Abstract
Introduction
Central nervous system lymphoma poses significant diagnostic challenges, with stereotactic biopsy being the gold standard for diagnosis. Intraoperative magnetic resonance imaging and intraoperative histological examination are utilized to enhance biopsy yield, yet their comparative efficacy remains unclear.
Research question
This study aims to compare the diagnostic yield of intraoperative magnetic resonance imaging and intraoperative histological examination in stereotactic brain biopsies for central nervous system lymphoma.
Materials and methods
A retrospective analysis was conducted on 115 patients who underwent stereotactic brain biopsies for central nervous system lymphoma. Diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of intraoperative magnetic resonance imaging and intraoperative histological examination were assessed and compared.
Results
Out of 125 surgeries, frameless biopsies were the most common, accounting for 74.4 percent. Intraoperative magnetic resonance imaging demonstrated a sensitivity of 80.00 percent and a specificity of 98.51 percent (AUC = 0.893, p = 0.004), whereas intraoperative histological examination showed a sensitivity of 66.67 percent and a specificity of 59.09 percent (AUC = 0.629, p = 0.459).
Discussion and conclusions
The study emphasizes the critical role of intraoperative examinations, thus improving precision and diagnostic yield in the surgical management of central nervous system lymphoma. Intraoperative magnetic resonance imaging outperforms intraoperative histological examination in terms of sensitivity and specificity for confirming positive biopsy yields in central nervous system lymphoma, thereby reducing the need for additional surgeries. These findings support the routine use of intraoperative magnetic resonance imaging in the surgical strategy for central nervous system lymphoma to improve diagnostic accuracy and patient outcomes.
Keywords: Central nervous system lymphoma, Intraoperative MRI, Intraoperative histological examination, Stereotactic biopsy, Diagnostic accuracy, Neurooncology
Highlights
-
•
Study provides the first direct comparison of iMRI and iHE in CNS lymphoma.
-
•
IMRI showed superiority over intraoperative histological examination.
-
•
Preoperative ECOG status significantly impacts post-biopsy survival rates.
Abbreviation list:
- ALA -
Aminolevulinic Acid
- AUC -
Area Under the Curve
- CNS -
Central Nervous System
- ECOG -
Eastern Cooperative Oncology Group
- iHE -
Intraoperative Histological Examination
- iMRI -
Intraoperative Magnetic Resonance Imaging
- MRI -
Magnetic Resonance Imaging
- NPV -
Negative Predictive Value
- PPV -
Positive Predictive Value
1. Introduction
Central nervous system (CNS) lymphoma, encompassing both primary and secondary brain involvement, poses significant diagnostic and therapeutic challenges. An essential step at the beginning of the treatment process is establishing the diagnosis using tissue sample acquired through biopsy. Stereotactic Biopsy is the gold standard for diagnosing brain lymphoma (Hasner et al., 2024). Lymphoma is diagnosed through the presence of neoplastic lymphoid cells on histological analysis. The diagnosis is further confirmed by detecting specific markers, such as CD20, CD79a, and CD45, which are commonly expressed in cells of B-cell lymphomas, the most common lymphoproliferative disease of CNS (Boyd et al., 2013).
Achieving a high diagnostic yield in stereotactic biopsies for brain lymphoma necessitates exceptional precision to minimize the need for repeat procedures. Two primary techniques exist for accurate lesion targeting: (1) frame-based and (2) frameless stereotaxy.
The frame-based technique involves securing a rigid frame to the patient's skull, providing a fixed coordinate system for guiding the biopsy needle to the targeted lesion. Conversely, the frameless stereotactic biopsy technique utilizes advanced computer technology and neuronavigation to guide the biopsy needle in real-time without needing a physical frame attached to the skull. Both stereotactic frame-based and frameless techniques offer a comparable safety profile and accuracy in targeting brain lesions (Ungar et al., 2022; Georgiopoulos et al., 2018; Dhawan et al., 2019; Woodworth et al., 2006).
We have several options during surgery to verify the adequacy of the sample collection, namely Intraoperative histological examination (iHE) and Intraoperative magnetic resonance imaging (iMRI) (Moriarty et al., 2000; Bradac et al., 2017).iHE, including frozen section analysis, has traditionally been employed to provide an immediate assessment of biopsy yield during surgery (Mathon et al., 2019a; Livermore et al., 2014) but more recently, iMRI has emerged as a revolutionary tool, offering real-time and high-resolution imaging that allow the visualization of the trajectory of biopsy needle and the targeted lesion. The use of both iMRI and iHE has been shown to enhance the diagnostic yield of brain biopsies (Bradac et al., 2017).
This study examines the comparative predictive value of intraoperative histological examination and intraoperative MRI for diagnostic biopsy yield in brain lymphoma, with a view to elucidate their respective roles in enhancing surgical precision and patient outcomes. To the best of our knowledge, no previous paper has compared these two intraoperative examinations.
2. Methods
2.1. Data collection
This study comprises a retrospective analysis of patient data extracted from the hospital information system, focusing on individuals who underwent surgery for brain lymphoma at the Military University hospital between January 1, 2012, and December 31, 2023. The data were manually extracted and anonymized to maintain patient confidentiality. This study was reviewed and approved by the institutional ethics committee with approval number 108/19–20/2024.
2.2. Inclusion criteria
-
1.
Diagnosis: Patients with a confirmed diagnosis of primary or secondary brain lymphoma based on histology results.
-
2.
Time Frame: Patients treated between January 1, 2012, and December 31, 2023, during the routine use of the iMRI suite in our hospital.
2.3. Exclusion criteria
-
1.
Incomplete Data: Patient records missing essential data points such as the date of surgery, ECOG performance status, or definitive biopsy yield.
-
2.
Non-Surgical Treatment: Patients treated exclusively with non-surgical methods such as chemotherapy or radiation therapy without any surgical intervention.
-
3.
Co-existing CNS Disorders: Patients with concurrent primary central nervous system disorders that could confound the assessment of brain lymphoma treatment outcomes.
-
4.
Lost to Follow-Up: Patients lost to follow-up immediately after surgery, preventing the assessment of postoperative outcomes and overall survival.
We studied various variables, including the following.
-
•
Patient demographics, which included sex, age at diagnosis, significant comorbidities, and any history of lymphoma outside the central nervous system.
-
•
Clinical presentation, documenting clinical symptoms and the Eastern Cooperative Oncology Group (ECOG) performance scale to assess preoperative functional status.
-
•
Surgical data, detailing the date of surgery, tumor location within cerebral regions (frontal, parietal, temporal, occipital, deep structures, and posterior fossa), surgical approach (frame-based biopsy, frameless biopsy, resection <90%, resection >90%), and preoperative corticosteroid use within 14 days of biopsy.
-
•
Intraoperative diagnostic tools, evaluating the efficacy of intraoperative histological examination (frozen section) and intraoperative MRI in predicting final diagnostic yield.
-
•
Biopsy analysis, detailing the definitive biopsy yield, including successful extraction of diagnostic tissue and the need for subsequent biopsies.
-
•
Surgical outcomes, classifying postoperative complications based on the need for revision surgery.
-
•
Survival data, documenting the interval from biopsy to death to calculate overall survival.
-
•
Histological assessment, classifying lymphoma subtype according to established histological criteria.
2.4. Surgical procedure description
All stereotactic procedures were performed under general anesthesia using either the BrainLab VarioGuide Alignment System (BrainLAB AG) or the CRW Precision Arc system (Integra NeuroSciences). A single tissue sample was sent for iHE. All iHE procedures were conducted in a standardized manner, following the pathology department's internal guidelines. Briefly, the sample was placed on a 25 mm cryostat chuck using frozen section gel and transferred to the cryotome. After freezing the sample to −44 °C, at least three 4-μm thick tissue sections were cut and placed on glass slides. The slides were stained with Weigert's hematoxylin for 2 min and eosin solution for 30 s. Afterwards, the slides were mounted and delivered to the pathologist for diagnostic assessment. An additional 2–6 tissue samples were sent for definitive histological examination, fixed in 4% formaldehyde solution, paraffin-embedded, and cut for definitive histology slides according to the department's routine procedure. iMRI was conducted after the surgical procedure on the anesthetized patient using a 3T GE 750w MR system (GE Healthcare).
2.5. iMRI and iHE interpretation
The results of intraoperative examinations (iMRI and iHE) were compared to the definitive histological diagnosis, categorized as follows.
-
-
Positive: The intraoperative examination confirmed that the biopsy needle reached the designated target lesion (iMRI) or the pathologist confirmed the presence of tumorous tissue (not necessarily lymphoma).
-
-
Negative: The needle trajectory deviated from the target (iMRI) or the pathologist reported normal brain tissue (iHE).
-
-
Inconclusive: The needle trajectory was at the border of the target lesion (iMRI) or the pathologist reported non-specific alterations of the brain parenchyma such as the presence of reactive glial tissue or infiltrates of foamy histiocytes but no presence of unequivocal tumor tissue.
-
-
Not Done: The examination was not performed.
After reviewing the results from iMRI and/or iHE, the surgeon was responsible for determining whether to conclude the surgery or make a second attempt of the biopsy.
2.6. Statistical analysis
The data were analyzed to identify patterns and associations between various clinical and demographic factors and surgical outcomes. Descriptive statistics were used to summarize the frequency and percentage distributions of categorical variables, such as tumor localization, type of surgery, and surgical complications. The chi-square test (with adjustment of p-value using Monte-Carlo resampling with 10000 samples) was employed to compare categorical outcomes, such as the effect of corticosteroid administration and type of surgery on biopsy yield. Kaplan-Meier survival analysis with log-rank test was employed to estimate overall survival and compare factors such as sex, ECOG scale and type of surgery. A multivariate Cox proportional hazards model was then applied to determine the independent effects of various predictors on survival. Additionally, area under curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to assess the diagnostic accuracy of iHE and iMRI. All statistical tests were two-tailed, and a p-value of less than 0.05 was considered indicative of statistical significance.
3. Results
There were a total of 125 surgeries performed for brain lymphoma, involving 115 unique patients, including 10 patients who underwent surgery twice (See Table 1 for detailed information).
Table 1.
Descriptive table of sample characteristics. Data are presented as median (IQR) for continuous measures, while for categorical variables count (percentage) are provided. Abbreviations: PCNSL, primary central nervous system lymphoma; SCNSL, secondary central nervous system lymphoma; DLBCL, diffuse large B-cell lymphoma; LG B-NHL, low grade B-non Hodgkin lymphoma; GC, germinal center.
| Patients | n = 115 |
|---|---|
| Sex: Male | 63 (54.6 %) |
| Age (years) | 68 (60–74) |
| Symptoms | |
| focal deficit | 56 (48.7%) |
| confusion | 34 (29.6%) |
| epileptic seizure | 9 (7.8%) |
| raised ICP | 8 (7.0%) |
| other | 8 (7.0%) |
| ECOG scale | 2 (1–2) |
| Immunity status | |
| immunocompetent | 111 (96.5%) |
| immunosupression after Tx | 2 (1.7%) |
| chronic corticoteroid therapy | 2 (1.7%) |
| History of non-CNS lymphoma: Yes | 19 (16.5%) |
| Histological classifciation | |
| PCNSL | 112 (97.4%) |
| SCNSL | 3 (2.6%) |
| DLBCL | 112 (97.4%) |
| LG B-NHL | 2 (1.7%) |
| not classified | 1 (0.9%) |
| GC lymphoma | 32 (27.8%) |
| non-GC lymphoma | 83 (72.2%) |
| Surgical procedures | n = 125 |
|---|---|
| Location | |
| frontal | 44 (35.2%) |
| deep supratentorial | 37 (29.6%) |
| temporal | 17 (13.6%) |
| parietal | 14 (11.2%) |
| occipital | 9 (7.2%) |
| infratentorial | 4 (3.2%) |
| Type of procedure | |
| frameless biopsy | 93 (74.4%) |
| framebased biopsy | 12 (9.6%) |
| resection more than 90% | 15 (12.0%) |
| resection less than 90% | 5 (4.0%) |
| Corticosteroids prior the surgery: Yes | 42 (33.6%) |
| Surgical complication | |
| no | 116 (92.8%) |
| yes, without revision | 7 (5.6%) |
| yes, with revision | 2 (1.6%) |
The localization of brain lymphoma in the studied cohort was predominantly in the frontal lobe (F) with 44 cases (35.2%), followed by deep supratentorial structures (deep) with 37 cases (29.6%), temporal lobe (T) with 17 cases (13.6%), parietal lobe (P) with 14 cases (11.2%), occipital lobe (O) with 9 cases (7.2%), and posterior fossa with 4 cases (3.2%).
The types of surgeries varied, with frameless biopsies being the most common (93 cases, 74.4%), followed by resection surgeries (15 cases with more than 90% extent of resection and 5 with less than 90% extent of resection), and framebased biopsies (12 cases).
Surgical complications were relatively uncommon, with 116 surgeries not experiencing any complications, 7 resulting in complications without requiring revision (4 small hematoma in biopsy target, 2 surgical site infection and 1 epileptic seizure), and 2 necessitating revision (1 subdural hematoma and 1 surgical site infection).
The histological classification of brain lymphomas showed that 112 cases (97.4%) were primary central nervous system lymphomas (PCNSL) and 3 cases (2.6%) were secondary central nervous system lymphomas (SCNSL). Among all cases, 112 cases (97.4%) were classified as diffuse large B-cell lymphoma (DLBCL), 2 cases (1.7%) as low-grade B-cell non-Hodgkin lymphoma (LG B-NHL), and 1 case (0.9%) was not further classified because of early death of the patient. Additionally, 32 cases (27.8%) were of the germinal center (GC) subtype, and 83 cases (72.2%) were non-germinal center (non-GC).
3.1. Survival
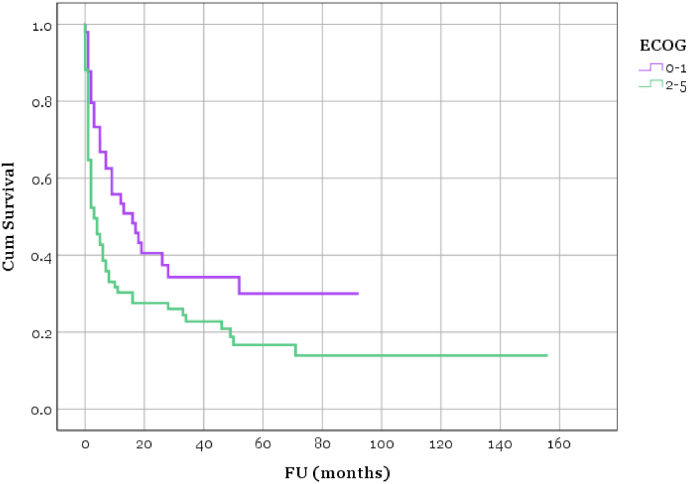
In the Kaplan-Meier analysis using the ECOG scale, we observed statistically significant disparities in outcomes between Group 1 (ECOG performance status 0–1) and Group 2 (ECOG performance status 2–5) (p = 0.010). For instance, one month post-biopsy, the survival rate was 88% in Group 1, in stark contrast to just 65% in Group 2 (see Fig. 1).
Fig. 1.
Kaplan-Meier survival curve: Survival of Group 1 (preoperative ECOG 0–1) and Group 2 (preoperative ECOG 2–4) (p = 0.010).
The survival curves hint at a potential difference in survival between the biopsy and resection groups, even though the statistical test for overall (mean) survival indicates a lack of significance (p = 0.498), likely due to a smaller sample size in the resection group.
The multivariate Cox regression model has revealed that the ECOG performance status remained a significant predictor of survival (HR = 1.680, 95% CI [1.051–2.684], p = 0.030), even after adjusting for other significant covariates/confounders (age and corticosteroid use).
3.2. Corticosteroids
In 42 cases, the corticosteroids had been administered during the span of 14 days prior to the procedure. Two patients in the failed definitive biopsy group received corticosteroids, but effect of corticosteroids on failed biopsy was not statistically significant (p = 0.318)
Regarding iHE, effect of corticosteroids on inconclusive and negative iHE was not found (p = 1.000).
3.3. Failed biopsy
In 10 cases (8.0%), definitive histology smears failed to identify the presence of tumorous tissue (i.e. negative biopsy yield) and second surgery had to be performed.
Failed procedures were frameless biopsy in 9 cases and framebased biopsy in 1 case, effect of surgery type was not significant (p = 1.000).
Two patients received corticosteroids in the 14 days prior to the failed biopsy, but effect of corticosteroids was not statistically significant (p = 0.318). One patient, whose definitive histology failed to provide a diagnosis, discontinued corticosteroids seven days before surgery. He subsequently underwent an open biopsy. Another patient continued receiving corticosteroids up until the day of surgery. The procedure was performed because the navigation MRI on the same day revealed an enhancing lesion with no regression compared to the previous scan.
Both, intraoperative MRI and intraoperative histopathological examination, showed one false positive results. In another case, both examinations showed true negative results, but surgery was concluded, which we consider retrospectively as surgeons failure. Detailed information about failed biopsies are summarized in Table 2.
Table 2.
Failed biopsy cases.
| ID | Sex | Age | ECOG | Location | Procedure | Corticosteroids | iHE | iMRI |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 80 | 2 | parietal | frameless biopsy | N | negative | negative |
| 2 | F | 50 | 3 | deep | frameless biopsy | Y | not done | not done |
| 3 | F | 68 | 3 | temporal | frameless biopsy | N | positive | inconclusive |
| 4 | F | 61 | 2 | deep | frameless biopsy | N | inconclusive | not done |
| 5 | M | 57 | 4 | frontal | frameless biopsy | Y | not done | not done |
| 6 | F | 73 | 1 | deep | frameless biopsy | N | not done | positive |
| 7 | M | 62 | 2 | occipital | frameless biopsy | N | not done | not done |
| 8 | F | 53 | 0 | deep | framebased biopsy | N | not done | not done |
| 9 | F | 73 | 1 | parietal | frameless biopsy | N | not done | inconclusive |
| 10 | M | 62 | 2 | frontal | frameless biopsy | N | not done | inconclusive |
3.4. Intraoperative histological examination
iHE were not conducted in 78 surgeries. When performed, the outcomes were positive of lymphoma in 27 cases, negative in 8 cases, and inconclusive in 12 cases.
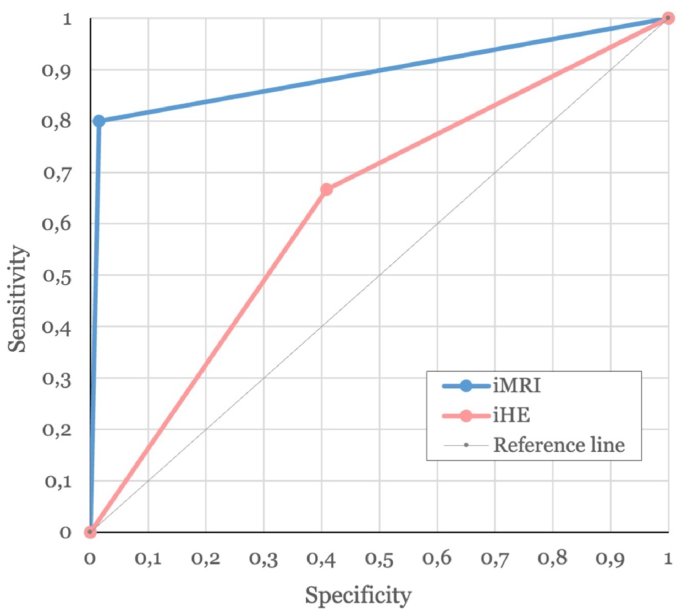
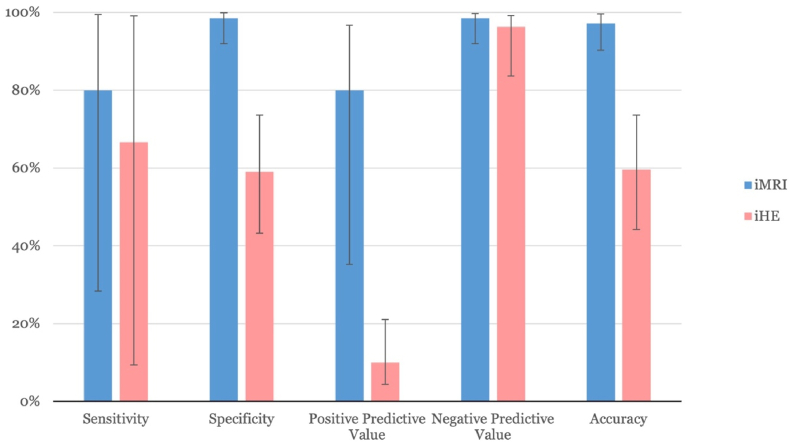
iHE demonstrated a sensitivity of 66.67% and a specificity of 59.09% in identifying positive biopsy yields (AUC = 0.629, 95% CI [0.305–0.953], p = 0.459, see Fig. 2). The positive predictive value was 10.00%, while the negative predictive value was 96.30% (see Fig. 3).
Fig. 2.
ROC Curve: Comparison of diagnostic performance between iMRI (blue) and iHE (pink) classifiers. The diagonal reference line indicates no discrimination. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Diagnostic performance metrics: Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for iMRI (blue) and iHE (pink) with 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Intraoperative MRI
iMRI was reported as positive (i.e. needle reached designated target) in 67 surgeries, not performed in 53 cases, inconclusive in 4, and negative in 1 case. A re-biopsy following iMRI during the one procedure (and one general anesthesia) was noted in 3 cases.
The sensitivity of iMRI for detecting positive biopsy yield was 80.00%, with a specificity of 98.51% (AUC = 0.893, 95% CI [0.683–1.000], p = 0.004, see Fig. 2). The positive predictive value was recorded at 80.00%, and the negative predictive value at 98.51% (see Fig. 3).
3.6. Inconclusive cases
In instances where either iMRI or iHE provided an inconclusive report, it was the surgeon's responsibility to decide how to proceed. iMRI produced inconclusive results in four cases, with a second biopsy attempt during the same surgery performed in one of those cases. Interestingly, the remaining three cases were concluded, and the definitive histology yield was not diagnostic.
iHE yielded inconclusive results in 12 cases. In two of these, a second biopsy attempt was made during the same surgery. Notably, iMRI was conducted in 10 of the 12 cases, all of which returned positive results. In these 10 cases, the definitive histology yield was diagnostic.
4. Discussion
The primary goal of surgically treating suspected brain lymphoma is to obtain a definitive tissue diagnosis, usually through stereotactic biopsy. To increase diagnostic accuracy and minimize the need for additional surgery, intraoperative assessment methods like intraoperative MRI and histopathological examination are used. Our study compared these two techniques, finding that iMRI has superior sensitivity and specificity, leading to a higher rate of accurate positive biopsies. To the best of our knowledge, this paper is the first to compare these two intraoperative methods.
4.1. Intraoperative MRI
The advent of intraoperative MRI has revolutionized numerous neurooncological surgical procedures, including glioma and pituitary adenoma surgeries. In stereotactic brain biopsies, iMRI enables confirmation of the sample collection point during the procedure. iMRI systems can be broadly categorized into high-field and low-field systems, each with distinct advantages and limitations. High-field iMRI, as utilized in our study, provides superior image quality comparable to standard MRI examinations. It is typically used after sample collection to confirm that the target lesion has been reached and to rule out possible complications (Lu et al., 2019). However, this system typically necessitates moving the anesthetized patient into a separate MRI scanner, which can prolong the procedure and requires careful coordination to avoid disrupting the surgical setup. Conversely, low-field iMRI systems offer the advantage of real-time needle position verification directly in the operating room, albeit with compromised image quality. Quinn et al. reported that low-field iMRI facilitated real-time correction of biopsy needle trajectory (Quinn et al., 2011).
While our study emphasizes the diagnostic and procedural advantages of iMRI over iHE, it is essential to acknowledge the significant practical challenges associated with its routine implementation. iMRI, particularly high-field systems, demands extensive infrastructure investments, including the necessary space, specialized equipment, and technical expertise. Furthermore, anesthesia management during iMRI requires additional precautions due to the confined space and magnetic environment, potentially prolonging the operation time and complicating intraoperative workflows. These factors can be prohibitive for smaller centers or institutions without access to iMRI facilities. It is also crucial to delineate the clinical contexts where the benefits of iMRI, in terms of diagnostic precision and patient outcomes, justify its higher cost and resource allocation compared to iHE. Understanding these trade-offs between diagnostic accuracy, operating times, and financial feasibility will aid in tailoring iMRI use to specific patient populations and institutional capabilities, thereby optimizing resource utilization.
4.2. Intraoperative histological examination
Intraoperative histological examination, particularly frozen section analysis, is another technique employed to verify biopsy adequacy during surgery. Many neurosurgeons consider iHE as a standard examintation during stereotactic brain biopsy (Tilgner et al., 2005; Mathon et al., 2019b).
However, the frozen section technique yields histological smears of limited quality compared to definitive histology smears, which can sometimes make interpretation challenging. Tilgner et al. retrospectively analyzed 5000 consecutive stereotactic brain biopsies from 4589 patients. They found, that iHE diagnosis was correct in 90.3% of biopsies. This included complete correlation in 81.3% of the biopsies and partial correlation in 9% of the biopsies. In the subgroup of brain lymphoma biopsies (n = 210), complete correlation was found in 88.6% of cases (Tilgner et al., 2005).
In this present paper, we observed much lower rate of concordant results (55,3%; 26/47 cases) compared to the literature and several factors may play a role to this. First, there is an inherent bias to the group of negative iHE. In the instances reported as negative (i.e. showing normal brain tissue), this likely meant that the tumor was missed at first sampling. However, as this was reported to the neurosurgeon for consideration, additional sampling of diagnostic tissue was likely performed from different area adjacent to the primary biopsy site. Thus, negative report effectively prompted sampling from different location. The situation is more interesting in inconclusive category, standing for the situations of cleraly pathological microscopic findings falling short of the lymphoma diagnosis. One basic scenario includes presence of limited lymphocytic infiltrates around the vessels that are considered suspicious or pathological, but not diagnostic of the lymphoma. As the additional immunohistochemistry tests allows for precise detection of pathological lymphoid cells even in scant numbers, this is usually resolved as a diagnostic result in definitive histology report. Second scenario involves presence of reactive glial cells and/or foamy macrophages and small lymfocytes, usually as a result of previous corticosteroid treatment. In such situation, the number of neoplastic cells is highly reduced, although, again, these are usually identifiable on subsequent immunohistochemical work-up in definitive sample. Furthermore, presence of foamy cells together with non-neoplastic small lymphocytes arises diagnostic question of demyelinating disorder or infection, while exuberant reactive glial cells may arise suspicion on glial neoplasm. Finally, it is widely understood, that frozen section technique yields slides of limited quality, compared to the formalin-fixed paraffin-embedded tissue sections. This may lead to uncertainities and more conservative approach to biopsy interpretation.
4.3. ALA
Another intraoperative diagnostic tool for high-grade glioma is the detection of a fluorescence signal resulting from the metabolization of aminolevulinic acid (ALA). Millesi et al. concluded that the diagnostic accuracy in cases exhibiting strong fluorescence, without intraoperative histopathological analysis, was comparable to those showing weak or no fluorescence where intraoperative histopathology was employed (Millesi et al., 2020). However, this technique is specifically applicable to high-grade gliomas and requires the preoperative administration of ALA and the use of specialized detection equipment, typically a microscope or exoscope.
4.4. Overall survival
In the comparative analysis using the ECOG scale, we observed statistically significant disparities in outcomes between Group 1 (ECOG performance status 0–1) and Group 2 (ECOG performance status 2–4) (p = 0.010). For instance, one month post-biopsy, the survival rate was 88% in Group 1, in stark contrast to just 65% in Group 2. When considering only patients with an ECOG performance status of 3 or 4, only 44% of these patients survive the first month.
The difference in survival rates is not surprising; however, it highlights a critical issue: many patients in poor condition at the time of surgery do not live long enough to commence oncological treatment. This underscores the importance of rapid and accurate diagnosis, and we advocate for the routine use of intraoperative examinations such as iMRI and iHE.
4.5. Corticosteroids
The statistical analysis of the effect of corticosteroids therapy (CST) on negative biopsy outcomes in our series was not significant. Common consensus among neurosurgeons is, that preoperative CST should be avoided as it seems to diminish the diagnostic rate of biopsy in CNS lymphoma patients (Scheichel et al., 2021). It is known, that corticosteroids exert a profound apoptotic effect on lymphoma cells, often leading to their rapid disappearance, a phenomenon referred to as vanishing lymphoma (Giannini et al., 2014). Many papers report that prior corticosteroid treatment complicates histological interpretation in as many as 50% of subsequent CNS biopsies, increasing subjectivity and diagnostic uncertainty (Önder et al., 2015; Kan et al., 2011; Brück et al., 2013). On the other hand other papers did not find effect of steroids on biopsy yield (Binnahil et al., 2016; Bullis et al., 2020).
Our finding that CST does not influence biopsy yield might be subject to bias, as navigation MRI is routinely performed a day before surgery. If there is a marked reduction in the enhanced lesion, the surgical procedure is typically deferred. Scheichel et al. argue that if CST has been administered preoperatively and there is still a contrast enhancing lesion to target for biopsy, surgeons should try to keep the diagnostic delay to a minimum as the likelihood for acquiring diagnostic tissue seems sufficiently high (Scheichel et al., 2021).
4.6. Anesthesiological considerations
Two primary anesthesiological challenges arise during brain lymphoma surgeries. First, the frame-based biopsy technique often necessitates fiberoptic intubation for airway management, requiring a skilled professional. Second, iMRI requires the anesthesiologist to manage patient transport and safety, ensuring the removal of all ferromagnetic objects and using earplugs to protect patients hearing. Adequate preparation of medications for all possible scenarios inside the iMRI suite is essential.
4.7. Limitations
Our study's retrospective nature limits control over confounding variables and biases that are better managed in a prospective analysis. Using retrospective data prevents standardization of procedures and patient selection, which may have impacted surgical outcomes and biopsy diagnostic yields.
As a single-center study, our results reflect a specific institutional protocol and patient population, which may not represent the broader clinical community. Differences in practices, patient demographics, and surgical techniques at other centers may limit the generalizability of our findings.
Additionally, not all surgeries used both iMRI and iHE together. The subset where both modalities were used may not be representative of the entire sample, introducing selection bias in the comparative analysis of iMRI and iHE. Surgeon preference largely dictated the use of either or both tools, reflecting a potential selection bias.
5. Conclusion
This study analyzes the diagnostic yield in brain lymphoma surgeries, comparing the effectiveness of intraoperative MRI (iMRI) and intraoperative histological examination (iHE) for obtaining conclusive biopsy samples. Our findings highlight the important role of accurate intraoperative diagnostics in improving patient outcomes. iMRI demonstrated higher sensitivity (80%) and specificity (98.51%) compared to iHE, which had sensitivity and specificity of 66.67% and 59.09%, respectively. These results emphasize the superiority of iMRI in confirming positive biopsy yields, reducing the need for additional surgeries. This technology allows surgeons to verify sample adequacy in real-time, increasing the likelihood of diagnostic success on the first attempt. Therefore, we recommend prioritizing iMRI as part of the surgical strategy for brain lymphoma when available.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Netuka reports financial support was provided by Charles University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by Institutional support MO1012 by Ministry of Defence of the Czech Republic and Cooperatio Neuroscience by Charles University. The funders of the study had no role in the study design nor the collection, analysis, and interpretation of data, writing of the report, or decision to submit the manuscript for publication.
During the preparation of this work the author(s) used ChatGPT-4 (OpenAI) to improve readability and language. After using this tool, the author(s) reviewed and edited the content as needed and take full responsibility for the content of the publication.
Handling Editor: Dr W Peul
References
- Binnahil M., Au K., Lu J.Q., Wheatley B.M., Sankar T. Preprint at Cambridge University Press; 2016. The Influence of Corticosteroids on Diagnostic Accuracy of Biopsy for Primary Central Nervous System Lymphoma; p. 255. [DOI] [PubMed] [Google Scholar]
- Boyd S.D., Natkunam Y., Allen J.R., Warnke R.A. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Appl. Immunohistochem. Mol. Morphol. 2013;21 doi: 10.1097/PAI.0b013e31825d550a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradac O., Steklacova A., Nebrenska K., Vrana J., de Lacy P., Benes V. Accuracy of VarioGuide frameless stereotactic system against frame-based stereotaxy: prospective, randomized, single-center study. World Neurosurg. 2017;104:831–840. doi: 10.1016/J.WNEU.2017.04.104. [DOI] [PubMed] [Google Scholar]
- Brück W., Brunn A., Klapper W., Kuhlmann T., Metz I., Paulus W., Deckert M. Differential diagnosis of lymphoid infiltrates in the central nervous system: experience of the Network Lymphomas and Lymphomatoid Lesions in the Nervous System. Pathologe. 2013;34:186–197. doi: 10.1007/s00292-013-1742-9. [DOI] [PubMed] [Google Scholar]
- Bullis C.L., Maldonado-Perez A., Bowden S.G., Yaghi N., Munger D., Wood M.D., Barajas R.F., Ambady P., Neuwelt E.A., Han S.J. Diagnostic impact of preoperative corticosteroids in primary central nervous system lymphoma. J. Clin. Neurosci. 2020;72:287–291. doi: 10.1016/j.jocn.2019.10.010. [DOI] [PubMed] [Google Scholar]
- Dhawan S., He Y., Bartek Jr J., Alattar A.A., Chen C.C. Comparison of frame-based versus frameless intracranial stereotactic biopsy: systematic review and meta-analysis. World Neurosurg. 2019;127:607–616. doi: 10.1016/j.wneu.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Georgiopoulos M., Ellul J., Chroni E., Constantoyannis C. Efficacy, safety, and duration of a frameless fiducial-less brain biopsy versus frame-based stereotactic biopsy: a prospective randomized study. J. Neurol. Surg. Cent. Eur. Neurosurg. 2018;79:31–38. doi: 10.1055/s-0037-1602697. [DOI] [PubMed] [Google Scholar]
- Giannini C., Dogan A., Salomão D.R. CNS lymphoma: a practical diagnostic approach. J. Neuropathol. Exp. Neurol. 2014;73:478–494. doi: 10.1097/NEN.0000000000000076. [DOI] [PubMed] [Google Scholar]
- Hasner M.C., van Opijnen M.P., van der Meulen M., Verdijk R.M., Maas S.L.N., te Boome L.C.J., Broekman M.L.D. Diagnostics and treatment delay in primary central nervous system lymphoma: what the neurosurgeon should know. Acta Neurochir. 2024;166:261. doi: 10.1007/s00701-024-06138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan E., Levi I., Benharroch D. Alterations in the primary diagnosis of lymphomas pretreated with corticosteroid agents. Leuk. Lymphoma. 2011;52:425–428. doi: 10.3109/10428194.2010.544049. [DOI] [PubMed] [Google Scholar]
- Livermore L.J., Ma R., Bojanic S., Pereira E.A.C. Yield and complications of frame-based and frameless stereotactic brain biopsy-The value of intra-operative histological analysis. Br. J. Neurosurg. 2014;28:637–644. doi: 10.3109/02688697.2014.887657. [DOI] [PubMed] [Google Scholar]
- Lu C.Y., Xu Z.S., Ye X. Evaluation of intraoperative MRI-assisted stereotactic brain tissue biopsy: a single-center experience in China. Chin Neurosurg J. 2019;5 doi: 10.1186/s41016-019-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathon B., Amelot A., Mokhtari K., Bielle F. Increasing the diagnostic yield of stereotactic brain biopsy using intraoperative histological smear. Clin. Neurol. Neurosurg. 2019;186 doi: 10.1016/j.clineuro.2019.105544. [DOI] [PubMed] [Google Scholar]
- Mathon B., Amelot A., Mokhtari K., Bielle F. Increasing the diagnostic yield of stereotactic brain biopsy using intraoperative histological smear. Clin. Neurol. Neurosurg. 2019;186 doi: 10.1016/j.clineuro.2019.105544. [DOI] [PubMed] [Google Scholar]
- Millesi M., Kiesel B., Wöhrer A., Mercea P.A., Bissolo M., Roetzer T., Wolfsberger S., Furtner J., Knosp E., Widhalm G. Is intraoperative pathology needed if 5-aminolevulinic-acid-induced tissue fluorescence is found in stereotactic brain tumor biopsy? Neurosurgery. 2020;86 doi: 10.1093/neuros/nyz086. [DOI] [PubMed] [Google Scholar]
- Moriarty T.M., Quinones-Hinojosa A., Larson P.S., Alexander E.I.I.I., Gleason P.L., Schwartz R.B., Jolesz F.A., Black P.McL. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: stereotactic brain biopsy. Neurosurgery. 2000;47 doi: 10.1097/00006123-200011000-00023. [DOI] [PubMed] [Google Scholar]
- Önder E., Arıkök A.T., Önder S., Han Ü., Sorar M., Kertmen H., Yılmaz E.D., Fesli R., Alper M. Corticosteroid pre-treated primary CNS lymphoma: a detailed analysis of stereotactic biopsy findings and consideration of interobserver variability. Int. J. Clin. Exp. Pathol. 2015;8:7798. [PMC free article] [PubMed] [Google Scholar]
- Quinn J., Spiro D., Schulder M. Stereotactic brain biopsy with a low-field intraoperative magnetic resonance imager. Neurosurgery. 2011;68 doi: 10.1227/NEU.0b013e31820826c2. [DOI] [PubMed] [Google Scholar]
- Scheichel F., Marhold F., Pinggera D., Kiesel B., Rossmann T., Popadic B., Woehrer A., Weber M., Kitzwoegerer M., Geissler K., et al. Influence of preoperative corticosteroid treatment on rate of diagnostic surgeries in primary central nervous system lymphoma: a multicenter retrospective study. BMC Cancer. 2021;21 doi: 10.1186/s12885-021-08515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilgner J., Herr M., Ostertag C., Volk B. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: intraoperative versus final diagnosis - influence of clinical factors. Neurosurgery. 2005;56:257–263. doi: 10.1227/01.NEU.0000148899.39020.87. [DOI] [PubMed] [Google Scholar]
- Ungar L., Nachum O., Zibly Z., Wohl A., Harel R., Attia M., Spiegelmann R., Zaubermann J., Feldman Z., Knoller N., et al. Comparison of frame-based versus frameless image-guided intracranial stereotactic brain biopsy: a retrospective analysis of safety and efficacy. World Neurosurg. 2022;164:e1–e7. doi: 10.1016/j.wneu.2021.07.063. [DOI] [PubMed] [Google Scholar]
- Woodworth G.F., McGirt M.J., Samdani A., Garonzik I., Olivi A., Weingart J.D. Frameless image-guided stereotactic brain biopsy procedure: diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J. Neurosurg. 2006;104:233–237. doi: 10.3171/jns.2006.104.2.233. [DOI] [PubMed] [Google Scholar]