Abstract
Objectives
Todetermine the association of anti-seizure medication (ASM) treatment with outcomes in acute ischemic stroke (AIS) patients undergoing continuous electroencephalography (cEEG).
Methods
Retrospective analysis of AIS patients admitted between 2012 and 2019. The following are the inclusion criteria: age ≥ 18 years and ≥ 16 h of cEEG within the first 7 days of admission. ASM treatment exposure was defined as > 48 h of treatment after the first 24 h of cEEG. The primary outcome measure was 90-day mortality, and the secondary outcome was 90-day functional recovery (Modified Ranking Scale 0–3). Propensity scores were used to adjust for baseline covariates and presence of epileptiform abnormalities (seizures, periodic and rhythmic patterns).
Results
One hundred thirteen patients met the inclusion criteria; 39 (34.5%) were exposed to ASM. ASM treatment was not associated with 90-day mortality (propensity adjusted HR 1.0 [0.31–3.27], p = 0.999) or functional outcomes (adjusted HR 0.99 [0.32–3.02], p = 0.989), compared to no treatment.
Conclusions
In our study, ASM treatment in AIS patients with cEEG abnormalities was not significantly associated with a change in 90-day mortality and functional recovery. Larger comparative effectiveness studies are indicated to identify which acute ischemic stroke patients with cEEG abnormalities benefit most from ASM treatment.
Keywords: Ischemic stroke, Seizure, Epileptiform abnormalities, Anti-seizure medications, Outcomes
Introduction
Continuous electroencephalography (cEEG) is often utilized for the detection of electrographic seizures in patients with acute ischemic stroke and a clinical concern for seizures or unexplained encephalopathy [1–3]. Epileptiform abnormalities (EAs), including seizures and periodic and rhythmic patterns, can be seen in up to 40% of patients presenting with post-stroke neurological deterioration [4]. Prior studies have shown conflicting results on whether EAs are associated with worse discharge outcomes in patients with acute ischemic stroke [1, 3]. At the same time, anti-seizure medications (ASMs) are frequently prescribed in response to cEEG findings, and there is limited evidence on whether treatment improves outcomes [1, 3]. The goal of this study was to describe ASM treatment patterns and estimate the effect of treatment on 3-month mortality and functional outcomes, in AIS patients undergoing cEEG monitoring.
Methods
This is a retrospective cohort study of patients with AIS admitted to a single center between January 2012 and December 2019. The study was approved by the Institutional Review Board. Informed consent was not required. The results are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies [5]. The data that support the findings of this study are available from the senior author upon reasonable request.
The inclusion criteria were (1) admission for acute ischemic stroke, (2) age > 18 years, (3) cEEG monitoring initiated within 7 days of admission, and (4) ≥ 16 h of cEEG monitoring. We restricted our cEEG exposure to the first 7 days of admission as this defines the risk period for acute symptomatic seizures [6, 7]. We restricted our inclusion criteria to patients with 16 h of cEEG as the probability of seizures drops to < 5% if no seizures have occurred in the first 16 h of recording [8]. We excluded patients with prior diagnosis of epilepsy or those on chronic ASM treatment prior to admission for AIS. We also excluded patients admitted for less than 7 days.
ASM treatment exposure definition
Indications for ASM treatment included clinical seizures and EAs. ASM type, dosage, and duration of treatment were at the discretion of the treating team. We defined ASM treatment exposure as the initiation or continuation of ASMs for ≥ 48 h after the first 24 h of cEEG and through day 7 of admission. The unexposed group consisted of patients who did not receive any ASMs or received less than 48 h of ASM exposure within the first 7 days of admission. Follow-up for outcomes started after day 7 of admission and continued in an intention-to-treat scheme until 90 days from start of follow-up.
Clinical covariates
Demographic and clinical variables were abstracted from the electronic health record. Comorbidities were quantified using the Charlson Comorbidity Index (CCI) [9]. We measured stroke severity using the NIH Stroke Scale (NIHSS) [10], and critical illness severity using the Acute Physiology and Chronic Health Evaluation (APACHE II) score [11]. Stroke etiology was classified using the Trial of ORG 10,172 in acute stroke treatment (TOAST) criteria [12]. Stroke location was characterized based on cortical involvement and vascular territory involved. Stroke treatment modality (i.e., intravenous thrombolysis and/or intraarterial thrombectomy) was recorded. Occurrence of post-stroke complications including hemorrhagic transformation, need for decompressive craniectomy, and hospital-acquired infections were also recorded.
cEEG recording and features
The primary indications for cEEG monitoring were screening for non-convulsive seizures (NCS) and unexplained altered mental status. Continuous video EEG was recorded using 21 electrodes and the International 10–20 system. Two clinical neurophysiologists reviewed and reported the cEEG findings at least twice daily during the hospitalization. All cEEG findings were reported using the American Clinical Neurophysiology Society nomenclature (ACNS) [13]. cEEG features and EA burden data were abstracted from the clinical EEG reports.
Epileptiform abnormalities (EAs) were defined as any of the following patterns: electrographic seizures, lateralized periodic discharges (LPDs), generalized periodic discharges (GPDs), bilateral independent periodic discharges (BiPDs), and lateralized rhythmic delta activity (LRDA). Electrographic seizures were defined as spikes and sharp wave or sharp-slow wave complexes lasting for 10 s or more at a frequency of 3 Hz or at a frequency of 1 Hz but with clear evolution in frequency, morphology, or location [14]. Generalized rhythmic delta activity (GRDA) and sporadic discharges are not associated with a high risk for poor outcomes and hence were excluded from our definition of EAs [3, 15].
EA burden was quantified using the ACNS terminology: rare < 1%, occasional 1–9%, frequent 10–49%, abundant 50–89%, or continuous > 90% [13]. We quantified the EA burden as follows:
First 24 h EA burden—defined as the percentage of the first 24 h of recording occupied by EAs. For patients with less than 24 h of recording, all available cEEG data was considered as the first 24-h epoch.
Peak EA burden—defined as maximum EA burden measured within any 12-h window. Peak EA burden was calculated as the percentage of the 12-h epoch occupied by EA patterns.
Primary and secondary outcomes
Our primary outcome measure was 90-day mortality. The secondary outcome was 90-day functional recovery (good outcome) as measured by the Modified Rankin Scale (mRS): 0, no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death [16]. For analysis, we dichotomized functional status into good outcome (mRS of 0 to 3) or poor (mRS of 4 to 6). mRS was abstracted from physician and physical and occupational therapy clinical examinations by two reviewers (PS and SFZ) who were blinded to the EEG findings and ASM treatment exposure status at the time of outcome abstraction. Additionally, we examined the time to late onset clinical seizures, defined as seizures occurring after day 7 of admission [17].
Statistical analysis
Fisher’s exact test was used for comparison of dichotomized and categorical variables, and the Mann–Whitney U test for continuous variables. Significance was set at 0.05, and 2-sided p values are reported. Equality of survival functions was assessed using the log-rank test.
We used propensity score–adjusted analysis to address the potential clinical differences between ASM treatment exposed and non-exposed patients. The propensity score regression model included variables likely to be associated with ASM treatment, acute symptomatic seizures, and poor outcomes [3, 18–22]. These variables included NIHSS, APACHE II score, CCI, acute stroke treatment (thrombolysis and/or thrombectomy), clinical seizures on presentation, and first 24-h EA burden. We selected variables that captured illness severity, EEG findings, and comorbidities and were measured prior to the exposure window. The area under the receiver operating curve (ROC) for the propensity score model was 0.92. The estimated propensity score was included in a Cox proportional hazard model to assess the association between ASM treatment and primary and secondary outcomes. The proportional hazards assumption was evaluated using Schoenfeld residuals and log–log survival plots. Hazard ratios are presented with 95% confidence intervals as HR [95% CI]. Patients lost to follow-up were right censored in the analysis.
Results
During the study period, there were on average 825 ischemic stroke admissions per year (range 790–895/year). One hundred thirteen patients met the inclusion criteria. Thirty-nine (34.5%) patients received ASM treatment exposure, and 74 (65.5%) were non-exposed. Table 1 summarizes the clinical and demographic characteristics of this cohort. There were no missing data. There were no significant differences in NIHSS, APACHE II score, and rates of hemorrhagic transformation and hemicraniectomy between the two groups. Patients receiving ASM exposure were monitored with cEEG for longer duration and were more likely to have clinical seizures and higher first 24-h and peak EA burden.
Table 1.
Clinical and demographic characteristics
| ASM treatment exposure, N = 39 (34.5%) | No ASM treatment exposure, N = 74 (65.5%) | p-value | |
|---|---|---|---|
|
| |||
| Age, median (Q1-Q3) | 68 (60–76) | 69 (56–77) | 0.9110 |
| Gender, female (%) | 22 (56.4) | 36 (48.6) | 0.5529 |
| CCI, median (Q1-Q3) | 4 (3–6) | 2 (2–6) | 0.7843 |
| NIHSS, median (Q1-Q3) | 12 (7–23) | 14 (9–18) | 0.7856 |
| APACHE II, median (Q1-Q3) | 18 (13–25) | 17 (12–22) | 0.6328 |
| Stroke etiology | 0.927 | ||
| Large-artery | 8 (20.5%) | 21 (28.3%) | |
| Cardioembolic | 20 (51.3%) | 33 (44.6%) | |
| Small-vessel | 2 (5.1%) | 5 (6.8%) | |
| Other determined etiologies | 4 (10.3%) | 7 (9.6%) | |
| Undetermined | 5 (12.8%) | 8 (10.8%) | |
| Stroke location | |||
| Cortical | 32(82.1%) | 60 (81.1%) | 1.000 |
| Subcortical | 7 (17.9%) | 14 (18.9%) | |
| Anterior circulation | 33 (84.6%) | 57 (77.0%) | 0.531 |
| Posterior circulation | 6 (15.4%) | 14 (18.9%) | |
| Multiple vascular territories | 0 (0.0%) | 3 (4.1%) | |
| Stroke treatment modality | |||
| Intravenous thrombolysis | 7 (17.9%) | 17 (23.0%) | 0.357 |
| Intra-arterial thrombectomy | 3 (7.7%) | 17 (23.0%) | 0.0647 |
| Hemorrhagic transformation | 10 (25.6%) | 24 (32.4%) | 0.522 |
| Hemicraniectomy | 4 (10.3%) | 6 (8.1%) | 0.735 |
| Clinical seizures on presentation | 17 (43.6%) | 2 (2.7%) | < 0.0001 |
| cEEG duration in hours, median (Q1–Q3) | 42 (24–50) | 24(21–28) | 0.0002 |
| cEEG findings | |||
| Electrographic seizures | 11 (28.2%) | 0 (0.0%) | < 0.0001 |
| Lateralized periodic discharges | 18 (46.2%) | 13(76.6%) | 0.002 |
| Generalized periodic discharges | 8 (20.5%) | 6 (8.1%) | 0.074 |
| Lateralized rhythmic delta activity | 2 (5.1%) | 5 (6.8%) | 1.000 |
| Generalized rhythmic delta activity | 10 (25.6%) | 12 (16.2%) | 0.317 |
| Sporadic discharges | 6 (15.4%) | 10 (13.5%) | 0.744 |
| First 24-h epileptiform abnormality burden | < 0.0001 | ||
| None | 10 (25.6%) | 52 (70.3%) | |
| Rare (< 1%) | 7 (17.9%) | 0 (0.0%) | |
| Occasional (1–9%) | 4 (10.3%) | 11 (14.9%) | |
| Frequent (10–49%) | 10 (25.6%) | 8 (10.8%) | |
| Abundant (50–89%) | 5 (12.8%) | 2 (2.7%) | |
| Continuous (≥ 90%) | 3 (7.7%) | 1 (1.4%) | |
| Peak epileptiform abnormality burden | < 0.0001 | ||
| None | 9 (23.1%) | 52 (70.3%) | |
| Rare (< 1%) | 7 (17.9%) | 0 (0.0%) | |
| Occasional (1–9%) | 3 (7.7%) | 11 (14.9%) | |
| Frequent (10–49%) | 9 (23.1%) | 8 (10.8%) | |
| Abundant (50–89%) | 6 (15.4%) | 2 (2.7%) | |
| Continuous (≥ 90%) | 5 (12.8%) | 1 (1.4%) | |
| Hospital-acquired pneumonia | 4 (10.3%) | 17 (23.0%) | 0.129 |
| Tracheostomy | 9 (23.1%) | 7 (9.5%) | 0.085 |
| Percutaneous gastrostomy | 13 (33.3%) | 24 (32.4%) | 1.000 |
| Length of hospital stay (days) | 13 (9–25) | 16 (10–27) | 0.3170 |
ASM anti-seizure medication, CCI Charlson Comorbidity Index, APACHE II Acute Physiology and Chronic Health Evaluation II, NIHSS National Institutes of Health Stroke Scale
Patients with ASM exposure received a median of 9 days of inpatient ASM treatment (IQR 6–19). Among treatment-exposed patients, 37 (94.9%) received levetiracetam treatment, 7 (17.9%) received phenytoin, 4 (10.3%) received lacosamide, and 3 (7.7%) received valproic acid (these percentages are not mutually exclusive). Eleven patients in the unexposed group received < 48 h of ASM (levetiracetam only) treatment. In all eleven unexposed patients, ASMs were initiated due to an initial clinical concern for seizures and discontinued within 48 h of cEEG monitoring. Of the 33 ASM treatment-exposed patients alive at discharge, 24 (72.7%) were discharged with ASM prescriptions.
Primary and secondary outcomes
Figure 1 shows the Kaplan–Meier and adjusted survival curves. At 90 days, mortality was 7/39 (18%) in the ASM treatment-exposed group and 23/74 (31%) in the non-exposed group. Twenty-five of 30 (83.3%) patients who died had withholding of life-sustaining therapy or were discharged to hospice. The unadjusted HR for 90-day mortality in the ASM treatment-exposed group was 0.55 [0.23–1.28]. After adjusting for the propensity score, the HR was 1.0 [0.31–3.27].
Fig. 1.
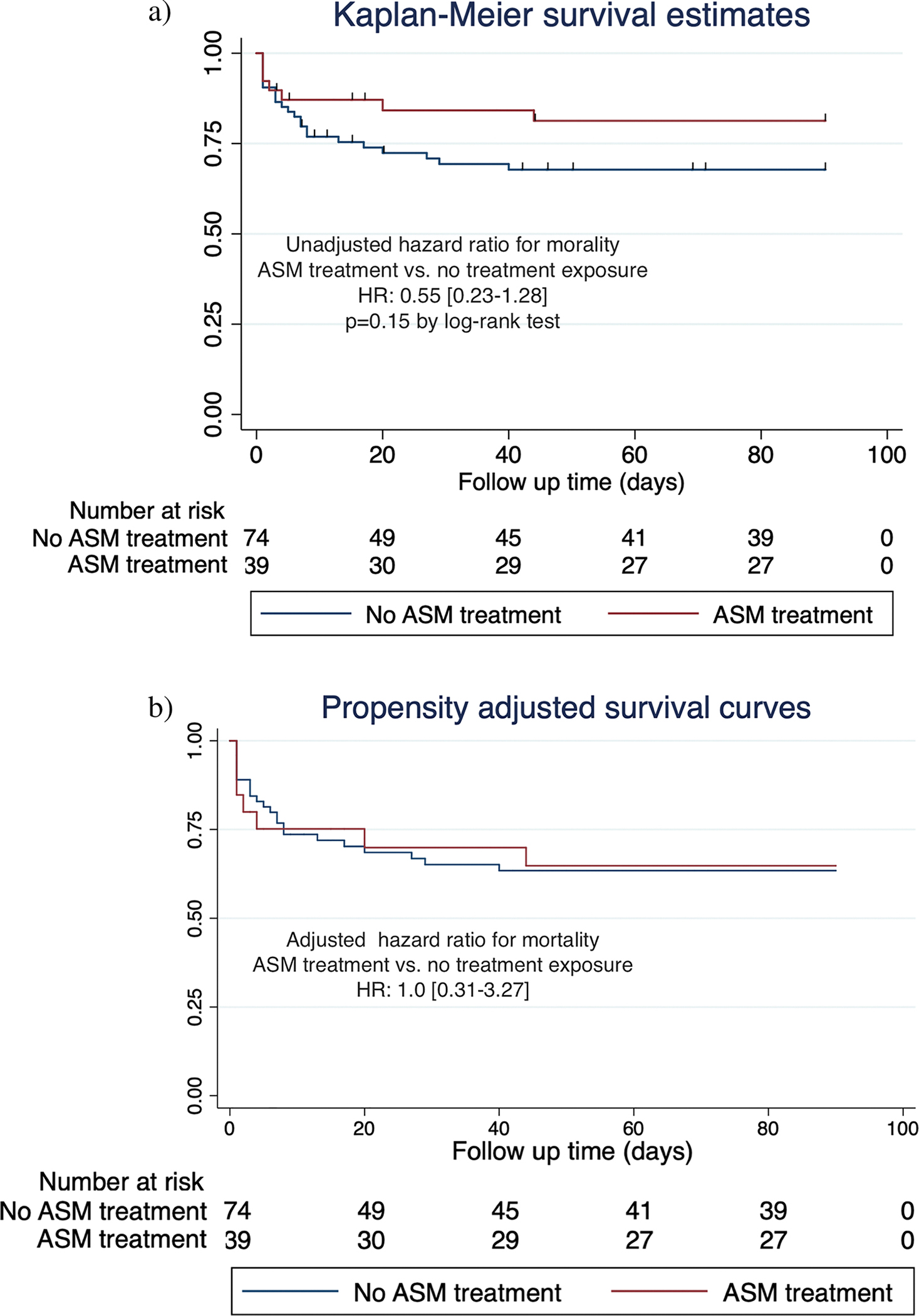
Ninety-day mortality. a Kaplan–Meier curves for time to death comparing ASM treatment exposure (red curve) vs. non-exposure (blue curve) in acute ischemic stroke patients undergoing cEEG monitoring. b Propensity-adjusted survival curves for time to death comparing ASM treatment exposure (red curve) vs. non-exposure (blue curve) in acute ischemic stroke patients undergoing cEEG monitoring. ASM, anti-seizure medication; cEEG, continuous electroencephalography
Figure 2 shows the Kaplan–Meier and adjusted curves for good outcomes defined as mRS 0–3. At 90 days, 10 (26%) patients in the ASM treatment-exposed group had good functional outcomes vs. 29 (39%) in the non-exposed group. The unadjusted HR for good functional outcome in the ASM treatment group was 1.19 [0.53–2.69] compared to the non-exposed group. After adjusting for the propensity score, ASM treatment exposure was not significantly associated with functional outcomes: HR 0.99 [0.32–3.02]. Survival data, including loss to follow-up rates, are summarized in Supplementary Tables S1 and S2.
Fig. 2.
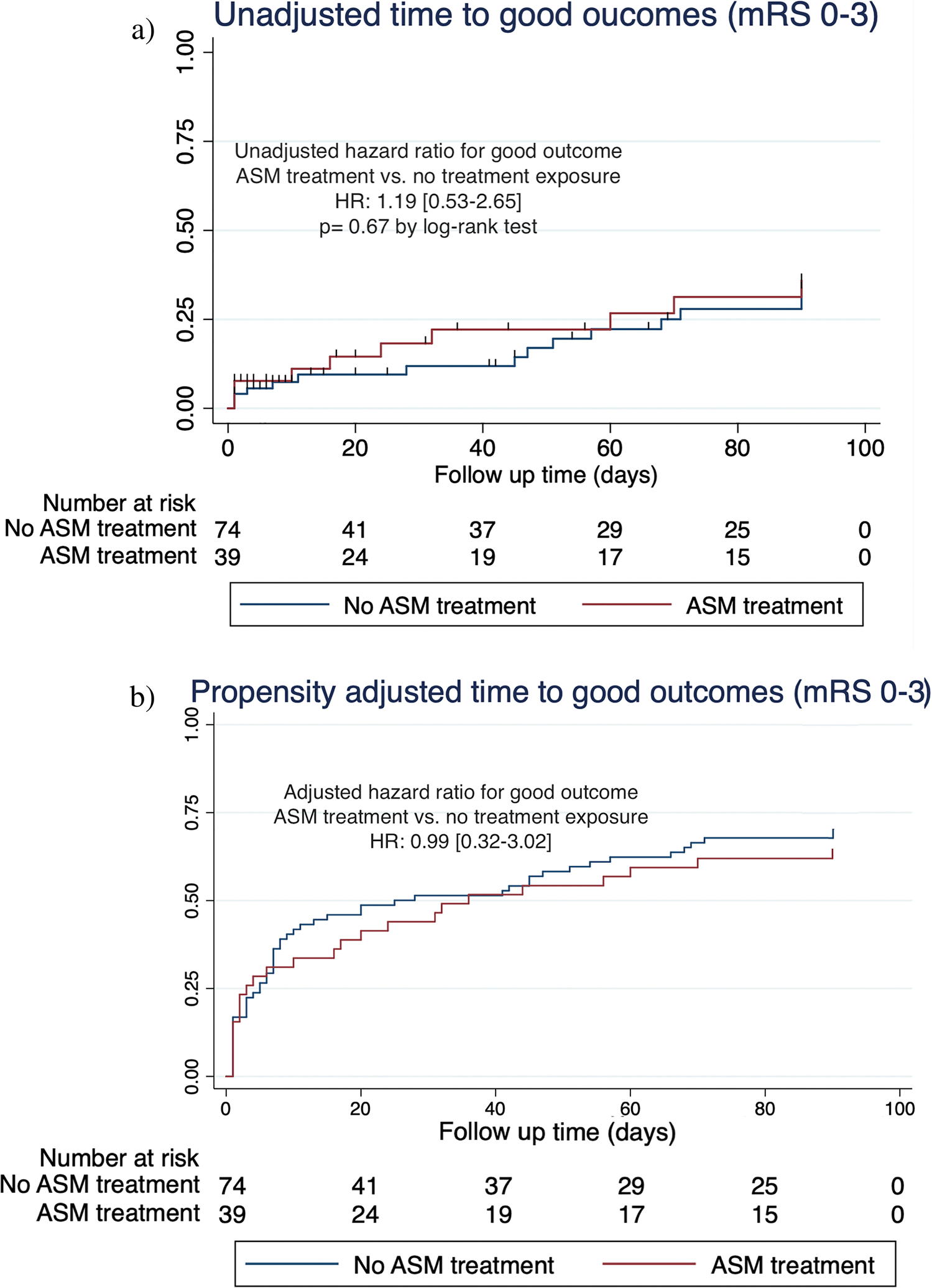
Ninety-day functional recovery. a Kaplan–Meier curves for time to good functional outcome (mRS 0–3) comparing ASM treatment exposure (red curve) vs. non-exposure (blue curve). b Propensity adjusted curves for time to good functional outcome (mRS 0–3) comparing ASM treatment exposure (red curve) vs. non-exposure (blue curve)
Three patients in the ASM treatment-exposed group had late seizures, compared with 2 patients in the unexposed group.
Sensitivity analysis
In a sensitivity analysis, we excluded all patients with clinical seizures on admission as these patients have a higher likelihood of being continued on ASMs regardless of cEEG findings. After exclusion of patients with clinical seizures, the ASM exposure arm only included patients in whom treatment was prescribed in response to cEEG findings. In this analysis, there continued to be no significant association between ASM treatment exposure and 90-day mortality (propensity adjusted HR 0.77 [0.023–2.66], p = 0.70) or functional outcomes (propensity adjusted HR 0.49 [0.09–2.80], p = 0.42).
In an additional sensitivity analysis, we excluded the 11 patients with < 48 h of ASM treatment in the unexposed group. In this analysis, ASM treatment exposure was not significantly associated with mortality (HR 0.539 [0.21–1.36], p = 0.184) or functional outcomes (HR 1.44 [0.66 [0.59–3.52]], p = 0.425). Finally, we performed a sensitivity analysis using fewer covariates in the propensity score model to address any potential impact of over fitting in the main propensity model and found no difference in our primary results (supplemental material).
Discussion
In our cohort of acute ischemic stroke patients undergoing cEEG monitoring, sustained exposure to ASMs was not associated with significant changes in mortality or functional outcomes. Our study also demonstrated the utility of early cEEG for the characterization of clinical symptoms concerning for seizures and de-escalation of unnecessary and potentially harmful ASM treatment in stroke.
Concerning EA, not all patterns may warrant sustained or high-dose ASM treatment. Within acute ischemic stroke patients, there are conflicting reports on the impact of EAs on outcomes [1, 3]. In one study of 132 acute ischemic stroke patients, EAs were not associated with worse discharge outcomes [1]. However, the authors did not quantify and account for EA burden. In 143 acute ischemic stroke patients, we have previously shown that increasing EA burden was associated with worse discharge functional outcomes [3]. Additionally, in prior work, we have demonstrated increasing burden of epileptiform abnormalities is associated with worse discharge outcomes across a larger cohort of medical, surgical, and neurological patients [23]. All aforementioned studies have shown frequent prescription of ASMs in patients with EAs [1, 3, 23]. Future randomized studies including acute ischemic stroke patients with high burden EAs, and excluding low burden EAs, could provide evidence on the impact of ASM treatment on outcomes. Furthermore, such studies should evaluate long-term outcomes in multiple domains including functional, cognitive, and health-related quality of life.
In addition to a clear clinical correlate and high burden, EA pattern type, and frequency can also serve as potential treatment targets in future studies [24]. Specifically, prospective studies investigating the impact of ASM treatment on both seizure risk and outcomes in patients with high frequency (> 1.5 Hz) LPDs, GPDs, and LRDA are warranted as these patterns have the highest association with both seizures and secondary brain injury [15, 25]. Periodic discharges are associated with metabolic stress as evidenced by markers of brain metabolism including reduction in brain tissue oxygenation, elevation in lactate/pyruvate ratio, and PET hypermetabolism [25–27]. Biomarker-guided treatment may also help optimize the potential impact of ASM on long-term outcomes.
Within our cohort, majority of patients with treatment exposure were also discharged on ASMs (72.7% of all treatment exposed patients). A similar finding of long-term continuation of ASMs in stroke patients with EAs has been reported in another study [28]. ASMs have the potential to impair functional and cognitive recovery in neurocritical care patients as highlighted by several studies [29, 30]. ASMs can also impair neurocognitive recovery and affect the quality of life of patients with post-stroke epilepsy (PSE) [31–33]. Therefore, further work is needed to investigate the proposed benefit of treatment during the acute phase and timely discontinuation or reduction of ASMs in the post-acute phase [34]. The post-acute symptomatic seizure (PASS) clinic model provides continuity of care for such patients and ensures appropriate ASM weaning when long-term treatment is not warranted [35]. A single-center PASS clinic model resulted in the reduction or discontinuation of ASMs in 44.6% of patients [35].
An indication for cEEG is the characterization and diagnostic clarification of paroxysmal events concerning for seizures [36]. Our study highlights the utility of cEEG for this indication in patients with acute ischemic stroke. In our cohort, 11 patients in the unexposed group were initially started on ASMs for clinical concern, with discontinuation shortly after initiation of cEEG monitoring. All 11 patients (10% of the total cohort) received ASMs for < 48 h and were therefore counted in the unexposed group. In a separate cohort of acute ischemic stroke patients, cEEG-guided de-escalation of treatment was reported in 4.5% of patients [1]. Paroxysmal events including rhythmic and repetitive motor movements, tremors, gaze deviation, and autonomic symptoms are frequently encountered in neurocritical care [37, 38]. These paroxysmal symptoms are not always seizures but can be misdiagnosed as seizures resulting in initiations of ASMs [37, 38]. In such patients, risk of ASM adverse effects outweigh benefits and cEEG data can provide guidance on discontinuation of unnecessary treatment.
Limitations of our study include the lack of randomization and the use of information from a single-center which may limit generalizability. There was limited sample size and number of patients in the exposed group. Our study may, therefore, be underpowered to find smaller differences. Restricting the eligibility criteria to 16 h of cEEG, may create a selection bias. Our cohort was also restricted to patients with higher stroke severity given our eligibility criteria of admission length ≥ 7 days, and need for prolonged cEEG. Finally, although we used propensity scores, our results may still be prone to residual or unmeasured confounding.
In conclusion, acute ischemic stroke patients undergoing cEEG monitoring are often exposed to ASM treatment. However, it is unclear whether ASM treatment improves the outcomes. In our study, we found no significant differences in the outcomes between patients exposed to ASMs vs. not. Future larger comparative effectiveness studies and randomized investigations are indicated to identify patients that may benefit the most from ASM treatment and to define the optimum duration of treatment. Further work is also needed to determine whether cEEG-guided de-escalation of treatment in patients with a clinical concern for seizures could minimize the harmful effects of ASMs, such as cognitive impairment and sedation, and improve outcomes.
Supplementary Material
Acknowledgements
This research was supported by funding from NIH-NINDS K23NS114201 (PI- SFZ).
Funding
Dr. Zafar is supported by funding from NIH K23NS114201 and American Epilepsy Society Infrastructure grant. Dr. Moura is supported by funding from NIH (1K08AG053380–01A1, 1R01AG062282–01) and the Epilepsy Foundation (Epilepsy Learning Healthcare System) and is the Director of the Data Coordinating Center. Dr. Rosenthal is supported by NIH (K23NS105950 and R01NS117904). Dr. Hsu is supported by NIH (P01AG032952 and R01AG062282). Dr. Patorno is supported by multiple grants from NIH (K08AG055670, R01HD097778, 5R01HL141505, U01FD007213), the FDA (5U01FD007213, 75F40119D10037), and PCORI (DB-2020C2–20326). She is an investigator of a research grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not related to this work. Dr. Westover is supported by the Glenn Foundation for Medical Research and American Federation for Aging Research (Breakthroughs in Gerontology Grant); American Academy of Sleep Medicine (AASM Foundation Strategic Research Award); Football Players Health Study (FPHS) at Harvard University; Department of Defense through a subcontract from Moberg ICU Solutions, Inc.; and NIH (1R01NS102190, 1R01NS102574, 1R01NS107291, 1RF1AG064312).
Footnotes
Conflict of interest Dr. Zafar is a clinical neurophysiologist for Corticare, unrelated to this work. Dr. Rosenthal received consulting fees from UCB Pharma, Inc. and Ceribell, Inc., unrelated to this work. Dr. Westover is cofounder of Beacon Biosignals unrelated to this work.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10072-022-06183-9.
Ethical approval None.
The study was approved by the Institutional Review Board of Mass General Brigham.
References
- 1.Dhakar M, Sheikh Z, Kumari P, Lawson E, Jeanneret V, Desai D, Rodriguez Ruiz A, Haider H (2020) Epileptiform abnormalities in acute ischemic stroke: impact on clinical management and outcomes. J Clin Neurophysiol. 10.1097/WNP.0000000000000801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavvala J et al. (2014) Continuous EEG monitoring: a survey of neurophysiologists and neurointensivists. Epilepsia 55(11):1864–71. 10.1111/epi.12809 [DOI] [PubMed] [Google Scholar]
- 3.Tabaeizadeh M et al. (2020) Burden of epileptiform activity predicts discharge neurologic outcomes in severe acute ischemic stroke. Neurocrit Care. 10.1007/s12028-020-00944-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scoppettuolo P, Gaspard N, Depondt C, Legros B, Ligot N, Naeije G (2019) Epileptic activity in neurological deterioration after ischemic stroke, a continuous EEG study. Clin Neurophysiol. 10.1016/j.clinph.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61(4):344–349. 10.1371/journal.pmed.0040297 [DOI] [PubMed] [Google Scholar]
- 6.Leung T, Leung H, Soo YOY, Mok VCT, Wong KS (2017) “The prognosis of acute symptomatic seizures after ischaemic stroke,” J. Neurol. Neurosurg. Psychiatry 88 (1) 10.1136/jnnp-2015-311849 [DOI] [PubMed] [Google Scholar]
- 7.Wang JZ, Vyas MV, Saposnik G, Burneo JG (2017) Incidence and management of seizures after ischemic stroke. Neurology 89(12):1220–1228. 10.1212/WNL.0000000000004407 [DOI] [PubMed] [Google Scholar]
- 8.Westover MB et al. (2015) The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol. 10.1016/j.clinph.2014.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 10.Adams HP et al. (1999) “Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST),” Neurology 53 (1) 10.1212/WNL.53.1.126 [DOI] [PubMed] [Google Scholar]
- 11.Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 12.Love BB and Bendixen BH (1993) “Classification of subtype of acute ischemic stroke definitions for use in a multicenter clinical trial,” Stroke, vol. 24, no. 1. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 13.Hirsch LJ et al. (2013) American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 30(1):1–27. 10.1097/WNP.0b013e3182784729 [DOI] [PubMed] [Google Scholar]
- 14.De Marchis GM et al. (2016) Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 10.1212/WNL.0000000000002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz AR et al. (2017) Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 10.1001/jamaneurol.2016.4990 [DOI] [PubMed] [Google Scholar]
- 16.Quinn TJ, Dawson J, Walters MR, Lees KR (2009) “Functional outcome measures in contemporary stroke trials,” International Journal of Stroke 4 (3) 10.1111/j.1747-4949.2009.00271.x [DOI] [PubMed] [Google Scholar]
- 17.Galovic M et al. (2018) Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. 10.1016/S1474-4422(17)30404-0 [DOI] [PubMed] [Google Scholar]
- 18.Fischer U et al. (2006) “Impact of comorbidity on ischemic stroke outcome,” Acta Neurol. Scand, 113 (2) 10.1111/j.1600-0404.2005.00551.x [DOI] [PubMed] [Google Scholar]
- 19.Mistry EA et al. (2017) “Mechanical thrombectomy outcomes with and without intravenous thrombolysis in stroke patients: a meta-analysis,” Stroke 48 (9) 10.1161/STROKEAHA.117.017320 [DOI] [PubMed] [Google Scholar]
- 20.Moon BH, Park SK, Jang DK, Jang KS, Kim JT, Han YM (2015) Use of APACHE II and SAPS II to predict mortality for hemorrhagic and ischemic stroke patients. J Clin Neurosci. 10.1016/j.jocn.2014.05.031 [DOI] [PubMed] [Google Scholar]
- 21.Rost NS et al. (2016) Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc. 10.1161/JAHA.115.002433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reith J, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS (1997) Seizures in acute stroke: predictors and prognostic significance: the Copenhagen Stroke Study. Stroke. 10.1161/01.STR.28.8.1585 [DOI] [PubMed] [Google Scholar]
- 23.Zafar SF et al. (2021) Automated annotation of epileptiform burden and its association with outcomes. Ann Neurol 90(2):300–311. 10.1002/ana.26161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivaraju A, Gilmore EJ (2016) Understanding and managing the ictal-interictal continuum in neurocritical care. Curr Treat Options Neurol 18(2):1–13. 10.1007/s11940-015-0391-0 [DOI] [PubMed] [Google Scholar]
- 25.Witsch J et al. (2017) “Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury,” JAMA Neurol, 74 (3) 10.1001/jamaneurol.2016.5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vespa PM et al. (2007) “Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis,” Crit. Care Med 35 (12) 10.1097/01.CCM.0000295667.66853.BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES (2016) Metabolic correlates of the ictal-interictal continuum: FDG-PET during continuous EEG. Neurocrit Care. 10.1007/s12028-016-0245-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punia V et al. (2021) “Long-term continuation of anti-seizure medications after acute stroke,” Ann. Clin. Transl. Neurol acn3.51440. 10.1002/acn3.51440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naidech AM et al. (2005) Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 10.1161/01.STR.0000141936.36596.1e [DOI] [PubMed] [Google Scholar]
- 30.Yoon SJ, Joo JY, Kim YB, Hong CK, Chung J (2015) “Effects of prophylactic antiepileptic drugs on clinical outcomes in patients with a good clinical grade suffering from aneurysmal subarachnoid hemorrhage,” J. Cerebrovasc. Endovasc. Neurosurg 17 (3) 10.7461/jcen.2015.17.3.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein LB, Matchar DB, Morgenlander JC, Davis JN (1990) “Influence of drugs on the recovery of sensorimotor function after stroke,” Neurorehabil. Neural Repair 4 (3) 10.1177/136140969000400303 [DOI] [Google Scholar]
- 32.Goldstein LB (1995) Common drugs may influence motor recovery after stroke. Neurology. 10.1212/WNL.45.5.865 [DOI] [PubMed] [Google Scholar]
- 33.Winter Y, Daneshkhah N, Galland N, Kotulla I, Krüger A, Groppa S (2018) Health-related quality of life in patients with poststroke epilepsy. Epilepsy Behav 80:303–306. 10.1016/j.yebeh.2017.12.037 [DOI] [PubMed] [Google Scholar]
- 34.Jones FJS et al. (2021) Anticonvulsant primary and secondary prophylaxis for acute ischemic stroke patients: a decision analysis. Stroke. 10.1161/STROKEAHA.120.033299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Punia V, Chandan P, Fesler J, Newey CR, Hantus S (2020) “Post-acute symptomatic seizure (PASS) clinic: a continuity of care model for patients impacted by continuous EEG monitoring,” Epilepsia Open 5 (2) 10.1002/epi4.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman ST et al. (2015) Consensus statement on continuous EEG in critically Ill adults and children, part I: indications. J Clin Neurophysiol. 10.1097/WNP.0000000000000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benbadis SR, Chen S, Melo M (2010) “Whats shaking in the ICU? The differential diagnosis of seizures in the intensive care setting,” Epilepsia 51 (11) 10.1111/j.1528-1167.2010.02683.x [DOI] [PubMed] [Google Scholar]
- 38.Boesebeck F, Freermann S, Kellinghaus C, Evers S (2010) “Misdiagnosis of epileptic and non-epileptic seizures in a neurological intensive care unit,” Acta Neurol. Scand 122 (3) 10.1111/j.1600-0404.2009.01287.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


