in the recruited patients, considering pH, PaCO2, and bicarbonate values obtained by emo-gas analysis performed at hospital admission. Within the population, approximately two-thirds presented an alkalosis state, detected by pH alterations (greater than 7.45). When the acid-base disorder is caused by an abnormality in respiratory function, a decrease of PaCO2 occurs, and the condition is classified as “respiratory” alkalosis, while when the primary change is ascribed to alterations of bicarbonate concentrations it is defined as “metabolic” alkalosis [14]. Herein, in the alkalosis group, low PaCO2 was revealed in 43% of patients, indicating a respiratory alkalosis state. The remaining 25% is affected by metabolic alkalosis, confirmed by higher bicarbonate levels. In accordance with our results, respiratory and metabolic alkalosis has been found as the most frequent acid-base disorders during COVID-19 disease [11,13], which have been associated with increased mortality [19,45,46]. On the other hand, also mixed respiratory and metabolic acidosis has been recently related to a higher risk factor for mortality [26]. As well as metabolic acidosis [47] evidencing the need for more data to better explain the role of acid-base alteration in COVID-19 patient outcomes.
Respiratory alkalosis state usually occurs secondary to hyperventilation, in response to the hypoxic state. At the early stage, the hypocapnia resulting from hyperventilation leads to a depression of the respiratory stimuli, and dyspnoea could be not felt or be absent. The worsening of hypoxemia leads to a state called “silent hypoxia” until the point of decompensation when patients perceive a state of lung distress [8,17,18]. Interestingly, no relationship between hypoxemia and acid-base disorders has been found in COVID-19 patients [11]. Accordingly, our data showed no oxygen saturation, PaO2, and hemoglobin level alterations among the three groups. However, patients with metabolic alkalosis presented significantly lower 2,3-BPG levels compared to the other two groups. In accordance, increased production of the 2,3-BPG during a respiratory alkalosis state has been also reported in the literature [7,20]. Interestingly, the patients with metabolic alkalosis showed significantly higher blood glucose concentrations compared to the other two groups. The increase in glucose levels has been related to an increase in glycolysis rate suggesting a consequent reduction of the side-metabolite 2,3BPG production supporting its lower levels in the metabolic alkalosis group.
On the other hand, no differences in CALIPER ILD and VRS were revealed in COVID-19 patients with respiratory alkalosis and the other groups within the first day after hospital admission. The data was in accordance with literature in which similar lung CT abnormalities extension has been found in patients with respiratory alkalosis compared with patients without alkalosis [12]. However, a trend of increase in hospitalization days was observed in patients with respiratory alkalosis compared to those with metabolic alkalosis, who in turn were hospitalized longer than patients without alkalosis.
5. Conclusion
Overall, the data confirm CALIPER ILD and VRS percentage as predictors of the disease severity and reveal a different pattern of activation of blood oxygenation compensatory mechanisms reflecting a different course of the disease. COVID-19 patients that do not develop alkalosis seem to have higher levels of 2,3-BPG, which leads to restrained damage and consequently shorter hospitalization by promoting tissue oxygenation. On the other hand, patients with metabolic alkalosis showed lower levels of 2,3-BPG, probably counterbalanced by higher PaCO2 values, which could prompt a left shift of the ODC, therefore increasing peripheral oxygen release. Furthermore, patients who have developed respiratory alkalosis during SARS-CoV-2 infection, have higher levels of 2,3-BPG and lower PaCO2 with respect to those with metabolic alkalosis. Of note, in these patients, the decrease of PaCO2 suggests the insurgence of a wrong perception of oxygenation state with the insurgence of silent hypoxia condition. This state could explain the observed increase in the time spent at home before the hospitalization, with the increase in lung injury and, consequently, the total hospitalization days. Despite other studies being mandatory to better highlight the modulation of blood oxygen availability and release after the SARS-CoV-2 infection, this study evidences the specific modulation of several parameters that must be considered to improve not only the knowledge about COVID-19 infection but also its diagnostic capability.
Funding
This research project is funded by Tuscany Region “Bando ricerca covid-19” OPTIMISED.
Declaration of competing interest
The authors declared no potential conflict of interests.
Acknowledgements
This work was supported by Optimised Study Group (Pisa University Hospital, AOUP).
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Chiara Romei, Email: chiara.romei@gmail.com.
Chiara Giacomelli, Email: chiara.giacomelli@unipi.it.
References
- 1.World health organization coronavirus (COVID-19) dashboard, https://covid19.who.int/.
- 2.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomelli C, Piccarducci R, Marchetti L, Romei C, Martini C. Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: lessons from post-COVID-19 patients. Biochem Pharmacol. 2021;193 doi: 10.1016/j.bcp.2021.114812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romei C, Tavanti LM, Taliani A, De Liperi A, Karwoski R, Celi A, et al. Automated computed tomography analysis in the assessment of idiopathic pulmonary fibrosis severity and progression. Eur J Radiol. 2020;124 doi: 10.1016/j.ejrad.2020.108852. [DOI] [PubMed] [Google Scholar]
- 5.Romei C, Falaschi Z, Danna PSC, Airoldi C, Tonerini M, Rocchi E, et al. Lung vessel volume evaluated with CALIPER software is an independent predictor of mortality in COVID-19 patients: a multicentric retrospective analysis. Eur Radiol. 2022;32(6):4314–4323. doi: 10.1007/s00330-021-08485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grieb P, Swiatkiewicz M, Prus K, Rejdak K. Hypoxia may be a determinative factor in COVID-19 progression. Curr Res Pharmacol Drug Discov. 2021;2 doi: 10.1016/j.crphar.2021.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald R. Red cell 2,3-diphosphoglycerate and oxygen affinity. Anaesthesia. 1977;32(6):544–553. doi: 10.1111/j.1365-2044.1977.tb10002.x. [DOI] [PubMed] [Google Scholar]
- 8.Cajanding RJM. Silent hypoxia in COVID-19 pneumonia: state of knowledge, pathophysiology, mechanisms, and management. AACN Adv Crit Care. 2022;33(2):143–153. doi: 10.4037/aacnacc2022448. [DOI] [PubMed] [Google Scholar]
- 9.Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2014;371(15):1434–1445. doi: 10.1056/NEJMra1003327. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton PK, Morgan NA, Connolly GM, Maxwell AP. Understanding acid-base disorders. Ulster Med J. 2017;86(3):161–166. [PMC free article] [PubMed] [Google Scholar]
- 11.Chiumello D, Pozzi T, Fratti I, Modafferi L, Montante M, Papa GFS, et al. Acid-base disorders in COVID-19 patients with acute respiratory distress syndrome. J Clin Med. 2022;11(8):2093. doi: 10.3390/jcm11082093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Wang G, Zhang Q, Yu B, Lv J, Zhang S, et al. Association between respiratory alkalosis and the prognosis of COVID-19 patients. Front Med. 2021;8 doi: 10.3389/fmed.2021.564635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfano G, Fontana F, Mori G, Giaroni F, Ferrari A, Giovanella S, et al. Acid base disorders in patients with COVID-19. Int Urol Nephrol. 2022;54(2):405–410. doi: 10.1007/s11255-021-02855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2014;371(15):1434–1445. doi: 10.1056/NEJMra1003327. [DOI] [PubMed] [Google Scholar]
- 15.Komorowski M, Aberegg SK. Using applied lung physiology to understand COVID-19 patterns. Br J Anaesth. 2020;125(3):250–253. doi: 10.1016/j.bja.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer BF. Evaluation and treatment of respiratory alkalosis. Am J Kidney Dis. 2012;60(5):834–838. doi: 10.1053/j.ajkd.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Rahman A, Tabassum T, Araf Y, Al Nahid A, Ullah MA, Hosen MJ. Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol Biol Rep. 2021;48(4):3863–3869. doi: 10.1007/s11033-021-06358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swenson KE, Ruoss SJ, Swenson ER. The pathophysiology and dangers of silent hypoxemia in COVID-19 lung injury. Ann Am Thorac Soc. 2021;18(7):1098–1105. doi: 10.1513/AnnalsATS.202011-1376CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondal S, Kumar Das T, Bhattacharya S, Banerjee S, Hazra D. Blood gas analysis among COVID-19 patients: a single centre retrospective observational study. J Clin Diagn Res. 2021;15(8):LC01–4.. [Google Scholar]
- 20.Duhm J, Gerlach E. On the mechanisms of the hypoxia-induced increase of 2,3-diphosphoglycerate in erythrocytes. Studies on rat erythrocytes in vivo and on human erythrocytes in vitro. Pflügers Archiv. 1971;326(3):254–269. doi: 10.1007/BF00592506. [DOI] [PubMed] [Google Scholar]
- 21.Böning D, Kuebler WM, Bloch W. The oxygen dissociation curve of blood in COVID-19. Am J Physiol Lung Cell Mol Physiol. 2021;321(2):L349–57. doi: 10.1152/ajplung.00079.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Böning D, Kuebler WM, Vogel D, Bloch W. The oxygen dissociation curve of blood in COVID-19-An update. Front Med. 2023;10 doi: 10.3389/fmed.2023.1098547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceruti S, Minotti B, Glotta A, Biggiogero M, Bona G, Marzano M, et al. Temporal changes in the oxyhemoglobin dissociation curve of critically ill COVID-19 patients. J Clin Med. 2022;11(12):3376. doi: 10.3390/jcm11030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel Y, Hunt BJ, Retter A, Henderson K, Wilson S, Sharpe CC, et al. Haemoglobin oxygen affinity in patients with severe COVID-19 infection. Br J Haematol. 2020;190(3):e126–7. doi: 10.1111/bjh.16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans JD. Brooks/Cole Pub. Co.; Pacific Grove: 1996. Straightforward statistics for the behavioural sciences. [Google Scholar]
- 26.Al-Azzam N, Khassawneh B, Al-Azzam S, Karasneh RA, Aldeyab MA. Acid-base imbalance as a risk factor for mortality among COVID-19 hospitalized patients. Biosci Rep. 2023;43(3) doi: 10.1042/BSR20222362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022;20(5):270–284. doi: 10.1038/s41579-022-00713-0. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 29.Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172(4):488–493. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 30.Wu X, Kim GH, Salisbury ML, Barber D, Bartholmai BJ, Brown KK, et al. Computed tomographic biomarkers in idiopathic pulmonary fibrosis. The future of quantitative analysis. Am J Respir Crit Care Med. 2019;199(1):12–21. doi: 10.1164/rccm.201803-0444PP. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Zhou H, Zhou Y, Wu X, Zhao Y, Lu Y, et al. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect. 2020;81(1):e95–7. doi: 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serebrovska ZO, Chong EY, Serebrovska TV, Tumanovska LV, Xi L. Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharmacol Sin. 2020;41(12):1539–1546. doi: 10.1038/s41401-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J, Covassin N, Fan Z, Singh P, Gao W. Li G,et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie C, Deng J, Li F, Wu C, Xu M, Yu B, et al. The association between alveolar-arterial oxygen tension difference and the severity of COVID-19 in patients. Infect Dis Ther. 2023;12(2):577–587. doi: 10.1007/s40121-022-00752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel J, Franklin C. In: Common surgical diseases. Myers JA, Millikan KW, Saclarides TJ., editors. Springer; New York: 2008,. Hypoxemia and hypoxia; p. 391.. [Google Scholar]
- 36.Serrano R, Corbella X, Rello J. Management of hypoxemia in SARS-CoV-2 infection: lessons learned from one year of experience, with a special focus on silent hypoxemia. J Intensive Med. 2021;1(1):26–30. doi: 10.1016/j.jointm.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas HM 3rd, Lefrak SS, Irwin RS, Fritts HW, Jr., Caldwell PR. The oxyhemoglobin dissociation curve in health and disease. Role of 2,3-diphosphoglycerate. Am J Med. 1974;57(3):331–348. doi: 10.1016/0002-9343(74)90129-6. [DOI] [PubMed] [Google Scholar]
- 38.Bremner K, Bubb WA, Kemp GJ, Trenell MI. Thompson CH.The effect of phosphate loading on erythrocyte 2,3-bisphosphoglycerate levels. Clin Chim Acta. 2002;323(1–2):111–114. doi: 10.1016/s0009-8981(02)00165-1. [DOI] [PubMed] [Google Scholar]
- 39.Lichtman MA, Miller DR, et al. Reduced red cell Glycolysis 2,3-Diphosphoglycerate and adenosine Triphosphate concentration, and increased Hemoglobin -Oxygen affinity caused by hyposphatemia. Ann Intern Med. 1974;74(4):562–568. doi: 10.7326/0003-4819-74-4-562. [DOI] [PubMed] [Google Scholar]
- 40.Thomas T, Stefanoni D, Dzieciatkowska M, Issaian A, Nemkov T, Hill RC, et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J Proteome Res. 2020;19(11):4455–4469. doi: 10.1021/acs.jproteome.0c00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purcell Y, Brozović B. Red cell 2,3-diphosphoglycerate concentration in man decreases with age. Nature. 1974;251(5475):511–512. doi: 10.1038/251511a0. [DOI] [PubMed] [Google Scholar]
- 42.Kaminsky YG, Reddy VP, Ashraf GM, Ahmad A, Benberin VV, Kosenko EA, et al. Age-related defects in erythrocyte 2,3-diphosphoglycerate metabolism in dementia. Aging Dis. 2013;4(5):244–255. doi: 10.14336/AD.2013.0400244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel S, Jose A, Mohiuddin SS. Physiology, oxygen transport and carbon dioxide dissociation curve. 2023 mar 27. StatPearls [internet]. Treasure Island (FL) Publishing; StatPearls: 2023.. [PubMed] [Google Scholar]
- 44.Renoux C, Fort R, Nader E, Boisson C, Joly P, Stauffer E, et al. Impact of COVID-19 on red blood cell rheology. Br J Haematol. 2021;192(4):e108–11. doi: 10.1111/bjh.17306. [DOI] [PubMed] [Google Scholar]
- 45.Zanella A, Florio G, Antonelli M, Bellani G, Berselli A, Bove T, et al. Time course of risk factors associated with mortality of 1260 critically ill patients with COVID-19 admitted to 24 Italian intensive care units. Intensive Care Med. 2021;47(9):995–1008. doi: 10.1007/s00134-021-06495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanghani H, Bansal S, Parmar V, Shah R. Study of arterial blood gas analysis in moderate-to-severe COVID-19 patients. Cureus. 2022;14(7) doi: 10.7759/cureus.26715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zemlin AE, Sigwadhi LN, Wiese OJ, Jalavu TP, Chapanduka ZC, Allwood BW, et al. The association between acid-base status and clinical outcome in critically ill COVID-19 patients admitted to intensive care unit with an emphasis on high anion gap metabolic acidosis. Ann Clin Biochem. 2023;60(2):86–91. doi: 10.1177/00045632221134687. [DOI] [PMC free article] [PubMed] [Google Scholar]