Abstract
Background
Pulmonary fibrosis is a progressive diffuse parenchymal lung disorder with a high mortality rate. Studies have indicated that injured lung tissues release various pro-inflammatory factors, and produce a large amount of nitric oxide. There is also accumulation of collagen and oxidative stress-induced injury, collectively leading to pulmonary fibrosis. Antrodia cinnamomea is an endemic fungal growth in Taiwan, and its fermented extracts exert anti-inflammatory effects to alleviate liver damages. Hence, we hypothesized and tested the feasibility of using A. cinnamomea extracts for treatment of pulmonary fibrosis.
Methods
The TGF-β1-induced human lung fibroblast cells (MRC-5) in vitro cell assay were used to evaluate the effects of A. cinnamomea extracts on the collagen production in MRC-5. Eight-week-old ICR mice were intratracheally administered bleomycin and then fed with an A. cinnamomea extract on day 3 post-administration of bleomycin. At day 21 post-bleomycin administration, the pulmonary functional test, the expression level of inflammation- and fibrosis-related genes in the lung tissue, and the histopathological change were examined.
Results
The A. cinnamomea extract significantly attenuated the expression level of collagen in the TGF-β1-induced MRC-5 cells. In the A. cinnamome-treated bleomycin-induced lung fibrotic mice, the bodyweight increased, pulmonary functions improved, the lung tissues expression level of inflammatory factor and the fibrotic indicator were decreased, and the histopathological results showed the reduction of thickening of the inter-alveolar septa.
Conclusions
The Antrodia cinnamomea extract significant protects mice against bleomycin-induced lung injuries through improvement of body weight gain and lung functions, and attenuation of expression of inflammatory and fibrotic indicators.
Keywords: Antrodia cinnamomea, Bleomycin-induced pulmonary fibrosis, mTOR
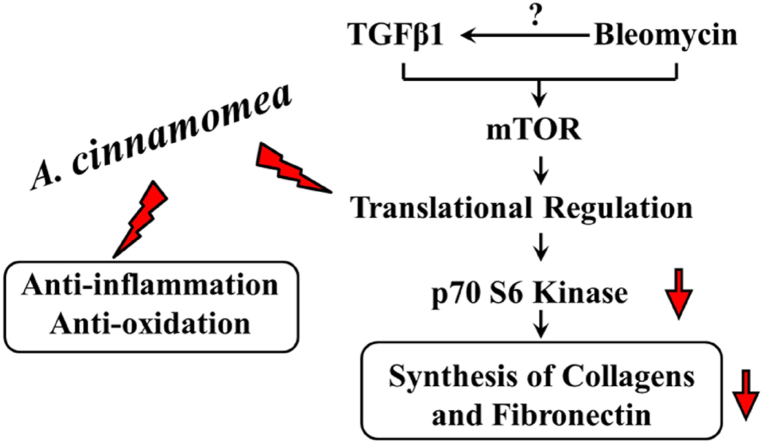
Graphical abstract
Highlights
-
•
A. C extracts attenuates inflammatory effects and protects against oxidative stress.
-
•
A. C extracts inhibits the production of ECM protein through mTOR-mediated pathway.
-
•
A. C extracts alleviates Bleomycin-induced pulmonary fibrosis in Mice
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and almost invariably lethal interstitial lung disease characterized by the involvement of an intricate cytokine network and abnormal accumulation of scar tissues [1,2]. Although two recent clinical trials have demonstrated that slowing IPF progression with medication is possible [3], IPF remains the most life-threatening pulmonary disease with a global median survival of about 3–5 years since initial diagnosis [4].
The fungus Niuchangchih, or Antrodia cinnamomea, was historically used by native Taiwanese as a folk medicine for alleviating discomforts caused by excessive consumption of alcohol or exhaustion. Niuchangchi/A. cinnamomea is currently used as a food supplement; the fruiting bodies and mycelia of A. cinnamomea have been reported to exhibit a number of bioactive activities, including anti-cancer [5], anti-inflammatory and anti-oxidant effects [6,7], and hepato- and neuroprotective functions [8]. The fungus has also been suggested for treatment of diabetes and cardiovascular diseases. Among its diverse pharmacological activities, evidences for hepatoprotective activities, including inhibiting the hepatitis B virus, preventing ethanol- and CCl4-induced liver injuries and anti-hepatocarcinoma, are more convincing and highly recognized [9].
To date, researchers have identified more than a hundred secondary metabolites from A. cinnamomea. These compounds have been shown to protect liver cells against free radical-induced apoptosis via suppressing ROS generation and up-regulation of Bcl-2 in in vitro and in vivo studies [10]. In rat models, CCl4-induced liver injuries, liver fibrosis and lipid peroxidation are both reduced by treatment with an A. cinnamomea extract [[11], [12], [13]]. Wu et al. [14] further demonstrated beneficial effects of A. cinnamomea essence extracts on alcohol-induced liver fibrosis. The protective effects may account for the accelerated alcohol clearance and the suppression of ethanol-induced elevation of pro-inflammatory and fibrotic factors MMP-9, TNF-α, KLF-6 and TGF-β1. However, there have been no published studies on using A. cinnamomea essence extract on treatment of pulmonary fibrosis.
In this work, we investigated if A. cinnamomea essence extract would lead to comprehensive regulation that was directed against oxidative stress-induced cell death, modulation of inflammatory response, and attenuation of extracellular matrix (ECM) production, resulting in inhibited inflammatory response, fibrotic factor production and significant improvements in lung functions in a lung fibrosis mouse model.
2. Material and methods
2.1. Chemicals
Bleomycin sulfate (BLM) from Streptomyces verticillus was obtained from Sigma-Aldrich (St Louis, MO, USA).
2.2. Antrodia cinnamomea essence extract
Antrodia Cinnamomea (Niuchangchih) Liquid Pure Extract was purchased from Chang Gung Biotechnology (Taipei, Taiwan). The extract was isolated from optimal combinations of fruiting bodies and mycelia of the inner trunk cavities of the rare and indigenous tree C. kanehiral. the strain was identified by PCR analysis and sequencing of 5.8s rDNA and flanked internal transcribed spacers (ITS-1 and ITS-2) and showed 99.7% homology with a type strain (BCRC strain AJ496398; Bioresource Collection and Research Center, Hsinchu, Taiwan).
2.3. Cell lines
Human 14-week male embryonal lung cell line MRC-5 (BCRC-60023) was purchased from Bioresource Collection and Research Center, Hsinchu, Taiwan. MRC-5 cells were maintained in Eagle's Minimal Essential Medium (MEM; Life Technologies) supplemented with 10% FBS (Life Technologies) and 1% penicillin/streptomycin (Life Technologies). Mouse macrophage-like cell line RAW264.7 was purchase from American Type Culture Collection. RAW264.7 cells were maintained in DMEM supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin and was incubated in 37 °C in a 5% CO2 incubator.
2.4. Cell viability assay
Cells were fixed in 10% formalin for 30 min and then stained with 0.05 % crystal violet for 30 min at room temperature, washed several times with PBS, and air-dried. An appropriate volume of methanol was then added to the cells to solubilize the dye, followed by measurements at 550 nm. Cell viability was calculated from relative dye intensity compared to that of the control.
2.5. A. cinnamomea essence extract and MRC-5 co-culture assay
The co-culture assay was performed and mild modified as described previously [15]. MRC-5 cells were plated at a density of 2 × 105 cells/well in transwells (BD Biosciences) and 6-well culture plates (BD Biosciences) and the cells were cultured overnight. MRC-5 cells were treated with or without 2.5 ng/mL TGF-β1 (Sino Biological Inc., Beijing, China) for 24 h. After removing the medium, the MRC-5 cells were incubated with 0.6 % or 1.2 % A. cinnamomea essence extract, or with PBS as a control, for 24 h. The cells were harvested for detection of fibronectin mRNA expression level by quantitative real-time RT-PCR.
2.6. RNA isolation and quantitative real-time RT-PCR
Total RNA was prepared from the cell lines and was treated with DNase I (New England BioLabs, Ipswich, MA, USA). RNAs were reverse transcribed into cDNAs at 42 °C for 60 min using Moloney Murine Leukemia Virus Reverse Transcriptase (Life Technologies). After the oligo (dT)-primed reverse transcription reaction, quantitative real-time RT-PCR was performed using LightCycler 480 SyberGreen I Master Mix in LightCycler® 480 Instrument (Roche, Mannheim, Germany) as previously described [16]. Sequences of the mouse gene-specific primers used and for the human fibronectin gene are listed in [Table 2]. For normalization, the GADPH mRNA level in each RNA preparation was also determined. Relative gene expression was determined by the △△Ct method, where Ct is threshold cycle. The relative mRNA levels were normalized to the mRNA level of the reference GADPH gene. The melting curve of the amplification product was always checked to ensure a single clean peak to ensure good-quality quantitative real-time RT-PCR data.
Table 2.
Primer sequences.
| Gene | Primer Sequence |
|---|---|
| iNOS | Forward: CACCTTGGAGTTCACCCAGT |
| Reverse: ACCACTCGTACTTGGGATGC | |
| Ltbp2 | Forward: AACAGCACCAACCACTGTATC |
| Reverse: CCTGGCATTCTGAGGGTCAAA | |
| TIMP-1 | Forward: GCAACTCGGACCTGGTCATAA |
| Reverse: CGGCCCGTGATGAGAAACT | |
| SOD1 | Forward: GCGACGAAGGCCGTGTGCGTG |
| Reverse: CAGTGTGCGGCCAATGATGCA | |
| Fibronectin | Forward: CCCACCGTCTCAACATGCTTAG |
| Reverse:CTCGGCTTCCTCCATAACAAGTAC | |
| Pro-IL-1β | Forward: GCTCATCTGGGATCCTCTCC |
| Reverse: CCTGCCTGAAGCTCTTGTTG | |
| IL-6 | Forward: CCACTTCACAAGTCGGAGGCTTA |
| Reverse: GCAAGTGCATCATCGTTGTTCATAC | |
| Col III | Forward: GTTCTAGAGGATGGCTGTACTAAACACA |
| Reverse: TTGCCTTGCGTGTTTGATATTC | |
| CTGF | Forward: ACCTGGAGGAAAACATTAAGAAGG |
| Reverse: AGCCCTGTATGTCTTCACACTG | |
| β-Actin | Forward:GCGAGAAGATGACCCAGATC |
| Reverse:CCAGTGGTACGGCCAGAGG | |
| GAPDH | Forward: TCCCACCCAGAGACAGCCGC |
| Reverse: CCGTTCACACCGACCTTCAC |
Abbreviations: Col III:collagen type llI;CTGF:connective tissue growth factor;IL6:interleukin 6;iNOS:inducible nitric oxide synthase;Ltbp2:latent-transforming growth factor beta-binding protein 2;Pro-IL-1β:Interleukin-1β precursor;SOD1:copper/zinc superoxide dismutase;TIMP:tissue inhibitor of metalloproteinase;β-Actin:Beta-Actin;GAPDH:glyceraldehyde-3-phosphate dehydrogenase.
2.7. Western blot analysis
Total cellular proteins were isolated from cell lines by the PRO-PREP™ Protein Extraction Solution (Intron Biotechnology, Kyonggi-do, Korea) and Western blot analysis was performed as described previously [17]. Briefly, 25 or 50 μg total proteins from cell lysates or conditioned media was separated in sodium dodecyl sulfate polyacrylamide electrophoresis. After electrophoresis, the resolved proteins were transferred to a PVDF membrane (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk powder (Anchor, Kowloon, Hong Kong) in phosphate buffered saline-Tween (PBS-T), which was composed of phosphate buffered saline (PBS, Sigma-Aldrich) containing 0.1 % Tween-20 in (Sigma-Aldrich), for 1 h and probed overnight with the following antisera at appropriate dilutions in PBS-T: 1:1000 dilution of the anti-Fibronectin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) antiserum and primary antibodies detecting pan-AKT or phospho-AKT S473 (Cell Signaling Technology, Beverly, MA, USA), a 1: 2000 dilution of phospho-p70S6K and total p70S6K, and a 1:10,00 dilution of the anti β-actin (Millipore) antisera. Identification of each protein was achieved with the Western Lighting Plus Reagent (PerkinElmer, Waltham, MA, USA) using an appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson Immuno Research Laboratories, West Grove, PA, USA). Protein levels in the Western blot analysis were detected and quantified by the LAS-3000 chemiluminescence detection device (Fujifilm, Valhalla, NY, USA). To adjust for loading differences, the optical density of each protein was normalized to that of the β-actin band.
2.8. Animal model of bleomycin-induced lung fibrosis
Eight-week old male ICR mice were purchased from BioLASCO Taiwan (Taipei, Taiwan). The mice were maintained in an air-conditioned animal facility under constant temperature and humidity conditions with a 12:12 light-dark cycle and were allowed ad libitum diet and drinking water. All experimental procedures were conducted under the Institutional Animal Care and Use Committee protocols approved by Chang Gung University and in compliance with the Animal Welfare Act and the principles set forth in the Guide for the Care and Use of Laboratory Animals National Research Council, National Academies Press, 1996. Mice were randomly divided into four groups (n = 6 per group): (1) normal control (PBS) group, (2) BLM group, (3) PBS + A. cinnamomea group, and (4) BLM + A. cinnamomea group.
BLM sulfate (Sigma-Aldrich) stock was prepared by dissolving in sterile phosphate-buffered saline (PBS) at 10 mg/mL and storing in small aliquots at 4 °C. Mice were anesthetized by isoflurane (Abbott Laboratories, Abbott Park, IL, USA) inhalation and bleomycin was instilled intratracheally at 1.5 mg/kg bodyweight in 50 μL of sterile PBS. All animals received intratracheal instillation of either bleomycin or PBS on day 0. On day 3, mice were randomly selected for orally gavaged 200 μl of A. cinnamomea extracts or PBS five times a week. On day 18 after A. cinnamomea treatment, animals were placed in the whole-body plethysmograph tanks for analysis of pulmonary functions. At each time point, the mice were sacrificed by an overdose of 2.5 % avertin (Sigma-Aldrich) and tissues were prepared for morphological and biochemical analysis.
2.9. Noninvasive measurement of pulmonary functions by whole-body plethysmography (WBP)
The mice were placed in the main WBP chamber (Buxco Electronic Inc, Wilmington, NC, USA) for functional in vivo measurement. The WBP is used to measure ventilatory parameters by monitoring the box flow pattern by animal's spontaneous breathing. In a period of approximately 5 min, unrestrained mice were monitored and the respiratory parameters, i.e. enhanced pause (Penh), was determined by the use of the WBP software.
2.10. Lung morphometry
Left lungs were fixed with 10 % formalin and were embedded in paraffin sections before staining with hematoxylin and eosin (H&E) (Sigma-Aldrich) according to standard protocols [18]. Briefly, 4-μm serial step sections were taken along the longitudinal axis of the lobe. The fixed distance between the sections was calculated to allow systematic sampling of 10 sections across the whole lobe. The Ashcroft score was used for semi-quantitative assessment of lung fibrotic changes [19]. For evaluation of collagen deposition, Masson's trichrome staining (Trichrome Stain Kit, Sigma-Aldrich) was performed to identify the density and magnitude of collagen fibers, an index of lung fibrosis.
2.11. Semi-quantitative total collagen staining
MRC-5 cells were seeded and treated with TGF-β1 with or without A. cinnamomea for 24 h. At the end of the incubation, the Semi-Quantitative Collagen Assay Kit (Chondrex, Inc., Redmond, WA, USA) was used to detect the amounts of collagen and non-collagen proteins. The stained cells were extracted and the cell extracts were measured by spectrophotometry. The amounts (in μg) of collagen and non-collagenous proteins in each section were calculated based on measurements at OD540 (Sirius Red) and OD605 (Fast Green).
2.12. Statistical analysis
Data are shown as individual data points in a vertical scatter dot plot, with a line to indicate the mean; or the data are shown as bar graphs showing mean ± standard deviation (SD). Comparisons between two groups were analyzed using the two-tailed Student's t-test. For multiple comparisons, one-way analysis of variance analysis was used, followed by the Tukey's post hoc test for analyzing parametric data. All statistical analyses were performed using Graph Pad Prism (GraphPad Software, Inc., San Diego, CA). ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 are considered statistically significant.
3. Results
3.1. A. cinnamomea essence extract attenuated oxidative stress-induced cell death in MRC5 cells
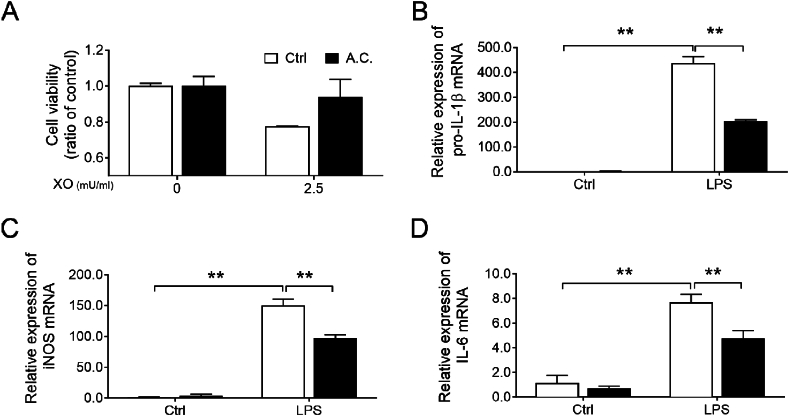
Superoxide generated from xanthine and xanthine oxidase (X/XO) activates lung fibroblasts, which elicits specific toxicity to human lung fibroblasts and plays an important role in the development of pulmonary fibrosis [20]. In this study, X/XO caused about 20 % of MRC5 cell death compared to the untreated control. On treatment with the A. cinnamomea extract, there were significant degrees of protection against X/XO-induced cell death [Fig. 1A], indicating anti-oxidative stress properties of A. cinnamomea.
Fig. 1.
Characterization of the anti-oxidative and anti-inflammatory properties of A. cinnamomea extract in vitro. (A) MRC-5 cells were incubated without or with xanthine (2.5 mU.mL)-xanthine oxidase (XO) and co-treated with A. cinnamomea (A.C.) extract for 2 h (filled bar), or without A. cinnamomea treatment (empty bar). At the end of treatment, cells were harvested for cell viability measurement by crystal violet staining. (B–D) Effects of A. cinnamomea on the pro-inflammatory cytokines expression of LPS-induced RAW264.7 cells. The cells were treated with LPS (10 ng/ml) in the presence of A. cinnamomea for 4 h (emtpy bars) and or 18 h (filled bars). The mRNA levels of pro-IL-1β (B), iNOS (C) and IL-6 (D) were determined by quantitative real-time RT-PCR. Values were normalized to β-actin and are expressed relative to the respective control group. ∗∗p < 0.01.
3.2. A. cinnamomea extract reduced LPS-induced pro-inflammatory cytokine levels and mediator production in RAW264.7 cells
IL-1β, IL-6 and iNOS are pro-inflammatory and pro-fibrotic cytokines involved in the pathogenesis of lung diseases [[21], [22], [23]]. After LPS stimulation, transcription levels of pro-IL-1β, IL-6, and iNOS were up-regulated 400-, 8- and 150-fold, respectively, in RAW264.7 cells. Moreover, these increments were significantly decreased by treatment with the A. cinnamomea extract [Fig. 1B–D], presenting anti-inflammatory properties of A. cinnamomea.
3.3. A. cinnamomea attenuates extracellular matrix production through TGF-β1-mediated AKT-mTOR signaling
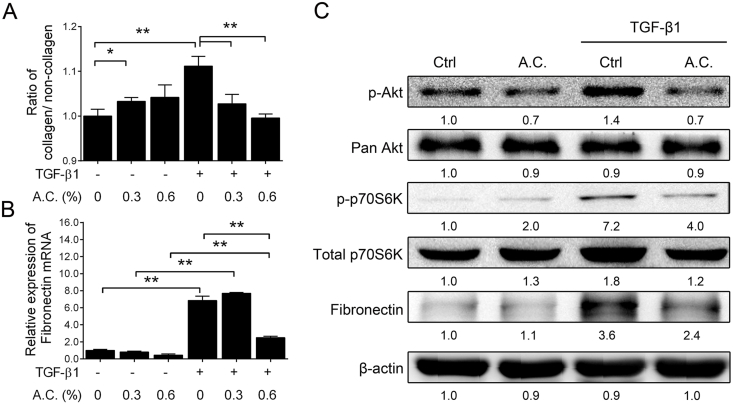
Previous studies have shown that TGF-β1 induces the synthesis of extracellular matrix (ECM) components, fibronectin and collagen as a result of increased phosphorylation of Akt and its downstream signaling in fibroblast cells [24,25]. To assess the anti-fibrotic effects of the A. cinnamomea extract, the production of the representative ECM components, collagen and fibronectin, and downstream signaling molecules Akt in it phosphorylated form, pAkt, and Pan Akt [24], and phosphorylated and total p70S6K [25] in the TGF-β1-stimulated MRC5 fibroblasts was examined. Firstly, TGF-β1 treatment promoted collagen production, and increased both the fibronectin mRNA and protein expression levels in MRC5 cells [Fig. 2B & C]. Likewise, TGF-β1 also up-regulated expression of phosphorylated and Pan Akt as well as phosphorylated and total p70S6K [Fig. 2C, columns 1 & 3], indicating TGF-β1-induced activation of the Akt-mTOR signaling pathway.
Fig. 2.
Attenuation of ECM production through TGF-β1 stimulation of Akt-mTOR signaling on A. cinnamomea treatment. (A) Expression of collagen. MRC-5 fibroblasts were treated with TGF-β (1 ng/ml) in the presence of different concentrations of A. cinnamomea (A.C.) for 24 h. After treatment, cells were harvested for determination of cellular collagen contents by using Sirius Red/Fast Green Collagen Staining Kit to determine the ratio of collagen/non-collagen. (B) Expression of the fibrotic marker, fibronection. The mRNA levels of fibronection were determined by quantitative real-time RT-PCR. (C) Western blot analysis of the signaling molecules in the Akt-mTOR pathway after 24 h A. cinnamomea treatment. Values were normalized to β-actin and are expressed relative to the respective control group. ∗p < 0.05, ∗∗p < 0.01.
As anticipated, synthesis of the ECM components collagen and fibronectin was significantly attenuated in an apparent dose-dependent manner on A. cinnamomea treatment [Fig. 2A & B]. Western blot analysis also showed that A. cinnamomea down-regulated the expression of phosphorylated AKT and the mTOR downstream mediator, phopshorylated p70S6K [Fig. 2C, columns 3 & 4], leading to eventual attenuated synthesis of fibronectin [Fig. 2C].
3.4. A. cinnamomea treatment improved pulmonary respiratory functions in the bleomycin-induced pulmonary fibrosis mouse model
Since bleomycin was used to induce lung fibrosis to test the in vivo effects of A. cinnamomea in mice, effects of A. cinnamomea treatment on bleomycin-induced bodyweight loss was first examined. Due to the bleomycin-induced acute lung damage, a significant bodyweight loss of about 10% was observed in the bleomycin-treated mice compared to the PBS-treated control groups on days 7, 14 and 21 ([Table 1], rows 1 & 3). However, A. cinnamomea extract-fed mice show no significant loss in bodyweight [Table 1]. Instead, the mice treated with both bleomycin and A. cinnamomea exhibited a slight (<10 %) but significant gain in bodyweight despite bleomycin treatment ([Table 1], rows 3 & 4).
Table 1.
Change of bodyweight of mice treated with A. cinnamomea extract and/or bleomycin.
| Weight (g) | |||
|---|---|---|---|
| Days of A.C. treatment | 7d | 14d | 21d |
| PBS | 31.89 ± 1.85 | 33.23 ± 1.61 | 34.33 ± 1.14 |
| PBS + A.C. | 33.60 ± 1.67 | 33.92 ± 1.07 | 34.22 ± 0.73 |
| BLM | 28.24 ± 1.95∗ | 30.63 ± 0.97∗ | 32.03 ± 0.78∗ |
| BLM + A.C. | 29.96 ± 1.96∗,# | 32.52 ± 1.30# | 34.28 ± 1.65# |
Abbreviations: PBS:phosphate-buffered saline control;A.C.:A. cinnamomea extract-treated;BLM:bleomycin-treated. ∗p < 0.05 vs PBS control group; #p < 0.05 vs BLM group.
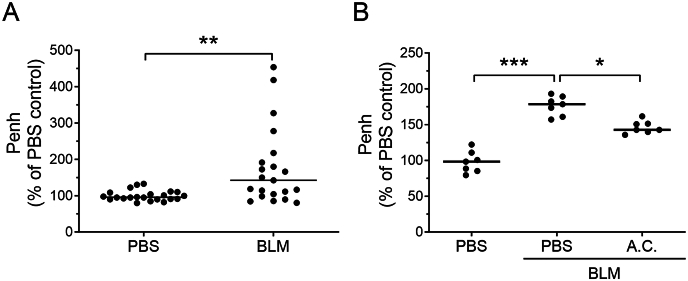
To evaluate the therapeutic efficacy of A. cinnamomea extract in the bleomycin-induced pulmonary fibrosis mouse model, whole-body barometric plethysmography was used to monitor lung functions of the treated mice. The respiratory parameter, in particularly the enhanced pause value, Penh, was measured as a noninvasive index of bleomycin-induced airway dysfunction [26], [27]. In the experiments, the Penh values showed a 1.5-fold increment on days 3 and 21 after bleomycin treatment of the mice compared with the PBS placebo groups [Fig. 3A]. However, mice fed with the A. cinnamomea extract showed significant lower Penh values when compared with bleomycin control, indicating significantly improved lung functions after suffering 21 days of bleomycin damage [Fig. 3B].
Fig. 3.
A. cinnamomea treatment improved pulmonary respiratory function index (Penh) in a bleomycin-induced pulmonary fibrosis mouse model. Mice were intratracheally administered bleomycin or PBS on day 0, and started orally gavaging A. cinnamomea (A.C.) or PBS on day 3, orally once a day, five times a week for 18 days. Whole-body plethysmograph (WBP) was employed and Penh was used as a noninvasive index of airway dysfunction on days 3 (A) and 21 (B) after BLM administration. Penh values were determined relative to those of the PBS treatment controls. Each dot represents an individual mouse with the mean shown for n ≥ 6 per group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.5. Down-regulated expression of inflammatory and fibrotic factor genes in A. cinnamomea-treated bleomycin-induced pulmonary fibrosis mice
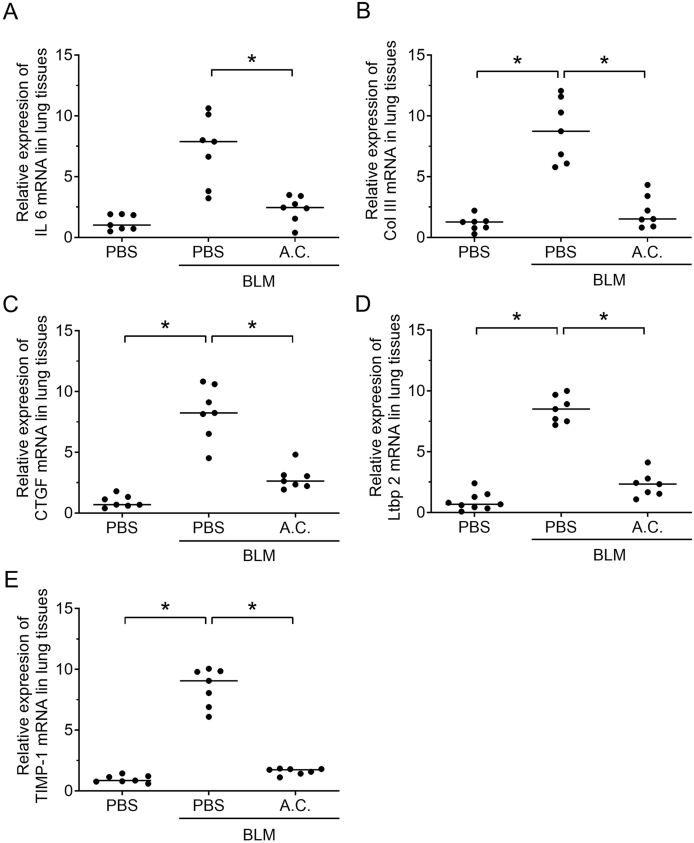
To determine the effects of A. cinnamomea on inflammation and fibrosis of bleomycin-induced pulmonary fibrosis, the expression levels of pro-inflammatory cytokine and fibrotic factors were determined. The mRNA level of the inflammation-mediating IL-6 was up-regulated 21 days post-bleomycin treatment compared with the PBS placebo group [Fig. 4A]. A. cinnamomea treatment resulted in a significant reduction in the expression of the IL-6 inflammatory mediators [Fig. 4A]. Furthermore, expression of collagen type III, tissue inhibitor of metalloproteinase-1 (TIMP-1), connective tissue growth factor (CTGF), and Latent-transforming growth factor beta-binding protein 2 (Ltbp2) that are known to mediate fibrosis [26] was significantly up-regulated on day 21 post bleomycin treatment; expression of these fibrotic mediators was clearly reduced on A. cinnamomea treatment [Fig. 4]. Taken together, the data showed that treatment with the A. cinnamomea essence extract inhibited up-regulation of pro-inflammatory and fibrotic factors in the bleomycin-induced pulmonary fibrosis model.
Fig. 4.
Down-regulated expression of inflammatory factor and fibrotic Indicators' genes on A. cinnamomea treatment of bleomycin-induced pulmonary fibrosis mice. Bleomycin and A. cinnamomea treatment of the mice was as in [Fig. 3]. The mice were sacrificed on 21 days after BLM administration, and total RNA was extracted from lung tissues of the mice for real-time RT-PCR analysis using specific primers set for inflammatory factor gene, IL-6 (A), and fibrotic indicator genes Col III (B), CTGF(C), Ltbp2 (D), and TIMP-1 (E). Each dot represents an individual mouse with the mean shown for n ≥ 6 per group. ∗p < 0.05.
3.6. A. cinnamomea treatment reduces histological changes in the lungs of bleomycin-induced pulmonary fibrosis mice
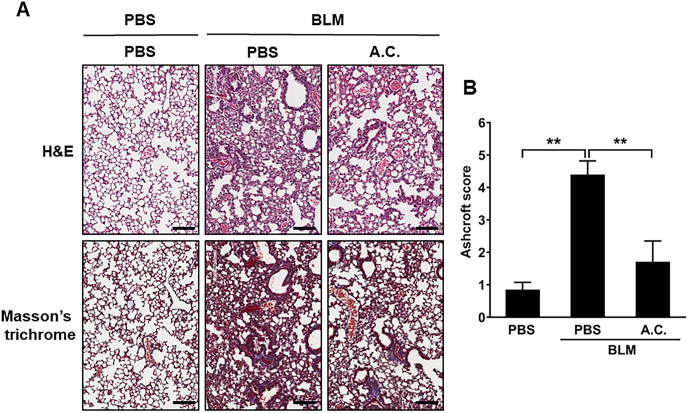
To further investigate A. cinnamomea-induced protection of the fibrotic lung, lung histopathologic sections from each experimental group on days 3 and 21 after bleomycin administration were examined [Fig. 5]. There were no obvious lesions or inflammatory infiltration in the lung of the PBS placebo group with or without A. cinnamomea treatment [Fig. 5]. In the bleomycin-only group, H&E staining showed that inflammatory cellular infiltration into the alveolar septa at the early stage of the disease (data not shown). Moreover, the pulmonary alveolus cavities decreased in size, the alveolar wall was thicken, and there was an accumulation of inflammatory cells as well as increased collagen deposition on day 21 post bleomycin administration by H&E staining and Masson's trichrome staining [Fig. 5A]. However, lungs of the mice fed with A. cinnamomea showed lesser extents of infiltration of inflammatory cells, and there was a significant reduction of the alveolar wall thickness and accumulation of collagen in the lung interstitium [Fig. 5]. Consequently, Ashcroft score was used to quantify the overall grade of the fibrotic changes in the lungs [Fig. 5B]. The scores of the mice administered with BLM were significantly elevated compared to the normal control group. A. cinnamomea treatment significantly reduced the Ashcroft score in BLM-treated mice. Thus, our data indicated that A. cinnamomea had therapeutic effects and improved lung functions in bleomycin-induced pulmonary fibrotic mice.
Fig. 5.
Effects of A. cinnamomea extract on histopathologic changes in bleomycin-induced pulmonary fibrosis mice. Mice were administered bleomycin (BLM) or PBS on day 0, The treatment groups received orally with A. cinnamomea (A.C.), or PBS on day 3, orally once a day, five times a week for 18 days. Lung tissues were collected 21 days after bleomycin administration for sectioning and H&E staining. Mice were sacrificed on day 21 and lung samples were collected for further analysis. (A) Representative photographs of H&E and Masson's trichrome staining of lung tissue sections in the indicated groups. Scale bar: 100 μm. (B) Ashcroft fibrosis scores were used to evaluate the degree of lung fibrosis. Data are represented as the mean ± SEM (n ≥ 6 per group). #p < 0.05 compared with vehicle-treated control group. ∗p < 0.05 compared with BLM group.
4. Discussion
Study aimed to investigate the effectiveness of A. cinnamomea essence extract in the prevention of lung fibrosis using a single-dose bleomycin-induced mouse model. Administration of bleomycin resulted in progressive inflammation, exacerbated fibrosis and excessive ECM accumulation in the lung of the treated mice. However, treatment of the mice with A. cinnamomea essence extract decreased inflammatory response and inhibited ECM production by targeting the Akt/p70S6K1 pathways, as shown in in vitro studies. Notably, the molecular changes induced by A. cinnamomea led to improved lung functions, reduced lung edema and the levels of fibrotic factors in the bleomycin-treated mice.
Previous works have shown that an ethanol extract from solid-state cultured A. cinnamomea essence extract inhibits the growth of cancer cells, such as A549 and HL-60; however, no A. cinnamomea-induced cytotoxicity was found in MRC5, Chang liver cell, Detroit 551 and WS-1 [28,29]. Notably in this work, the dosages of the A. cinnamomea extract used did not induce side effects on the mice during the experimental period.
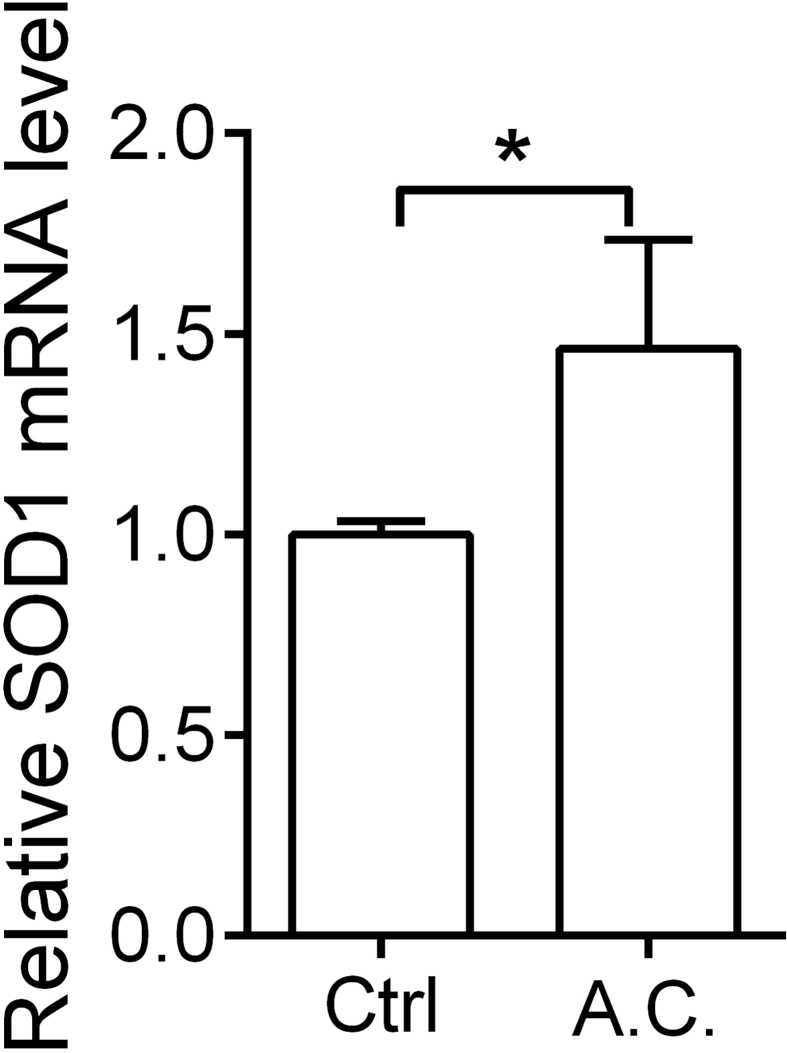
The pathogenesis of induced pulmonary fibrosis is known to be a combination of alterations of lung fibroblasts, loss of alveolar epithelial cells, accumulation of ECM, induction of oxidative stress and activation of inflammatory response [30]. It has been reported that reactive oxygen species (ROS) and additional oxidants including hydroxyl radicals and reactive nitrogen species (RNS) can be augmented in the microenvironment of damaged lungs [31]. However, numerous studies also showed that appropriate antioxidant strategies may be potential therapy for treating fibroproliferating disorders [32,33]. Recently, much attention has been paid to the antioxidant properties of A. cinnamomea essence extract [6], which may enhance several antioxidant activities [34] of enzymes including catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) [35] and glutathione S-transferase (GST) [36], and also improve ROS scavenging abilities [37]. A previous study demonstrated that superoxide anion (O2−) is produced by xanthine/xanthine oxidase (X/XO) that massively increases the amounts of intracellular ROS before initiating cell death [38]. However, SOD is a well-known enzymatic scavenging of O2− [39]. Echoing the abovementioned published data, results in this work found that the A. cinnamomea essence extract used may also attenuate the X/XO-induced cell death, which may be due to up-regulated expression of SOD1 in the treated cells [Supplement Fig. S1].
Furthermore, mounting evidence has reported that both water and alcohol extracts of A. cinnamomea possess anti-inflammatory effects. Hseu et al. demonstrated that a water extract of A. cinnamomea inhibited the in vitro production of TNF-α, IL-1β, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) [40]; the same group also reported that A. cinnamomea essence extract suppressed the expression levels of TNF-α, IL-1β, and NF-κB in various organs of LPS-treated mice [41]. On the other hand, the alcohol extract of A. cinnamomea attenuated the production of LPS-induced pro-inflammatory cytokines TNF-α and IL-6, and mediators NO and PGE2 in mice and human inflammatory cells [42]. Huang et al. further reported that alcohol extract of A. cinnamomea reduced the secretion IL-1β and IL-18 through suppressing the activation of NF-κB, MAPK and NLRP3 inflammasome complex in human macrophage [43]. Hence, in vivo studies also showed that alcohol extract of A. cinnamomea essence extract strongly inhibits endotoxin- or carrageenan-induced inflammatory mediators’ production [42,44]. The A. cinnamomea essence extract we used, indeed, also clearly inhibited the expression of IL-1β, IL-6 and NO in vitro [Fig. 1B–D], and significantly reduced IL-6 expression in bleomycin-treated mice [Fig. 4A].
TGF-β1 is a most important cytokine that promotes the development of fibrosis in all parenchymal organs such as liver [45], lung [46] and renal fibrosis [47]. Therefore, TGF-β1-associated genes or signals are highlighted as potential therapeutic targets in pulmonary fibrosis [48]. The primary collagen-producing cell responses, i.e. the fibroblasts, elicited by TGF-β involve both Smad-dependent and -independent signal pathway [49]. Phosphorylated Smad2/3 subsequently interacts with Smad4 and is translocated into the nucleus to initiate expression of target genes, such as type I and III collagen [50,51]. Hence, mounting evidence has shown that either bleomycin or downstream pathways of TGF-β1 may induce ECM synthesis, and ECM deposition is mediated through phosphoinositide-3 kinase (PI3K)-Akt-S6K1 signaling in fibroblasts [25,30]. It was observed in this work that the A. cinnamomea attenuated the production of fibronectin through down-regulation of the Akt-S6K1 pathway in MRC5 fibroblasts by attenuating Akt and p70S6K1 phosphorylation levels [Fig. 2C]. The data thereby indicate that the Akt-S6K1-ECM signaling pathway may, at least in part, explain the inhibitory effects of A. cinnamomea extract on ECM production [Fig. 2].
The extracellular glycoproteins, viz. latent TGF-β binding proteins (LTBPs), play important roles in ECM; perturbations of the function of LTBPs is manifested in a wide range of diseases [52]. It is believed that LTBP-2 is more likely a component of the mature ECM and that matrix association of LTBP-2 is dependent on a fibrillin-1 network [53]. Therefore, recent studies demonstrated that fibrillin-1 may indirectly affect TGF-β activity in fibroblasts and other cell types [54,55]. Hence, Gibson MA et al. mentioned that LTBP-2 contributes to the development of fibrosis and modulates elastic fiber assembly in fibrotic skin lesions such as keloids and hypertrophic scars [56]. Besides LTBP-2, our results suggested that the expression of other fibrotic mediators TIMP-1, CTGF and ColIII, and the pro-fibrotic cytokine, IL-6, was significantly down-regulated in bleomycin-induced lung fibrotic mice on A. cinnamomea treatment [Fig. 4].
5. Conclusions
We propose here that the cytoprotective mechanism of the A. cinnamomea essence extract in bleomycin-induced pulmonary fibrosis mice is via attenuation of inflammatory effects and decreases oxidative stress-induced cell death. Furthermore, A. cinnamomea essence extract also inhibits the production of ECM proteins, such as collagens and fibronectin, through down-regulation of AKT-mediated p70-S6 kinase pathway.
Funding
This work was supported in parts by grants from the National Science and Technology Council, Taiwan (MOST-108-2314-B-182-052-MY3, NSTC-111-2314-B-182-057 and NSTC-112-2314-B-182-059), Chang Gung Memorial Hospital, Taiwan (BMRP-861, CORPD-1K-0021, and CMRPD-1N-0251) and the Chang Gung University, Taiwan (UMRPD-1M − 0281) (K-YC). In addition, partially supported by the iEGG and Animal Biotechnology Center from the Feature Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan (MOE-113-S-0023-A) (C-MC).
Conflicts of interest
The authors declare no competing interests.
Acknowledgements
We acknowledge the Pathology core of Molecular Medicine Research Center, Chang Gung University, for help with imaging of tissue slides, and Professor Ming-Ling Kuo, Department of Microbiology and Immunology, Chang Gung University, for technical advice on noninvasive measurement of pulmonary function by Whole-body Plethysmography (WBP).
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2024.100720.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Supplementary Fig.1Effects of A. cinnamomea extract on anti-oxidative gene expressions. MRC-5 cells were incubated without or with A. cinnamomea (A.C.) extract for 24 h. Quantitative real-time RT-PCR was performed to examine the relative SOD1 mRNA expression levels. Values were normalized to β-actin and are expressed relative to the respective control group. ∗p < 0.05.
References
- 1.Luzina IG, Todd NW, Sundararajan S, Atamas SP. The cytokines of pulmonary fibrosis: much learned, much more to learn. Cytokine. 2015;74(1):88–100. doi: 10.1016/j.cyto.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Rafii R, Juarez MM, Albertson TE, Chan AL. A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis. 2013;5(1):48–73. doi: 10.3978/j.issn.2072-1439.2012.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spagnolo P, Wells AU, Collard HR. Pharmacological treatment of idiopathic pulmonary fibrosis: an update. Drug Discov Today. 2015;20(5):514–524. doi: 10.1016/j.drudis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Renzoni E, Srihari V, Sestini P. Pathogenesis of idiopathic pulmonary fibrosis: review of recent findings. F1000Prime Rep. 2014;6:69. doi: 10.12703/P6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hseu YC, Tsou HT, Kumar KJ, Lin KY, Chang HW, Yang HL. The antitumor activity of Antrodia camphorata in melanoma cells: modulation of wnt/beta-catenin signaling pathways. Evid Based Complement Alternat Med. 2012;2012:197309. doi: 10.1155/2012/197309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar KJ, Chu FH, Hsieh HW, Liao JW, Li WH, Lin JC, et al. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J Ethnopharmacol. 2011;136(1):168–177. doi: 10.1016/j.jep.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Chen YF, Lu WL, Wu MD, Yuan GF. Analysis of taiwan patents for the medicinal mushroom “Niu-Chang- chih”. Recent Pat Food, Nutr Agric. 2013;5(1):62–69. doi: 10.2174/2212798411305010010. [DOI] [PubMed] [Google Scholar]
- 8.Ao ZH, Xu ZH, Lu ZM, Xu HY, Zhang XM, Dou WF. Niuchangchih (Antrodia camphorata) and its potential in treating liver diseases. J Ethnopharmacol. 2009;121(2):194–212. doi: 10.1016/j.jep.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Yue PY, Wong YY, Wong KY, Tsoi YK, Leung KS. Current evidence for the hepatoprotective activities of the medicinal mushroom Antrodia cinnamomea. Chin Med. 2013;8(1):21. doi: 10.1186/1749-8546-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokila Vani M, Kumar KJ, Liao JW, Chien SC, Mau JL, Chiang SS, et al. Antcin C from Antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through nrf2-dependent mechanism. Evid Based Complement Alternat Med. 2024;2013 doi: 10.1155/2013/296082. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsiao G, Shen MY, Lin KH, Lan MH, Wu LY, Chou DS, et al. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J Agric Food Chem. 2003;51(11):3302–3308. doi: 10.1021/jf021159t. [DOI] [PubMed] [Google Scholar]
- 12.Hseu YC, Chen SC, Yech YJ, Wang L, Yang HL. Antioxidant activity of Antrodia camphorata on free radical-induced endothelial cell damage. J Ethnopharmacol. 2008;118(2):237–245. doi: 10.1016/j.jep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen YR, Chang KT, Tsai MJ, Lee CH, Huang KJ, Cheng H. et al.Antrodia cinnamomea profoundly exalted the reversion of activated hepatic stellate cells by the alteration of cellular proteins. Food Chem Toxicol. 2014;69:150–162. doi: 10.1016/j.fct.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Wu MF, Peng FC, Chen YL, Lee CS, Yang YY, Yeh MY, et al. Evaluation of genotoxicity of Antrodia cinnamomea in the Ames test and the in vitro chromosomal aberration test. In. Vivo. 2011;25(3):419–423. [PubMed] [Google Scholar]
- 15.Lan YW, Chen CM, Chong KY. In vitro methods to evaluate the effects of mesenchymal stem cells on TGF-beta1-induced pulmonary fibrosis. Methods Mol Biol. 2021;2269:83–92. doi: 10.1007/978-1-0716-1225-5_6. [DOI] [PubMed] [Google Scholar]
- 16.Lan YW, Choo KB, Chen CM, Hung TH, Chen YB, Hsieh CH. et al.Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2015;6(1):97. doi: 10.1186/s13287-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YB, Lan YW, Chen LG, Huang TT, Choo KB, Cheng WT. et al.Mesenchymal stem cell-based HSP70 promoter-driven VEGFA induction by resveratrol alleviates elastase-induced emphysema in a mouse model. Cell Stress Chaperones. 2015;20(6):979–989. doi: 10.1007/s12192-015-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tung YT, Chen HL, Lai CW, Shen CJ, Lai YW, Chen CM. Curcumin reduces pulmonary tumorigenesis in vascular endothelial growth factor (VEGF)-overexpressing transgenic mice. Mol Nutr Food Res. 2011;55(7):1036–1043. doi: 10.1002/mnfr.201000654. [DOI] [PubMed] [Google Scholar]
- 19.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41(4):467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi S, den Hartog GJ, Bast A. Superoxide radicals increase transforming growth factor-beta1 and collagen release from human lung fibroblasts via cellular influx through chloride channels. Toxicol Appl Pharmacol. 2009;237(1) doi: 10.1016/j.taap.2009.02.019. 111-8. [DOI] [PubMed] [Google Scholar]
- 21.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito F, Tasaka S, Inoue K, Miyamoto K, Nakano Y, Ogawa Y, et al. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol. 2008;38(5):566–571. doi: 10.1165/rcmb.2007-0299OC. [DOI] [PubMed] [Google Scholar]
- 23.Kalayarasan S, Sriram N, Sudhandiran G. Diallyl sulfide attenuates bleomycin-induced pulmonary fibrosis: critical role of iNOS, NF-kappaB, TNF-alpha and IL-1beta. Life Sci. 2008;82(23-24):1142–1153. doi: 10.1016/j.lfs.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Fuentes-Calvo I, Blázquez-Medela AM, Eleno N, Santos E, López-Novoa JM, Martínez-Salgado C., et al. H-Ras isoform modulates extracellular matrix synthesis, proliferation, and migration in fibroblasts. Am J Physiol Cell Physiol. 2012;302(4):C686–97. doi: 10.1152/ajpcell.00103.2011. [DOI] [PubMed] [Google Scholar]
- 25.Goc A, Choudhary M, Byzova TV, Somanath PR. TGFbeta- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR) J Cell Physiol. 2011;226(11):3004–3013. doi: 10.1002/jcp.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25(2):164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 27.Shan B, Yao TP, Nguyen HT, Zhuo Y, Levy DR, Klingsberg RC, et al. Requirement of HDAC6 for transforming growth factor-beta1-induced epithelial-mesenchymal transition. J Biol Chem. 2008;283(30):21065–21073. doi: 10.1074/jbc.M802786200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang TY, Huang WW. Tissue culture and bioavailability of taiwan’s "Antrodia camphorata". J Gen Educ. 2003;(5):247–255. [Google Scholar]
- 29.Wang W, Liu F, Chen N. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonists attenuate the profibrotic response induced by TGF-beta1 in renal interstitial fibroblasts. Mediat Inflamm. 2007;2007:62641. doi: 10.1155/2007/62641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5(1):11. doi: 10.1186/1755-1536-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao F, Kinnula VL, Myllarniemi M, Oury TD. Extracellular superoxide dismutase in pulmonary fibrosis. Antioxidants Redox Signal. 2008;10(2):343–354. doi: 10.1089/ars.2007.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day BJ. Antioxidants as potential therapeutics for lung fibrosis. Antioxidants Redox Signal. 2008;10(2):355–370. doi: 10.1089/ars.2007.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta. 2013;1832(7):1028–1040. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang FC, Yang YH, Lu HC. Enhanced antioxidant and antitumor activities of Antrodia cinnamomea cultured with cereal substrates in solid state fermentation. Biochem Eng J. 2013;78:108–113. [Google Scholar]
- 35.Huang GJ, Deng JS, Huang SS, Shao YY, Chen CC, Kuo YH. Protective effect of antrosterol from Antrodia camphorata submerged whole broth against carbon tetrachloride-induced acute liver injury in mice. Food Chem. 2012;132(2):709–716. [Google Scholar]
- 36.Tsai MC, Song TY, Shih PH, Yen GC. Antioxidant properties of water-soluble polysaccharides from Antrodia cinnamomea in submerged culture. Food Chem. 2007;104(3):1115–1122. [Google Scholar]
- 37.Ker YB, Peng CC, Chang WL, Chyau CC, Peng RY. Hepatoprotective bioactivity of the glycoprotein, antrodan, isolated from Antrodia cinnamomea mycelia. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satoh T, Numakawa T, Abiru Y, Yamagata T, Ishikawa Y, Enokido Y, et al. Production of reactive oxygen species and release of L-glutamate during superoxide anion-induced cell death of cerebellar granule neurons. J Neurochem. 1998;70(1):316–324. doi: 10.1046/j.1471-4159.1998.70010316.x. [DOI] [PubMed] [Google Scholar]
- 39.Villamor E, Kessels CG, Fischer MA, Bast A, de Mey JG, Blanco CE. Role of superoxide anion on basal and stimulated nitric oxide activity in neonatal piglet pulmonary vessels. Pediatr Res. 2003;54(3):372–381. doi: 10.1203/01.PDR.0000077481.15081.C8. [DOI] [PubMed] [Google Scholar]
- 40.Hseu YC, Wu FY, Wu JJ, Chen JY, Chang WH, Lu FJ, et al. Anti-inflammatory potential of Antrodia Camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-kappaB pathway. Int Immunopharm. 2005;5(13-14):1914–1925. doi: 10.1016/j.intimp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Hseu YC, Huang HC, Hsiang CY. Antrodia camphorata suppresses lipopolysaccharide-induced nuclear factor-kappaB activation in transgenic mice evaluated by bioluminescence imaging. Food Chem Toxicol. 2010;48(8-9):2319–2325. doi: 10.1016/j.fct.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 42.Wen CL, Chang CC, Huang SS, Kuo CL, Hsu SL, Deng JS, et al. Anti-inflammatory effects of methanol extract of Antrodia cinnamomea mycelia both in vitro and in vivo. J Ethnopharmacol. 2011;137(1):575–584. doi: 10.1016/j.jep.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Huang TT, Wu SP, Chong KY, Ojcius DM, Ko YF, Wu YH, et al. The medicinal fungus Antrodia cinnamomea suppresses inflammation by inhibiting the NLRP3 inflammasome. J Ethnopharmacol. 2014;155(1):154–164. doi: 10.1016/j.jep.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 44.Wang HC, Chu FH, Chien SC, Liao JW, Hsieh HW, Li WH, et al. Establishment of the metabolite profile for an Antrodia cinnamomea health food product and investigation of its chemoprevention activity. J Agric Food Chem. 2013;61(36):8556–8564. doi: 10.1021/jf402849b. [DOI] [PubMed] [Google Scholar]
- 45.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatler AL, Jenkins G. TGF-beta activation and lung fibrosis. Proc Am Thorac Soc. 2012;9:130–136. doi: 10.1513/pats.201201-003AW. [DOI] [PubMed] [Google Scholar]
- 47.Lan HY, Chung AC. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012;32(3):236–243. doi: 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Kang HR, Lee JY, Lee CG. TGF-beta1 as a therapeutic target for pulmonary fibrosis and COPD. Expet Rev Clin Pharmacol. 2008;1(4):547–558. doi: 10.1586/17512433.1.4.547. [DOI] [PubMed] [Google Scholar]
- 49.Cheng X, Gao W, Dang Y, Liu X, Li Y, Peng X. et al.Both ERK/MAPK and TGF-Beta/Smad signaling pathways play a role in the kidney fibrosis of diabetic mice accelerated by blood glucose fluctuation. J Diabetes Res. 2013;2013:463740. doi: 10.1155/2013/463740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhattacharyya S, Chen SJ, Wu M, Warner-Blankenship M, Ning H, Lakos G, et al. Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma. Am J Pathol. 2008;173(4):1085–1099. doi: 10.2353/ajpath.2008.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zong L, Qu Y, Xu MY, Dong YW, Lu LG. 18 alpha-glycyrrhetinic acid down-regulates expression of type I and III collagen via TGF-Beta1/Smad signaling pathway in human and rat hepatic stellate cells. Int J Med Sci. 2012;9(5):370–379. doi: 10.7150/ijms.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Todorovic V, Rifkin DB. LTBPs, more than just an escort service. J Cell Biochem. 2012;113(2):410–418. doi: 10.1002/jcb.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vehvilainen P, Hyytiainen M, Keski-Oja J. Matrix association of latent TGF-beta binding protein-2 (LTBP-2) is dependent on fibrillin-1. J Cell Physiol. 2009;221(3):586–593. doi: 10.1002/jcp.21888. [DOI] [PubMed] [Google Scholar]
- 54.Jelodari-Mamaghani S, Haji-Seyed-Javadi R, Suri F, Nilforushan N, Yazdani S, Kamyab K, et al. Contribution of the latent transforming growth factor-beta binding protein 2 gene to etiology of primary open angle glaucoma and pseudoexfoliation syndrome. Mol Vis. 2013;19:333–347. [PMC free article] [PubMed] [Google Scholar]
- 55.Kaartinen V, Warburton D. Fibrillin controls TGF-beta activation. Nat Genet. 2003;33(3) doi: 10.1038/ng0303-331. 331-2. [DOI] [PubMed] [Google Scholar]
- 56.Sideek MA, Menz C, Parsi MK, Gibson MA. LTBP-2 competes with tropoelastin for binding to fibulin-5 and heparin, and is a negative modulator of elastinogenesis. Matrix biol. 2014;34:114–123. doi: 10.1016/j.matbio.2013.10.007. [DOI] [PubMed] [Google Scholar]