Abstract
Background
With the widespread use of abdominal ultrasonography (US), incidental detection of common bile duct (CBD) dilatation is common in pediatric populations. This study investigated the causes and clinical significance of CBD dilatation in children without biliary symptoms, jaundice, or causative lesions in US.
Methods
We retrospectively reviewed pediatric patients with CBD dilatation from July 2013 to June 2023. All cases were detected via abdominal US. We analyzed the patients’ clinical manifestations, laboratory data, diagnosis, underlying diseases, and clinical course.
Results
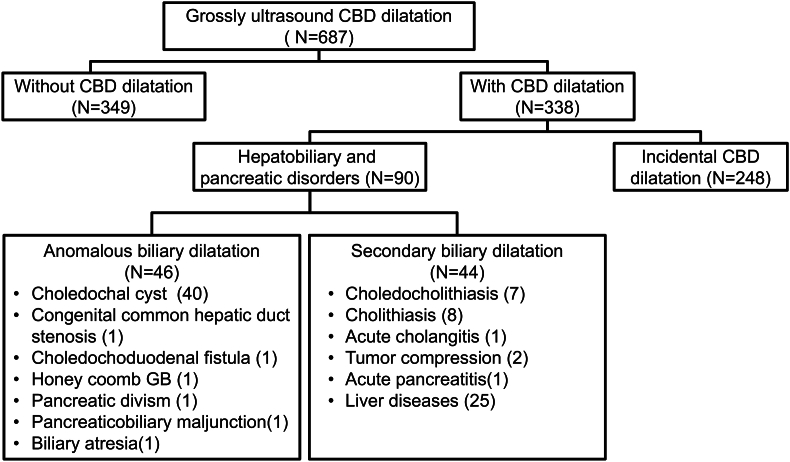
In a total of 687 patients enrolled, 338 met inclusion criteria (90 in hepatobiliary, 248 in CBD dilatation group). Of 128 patients with incidental CBD dilatation who underwent regular US examinations, 91 (71.1%) experienced resolution during follow-up. The proportion of patients with intrahepatic duct dilatation was significantly higher in the non-resolution group (p = 0.038). General health examination group had significant smaller CBD diameter compared to the gastrointestinal and infection groups. Correlation analysis found starting point of resolution decline at 3.24 mm (all-inclusive) and 2.51 mm (infant group) CBD diameter.
Conclusions
Most children with incidental CBD dilatation did not have abnormal hepatobiliary function or other sonographic abnormalities. They usually remained asymptomatic and experienced uneventful clinical courses.
Keywords: Incidental common bile duct dilatation, Ultrasonography, Clinical characteristics, Outcome, Children, Pediatric
Highlights
-
•
Most children with incidental CBD dilatation remained asymptomatic and experienced uneventful clinical courses. The high rate of spontaneous resolution supports a conservative approach to management.
-
•
In the infant group, the resolution rate began to decline when the CBD diameter exceeded 2.51 mm, whereas the children group experienced a decrease in the resolution rate with an abnormal CBD diameter >4 mm.
-
•
Despite GI and infection groups having larger CBD sizes than the GHE group, the resolution rates among the three groups remain similar.
1. Introduction
Common bile duct (CBD) dilatation is a frequently observed finding in ultrasonography (US). Although several studies have investigated CBD measurements in children, no consensus has been reached regarding the standard diameter of the CBD in different pediatric age groups [[1], [2], [3]]. A widely accepted cutoff indicating the need for further clinical investigations to avoid hepatobiliary disease is a CBD diameter >7 mm [[4], [5], [6], [7]]. Notably, however, the ambiguity of age and various conditions, such as previous hepatobiliary surgery, medication, fluid status, current disease, and operators’ subjectivity, can influence diameter measurements [8]. Nonetheless, identifying abnormal dilatation of the CBD is crucial because it may be associated with congenital anomalies and pathological conditions.
In adults, asymptomatic biliary dilatation is carefully evaluated because of the potential risk of malignancy [9,10]. However, its causes in the pediatric population differ from those in adults, with a higher incidence of congenital anomalies but a lower incidence of malignancy [11]. Despite these differences, understanding of the clinical outcomes and significance of asymptomatic biliary dilatation in both age groups remains limited [6].
US, a noninvasive and cost-effective examination, is widely used to monitor and diagnose hepatobiliary diseases in pediatric patients. It is a well-documented technique known for its sensitivity and specificity in detecting hepatobiliary diseases [12,13]. During US examinations, CBD may be incidentally observed. In this study, we investigated the natural course and clinical significance of incidentally discovered CBD dilatation in pediatric patients; we also evaluated the optimal cut-off CBD diameter for predicting the resolution of CBD dilatation.
2. Materials and methods
2.1. Study design
We retrospectively evaluated patients who received abdominal US from July 2013 to June 2023 at Chang Gung Memorial Hospital, Linkou Branch. At our institution, abdominal US is commonly used as a first-line tool to evaluate gastrointestinal illnesses (such as vomiting, diarrhea, abdominal pain, or fever), abdominal masses, malignancies, congenital anomalies, precocious puberty, short stature, and obesity and to perform newborn examinations. We analyzed data on age, sex, clinical symptoms and signs, liver function test results, diagnoses, associated diseases, complications, resolution rate, and duration of CBD dilatation.
2.2. Inclusion and exclusion criteria
The inclusion criteria were an age of 0–17 years and meeting the diagnostic criteria for CBD dilatation. CBD dilatation was defined as a CBD diameter >2 mm in infancy, >4 mm in childhood, or >7 mm in adolescence [3]. The exclusion criteria were a history of hepatobiliary surgery and an age ≥18 years.
2.3. US assessment of bile duct dilatation
We utilized two US machines (LOGIQ S8 XDclear and Logiq S7 Expert; GE HealthCare, Gyeonggi-do, Korea) with a 1–6 MHz or 2–11 MHz convex-array probe for the abdominal US examinations. CBD diameter was measured from the sagittal scan. All operators performing the procedures were trained and experienced pediatric gastroenterologists. CBD measurements were repeated three times, and the mean of the three values was calculated.
2.4. Categorization and evaluation of patients
The patients were divided into two categories based on clinical findings: the ‘hepatobiliary and pancreatic disorders’ (HPD) group (those with these disorders) and the incidental CBD dilatation group (those in whom CBD was discovered incidentally via US examination). Within the incidental group, we further divided the patients into four subgroups based on the context in which CBD was discovered: during a gastrointestinal work-up (GI group), during a general health examination (GHE group), during US imaging to assess treatment of an infection (Infection group), and during image analyses of other disorders (Other group). Then we analyzed the variation in CBD diameter across these subgroups. We also classified the patients in the incidental group into resolution and non-resolution groups to better understand the correlation between CBD diameter and resolution rate. Finally, we categorized the patients into three age groups (infants [<1 year old], children [1–12 years old], and adolescents [>12 years old]) to further analyze the correlations among CBD size, and resolution rate.
2.5. Definitions of anomalous biliary dilatation and resolution
The definitions of anomalous biliary dilatation include choledochal cysts, pancreaticobiliary maljunction, and biliary obstruction. Secondary biliary dilatation refers to dilatation of the bile ducts caused by conditions such as tumor compression, gallbladder stone obstruction, and pancreatitis [2,14]. Resolution was defined as the point at which the CBD returned to a diameter ≦2 mm during infancy, ≦4 mm during childhood, or ≦7 mm during adolescence with no clinical symptoms or signs [3].
2.6. Statistical analysis
All statistical analyses were conducted using Excel software for Windows, Version 2010. Patient demographic information was recorded, including age, sex, CBD diameter, other US findings, liver function test results [15,16], and follow-up outcomes. The results are presented as mean, median, standard deviation, and interquartile range. Several statistical analyses were performed to determine the differences between groups: the chi-square test, Student's t-test, polynomial regression analysis, and the R2 test. A statistically significant difference was defined as p < 0.05.
Ethics approval
The study protocol complied with the Declaration of Helsinki and was approved by the ethics committee of Chang Gung Memorial Hospital (IRB No. 202301570B0).
3. Results
3.1. Patients and subgroups
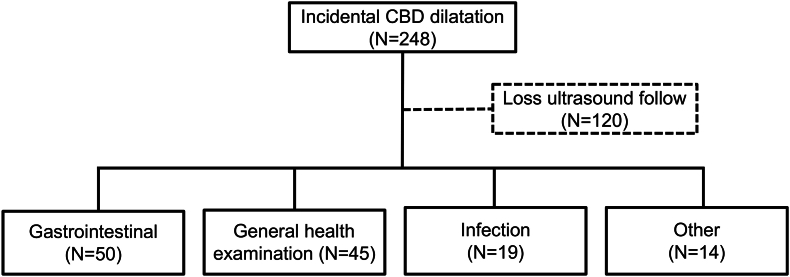
[Fig. 1, Fig. 2] show the algorithm for selecting and classifying patients. In total, 687 patients were found to have CBD dilatation. Among them, 338 met the inclusion criteria and were enrolled. These patients were categorized into the HPD group (n = 90, 26.6%) and the incidental CBD dilatation group (n = 248, 73.4%). In the former, 46 patients had anomalous biliary dilatation and 44 had secondary biliary dilatation. In the later, 128 patients underwent regular US examinations during their follow-up. Among these, 50 (39.1%) were assigned to the GI group, 45 (35.1%) were assigned to the GHE group, 19 (14.8%) were assigned to the Infection group, and 14 (11.0%) were assigned to the Other group. This last group included patients with congenital anomalies (n = 7), oncology and hematology disorders (n = 5), autoimmune disease (n = 1), and neurological disease (n = 1) [Fig. 2].
Fig. 1.
Flow chart illustrating patient grouping and selection process. Among 687 patients, a total of 338 patients met the inclusion criteria.
Fig. 2.
Patient distribution of incidental common bile duct (CBD) dilatation group. The incidental CBD dilatation group comprised 248 cases, of which 120 patients lost ultrasound follow-up. The remaining cases were divided into gastrointestinal, health examination, infection, and other groups.
3.2. Demographics and clinical characteristics between resolution and non-resolution groups
[Table 1] shows the differences in demographics and etiologies among patients with incidental CBD dilatation between the resolution and non-resolution groups. The resolution group included 91 (71.1%) patients, and the non-resolution group included 37 (28.9%) patients. In the non-resolution group, 15 (40.5%) patients had a decreased CBD diameter that did not return to the normal range, 13 (35.1%) had a stationary CBD diameter, and 9 (24.4%) had an increased CBD diameter at the last follow-up evaluation (mean follow-up period is 510 days, ranging from 5 to 2788 days).
Table 1.
Risk factors for incidentally discovered biliary tract dilatation.
| Resolution (N = 91) | Non-resolution (N = 37) | p-value | ||
|---|---|---|---|---|
| Age (mo) | 47.7 48.0 | 45.9 52.4 | 0.857a | |
| Male: Female |
33 : 58 |
19 : 18 |
0.115 b |
|
| Common bile duct size (mm) | 4.37 1.69 | 4.48 1.88 | 0.764a | |
| Gall bladder sludge (%) | 6.6 | 2.7 | 0.801 b | |
| Gall bladder wall thickening (%) | 5.5 | 5.4 | 0.498 b | |
| Hepatomegaly (%) | 9.9 | 10.8 | 0.876 b | |
| IHD dilatation (%) | 1.1 | 8.1 | 0.038 b | ∗ |
aContinuous variables are expressed as mean ± standard deviation and were analyzed using Student's t-test. Mean values were significantly different between variables (∗p < 0.05). bDescriptive data were analyzed using the chi-square test; the number (percentage) was significantly different between variables (∗p < 0.05).
There were no statistically significant differences in age (p = 0.857), sex (p = 0.115), laboratory test, and predictive factor relative to CBD dilatation (i.e., CBD diameter (p = 0.764), the proportion of gallbladder sludge (p = 0.801), gallbladder wall thickening (p = 0.498), and hepatomegaly (p = 0.876)) observed between the two groups. The proportion of patients with intrahepatic duct (IHD) dilatation was significantly higher in the non-resolution group (p = 0.038).
[Table 2] summarizes the differences in liver function test results between these two groups. There were no significant differences for aspartate aminotransferase (value: p = 0.837, abnormal ratio: p = 0.502), alanine aminotransferase (p = 0.289, p = 0.663), γ-glutamyl transferase (p = 0.089, p = 0.369), alkaline phosphatase (p = 0.233, p = 0.127), direct bilirubin (p = 0.585, p = 0.115), or total bilirubin (p = 0.715, p = 0.082). The liver function recovery rate was higher in the resolution group but without statistical significance (p = 0.402).
Table 2.
Probable factors explaining the resolution rate in incidentally discovered biliary tract dilatation (based on liver function tests).
| Resolution (N = 91) median (IQR) | Non-resolution (N = 37) median (IQR) | p-value | |
|---|---|---|---|
| Aspartate transaminase (IU/L) | 38.0 (29.0–63.0) | 36.5 (27.8–56.8) | 0.837a |
| Alanine transaminase (IU/L) | 21.5 (13.7–80.5) | 22.0 (12.0–46.3) | 0.289a |
| γ-glutamyl transferas (IU/L) | 89 (11.2–196.8) | 16.0 (11.0–87.0) | 0.089a |
| Alkaline phosphatas (IU/L) | 237.0 (154.0–293.0) | 261.0 (206.8–333.5) | 0.233a |
| Direct bilirubin (IU/L) | 0.3 (0.2–0.5) | 0.3 (0.1–0.8) | 0.585a |
| Total bilirubin (IU/L) |
0.8 (0.4–3.4) |
0.8 (0.2–4.8) |
0.715a |
| Aspartate transaminase abnormal (%) | 25.0 | 18.5 | 0.502 b |
| Alanine transaminase abnormal (%) | 26.6 | 22.2 | 0.663 b |
| γ-glutamyl transferas abnormal (%) | 18.8 | 11.1 | 0.369 b |
| Alkaline phosphatas abnormal (%) | 3.1 | 11.1 | 0.127 b |
| Direct bilirubin abnormal (%) | 12.5 | 25.9 | 0.115 b |
| Total bilirubin abnormal (%) | 14.1 | 29.6 | 0.082 b |
| Liver function abnormal (%) | 40.6 | 44.4 | 0.736 b |
| Liver function recovery rate (%) | 92.3 | 83.3 | 0.402 b |
aContinuous variables are expressed as mean ± standard deviation and were analyzed using Student's t-test. Mean values were significantly different between variables (∗p < 0.05). bDescriptive data were analyzed using the chi-square test; the number (percentage) was significantly different between variables (∗p < 0.05).
3.3. Demographic and clinical characteristics among different diagnosis groups
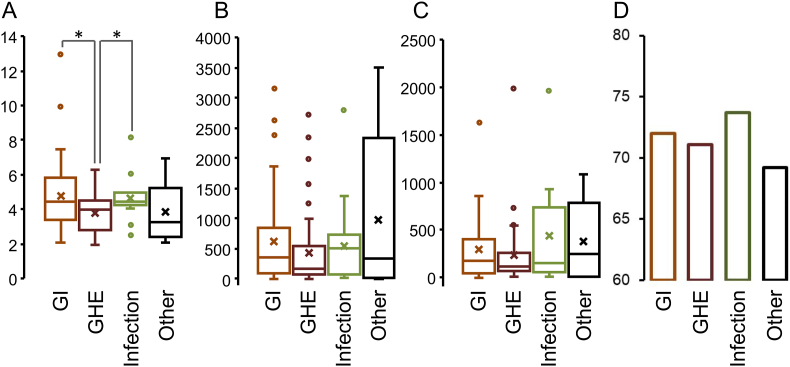
[Table 3] and [Fig. 3] compare demographic and clinical characteristics aomng the four diagnosis greoups using chi-square and t-tests. The GI group had the largest CBD diameter (mean, 4.8 ± 2.0 mm), and that of the GHE group was significantly smaller than those of the gastrointestinal and infection groups. There were no significant differences in the duration of resolution of CBD dilatation, follow-up period, or resolution rate among the groups.
Table 3.
Information on CBD diameter, resolution rate, resolution time, and ultrasound follow-up.
| Gastrointestinal (N = 50) | Health Examination (N = 45) | Infection (N = 19) | Others (N = 14) | |
|---|---|---|---|---|
| CBD size (mm) | 4.8 ± 2.0 | 3.8 ± 1.2 | 4.6 ± 1.2 | 3.9 ± 1.6 |
| Resolution rate (%) | 72.0 | 71.1 | 73.7 | 69.2 |
| Resolution time (Day) | 180 (49–399) | 118 (75–255) | 154 (67–714) | 248 (22–2339) |
| Ultrasound follow period (Day) | 366 (97–861) | 181 (89–550) | 527 (101–574) | 347 (22–2337) |
CBD size: Mean ± standard deviation.
Resolution time and Ultrasound follow period: Median (IQR).
Fig. 3.
Comparison of (A) CBD size (mm), (B) resolution time (day), (C) ultrasound follow period (day), and (D) resolution rate (%) of incidentally found biliary tract dilatation among designed diagnosis groups. Chi-square and t-tests were applied to perform statistical comparisons. ∗: p <0.05.
3.4. Correlation between resolution rate and CBD diameter
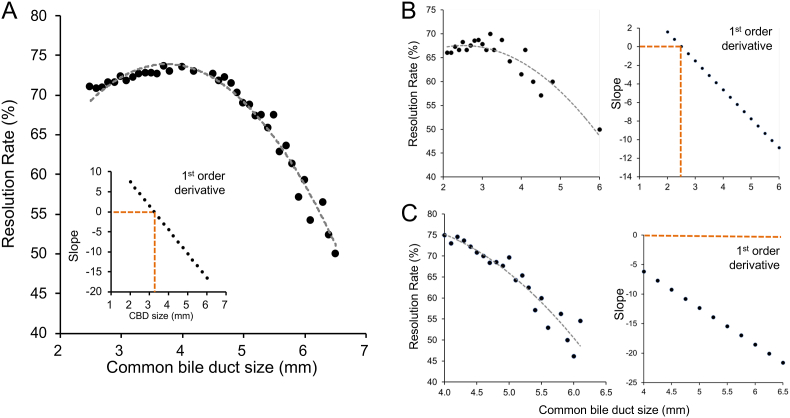
[Fig. 4] shows the correlation between the resolution rate and CBD diameter in different patient groups. The CBD diameter corresponding to a slope equal to zero represented the starting point of the decline of the resolution rate. The CBD diameter was 3.24 mm in the all-inclusive patient group and 2.51 mm in the infant group. No slope or CBD diameter interval was present in the children group.
Fig. 4.
Correlation between resolution rate and CBD size in (A) all-inclusive patients, (B) infant, and (C) children group. The regression curve and first-order differential plot are shown in all three groups. The CBD size corresponding to the slope equal to zero is determined as the starting point of the resolution rate decline. The CBD size was 3.24 mm in (A) the all-inclusive patient group and 2.51 mm in (B) the infant group. No slope and CBD size interval exist in (C) the children group.
4. Discussion
CBD dilatation is common in Asian children, particularly in girls [17,18]. It is often caused by congenital anomalies [11]. However, patients do not always exhibit symptoms, and they may have multiple symptoms such as jaundice, abnormal liver function, abdominal pain, or a palpable abdominal mass [19]. In addition, there is no standard range for what is considered a normal CBD diameter among different pediatric age groups [[1], [2], [3]]. Although an abnormal CBD diameter is commonly considered 7 mm [[4], [5], [6], [7]], prior studies highlight a diverse range of sizes based on the causes and patient age. For instance, Chan et al. [20] found that the mean diameter of choledochal cysts was 50 mm with a range of 20–120 mm. Lee et al. [21] observed that the mean extrahepatic bile duct diameter in patients with biliary atresia-associated biliary cysts was 7.9 ± 1.5 mm. When examining infantile non-biliary atresia-associated choledochal cyst, the mean diameter was 16.0 ± 3.7 mm. By contrast, late infantile non-biliary atresia-associated choledochal cysts exhibited a larger mean diameter of 21.5 ± 5.6 mm. Thus, there is variability in CBD dimensions across different clinical conditions and patient populations. Therefore, it is crucial to understand the natural course and clinical significance of incidentally discovered CBD dilatation in pediatric patients and evaluate the correlation between CBD diameter and the resolution rate among different ages.
In this study, 338 patients were enrolled, of whom 248 (73.3%) showed no causative lesions and maintained a benign course [14,22] during follow-up. The resolution rate in all included patients was 71.09%, similar to the rate of 71.10% in GHE group. Notably, our resolution rate is inconsistent with a previous study that reported that 87% of patients with fusiform dilatations (accounting for 98.12% of asymptotic neonates detected by US) resolved spontaneously [11]. We postulate that this discrepancy might be attributable to greater loss to follow-up among asymptomatic patients and the inclusion of a broader age range, including infants, children, and adolescents. This broader range may have introduced more variability in resolution rates due to the diverse developmental stages and physiological changes that occur across these age groups.
Among the various risk factors evaluated in this study, patients with IHD dilatation showed a significantly lower resolution rate of CBD dilatation. Although the mechanism of IHD dilatation is not fully understood, it might be attributable to bile duct constriction or irregularities caused by genetic factors, innate and adaptive immunity, or exposure to toxins. Another contributory factor might be pancreaticobiliary reflux induced by infection, inflammation, or obstruction [19,[23], [24], [25]]. Our observation is in agreement with earlier research, which similarly showed a higher risk for hepatobiliary disease and a lower resolution rate in patients with IHD dilatation [22]. Furthermore, the GHE group had significantly smaller CBD diameters than the GI and Infection groups, which could be attributable to the temporary disruption of bile flow caused by dehydration [22]. These findings highlight the complex relationship between physiological factors and bile duct dilatation in different patient groups.
We also investigated the correlation between CBD diameter and resolution rate in patients aged 0–17 years. The resolution rate decreased once the CBD diameter exceeded 3.24 mm. This aligns with a previous study that reported a lower resolution rate in patients with a CBD diameter ≥3 mm [11]. To refine our assessment of CBD size in different age groups, we divided the patients into three groups: infants, children, and a combined group of all ages. In the infant group, the resolution rate began to decline when the CBD diameter exceeded 2.51 mm, whereas the children group experienced a decrease in the resolution rate with an abnormal CBD diameter >4 mm. Although further research is needed for validation, this observation provides preliminary input for establishing specific CBD size ranges based on age.
In this study, we did not observe significant complications in the non-resolution groups, which might be due to the limited and wide range of follow-up periods, previous studies have shown CBD dilatation is possibly associated with biliary tract stones [26,27], acute pancreatitis [[28], [29], [30]] and biliary tract infection [9,22]. Moreover, various risk factors, including biliary tract cancer [26,[31], [32], [33]] and bile duct perforation [34], were reported to be associated with CBD dilatation. Thus, we believe further follow-up and evaluation on CBD size is needed.
Our study had several limitations. First, because of its retrospective design, the patients were investigated under various conditions. Second, although the results underwent review by experienced gastrologists, the variability in US results could be attributable to the subjectivity of the operators and the limitations of US technology. Finally, although we followed the patients for an average period of 29 months, a more extended follow-up duration might be necessary to build more robust and dependable outcomes.
5. Conclusions
We investigated the clinical significance of incidentally discovered CBD dilatation in pediatric populations. Most children with this condition remained asymptomatic and experienced uneventful clinical courses. The high rate of spontaneous resolution supports a conservative approach to management. Our observations also emphasize the importance of variability in CBD sizes across different age groups. Although our study had certain limitations, it provides valuable insight into the natural course of CBD dilatation in pediatric patients. We expect that further studies over a more extended period will determine the long-term outcomes of children with incidental CBD dilatation.
Authors’ contributions
HC and WH contributed to the conception and design of the work. WH, MW, and CC analyzed and interpreted the data. WH, MC, and PJ performed the statistical analysis. HC and WH wrote a draft of the manuscript. All authors contributed to manuscript revision and read and approved the submitted version. The corresponding author had full access to all of the data in this study and made the final decision to submit it for publication.
Consent for publication
All participants gave consent for publication.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics approval and consent to participate
This study protocol complied with the Declaration of Helsinki and was approved by the ethics committee of Chang Gung Memorial Hospital (IRB No. 202301570B0).
Language editing
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see:
Declaration of competing interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors thank all of the children and their parents or caregivers for participating in this study. The authors also thank the medical staff for their clinical care.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Hamada Y, Ando H, Kamisawa T, Itoi T, Urushihara N, Koshinaga T, et al. Diagnostic criteria for congenital biliary dilatation 2015. J Hepatobiliary Pancreat Sci. 2016;23(6):342–346. doi: 10.1002/jhbp.346. [DOI] [PubMed] [Google Scholar]
- 2.Ishibashi H, Shimada M, Kamisawa T, Fujii H, Hamada Y, Kubota M, et al. Japanese clinical practice guidelines for congenital biliary dilatation. J Hepatobiliary Pancreat Sci. 2017;24(1):1–16. doi: 10.1002/jhbp.415. [DOI] [PubMed] [Google Scholar]
- 3.Siegel M.J. In: Pediatric Sonography. fourth ed. Siegel M.J., editor. LIPPINCOTT WILLIAMS & WILKINS; Philadelphia: 2011. Gallbladder and biliary tract; pp. 275–304. [Google Scholar]
- 4.Bruno M, Brizzi RF, Mezzabotta L, Carucci P, Elia C, Gaia S, et al. Unexplained common bile duct dilatation with normal serum liver enzymes: diagnostic yield of endoscopic ultrasound and follow-up of this condition. J Clin Gastroenterol. 2014;48(8):e67–70. doi: 10.1097/MCG.0b013e3182a8848a. [DOI] [PubMed] [Google Scholar]
- 5.Bowie JD. What is the upper limit of normal for the common bile duct on ultrasound: how much do you want it to be? Am J Gastroenterol. 2000;95(4):897–900. doi: 10.1111/j.1572-0241.2000.01925.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim JE, Lee JK, Lee KT, Park DI, Hyun JG, Paik SW, et al. The clinical significance of common bile-duct dilatation in patients without biliary symptoms or causative lesions on ultrasonography. Endoscopy. 2001;33(6):495–500. doi: 10.1055/s-2001-15088. [DOI] [PubMed] [Google Scholar]
- 7.Carroll BA, Oppenheimer DA, Muller HH. High-frequency real-time ultrasound of the neonatal biliary system. Radiology. 1982;145(2):437–440. doi: 10.1148/radiology.145.2.7134449. [DOI] [PubMed] [Google Scholar]
- 8.Di Serafino M, Vitale V, Severino R, Barbuto L, Vezzali N, Ferro F, et al. Pediatric ultrasonography of the pancreas: normal and abnormal findings. J Ultrasound. 2019;22(3):261–272. doi: 10.1007/s40477-018-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon J, Song SY, Lee KT, Lee KH, Bae MH, Lee JK. Clinical significance and long-term outcome of incidentally found bile duct dilatation. Dig Dis Sci. 2013;58(11):3293–3299. doi: 10.1007/s10620-013-2792-9. [DOI] [PubMed] [Google Scholar]
- 10.Smith I, Monkemuller K, Wilcox CM. Incidentally identified common bile duct dilatation: a systematic review of evaluation, causes, and outcome. J Clin Gastroenterol. 2015;49(10):810–815. doi: 10.1097/MCG.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 11.Lin SF, Lee HC, Yeung CY, Jiang CB, Chan WT. Common bile duct dilatations in asymptomatic neonates: incidence and prognosis. Gastroenterol Res Pract. 2014;2014 doi: 10.1155/2014/392562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan SI, Rajan E, Adler DG, Baron TH, Anderson MA, Cash BD, et al. Role of EUS. Gastrointest Endosc. 2007;66(3):425–434. doi: 10.1016/j.gie.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Esparrach G, Ginès A, Sánchez M, Pagés M, Pellisé M, Fernández-Cruz L, et al. Comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the diagnosis of pancreatobiliary diseases: a prospective study. Am J Gastroenterol. 2007;102(8):1632–1639. doi: 10.1111/j.1572-0241.2007.01333.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee HC, Yeung CY, Chang PY, Sheu JC, Wang NL. Dilatation of the biliary tree in children: sonographic diagnosis and its clinical significance. J Ultrasound Med. 2000;19(3):177–182. doi: 10.7863/jum.2000.19.3.177. quiz 183-4. [DOI] [PubMed] [Google Scholar]
- 15.Tietz NW. Clinical guide to laboratory tests. 3rd ed. Saunders; Philadelphia: 1995. 20-11. [Google Scholar]
- 16.Soldin SJ, Brugnara C, Wong EC. Pediatric Reference Ranges. AACC Press; Washington DC: 2003. [Google Scholar]
- 17.Soares KC, Arnaoutakis DJ, Kamel I, Rastegar N, Anders R, Maithel S, et al. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg. 2014;219(6):1167–1180. doi: 10.1016/j.jamcollsurg.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi M. Congenital choledochal cyst. Analysis of 1,433 patients in the Japanese literature. Am J Surg. 1980;140(5):653–657. doi: 10.1016/0002-9610(80)90051-3. [DOI] [PubMed] [Google Scholar]
- 19.Hoilat GJ, John S . StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2024. Choledochal Cyst. [Google Scholar]
- 20.Chan KWE, Lee KH, Tsui SYB, Mou JWC, Tam YHP. Laparoscopic management of antenatally detected choledochal cyst: a 10-year review. Surg Endosc. 2016;30(12):5494–5499. doi: 10.1007/s00464-016-4912-z. [DOI] [PubMed] [Google Scholar]
- 21.Lee HC, Yeung CY, Fang SB, Jiang CB, Sheu JC, Wang NL. Biliary cysts in children--long-term follow-up in Taiwan. J Formos Med Assoc. 2006;105(2):118–124. doi: 10.1016/S0929-6646(09)60332-6. [DOI] [PubMed] [Google Scholar]
- 22.Son YJ, Lee MJ, Koh H, Kim S. Asymptomatic bile duct dilatation in children: is it a disease? Pediatr Gastroenterol Hepatol Nutr. 2015;18(3):180–186. doi: 10.5223/pghn.2015.18.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babbitt DP. Congenital choledochal cysts: new etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb. Ann Radiol. 1969;12(3):231–240. [PubMed] [Google Scholar]
- 24.Prokopič M, Beuers U. Management of primary sclerosing cholangitis and its complications: an algorithmic approach. Hepatol Int. 2021;15(1):6–20. doi: 10.1007/s12072-020-10118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh CW, Kim HW, Yi SW, Lee DK, Lee SJ. Common bile duct stones associated with pancreatobiliary reflux and disproportionate bile duct dilatation. Medicine (Baltim) 2017;96(34) doi: 10.1097/MD.0000000000007701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, et al. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003;10(5):345–351. doi: 10.1007/s00534-002-0741-7. [DOI] [PubMed] [Google Scholar]
- 27.Morine Y, Shimada M, Takamatsu H, Araida T, Endo I, Kubota M, et al. Clinical features of pancreaticobiliary maljunction: update analysis of 2nd Japan-nationwide survey. J Hepatobiliary Pancreat Sci. 2013;20(5):472–480. doi: 10.1007/s00534-013-0606-2. [DOI] [PubMed] [Google Scholar]
- 28.Jesudason SR, Jesudason MR, Mukha RP, Vyas FL, Govil S, Muthusami JC. Management of adult choledochal cysts--a 15-year experience. HPB (Oxford) 2006;8(4):299–305. doi: 10.1080/13651820500466715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swisher SG, Cates JA, Hunt KK, Robert ME, Bennion RS, Thompson JE, et al. Pancreatitis associated with adult choledochal cysts. Pancreas. 1994;9(5):633–637. [PubMed] [Google Scholar]
- 30.Lipsett PA, Pitt HA, Colombani PM, Boitnott JK, Cameron JL. Choledochal cyst disease. A changing pattern of presentation. Ann Surg. 1994;220(5):644–652. doi: 10.1097/00000658-199411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arima E, Akita H. Congenital biliary tract dilatation and anomalous junction of the pancreatico-biliary ductal system. J Pediatr Surg. 1979;14(1):9–15. doi: 10.1016/s0022-3468(79)80569-2. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H, Katayose Y, Rikiyama T, Onogawa T, Yamamoto K, Yoshida H, et al. Advanced bile duct carcinoma in a 15-year-old patient with pancreaticobiliary maljunction and congenital biliary cystic disease. J Hepatobiliary Pancreat Surg. 2008;15(5):554–559. doi: 10.1007/s00534-007-1310-x. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Kubota M, Yagi M, Okuyama N, Ohtaki M, Yamazaki S, et al. An 11-year-old male patient demonstrating cholangiocarcinoma associated with congenital biliary dilatation. J Pediatr Surg. 2006;41(1):e15–9. doi: 10.1016/j.jpedsurg.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 34.Fukuzawa H, Urushihara N, Miyakoshi C, Kajihara K, Kawahara I, Isono K, et al. Clinical features and risk factors of bile duct perforation associated with pediatric congenital biliary dilatation. Pediatr Surg Int. 2018;34(10):1079–1086. doi: 10.1007/s00383-018-4321-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.