Abstract
Background & Aims
Liver stiffness measurement (LSM) has been shown to adequately predict outcomes in patients with liver disease. However, the value of LSM as a predictor of disease progression in autoimmune hepatitis (AIH) remains to be determined. This study aimed to evaluate the role of LSM as a predictor of disease progression and decompensation of cirrhosis in patients with AIH.
Methods
This multicentre cohort study included 439 patients with histologically confirmed AIH and at least one LSM during follow-up. The association between the first LSM performed at least 6 months after treatment initiation (baseline LSM [BLSM]) and cirrhosis development and poor outcomes (decompensation, liver transplantation, and/or liver-related death) was assessed using Cox regression and its discriminating capacity with a receiver-operating characteristic curve.
Results
Most patients were female (n = 301, 70%), with a median age of 52 years. BLSM performed after a median of 2.18 (1.19-4.68) years had a median value of 6 kPa (4.5-8.5). At the time of BLSM, 332 (76%) patients had achieved a biochemical response and 57 (13%) had cirrhosis. During follow-up, eight patients (2%) presented with poor outcomes and 26 (7%) developed cirrhosis. BLSM was higher among patients with poor outcomes (13.5 kPa vs. 6 kPa; p <0.001) and was independently associated with cirrhosis development (hazard ratio 1.300; p <0.001), irrespective of the achievement of biochemical response. A cut-off of 8.5 kPa accurately predicted cirrhosis development and poor outcomes, with AUCs of 0.859 (95% CI 0.789-0.929) and 0.900 (95% CI 0.847-0.954), respectively.
Conclusion
BLSM could play a significant role in predicting AIH outcomes, potentially identifying a subgroup of patients at a high risk of progressing to cirrhosis and experiencing decompensation.
Impact and implications:
The value of liver stiffness measurement as a predictor of outcomes in patients with autoimmune hepatitis (AIH) remains to be determined. In this large multicentre study, liver stiffness measurement was found to be an independent predictive factor of adverse clinical outcomes and cirrhosis development in AIH, irrespective of the achievement of biochemical response. A cut-off of 8.5 kPa accurately predicted cirrhosis development and poor outcomes in AIH.
Keywords: autoimmune hepatitis, liver stiffness measurement, elastography, outcome, cirrhosis, decompensation
Graphical abstract
Highlights:
-
•
The value of liver stiffness measurement (LSM) as a predictor of outcome in patients with autoimmune hepatitis (AIH) remains to be determined.
-
•
In this large multicentre study, LSM was found to be an independent predictive factor of adverse clinical outcomes and cirrhosis development in AIH, irrespective of the achievement of biochemical response.
-
•
A cut-off of 8.5 kPa accurately predicted cirrhosis development and poor outcomes in AIH.
Introduction
Autoimmune hepatitis (AIH) is a chronic liver disease characterised by elevated transaminase and IgG levels, autoantibodies, and interface hepatitis on liver biopsy. In general, AIH responds favourably to immunosuppressive therapy, with complete biochemical response rates (CBR) of approximately 60%.1,2 However, in untreated patients or those with insufficient treatment response, liver inflammation can lead to fibrosis progression, cirrhosis development, and clinical decompensation.3,4
Furthermore, despite having normal transaminase levels, a considerable proportion of patients achieving CBR (with or without cirrhosis), have persistent histological activity and, as a result, a high risk of disease progression.5,6 These findings highlight the importance of monitoring the progression of fibrosis in patients with AIH. However, follow-up liver biopsies are not routinely performed in clinical practice because most patients are reluctant to undergo invasive procedures with a certain risk of complications, and their results are not free from biases related to sample size and representation.
In this context, liver stiffness measurement (LSM) using vibration-controlled transient elastography (VCTE) has been consolidated as a non-invasive tool for monitoring fibrosis progression and predicting clinical outcomes in patients with liver diseases.[7], [8], [9] In AIH, LSM has been shown to adequately correlate with fibrosis stage, with cut-off points ranging between 12.5 and 16 kPa for detecting cirrhosis[10], [11], [12] and 9 kPa for severe fibrosis.13 LSM is influenced by both fibrosis and inflammation, and thus, LSM within the first 3 months of treatment initiation correlates with inflammation rather than the fibrosis stage.10 Therefore, clinical practice guidelines recommend deferring LSM for at least 6 months after successful immunosuppressive treatment.14 However, a recent multicentre study found that currently used LSM cut-off points lack good discriminative capacity for diagnosing AIH-related cirrhosis (with or without portal hypertension), especially when LSM was performed long-term after treatment initiation.15 This is likely attributable to the resolution of inflammation and regression of fibrosis observed in patients with AIH during follow-up, particularly in those with CBR.16
Besides monitoring fibrosis progression, LSM has been found to be useful in predicting the risk of portal hypertension, cirrhosis decompensation, and liver-related mortality in various liver diseases. LSM has been shown to significantly improve the prediction of survival in patients with primary biliary cholangitis (PBC).9 However, its value as a predictor of outcome in patients with AIH remains to be determined. Therefore, in this large, multicentre study, we aimed to: 1) assess the use of LSM as a predictive factor for poor clinical outcomes in AIH, 2) determine the optimal cut-off point to identify patients at risk of disease progression and poor clinical outcomes, and 3) characterise the changes in LSM during follow-up and their association with disease progression and poor clinical outcomes.
Patients and methods
Study design
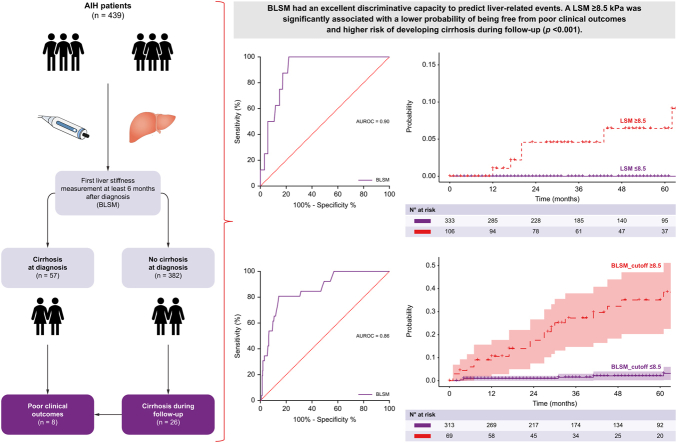
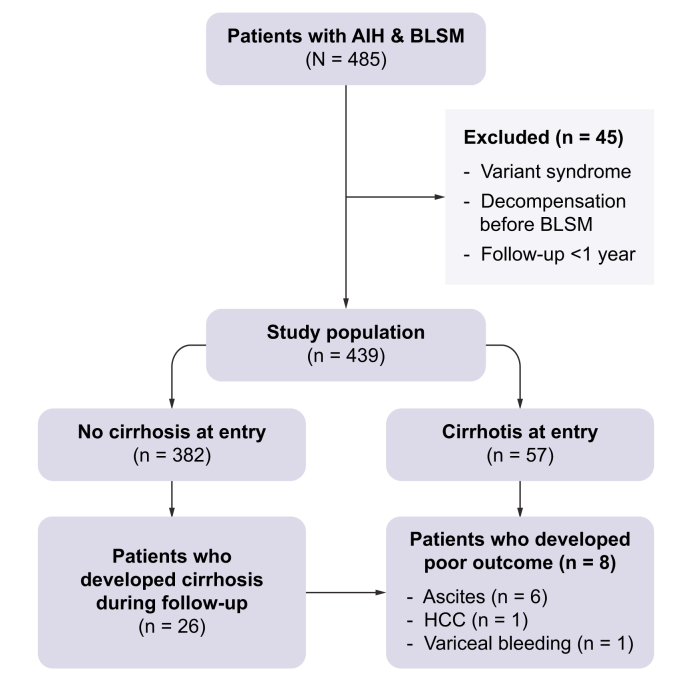
This was a retrospective, multicentre cohort study that enrolled 439 patients with AIH from four liver units (Hospital Clínic de Barcelona, Hospital Vall d’Hebron, Hospital Universitario Marqués de Valdecilla, and University Hospital of Larissa) (Fig. 1).
Fig. 1.
Study flowchart.
AIH, autoimmune hepatitis. BLSM, first liver stiffness measurement 6 months after diagnosis. HCC, hepatocellular carcinoma.
The inclusion criteria were: 1) age ≥16 years; 2) probable or definite diagnosis of AIH according to the simplified criteria established by the International Autoimmune Hepatitis Group (IAIHG);17 3) liver biopsy at diagnosis with compatible or typical findings of AIH; 4) at least one reliable LSM value using VCTE (FibroScan®, Echosens, Paris); and 5) a minimum of 1 year of follow-up after the first LSM. The exclusion criteria were: 1) AIH variants (AIH/PBC, AIH/primary sclerosing cholangitis or AIH/steatotic liver disease), 2) decompensated cirrhosis before the first LSM and 3) liver transplantation before the first LSM.
Data collection
Demographic and clinical data, including age, sex, date of AIH diagnosis, and immunosuppressive treatment were collected. Laboratory and serological data assessed at the time of diagnosis included: aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, alkaline phosphatase (recorded as -fold relative to the upper limit of normal), bilirubin, platelet count, IgG levels, international normalized ratio, and circulating autoantibodies. The original reports of the liver biopsies performed at diagnosis were also retrospectively analysed. H&E and Masson’s trichrome staining were used to determine portal and lobular necrosis and inflammation, and fibrosis staging was performed using the modified hepatitis activity index (mHAI) and Ishak scores, respectively. Cirrhosis at diagnosis was defined as Ishak stage 6 and advanced fibrosis as Ishak stages 3-5.18
The first LSM performed at least 6 months after treatment initiation in each patient was recorded as the baseline LSM (BLSM), and all subsequent LSMs performed during follow-up were also collected. LSMs were performed by experienced operators following the manufacturer’s recommendations, with the patients under fasting conditions. Only reliable LSMs (≥10 valid measurements, ≥60% success rate, and interquartile range [IQR]/median ratio ≤0.30) were included in the analysis. Liver tests performed within 2 months of each LSM were collected. Biochemical response (BR) was defined as normal transaminase levels and CBR was defined as normal transaminase and IgG levels. Study entry was the date of the BLSM. The time between study entry and development of poor clinical outcomes was calculated. Poor clinical outcomes were defined as cirrhosis decompensation (ascites, variceal bleeding and hepatic encephalopathy), development of hepatocellular carcinoma (HCC), need for liver transplantation (LT), and/or liver-related death.
In patients without cirrhosis at diagnosis, the time between study entry and development of cirrhosis was calculated. The diagnosis of cirrhosis during follow-up was based on clinical (decompensation of liver disease), histological, or endoscopic findings of portal hypertension, and/or ultrasound criteria (coarse echo pattern of the liver parenchyma along with irregular hepatic margins, spleen >12 cm, portal vein >16 mm).19
Statistical analysis
Continuous variables were expressed as median and IQR. The Mann-Whitney test was performed to compare continuous variables and analyse independent samples, and the Wilcoxon test was used for paired samples. Categorical variables were expressed as absolute numbers and percentages, and differences between proportions were compared by the Fisher’s exact test or the Chi square test, as appropriate.
The predictive factors for poor clinical outcomes and cirrhosis development were analysed using univariate and multivariate Cox regression analyses. For each covariate analysed, the association with the primary endpoint was expressed as a hazard ratio (HR) with a 95% CI. Sensitivity analyses were performed on different subpopulations of patients according to the different immunosuppressive regimens used and the treatment response.
The ability of LSM values to discriminate patients developing poor clinical outcomes and cirrhosis was evaluated using a receiver-operating characteristic curve. Optimal LSM cut-off values for predicting poor clinical outcomes and cirrhosis development were determined by estimating the AUC at the maximum total sensitivity and specificity (Youden’s index) and maximally selected rank statistics. Survival rates were estimated using Kaplan-Meier curves and compared using the log-rank test. Variations in LSMs during follow-up were assessed by repeated-measures analysis using a general linear model. Two-sided p values <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics 25 and Stata 18.0.
Ethics
The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of each participating centre.
Results
Baseline characteristics
The baseline characteristics of the 439 patients included in the study are summarized in Table 1. Briefly, most patients were women (n = 311, 71%), with a median age of 52 years (IQR 40-62) at the time of diagnosis. Fifty-seven (13%) patients had compensated cirrhosis at study entry and 433 (99%) received immunosuppressive treatment during follow-up. The most commonly used first-line treatment regimens were prednisone in combination with azathioprine (AZA, n = 243, 56%) or mycophenolate mofetil (MMF, n = 124, 28%). BLSM was performed a median of 2.18 years (IQR 1.19-4.68) after diagnosis, with a median value of 6 kPa (IQR 4.5-8.5) and 332 (76%) patients in BR at the time of measurement.
Table 1.
Baseline characteristics of the patients included in the study.
| Variable | n = 439 |
|---|---|
| Age at entry (years) | 56 (44 -65) |
| Female sex (n, %) | 311 (71%) |
| AST (U/L) | 315 (87-900) |
| ALT (U/L) | 419 (116-1,100) |
| Total bilirubin (mg/dl) | 1.7 (0.8- 8.8) |
| ALP (x ULN) | 1(0.64-1.48) |
| GGT (U/L) | 134 (56-267) |
| IgG (g/L)∗ | 17 (13 -22) |
| INR | 1.1 (1.0-1.3) |
| Platelets count (x109/L) | 219 (171-263) |
| mHAI | |
| Grading | 8 (6-10) |
| Staging | 1 (0-3) |
| Cirrhosis at diagnosis (n, %) | 49 (11%) |
| Cirrhosis at BLSM | 57 (13%) |
| Advanced fibrosis at diagnosis (n, %) | 99 (23%) |
| ANA (n, %) | 304 (69%) |
| SMA (n, %) | 338 (77%) |
| Anti-SLA/LP (n, %) | 27 (6%) |
| Anti-LKM1 (n, %) | 11 (2.5%) |
| Treatment (n, %) | 433 (99%) |
| Cs + AZA | 243 (56%) |
| Cs + MMF | 124 (28%) |
| Cs | 66 (15%) |
| BLSM (kPa) | 6.0 (4.5-8.5) |
| BR at BLSM (n, %) | 332 (76%) |
| Time from diagnosis to BLSM (years) | 2.18 (1.19-4.68) |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, antinuclear antibodies; Anti-LKM1, anti-liver kidney microsomal antibodies; anti-SLA/LP, anti-soluble liver antigen/liver pancreas antibodies; AST, aspartate aminotransferase; AZA, azathioprine; BLSM, first liver stiffness measurement after 6 months of treatment initiation; BR, biochemical response; Cs, corticosteroids; GGT, gamma-glutamyltransferase; mHAI, modified hepatitis activity index; MMF, mycophenolate mofetil; SMA, smooth muscle antibodies.
Data are expressed as median (IQR).
Available in 367 patients.
LSM predicts poor clinical outcomes in patients with AIH
After a median follow-up of 3.67 years (IQR 1.76-5.99), eight patients (2%) presented poor clinical outcomes: six patients developed ascites, one HCC, and one variceal bleeding. Two of these patients died and one underwent LT. During follow-up, five additional patients died due to a non-liver-related cause and were not considered in the following analysis. In these patients, the median time from AIH diagnosis to BLSM was 0.77 years (IQR 0.65-2.97), and the median time from BLSM to the event was 1.55 years (IQR 0.61-2.00). At the time of BLSM all patients had an abdominal ultrasound that ruled-out the presence of ascites or HCC.
Patients with poor clinical outcomes were more likely to have cirrhosis (p <0.001) or advanced fibrosis (p <0.001) at the time of diagnosis, a lower baseline platelet count (p = 0.009), higher BLSM values (p <0.001), and were less likely to have achieved BR at BLSM (p = 0.001) (Table 2). Multivariate analysis could not be performed due to the low number of events. However, the association between BLSM and poor clinical outcomes remained significant when the analysis was restricted to patients treated with AZA or MMF (data not shown).
Table 2.
Clinical, biochemical, and histological characteristics associated with the development of poor clinical outcomes in the univariate analysis.
| Variables | Poor clinical outcomes |
Univariate analysis |
||||
|---|---|---|---|---|---|---|
| No (n = 431) | Yes (n = 8) | p value | Wald | HR (95% CI) | p value | |
| Age at entry (yr) | 56 (44 – 65) | 63 (52-71) | 0.233 | |||
| Female sex (n, %) | 307 (70%) | 4 (50%) | 0.190 | |||
| AST (U/L) | 324 (89-906) | 82 (55-302) | 0.096 | |||
| ALT (U/L) | 430 (124-1,112) | 74 (52-192) | 0.016 | 2.778 | 0.996 (0.992–1.001) | 0.096 |
| Total bilirubin (mg/dl) | 1.7 (0.8-7.8) | 2.3 (0.8-3.7) | 0.907 | |||
| ALP (x ULN) | 0.9 (0.63-1.48) | 1.07 (0.75-216) | 0.381 | |||
| GGT (U/L) | 132 (55-266) | 220 (78-546) | 0.144 | |||
| IgG (g/L) | 17 (13-22) | 16 (11-22) | 0.684 | |||
| INR | 1.10 (1.01-1.25) | 1.1 (1.04-1.25) | 0.893 | |||
| Platelet count (x109/L) | 220 (171-263) | 141 (92-169) | 0.009 | 6.473 | 1.000 (1.000–1.000) | 0.011 |
| mHAI | ||||||
| Grade | 8 (6-10) | 6.5 (4.3-8.8) | 0.400 | 0.871 | 0.898 (0.717–1.125) | 0.351 |
| Stage | 1 (0-3) | 5 (3.5-6) | <0.001 | 12.899 | 2.053 (1.38 7–3.040) | <0.001 |
| Cirrhosis at diagnosis (n, %) | 43 (10%) | 6 (75%) | <0.001 | 15.043 | 23.733 (4.790–117.594) | <0.001 |
| Advanced fibrosis (n, %) | 92 (21%) | 7 (87%) | <0.001 | 7.858 | 20.022 (2.463–162.734) | 0.005 |
| ANA (n, %) | 298 (68%) | 6 (75%) | 0.75 | |||
| SMA (n, %) | 330 (75%) | 8 (100%) | 0.128 | |||
| Anti-SLA/LP (n, %) | 27/426 (6 %) | 0 | 0.462 | |||
| Anti-LKM1 (n, %) | 11 (2%) | 0 | 0.217 | |||
| Cs + AZA (n, %) | 239 (56%) | 4 (50%) | 0.753 | |||
| Cs + MMF (n, %) | 122 (28%) | 2 (25%) | 0.834 | |||
| BLSM (kPa) | 6 (4.5-8.2) | 13.5 (10-17.5) | <0.001 | 20.104 | 1.173 (1.094–1.258) | <0.001 |
| BR at BLSM (n, %) | 330 (77%) | 2 (25%) | 0.001 | 7.107 | 0.113 (0.023–0.562) | 0.008 |
| Time from diagnosis to BLSM (yr) | 2.19 (1.21-4.84) | 1.04 (0.58-3.24) | 0.073 | |||
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, antinuclear antibodies; Anti-LKM1, anti-liver kidney microsomal antibodies; anti-SLA/LP, anti-soluble liver antigen/liver pancreas antibodies; AST, aspartate aminotransferase; AZA, azathioprine; BLSM, first liver stiffness measurement after 6 months of treatment initiation; BR, biochemical response; Cs, corticosteroids; GGT, gamma-glutamyltransferase; HR, hazard ratio; INR, international normalised ratio; mHAI, modified hepatitis activity index; MMF, mycophenolate mofetil; SMA, smooth muscle antibodies.
Data are expressed as the median (IQR). Univariate analysis was performed by Cox regression.
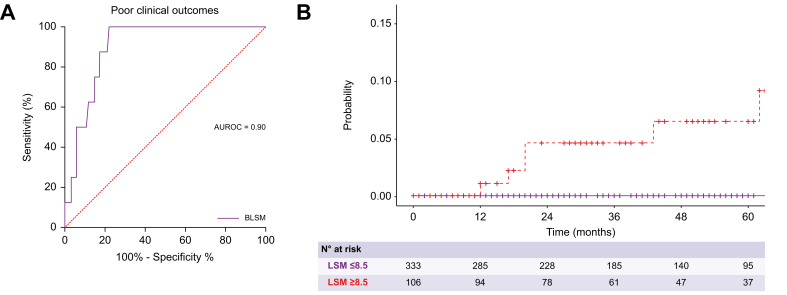
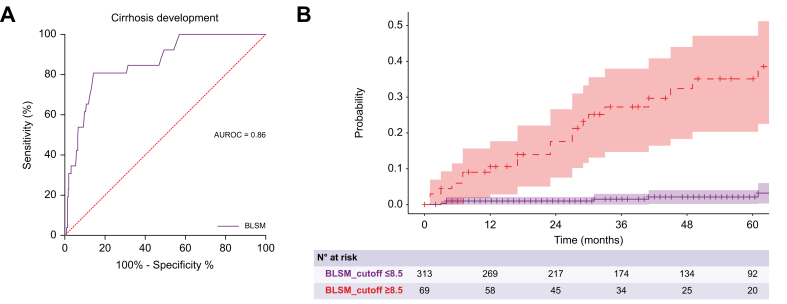
The predictive ability of BLSM for poor clinical outcomes demonstrated excellent performance with an AUC of 0.90 (95% CI 0.85–0.95) (Fig. 2A). According to Youden’s index, the best cut-off point of BLSM to predict clinical outcomes was 8.5 kPa (sensitivity: 100%, specificity: 76%, positive predictive value: 100%). As shown in Fig. 2B, patients with BLSM greater than 8.5 kPa had a significantly lower probability of being free from poor clinical outcomes (p <0.001). Baseline platelet count and mHAI also had excellent accuracy in the prediction of poor outcomes with AUCs of 0.93 (95% CI 1.00–0.84) and 0.86 (95% CI 0.76–0.99), respectively (Fig. S1).
Fig. 2.
Association of BLSM with poor clinical outcomes.
(A) ROC curve of BLSM for the prediction of poor clinical outcomes. (B) Probability of being free of poor clinical outcomes according to the BLSM. Patients with BLSM greater than 8.5 kPa had a significantly lower probability of being free from poor clinical outcomes, represented by a Kaplan-Meier curve and log-rank test (p <0.001). BLSM, baseline liver stiffness measurement.
Association between LSM and the risk of cirrhosis development during follow-up
Among the 382 patients without cirrhosis at baseline, 26 (7%) developed cirrhosis after BLSM. Cirrhosis was diagnosed by liver biopsy in eight patients, and ultrasound criteria in the remaining 18 patients, all of whom had a nodular liver surface and splenomegaly. The median time from AIH diagnosis to BLSM in patients who developed cirrhosis was 1.75 years (IQR 1.12-3.62), and the median time from BLSM to the diagnosis of cirrhosis was 1.65 (IQR 0.71-2.45) years.
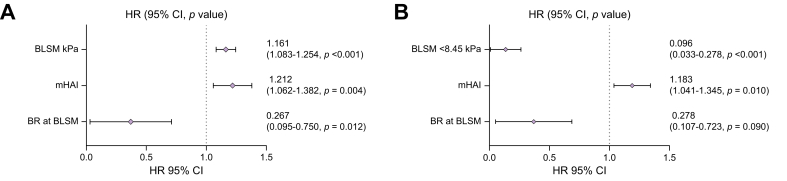
Patients who developed cirrhosis during follow-up had higher BLSM values (11.05 kPa vs. 5.4 kPa; p <0.001), mHAI index (10.5 vs. 8; p = 0.001), aspartate aminotransferase (563 U/L vs. 323 U/L; p = 0.045), and IgG (20 g/L vs. 17 g/L; p = 0.026) at diagnosis. In addition, patients who developed cirrhosis more frequently had advanced fibrosis at diagnosis (p = 0.017) and were less likely to have achieved BR at BLSM (p <0.001) (Table 3). Owing to the limited number of events, these variables were included in two different Cox regression multivariate analyses. As shown in Fig. 3, BLSM (Model 1 = HR 1.161; 95% CI 1.07408–1.24425; p <0.001), BLSM <8.5 kPa (Model 2 = HR 0.096; 95% CI (0.033–0.278), mHAI (Model 1 = HR 1.212; 95% CI 1.062–1.382; p = 0.004; Model 2 = HR 1.183; 95% CI 1.041–1.345; p = 0.010), and BR at the time of BLSM (Model 1 = HR 0.267; 95% CI 0.095–0.750; p = 0.012; Model 2 = HR 0.278; 95% CI 0.107–0.723; p = 0.090) were independently associated with the risk of developing cirrhosis during follow-up. IgG at diagnosis and time between diagnosis and BLSM were not associated with the risk of developing cirrhosis (Fig. S2). Of note, the association between BLSM and the risk of developing cirrhosis remained when the analysis was restricted to patients treated with AZA or MMF (Tables S1 and S2).
Table 3.
Predictive factors of cirrhosis development during the follow-up in patients without cirrhosis at baseline.
| Variables | Cirrhosis development |
Univariate analysis |
||||
|---|---|---|---|---|---|---|
| No (n = 356) | Yes (n = 26) | p value | Wald | HR (95% CI) | p value | |
| Age at entry (yr) | 55 (42-65) | 56 (44-62) | 0.768 | |||
| Female sex | 256 (72%) | 18 (69%) | 0.770 | |||
| AST (U/L) | 323 (85-957) | 563 (288-1,057) | 0.045 | |||
| ALT (U/L) | 455 (124-1,198) | 760 (323-1,170) | 0.138 | 0.022 | 1.000 (1.000–1.001) | 0.631 |
| Total bilirubin (mg/dl) | 1.7(0.80-7.80) | 6.4 (1-14.5) | 0.074 | |||
| ALP (x ULN) | 0.96 (0.61-1.44) | 1.26 (1.00-1.62) | 0.023 | 4.122 | 1.379 (1.011–1.879) | 0.018 |
| GGT (U/L) | 131 (49-226) | 132 (86-330) | 0.285 | |||
| IgG (g/L) | 17 (12-21) | 20 (14-35 | 0.026 | 5.834 | 1.03 (1.006–1.056) | 0.016 |
| INR | 1.10 (1.00-1.21) | 1.2 (1.03-1.58) | 0.088 | |||
| Platelets count (x109/L) | 227 (179-273) | 199 (147-27) | 0.079 | |||
| mHAI (n, %) | ||||||
| Grade | 8.00 (6.00-10.00) | 11 (8.00-12.00) | 0.001 | 6.681 | 1.181 (1.041–1.339) | 0.010 |
| Stage | 1 (0-2) | 1 (0-3) | 0.088 | |||
| Fibrosis (n, %) | 170 (48%) | 17 (65% | 0.050 | 2.779 | 2.115 (0.877–5.102) | 0.096 |
| Advanced fibrosis (n, %) | 42 (12%) | 7 (27%) | 0.017 | 5.799 | 3.052 (1.231–7.568) | 0.016 |
| ANA (n, %) | 241 (68%) | 24 (92%) | 0.010 | |||
| SMA (n, %) | 269 (76%) | 22 (85%) | 0.338 | |||
| Anti-SLA/LP (n, %) | 23 (6.5%) | 1 (3.8%) | 0.588 | |||
| Anti-LKM1 (n, %) | 10 (2.0%) | 0 (0%) | 0.378 | |||
| Cs + AZA (n, %) | 197 (55%) | 18 (69 %) | 0.168 | |||
| Cs + MMF (n, %) | 102 (29%) | 4 (15.4%) | 0.145 | |||
| BLSM (kPa) | 5.4 (4.3-7.08) | 11.05 (8.58-15.93) | <0.001 | 50.148 | 1.204 (1.144–1.267) | <0.001 |
| BR at BLSM (n, %) | 288 (81%) | 10 (39%) | <0.001 | 19. 108 | 0.172 (0.078–0.378) | <0.001 |
| Time from diagnosis to BLSM (yr) | 2.19 (1.21-4.98) | 1.75 (1.13-3.62) | 0.524 | |||
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, antinuclear antibodies; Anti-LKM1, anti-liver kidney microsomal antibodies; anti-SLA/LP, anti-soluble liver antigen/liver pancreas antibodies; AST, aspartate aminotransferase; AZA, azathioprine; BLSM, first liver stiffness measurement after 6 months of treatment initiation; BR, biochemical response; Cs, corticosteroids; GGT, gamma-glutamyltransferase; HR, hazard ratio; INR, international normalised ratio; mHAI, modified hepatitis activity index; MMF, mycophenolate mofetil; SMA, smooth muscle antibodies.
Data are expressed as the median (IQR). Univariate analysis was performed by Cox regression.
Fig. 3.
Multivariate Cox regression analysis for cirrhosis development according to BLSM.
Two models were performed including statistically significant parameters in the previous univariate analysis. BLSM (Model 1 = HR 1.161; 95% CI 1.07408–1.24425; p <0.001), BLSM <8.5 kPa (Model 2 = HR 0.096; 95% CI 0.033–0.278), mHAI (Model 1 = HR 1.212; 95% CI 1.062-1.382; p = 0.004; Model 2 = HR 1.183; 95% CI 1.041–1.345; p = 0.010), and BR at the time of BLSM (Model 1 = HR 0.267; 95% CI 0.095-0.750; p = 0.012; Model 2 = HR 0.278; 95% CI 0.107–0.723; p = 0.009) were independently associated with the risk of developing cirrhosis during follow-up. BLSM, baseline liver stiffness measurement; HR, hazard ratio; mHAI, modified hepatitis activity index.
Considering the critical role of BR in disease progression, the same analysis was performed, focusing on patients with a BR at BLSM. Ten (3%) of the 298 patients included in this analysis developed cirrhosis. Again, BLSM was significantly associated with the risk of developing cirrhosis (HR 1.300; 95% CI 1.153–1.465; p <0.001) (Table S3).
The AUC of BLSM in the prediction of cirrhosis development was 0.859 (95% CI 0.789-0.929) with an optimal cut-off point of 8.5 kPa (sensitivity: 81%, specificity: 86%, negative predictive value: 98%) (Fig. 4A). This cut-off point was confirmed by using maximally selected rank statistics (Fig. S3). As shown in Fig. 4B, patients with BLSM greater than 8.5 kPa had a significantly higher probability of developing cirrhosis during follow-up (p <0.001).
Fig. 4.
Association of BLSM with cirrhosis.
(A) ROC curve of BLSM for the prediction of cirrhosis development. (B) Probability of being free of cirrhosis during follow-up according to BLSM. Patients with BLSM greater than 8.5 kPa had a significantly higher probability of developing cirrhosis during follow-up, represented by a Kaplan-Meier curve and log-rank test (p <0.001). BLSM, baseline liver stiffness measurement.
Variation of LSM during follow-up
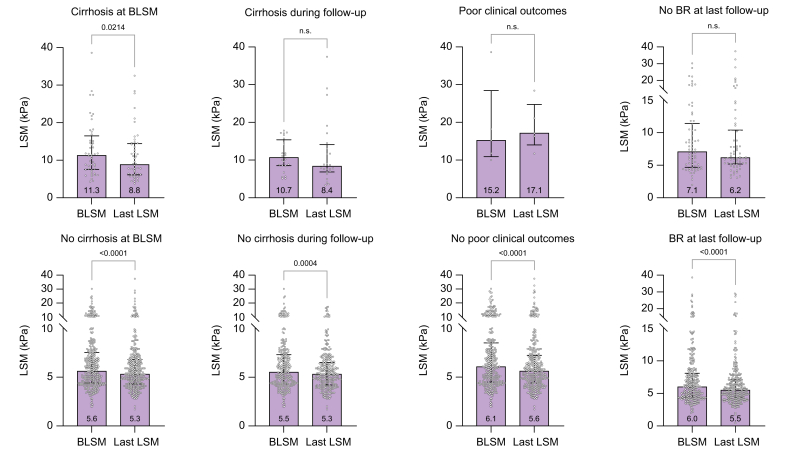
Three-hundred and seventy-one patients had at least a second LSM after BLSM, with a median number of LSMs per patient of 3 (range: 2-8). The time between BLSM and the last LSM was 3.5 years (IQR 1.95-5.79). There was a significant decrease in liver stiffness from BLSM to the last LSM (6.1 kPa vs. 5.6 kPa; p <0.001). The variation in liver stiffness (delta, Δ) was -0.6 kPa (IQR -2 to 0.9) with a progression rate (ΔLSM/year) of -0.13 kPa/year (IQR -0.63 to 0.28) This decrease in liver stiffness was observed in both patients with and without cirrhosis at BLSM (11.05 kPa vs. 8.8 kPa; p = 0.024 and 5.8 kPa vs. 5.4 kPa; p = 0.001, respectively), and with or without BR at the time of BLSM (from a median value of 5.5 kPa to 5.4 kPa; p = 0.007 and 8.5 kPa to 6.6 kPa; 0.005, respectively). However, liver stiffness did not significantly decrease in patients who developed cirrhosis during follow-up, patients with poor clinical outcomes, and those without BR at the last follow-up (10.6 kPa vs. 8.3 kPa; p = 1.000, 15.2 kPa vs. 17.1 kPa; p = 0.500, and 7.1 kPa vs. 6.1 kPa; p = 0.727, respectively) (Fig. 5).
Fig. 5.
Pairwise comparisons using a general linear model between BLSM and last LSM in each group.
Groups: cirrhosis at baseline, development of cirrhosis during follow-up, development of poor clinical outcomes, and BR at last follow-up. Dots represent individual LSM values in each group, bars represent median values, and error bars represent the 25th and 75th percentiles of the median (IQR). BLSM, baseline liver stiffness measurement; BR, biochemical response; LSM, liver stiffness measurement.
Discussion
In this large multicentre study, analysing over 400 patients with AIH, we demonstrated that elevated LSM values were associated with disease progression and unfavourable clinical outcomes. Furthermore, in patients without cirrhosis at the time of AIH diagnosis, LSM was also able to predict the development of cirrhosis during follow-up irrespective of the achievement of BR. Indeed, a cut-off point of 8.5 kPa for the LSM performed at least 6 months after treatment initiation had a high discriminative capacity for predicting poor clinical outcomes and cirrhosis development in patients with AIH.
A recent large retrospective study conducted by the IAIHG in a cohort of 1,700 patients with AIH determined that after a median follow-up of 10 years, 14% of patients experienced liver-related outcomes (death or LT). Non-white ethnicity, presence of cirrhosis at diagnosis, and lack of CBR at 6 months of treatment were associated with this composite endpoint.2 However, our study found a much lower rate of poor clinical outcomes, as less than 2% of patients decompensated, required LT, and/or died of a liver-related cause. While the shorter follow-up duration of our study may potentially explain the differences in outcomes, it is important to note that the enrolled patients were diagnosed with AIH after the publication of the clinical practice guidelines in 2010,20 which tightened the definition of CBR to complete normalization of transaminases and IgG levels, highlighting the excellent prognosis of AIH when CBR is achieved.
Despite the relatively small number of poor clinical outcomes, the findings of our study align with those of previous reports21,22 in which advanced fibrosis and/or cirrhosis at diagnosis, low platelet count, and lack of BR during follow-up were significantly associated with the development of poor clinical outcomes. In addition, we showed for the first time that a LSM >8.5 kPa, performed at least 6 months after the initiation of immunosuppressive treatment, was associated with poor clinical outcomes. Other variables such as platelet count and mHAI have also been shown to have excellent discriminative capacity to predict poor outcomes in our cohort. Unfortunately, multivariate and subgroup analyses were not feasible due to the low number of events. Overall, our findings suggest that similar to what has been demonstrated in patients with PBC,9 LSM seems to play a key role in predicting liver-related outcomes in AIH.
After having documented the role of LSM in predicting clinical outcomes, we wanted to evaluate the predictive factors of developing cirrhosis during follow-up and determine whether LSM could also predict the risk of fibrosis progression and cirrhosis development. Patients with advanced fibrosis, elevated mHAI at baseline liver biopsy, and insufficient response to immunosuppressive treatment were found to have a higher risk of developing cirrhosis. These results are in line with previous research indicating that patients with moderate to severe portal inflammation, interface hepatitis, and advanced fibrosis in the liver biopsy are slow responders23 and are thus at risk of fibrosis progression. Despite the relevance of the histological features in the prognosis of AIH, our study highlights the importance of LSM in predicting cirrhosis, since it was associated with the risk of cirrhosis development regardless of the presence of fibrosis in the initial liver biopsy. Thus, it could be speculated that despite BLSM being performed after the first 6 months of treatment, these values may still reflect not only fibrosis but also some degree of residual liver inflammation, which is a well-known predictive factor of poor clinical outcomes.6
Follow-up LSM revealed a significant decline in LSM values across the entire cohort regardless of the presence or absence of cirrhosis at BLSM. This observation suggests the influence of inflammation on LSM and highlights how the resolution of inflammation after starting immunosuppressive therapy improves LSM.10 Although the decrease in LSM could also be attributed to fibrosis regression, as previously described,24,25 the lack of follow-up biopsies does not allow this to be accurately demonstrated. Nonetheless, the decrease in LSM is consistent with our prior findings, showing that a significant proportion of patients with cirrhosis with or without portal hypertension exhibit low LSM values, especially after long-term treatment administration.15 However, it is essential to note that in patients with poor clinical outcomes and those who developed cirrhosis during follow-up, LSM did not significantly decrease (not even in patients with cirrhosis with a BR), suggesting that a lack of improvement in LSM may identify patients with more established fibrosis and a greater risk of developing negative outcomes.
We should acknowledge that this study has some limitations. First, it was a retrospective study, leading to variability in the timing of LSM across patients. However, the study entry date was the date of the first LSM performed at least 6 months after treatment initiation, establishing a uniform baseline time point for all participants, in order to limit the potential effect of liver inflammation on LSM values. Second, it lacks follow-up liver biopsies that could histologically confirm the development of cirrhosis during follow-up. However, these biopsies are not routinely performed in clinical practice, and several studies have shown good performance of liver ultrasound in the detection of cirrhosis with a sensitivity ranging between 54% and 84%, and a specificity of between 78%-100%.[26], [27], [28], [29] Third, the follow-up duration after BLSM was relatively short, a fact that could potentially underestimate the number of patients with poor clinical outcomes. Fourth, IgG levels were not considered for the definition of BR as recommended in current guidelines.3,4,30,31 This decision was made because the presence of cirrhosis may elevate IgG levels and, as a result, underestimate the BR rate, and secondly, recent evidence has shown that IgG normalization does not significantly increase the probability of being in histological remission when transaminases are normal.5,32 Lastly, BR was evaluated at the time of LSM and not at the specific timepoints recommended in the guidelines (6 or 12 months) because the aim was to investigate the potential impact of liver inflammation on LSM rather than the effect of BR on outcomes, which has already been established previously.
In conclusion, this comprehensive multicentre cohort study demonstrated that the incidence of poor clinical outcomes in patients with AIH managed in reference centres adhering to stringent response criteria remains notably low. However, LSM performed 6 months after treatment initiation serves as a predictive tool for both adverse clinical outcomes and progression to cirrhosis over a median follow-up period of 4 years. Furthermore, our findings suggest that failure to observe a decrease in LSM during follow-up could delineate a subgroup of patients at elevated risk for poor outcomes. Multicentre, prospective studies are needed to corroborate these results.
Abbreviations
AIH, autoimmune hepatitis; AZA, azathioprine; BLSM, baseline liver stiffness measurement; BR, biochemical response; CBR, complete biochemical response; HR, hazard ratio; LSM, liver stiffness measurement; LT, liver transplantation; MMF, mycophenolate mofetil; VCTE, vibration-controlled transient elastography.
Financial support
MCL received support from the Instituto de Salud Carlos III (ISCIII) through the project "PI21/00800" and co-funded by the European Union. IO was funded by Contractes Clínic de Recerca “Emili Letang - Josep Font” 2022, granted by the Hospital Clínic Barcelona. PA was supported by a grant from the Hellenic Association for the Study of the Liver (HASL).
Authors’ contributions
IO: study design, data collection, data analysis, manuscript writing; PA, SG, MDB, ADG, PE, MRB, SRT, and KZ: data collection, critical revision of the manuscript; EM: statistical analysis; GND and MCL: study design, data analysis, manuscript writing, critical revision.
Data availability statement
Data will be made available to other researchers upon any reasonable request and can be obtained through the principal investigator.
Conflict of interest
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Given their role as Editor, María-Carlota Londoño had no involvement in the peer-review of this article and had no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to the Guest Editor Annalisa Berzigotti.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101213.
Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
References
- 1.Dyson J.K., Wong L.L., Bigirumurame T., et al. Inequity of care provision and outcome disparity in autoimmune hepatitis in the United Kingdom. Aliment Pharmacol Ther. 2018;48(9):951. doi: 10.1111/APT.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slooter C.D., van den Brand F.F., Lleo A., et al. Lack of complete biochemical response in autoimmune hepatitis leads to adverse outcome: first report of the IAIHG retrospective registry. Hepatology. 2024;79(3):538–550. doi: 10.1097/HEP.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 3.Lohse A.W., Chazouillères O., Dalekos G., et al. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Sebode M., Hartl J., Vergani D., Lohse A.W. Autoimmune hepatitis: from current knowledge and clinical practice to future research agenda. Liver Int. 2018;38(1):15–22. doi: 10.1111/LIV.13458. [DOI] [PubMed] [Google Scholar]
- 5.Laschtowitz A., Zachou K., Lygoura V., et al. Histological activity despite normal ALT and IgG serum levels in patients with autoimmune hepatitis and cirrhosis. JHEP Rep. 2021;3(4) doi: 10.1016/J.JHEPR.2021.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhaliwal H.K., Hoeroldt B.S., Dube A.K., et al. Long-term prognostic significance of persisting histological activity despite biochemical remission in autoimmune hepatitis. Am J Gastroenterol. 2015;110(7):993–999. doi: 10.1038/AJG.2015.139. [DOI] [PubMed] [Google Scholar]
- 7.Sandrin L., Fourquet B., Hasquenoph J.M., et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Ferraioli G., Roccarina D. Update on the role of elastography in liver disease. Therap Adv Gastroenterol. 2022;15 doi: 10.1177/17562848221140657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corpechot C., Carrat F., Gaouar F., et al. Contribution of liver stiffness measurement by vibration-controlled transient elastography to outcome prediction in primary biliary cholangitis. J Hepatol. 2022;77(6):1545–1553. doi: 10.1016/J.JHEP.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Hartl J., Denzer U., Ehlken H., et al. Transient elastography in autoimmune hepatitis: timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65(4):769–775. doi: 10.1016/j.jhep.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Guo L., Zheng L., Hu L., et al. Transient elastography (FibroScan) performs better than non-invasive markers in assessing liver fibrosis and cirrhosis in autoimmune hepatitis patients. Med Sci Monit. 2017;23:5106. doi: 10.12659/MSM.907300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q., Sheng L., Bao H., et al. Evaluation of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis. J Gastroenterol Hepatol. 2017;32(3):639–644. doi: 10.1111/JGH.13508. [DOI] [PubMed] [Google Scholar]
- 13.Zachou K., Lygoura V., Arvaniti P., et al. FibroMeter scores for the assessment of liver fibrosis in patients with autoimmune liver diseases. Ann Hepatol. 2021;22 doi: 10.1016/J.AOHEP.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Mack C.L., Adams D., Assis D.N., et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology. 2020;72(2):671–722. doi: 10.1002/HEP.31065. [DOI] [PubMed] [Google Scholar]
- 15.Llovet L.P., Gratacós-Ginès J., Téllez L., et al. Noninvasive prediction of outcomes in autoimmune hepatitis–related cirrhosis. Hepatol Commun. 2022;6(6):1392. doi: 10.1002/HEP4.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartl J., Ehlken H., Sebode M., et al. Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J Hepatol. 2018;68(4):754–763. doi: 10.1016/j.jhep.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Hennes E.M., Zeniya M., Czaja A.J., et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 18.Everhart J.E., Wright E.C., Goodman Z.D., et al. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology. 2010;51(2):585–594. doi: 10.1002/HEP.23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy P., Bane O., Hectors S.J., et al. Noninvasive imaging assessment of portal hypertension. Abdom Radiol (New York) 2020;45(11):3473. doi: 10.1007/S00261-020-02729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manns M.P., Czaja A.J., Gorham J.D., et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 21.Miyake Y., Iwasaki Y., Terada R., et al. Persistent elevation of serum alanine aminotransferase levels leads to poor survival and hepatocellular carcinoma development in type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2006;24(8):1197–1205. doi: 10.1111/j.1365-2036.2006.03113.x. [DOI] [PubMed] [Google Scholar]
- 22.Verma S., Gunuwan B., Mendler M., et al. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004;99(8):1510–1516. doi: 10.1111/J.1572-0241.2004.30457.X. [DOI] [PubMed] [Google Scholar]
- 23.Engel B., Jaeckel E., Taubert R. 2022 International autoimmune hepatitis group non-response criteria in autoimmune hepatitis: quick vs. slow responders. J Hepatol. 2023;78(3):e113–e114. doi: 10.1016/j.jhep.2022.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Czaja A.J. Review article: the prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther. 2014;39(4):385–406. doi: 10.1111/APT.12592. [DOI] [PubMed] [Google Scholar]
- 25.Czaja A.J., Carpenter H.A. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40(4):646–652. doi: 10.1016/j.jhep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Lurie Y., Webb M., Cytter-Kuint R., et al. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21(41):11567–11583. doi: 10.3748/WJG.V21.I41.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saverymuttu S.H., Joseph A.E.A., Maxwell J.D. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J Clin Res Ed. 1986;292(6512):13–15. doi: 10.1136/BMJ.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harbin W.P., Robert N.J., Ferrucci J.T. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology. 1980;135(2):273–283. doi: 10.1148/RADIOLOGY.135.2.7367613. [DOI] [PubMed] [Google Scholar]
- 29.Gaiani S., Gramantieri L., Venturoli N., et al. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27(6):979–985. doi: 10.1016/S0168-8278(97)80140-7. [DOI] [PubMed] [Google Scholar]
- 30.Manns M.P., Czaja A.J., Gorham J.D., et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193–2213. doi: 10.1002/HEP.23584. [DOI] [PubMed] [Google Scholar]
- 31.Dalekos G.N., Koskinas J., Papatheodoridis G.V. Hellenic association for the study of the liver clinical practice guidelines: autoimmune hepatitis. Ann Gastroenterol. 2019;32(1):1–23. doi: 10.20524/AOG.2018.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz Gonzalez A., Carballo-Folgoso L., Álvarez-Navascués C., et al. Isolated IgG elevation is not associated with worse outcome in patients with autoimmune hepatitis. J Hepatol. 2022;77:S328. doi: 10.1016/S0168-8278(22)01020-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Data Availability Statement
Data will be made available to other researchers upon any reasonable request and can be obtained through the principal investigator.