Abstract
Otostegia fruticosa (Forssk.) is a shrub of the Lamiaceae family with a wide geographic distribution in Saudi Arabia, Western and Eastern Africa, Ethiopia, and the Middle East. The current study provides an overview of recent developments in the knowledge of O. fruticosa’s ethnobotanical, pharmacological, and phytochemical properties. In folkloric medicine, it has been used since ancient times for gastrointestinal disorders, oral health, ocular irritation, antiparasitic, diarrhea, tonsillitis, arthritis, respiratory complications, and sunstroke treatment. Pharmacological investigations of its antibacterial, antioxidant, anti-inflammatory, cytotoxic, analgesic, larvicidal, nephroprotective, and other effects further validated its folklore practice. A range of diterpenoids, triterpenes, flavonoids, and essential oils have been found in O. fruticosa, according to phytochemical studies, which are thought to be responsible for the pharmacological effects of this plant species. This scientific review summarizes the most important secondary metabolites isolated from the O. fruticosa. It also summarizes the biological activities, providing insights into further scientific exploration.
Keywords: Otostegia fruticosa, Folkloric use, Secondary metabolites, Pharmacology
1. Introduction
Since the beginning of recorded history, the quest for plants to treat human illnesses and afflictions has been a fundamental part of human culture. According to Borris (1996), 250,000 to 500,000 plant species exist worldwide. Humans and animal species consume one to ten percent of these as food. Possibly even more are used for medicinal purposes (Moerman, 1996). The pharmacopeias of many nations mention the use of therapeutic plants and herbal preparations, including herbal extracts (Hostettmann et al., 1995). These extracts include a variety of active ingredients, in contrast to modern medicines, which only contain a single active component. Interestingly, the natural ingredients in these “herbal cocktails” can act synergistically in the body to provide significant therapeutic benefits with few to no adverse effects (Kaufman et al., 1999). These plant-based pharmaceuticals are now referred to as herbal medicines and represent safety compared to synthetic medicines. Much of today’s research on medicinal plants has focused mainly on phytochemistry, pharmacology, and horticulture. The potentially bioactive constituents of medicinal plants were identified and isolated in phytochemistry before being subjected to a thorough structural investigation. The pharmacology of medicinal plants has also led to the discovery of probable mechanisms of action, target sites, and bioactivity assays. Horticultural research into medicinal plants is intended to create conditions for their cultivation so that they thrive to their full potential (Briskin, 2000). The Lamiaceae family includes the genus Otostegia and is sometimes referred to as the mint family; it is one of the most important flowering plant groups with worldwide distribution (Yuan et al., 2010). The family comprises about 236 genera, with an estimated 6,900–7,200 species in these genera (Tamokou et al., 2017, Ramasubramania, 2012, Ahmad et al., 2022). Most members of the Lamiaceae family are aromatic plants (Zinicovscaia et al., 2020, Nunez and de Castro, 1992). Some members of this family, such as Ajuga, Coleus, and Salvia, are cultivated for their aesthetic value, according to Khoury et al. (2016). This family also encompasses many plants with biological and medical applications. The most famous members of this family are aromatic spices such as mint, thyme, sage, oregano, basil, rosemary, savory, self-heal, lemon balm, hyssop, and others with more limited use (Uritu et al., 2018, Bekut et al., 2018). According to Ahmad et al., 2007, Sadeghi et al., 2014, and Khan et al. (2009), the genus Otostegia comprises 33 species mainly found in the Mediterranean region. Traditional healers commonly use these species to treat a variety of illnesses. A wide range of secondary metabolites with various activities, such as anxiolytic, antiulcer, depressive, sedative, and antispasmodic properties, have been discovered in this genus (Aboutabl et al., 1995, Vural et al., 1996, Anwar et al., 2004 Tesso and Konig, 2004. The chemical profile of the genus revealed the presence of phenolic acids, fatty acids, flavonoids and its glycosides, triterpenes, diterpenes, sesquiterpenes and monoterpenes (Ahmad et al., 2007, Al-Musayeib et al., 2000, Khan and Syed, 2013, Ahmad et al., 2022, Rosselli et al., 2019). O. fruticosa has been used extensively in traditional medicine in Saudi Arabia and globally. This review aims to shed some light on its potential to provide a novel therapeutic agent, discussing its secondary metabolites' diversity and biological activities to guide researchers in filling research gaps through further scientific investigations.
2. Taxonomic hierarchy
O. fruticose (Fig. 1) is a shrub that is widely distributed in Ethiopia, Saudi Arabia, and other countries in the Middle East as well as West and East Africa (Collenette, 1999, Ansari et al., 2020, Rosselli et al., 2019). The leaves of the plant have an oval to round shape, measure 5 to 12 cm in length, have hairy edges with round teeth, and may have a rounded base ending in a short petiole. The plant's branches are quite densely hairy. The flowers are cream in color, and up to 30 flowers can appear together. The fruit is 2–3 mm long and contains four nutlets (Bahta, et al., 2020). The scientific classification of O. fruticosa is kingdom: Plantae; Order: Lamiales; family: Lamiaceae; Genus: Otostegia; Species: fruticosa. It is known by various vernacular names, such as “sasa” in Tigray, Ethiopia (Kidane et al., 2013); “geram tungut” in central Ethiopia (Getaneh and Girma, 2014); “fesi hadima” in Eritrea (Andemariam, 2010); and “shakab, sharm” in Saudi Arabia (Rahman et al., 2004).
Fig. 1.

Otostegia fruticose (Al-Madinah Region, Bader Governorate, wadi Alanig).
3. Material and method
All information presented in this review was collected from the pertinent O. fruticosa publications. The literature was collected using scientific databases such as Scopus, Elsevier-Science Direct, Google Scholar, Web of Science, and other online sources, including the Saudi Arabian kingdom's flora. The following keywords were used for the literature search: chemical constituents of O. fruticosa, distribution of O. fruticosa, folkloric use of O. fruticosa, pharmacological activities of O. fruticosa, medicinal plants of Saudi Arabia, family Lamiaceae, Otostegia, and O. fruticosa. The tables summarize the chemical components isolated from O. fruticosa in addition to the compounds tentatively identified by GC-MC and HPLC-MS.
4. Folkloric use of O. fruticosa
O. fruticose has been used for generations in traditional and cultural medicinal practices. In Tigray, northern Ethiopia, the leaves are said to have mosquito-repellent properties (Kidane et al., 2013), while their juice is used to cure diarrhea in infants in the very nearby region of Delantia (Meragiaw et al., 2016). According to Davigdor et al. (2014), the smoke from burned leaves is used as a fumigant, disinfectant, and pesticide to treat inexplicable stomach aches. The branches are used for dental hygiene, and an infusion from the flowering branches treats sunstroke in Saudi Arabia (Rahman et al., 2004). In Yemen, it is said to possess antiparalytic effects (Mothana et al., 2011). Leaf decoction is used externally on animals’ eyes to relieve inflammation (Ali et al., 2017a; Mothana et al., 2011). Prior studies also report that the leaves of O. fruticosa are traditionally employed to treat feverish illnesses and respiratory problems (Enyew et al., 2013, Getaneh and Girma, 2014). Further research revealed that O. fruticosa aerial parts have been used to cure tonsillitis, asthma, arthritis, feverish illnesses, and gynecological disorders (Bahta et al., 2020, Andemariam, 2010).
5. Phytochemistry
5.1. Essential oils tentatively identified in. fruticosa by GC–MS
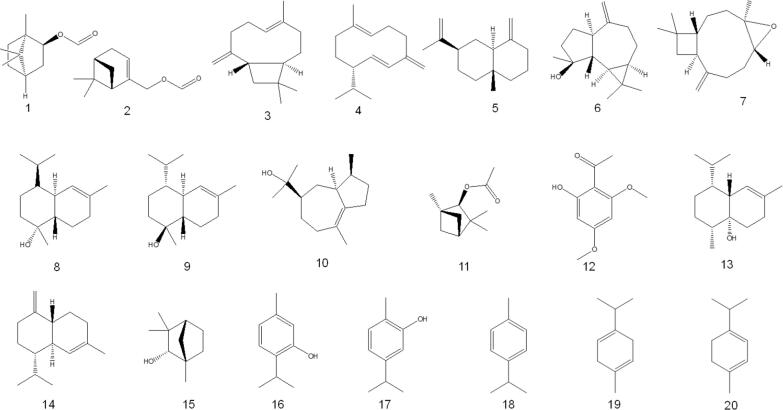
The essential oil obtained by hydrodistillation of air-dried leaves of O. fruticosa (growing in Taiz province, Yemen) and analyzed by GC–MS showed the presence of eighty-five compounds. The tentatively identified compounds are bornyl formate (5.2 %), myrtenyl formate (3.9 %), β-caryophyllene (8.8 %), germacrene D (2.6 %), β-selinene (3.0 %), spathulenol (3.2 %), caryophyllene oxide (2.5 %), τ-cadinol (9.3 %), α-cadinol (2.6 %), bulnesol (1.9 %), (E)-β-farnesene (1.67 %), endo-fenchyl acetate (1.46 %), xanthoxylin (Brevifolin) (1.68 %), 1, 10-di-epi-cubenol (1.03 %), γ-cadinene (1.36 %), δ-cadinene (1.13 %), endo-fenchol (1.44 %), and two major unidentified diterpenoids (12.7 % and 9.4 %) (Ali, et al., 2017b).
In another report, GC–MS analysis of hydro-distilled essential oil obtained from fresh aerial parts of O. fruticosa (a plant grown at the Experimental Station of the Faculty of Agriculture, El-Mansoura University, Egypt) showed thirty-six components representing 98.8 % of the oil, having almost equal amounts of oxygenated (47.6 %) and non-oxygenated (51.2 %) compounds. The most important oxygenated compounds tentatively identified are the phenols thymol (43.7 %) and carvacrol (0.8 %), while alcohols make up only 1.4 % of the oil. Twenty-one hydrocarbons represent 32.1 % of the oil and consist of monoterpenes (16.6 %) and sesquiterpenes (17.6 %). The primary monoterpene was p-cymene (12 %), while β-caryophyllene (9.5 %) was the primary sesquiterpene. Other identified constituents represented were γ −terpinene (16.4 %), trans-α-bergamotene (2.2 %), and β-selinene (2.6 %) (Aboutabl et al., 1995). Abdelshafeek et al., (2016a) reported that the GC–MS analysis of volatile oil extracted by hydrodistillation of the fresh aerial parts of O. fruticosa (grown in Sinai, Egypt) revealed the presence of volatile oil components: 2-heptenal, cis carveol, 4-terpineol, α-copaene, β-bourbonene, α-cis-bergamotene, β-bisablene, α-cadenen, pyridine-2-(1-methyl ethyl), caryophyllene oxide, and 4-decyne linalyl acetate, with caryophyllene oxide being the major component (60.86 %). GC–MS analysis also showed the presence of fatty alcohols. GLC analysis revealed the presence of long-chain hydrocarbons, long-chain fatty acids, and other miscellaneous compounds: lupeol, β-amyrin, cholesterol, campesterol, β-sitosterol, stigmasterol and α-amyrin.
GC–MS analysis of the hydroalcoholic extract of O. fruticosa revealed the presence of twenty three compounds: 2-methyl-benzaldehyde, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, e and z isomers of 1-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3,4,4-trimethyl-2-pent-ene, santalane, (+)-2-endo, 3-endo-dimethylbornane, lavandulyl acetate, 2, 8-dimethyl-4-(1-methylpropyl)-4, 6-decadiene, olean-12-en-28-al, trans-tetrahydroionone, dihydrotorulosol, peroxyergosterol, palustrol, peroxyergosterol, 2,5-dihydroxy-40-methoxy-flavanone, (all-e)-2, 6, 10, 14-tetramethyl-16-(phenylthio) hexadeca-2,6,10,14-tetraen-1-ol, and solanesol 9-octadecenoic acid (Ansari et al., 2020).
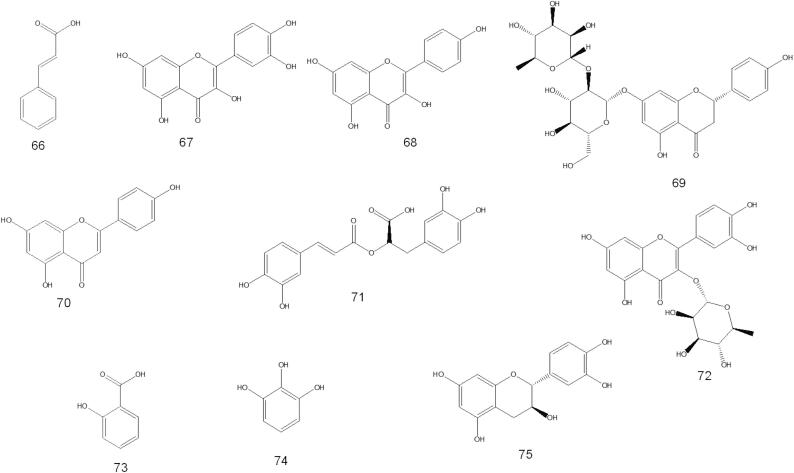
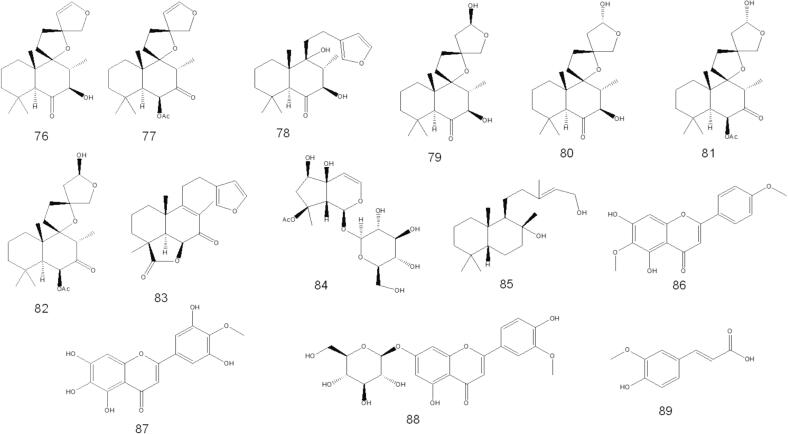
Al-Jumayi (2020) reported that essential oils from O. fruticosa leaves contain 2, 5-dimethyl-p-cymene, 2-allyl-4-methyl phenol, apiol, and isolongipholene in higher amounts, followed by isocalamendiol, β-caryophyllene, limonene, γ-terpineol, α-copaene, β-citronellol, pulegone and α-himachalene. The tentatively identified compounds identified in O. fruticosa by GC–MS are listed in Table 1 and structures are depicted in Fig. 2.
Table 1.
Significant chemical composition identified in O. fruticosa by GC–MS.
| S. No. | Compound’s name | Chemical class | Reference |
|---|---|---|---|
| 1 | bornyl formate (1) | monoterpenoid | Ali et al., 2017a, Ali, et al., 2017b |
| 2 | myrtenyl formate (2) | monoterpenoid | Ali et al., 2017a, Ali, et al., 2017b |
| 3 | β-caryophyllene (3) | sesquiterpene | Aboutabl et al., 1995, Al-Jumayi, 2020, Ali, et al., 2017b |
| 4 | germacrene D (4) | sesquiterpene | Ali, et al., 2017b |
| 5 | β-selinene (5) | sesquiterpene | Aboutabl et al., 1995, Ali, et al., 2017b |
| 6 | spathulenol (6) | sesquiterpenoid | Ali, et al., 2017b |
| 7 | caryophyllene oxide (7) | sesquiterpenoid | Ali, et al., 2017b |
| 8 | τ-cadinol (8) | sesquiterpenoid | Ali, et al., 2017b |
| 9 | α-cadinol (9) | sesquiterpenoid | Ali, et al., 2017b |
| 10 | bulnesol (10) | sesquiterpenoid | Ali, et al., 2017b |
| 11 | endo-fenchyl acetate (11) | monoterpenoid | Ali, et al., 2017b |
| 12 | xanthoxylin (12) | phenolic ketone | Ali, et al., 2017b |
| 13 | 1,10-di-epi-cubenol (13) | sesquiterpenoid | Ali, et al., 2017b |
| 14 | γ-cadinene (14) | sesquiterpene | Ali, et al., 2017b |
| 15 | endo-fenchol (15) | monoterpenoid | Ali, et al., 2017b |
| 16 | thymol (16) | monoterpenoid | Aboutabl, et al., 1995 |
| 17 | carvacrol (17) | monoterpenoid | Aboutabl, et al., 1995 |
| 18 | p-cymene (18) | monoterpene | Aboutabl, et al., 1995 |
| 19 | γ –terpinene (19) | monoterpene | Aboutabl, et al., 1995 |
| 20 | α-terpinene (20) | monoterpene | Aboutabl, et al., 1995 |
| 21 | α-humulene (21) | sesquiterpene | Ali, et al., 2017b |
| 22 | α-trans- bergamotene (22) | sesquiterpene | Aboutabl, et al., 1995 |
| 23 | cis-carveol (23) | monoterpenoid | Abdelshafeek, et al., 2016a |
| 24 | 4-terpineol (24) | monoterpenoid | Aboutabl et al., 1995, Abdelshafeek et al., 2016a |
| 25 | α-copaene (25) | sesquiterpene | Abdelshafeek et al., 2016a, Al-Jumayi, 2020 |
| 26 | β-bourbonene (26) | sesquiterpene | Abdelshafeek, et al., 2016a |
| 27 | α-cis-bergamotene (27) | sesquiterpene | Abdelshafeek, et al., 2016a |
| 28 | β-bisablene (28) | sesquiterpene | Abdelshafeek, et al., 2016a |
| 29 | α-cadinene (29) | sesquiterpene | Abdelshafeek, et al., 2016a |
| 30 | linalyl acetate (30) | monoterpenoid | Abdelshafeek, et al., 2016a |
| 31 | lupeol (31) | triterpenoid | Abdelshafeek, et al., 2016a |
| 32 | β–amyrin (32) | triterpenoid | Abdelshafeek, et al., 2016a |
| 33 | cholesterol (33) | steroid | Abdelshafeek, et al., 2016a |
| 34 | campesterol (34) | steroid | Abdelshafeek, et al., 2016a |
| 35 | β –sitosterol (35) | steroid | Abdelshafeek et al., 2016a, Aboutabl et al., 2000 |
| 36 | stigmasterol (36) | steroid | Abdelshafeek, et al., 2016a |
| 37 | α –amyrin (37) | triterpenoid | Abdelshafeek, et al., 2016a; Aboutabl et al., 2000 |
| 38 | (E) and (Z) isomers of 1-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3,4,4-trimethyl-2-pent-ene (38) |
sesquiterpene | Ansari, et al., 2020 |
| 39 | santalane (39) | sesquiterpene | Ansari, et al., 2020 |
| 40 | lavandulyl acetate (40) | monoterpenoid | Ansari, et al., 2020 |
| 41 | olean-12-en-28-al (41) | triterpenoid | Ansari, et al., 2020 |
| 42 | peroxyergosterol (42) | steroid | Ansari, et al., 2020 |
| 43 | palustrol (43) | sesquiterpenoid | Ansari, et al., 2020 |
| 44 | 2,5-dimethyl-p-cymene (44) | monoterpenoid | Al-Jumayi, 2020 |
| 45 | 2-allyl-4-methyl phenol (45) | monoterpenoid | Al-Jumayi, 2020 |
| 46 | apiol (46) | phenylpropene | Al-Jumayi, 2020 |
| 47 | isolongifolene (47) | sesquiterpene | Al-Jumayi, 2020 |
| 48 | isocalamendiol (48) | sesquiterpenoid | Al-Jumayi, 2020 |
| 49 | limonene (49) | monoterpene | Al-Jumayi, 2020 |
| 50 | γ-terpineol (50) | monoterpenoid | Al-Jumayi, 2020 |
| 51 | β-citronellol (51) | monoterpenoid | Al-Jumayi, 2020 |
| 52 | pulegone (52) | monoterpenoid | Al-Jumayi, 2020 |
| 53 | α-himachalene (53) | sesquiterpene | Al-Jumayi, 2020 |
Fig. 2.
Chemical composition identified in O. fruticosa by GC–MS.
5.2. Compounds tentatively identified in O. fruticosa by HPLC-MS/MS
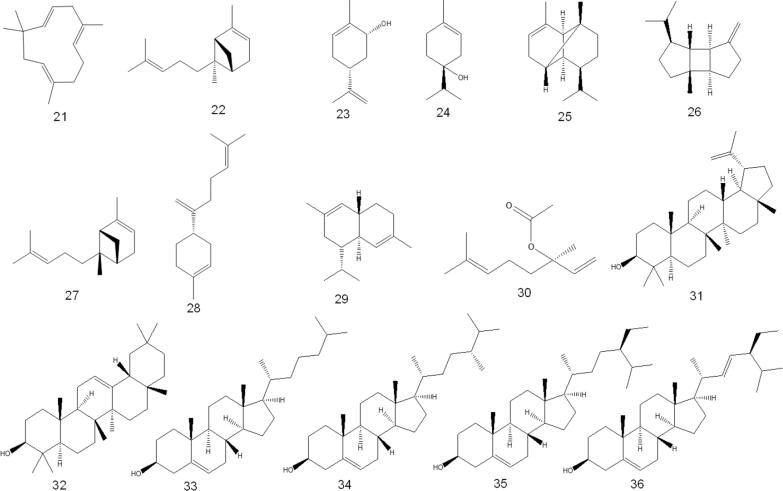
The HPLC-MS/MS profile of O. fruticosa leaves methanolic extract (Al-Madhagy, et al., 2022) revealed the presence of fifteen compounds belonging to phenolic acids and their derivatives, iridoids, xanthones, and flavonoids. The major identified compounds include neo chlorogenic acid, chlorogenic acid 5-, loganic acid, and caffeic acid (381.05, 231.57, 35.75, and 17.09 µg/g, respectively). The compounds identified in minor quantities are gallic acid, syringic acid, p-coumaric acid, ferulic acid, and 3,5-dicaffeoylquinic acid. Meanwhile, shikimic acid, vanillic acid, rutin, hyperoside, trans-cinnamic acid, and quercetin are found in traces.
In addition, HPLC analysis detected various flavonoids such as hesperetin, kaempferol, naringin, rutin, apigenin, rosmarinic, and quercetin, as well as various polyphenols such as vanillin, pyrogallol, salicylic and catechin (Al-Jumayi, 2020). Phytochemicals identified by HPLC-MS/MS are listed in Table 2 and structure are shown in Fig. 3.
Table 2.
O. fruticosa chemical composition identified by HPLC-MS/MS.
| S. No. | Compound’s name | Chemical class | Reference |
|---|---|---|---|
| 1 | chlorogenic acid (54) | phenolic acid | Al-Madhagy, et al., 2022 |
| 2 | neochlorogenic acid (55) | phenolic acid | Al-Madhagy, et al., 2022 |
| 3 | loganic acid (56) | monoterpenoid glycoside | Al-Madhagy, et al., 2022 |
| 4 | caffeic acid (57) | phenolic acid | Al-Madhagy, et al., 2022 |
| 5 | gallic acid (58) | phenolic acid | Al-Madhagy, et al., 2022 |
| 6 | p-coumaric acid (59) | phenolic acid | Al-Madhagy, et al., 2022 |
| 7 | syringic acid (60) | phenolic acid | Al-Madhagy, et al., 2022 |
| 8 | 3,5-dicaffeoylquinic acid (61) | phenolic acid | Al-Madhagy, et al., 2022 |
| 9 | shikimic acid (62) | phenolic acid | Al-Madhagy, et al., 2022 |
| 10 | vanillic acid (63) | phenolic acid | Al-Madhagy, et al., 2022;Al-Jumayi, 2020 |
| 11 | rutin (64) | flavonoid glycoside | Al-Jumayi, 2020, Al-Madhagy et al., 2022 |
| 12 | hyperoside (65) | flavonoid glycoside | Al-Madhagy, et al., 2022 |
| 13 | trans-cinnamic acid (66) | phenolic acid | Al-Madhagy, et al., 2022 |
| 14 | quercetin (67) | flavonoid | Al-Madhagy, et al., 2022 |
| 15 | kaempferol (68) | flavonoid | Al-Jumayi, 2020 |
| 16 | naringin (69) | flavonoid glycoside | Al-Jumayi, 2020 |
| 17 | apigenin (70) | flavonoid | Al-Jumayi, 2020 |
| 18 | rosmarinic acid (71) | phenolic acid | Al-Jumayi, 2020 |
| 19 | quercetrin (72) | flavonoid glycoside | Al-Jumayi, 2020 |
| 20 | salicylic acid (73) | phenolic acid | Al-Jumayi, 2020 |
| 21 | pyrogallol (74) | phenolic acid | Al-Jumayi, 2020 |
| 22 | catechin (75) | flavonoid | Al-Jumayi, 2020 |
Fig. 3.
O. fruticosa chemical composition identified by HPLC.
5.3. Compounds isolated from O. fruticosa by column chromatography
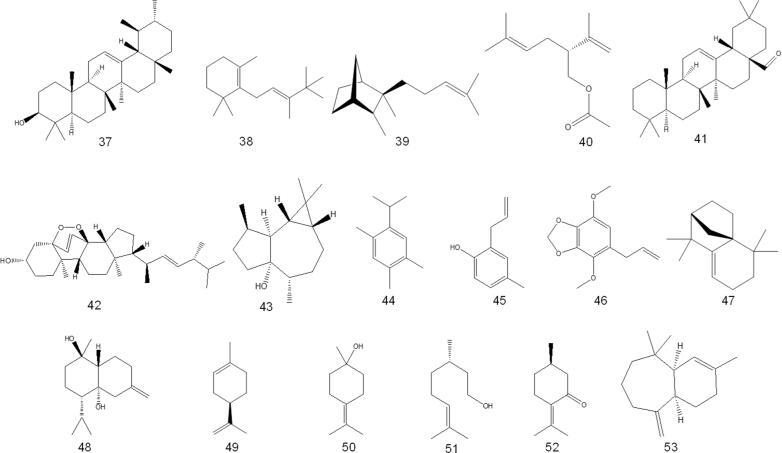
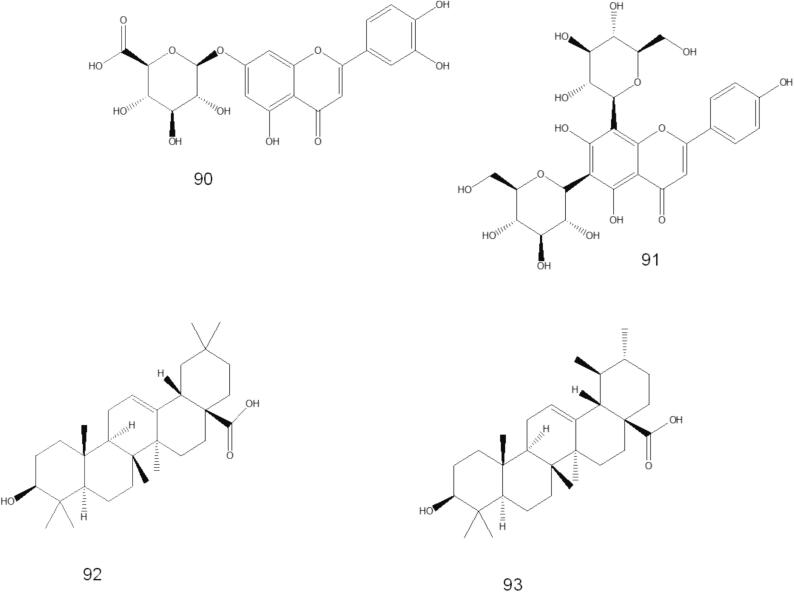
A few papers discuss isolating various chemical compounds from O. fruticosa aerial parts. In 2000, Al-Musayeb et al. isolated and elucidated labdane-type diterpenoids and iridoid glycosides from the ethyl acetate extract. In the same year, Aboutabl et al. isolated several chemical compounds belonging to flavonoids, triterpenes, and phenolic acids. Later, in 2016, Abdelshafeek et al. 2016b also isolated flavonoids and flavonoid glycoside from chloroform and ethyl acetate fractions of O. fruticosa methanolic extract. The details of the chemical structures of the compounds that have been isolated are shown in Table 3 and Fig. 4.
Table 3.
Phytochemicals isolated from O. fruticosa by column chromatography.
| S. No. | Compound’s name | Chemical class | Reference |
|---|---|---|---|
| 1 | preleoheterin (76) | diterpenoid | Al-Musayeib, et al., 2000 |
| 2 | otostegin A (77) | diterpenoid | Al-Musayeib, et al., 2000 |
| 3 | leoheterin (78) | diterpenoid | Al-Musayeib, et al., 2000 |
| 4 | leopersin C (79) | diterpenoid | Al-Musayeib, et al., 2000 |
| 5 | 15-epi-leopersin C (80) | diterpenoid | Al-Musayeib, et al., 2000 |
| 6 | otostegin B (81) | diterpenoid | Al-Musayeib, et al., 2000 |
| 7 | 15-epi-otostegin B (82) | diterpenoid | Al-Musayeib, et al., 2000 |
| 8 | ballonigrin (83) | diterpenoid | Al-Musayeib, et al., 2000 |
| 9 | 8-O-acetylharpagide (84) | monoterpenoid glycoside | Al-Musayeib, et al., 2000 |
| 10 | vulgarol (85) | diterpenoid | Al-Musayeib, et al., 2000 |
| 11 | pectolinarigenin (86) | flavonoid | Abdelshafeek, et al., 2016b |
| 12 | 5,6,7,3′,5′-pentahydroxy-4′-methoxy flavone (87) | flavonoid | Abdelshafeek, et al., 2016b |
| 13 | chrysoeriol-7-O-glucoside (88) | flavonoid glycoside | Abdelshafeek, et al., 2016b |
| 14 | ferulic acid (89) | phenolic acid | Abdelshafeek et al., 2016b, Al-Madhagy et al., 2022 |
| 15 | luteolin-7-O-β-D-glucuronide (90) |
flavonoid glycoside | Aboutabl, et al., 2000 |
| 16 | vicenin-2 (91) | flavonoid glycoside | Aboutabl,et al., 2000 |
| 17 | α-amyrin (37) | triterpenoid | Aboutabl, et al., 2000 |
| 18 | oleaonolic acid (92) |
triterpenoid | Aboutabl et al., 2000, Abdelshafeek et al., 2016a |
| 19 | ursolic acid (93) | triterpenoid | Aboutabl, et al., 2000 |
| 20 | β-sitosterol (35) | steroid | Aboutabl, et al., 2000 |
| 21 | caffeic acid (57) | phenolic acid | Aboutabl et al., 2000, Abdelshafeek et al., 2016a |
Fig. 4.
Phytochemicals isolated from O. fruticosa by column chromatography.
6. Pharmacological activities of O. fruticosa
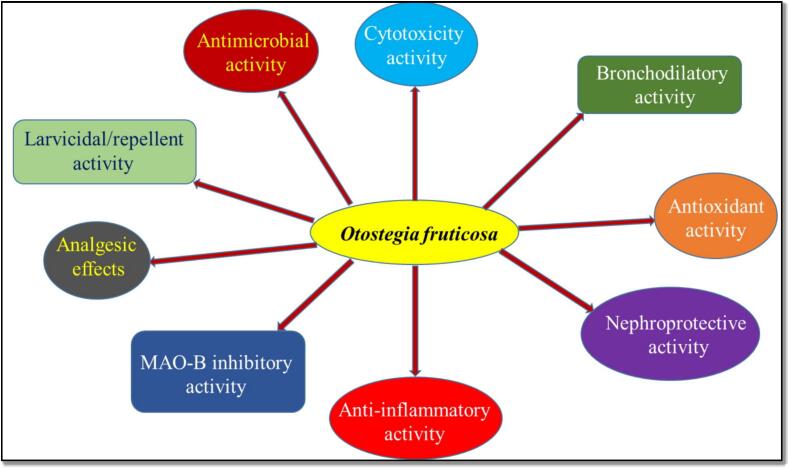
Several pharmacological activities have been carried out on various parts of O. fruticosa. The main activities reported are antimicrobial, cytotoxicity, bronchodilatory, antioxidant, nephroprotective, anti-inflammatory, MAO-B inhibitory, analgesic, and larvicidal/repellent activity (Fig. 5).
Fig. 5.
Schematic diagram of pharmacological activites reported on O. fruticosa.
6.1. Antimicrobial activity
The essential oil obtained from cultivated fresh aerial parts of O. fruticosa by the hydrodistillation method exhibited significant antimicrobial activity against gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus faecalis) and gram-negative bacteria (Escherichia coli and klebsiella aerogenes) as well as two pathogenic yeasts (Candida albicans and Saccharomyces cerevisiae). Except for S. cerevisiae, the results showed that all microorganisms tested were susceptible to essential oils. S. epidermidis and S. faecalis were the most sensitive (minimum inhibitory concentration [MIC] = 2.0 µg/mL), followed by B. subtilis and S. aureus (MIC = 6.0 µg/mL). The gram-negative bacteria E. coli and K. aerogenes showed MICs of 6.0 and 1.5 µg/mL, respectively. Yeast S. cerevisiae and C. albicans had MICs of 16.0 and 6.0 µg/mL, respectively (Aboutabl et al., 1995). Studies on the essential oil of the Yemeni species leaves of O. fruticosa s were equally successful (Ali, et al., 2017b). The essential oil was tested against three fungal strains (Aspergillus niger, C. albicans, and Botrytis cinerea), two gram-positive bacteria (Bacillus cereus, S. aureus), and two gram-negative bacteria (E. coli, Pseudomonas aeruginosa). The investigation revealed that the essential oil was ineffective against all tested strains of gram-negative bacteria and fungi. The study's results starkly contrast with the antibacterial activity of essential oils reported by Aboutabl et al. 1995. The antifungal activity was also tested against the fungal strains A. niger, Botrytis cinerea, and C. albicans and proved inactive against all the tested fungi, with an MIC value > 2500 μg/mL.
Abdelshafeek et al. (2016a) also tested the antimicrobial potential of O. fruticosa extracts (70 % methanol, chloroform, ethyl acetate petroleum ether, fatty alcohols, fatty acids, and unsaponifiable materials) against two gram-positive bacteria (S. aureus and B. subtilis), one gram-negative bacterium (E. coli), one fungus (A. niger), and one yeast (S. cerevisiae). The zone of inhibition (ZOI) was used to express the results. The zeolite extracts inhibited B. subtilis and fungi (ZOI 9–11 mm) and 70 % methanol and ethyl acetate extracts inhibited all microorganisms (ZOI 9–17 mm). The petroleum’s ether extract proved to be inactive against B. subtilis and S. cerevisiae and had ZOI values of 10 mm for S. aureus, 12 mm for E. coli, and 16 mm for A. niger. With a ZOI of 12 mm, the fatty alcohol extract proved to be active only against A. niger; in contrast, the fatty acid extract demonstrated activity against all tested stains, with a ZOI range from 12 to 16 mm, except A. niger. Unsaponifiable materials are ineffective against the tested gram-positive bacteria E. coli, and S. cerevisiae. Mothana et al. (2011) conducted tests to determine the antibacterial activity of methanol and hot aqueous extract of air-dried plant material of O. fruticosa against three gram-positive bacteria (S. aureus, B. subtilis, and Micrococcus flavus), two gram-negative bacteria (E. coli and P. aeruginosa), and one yeast (Candida maltosa SBUG). In addition, multi-resistant S. epidermidis 847, multi-resistant Staphylococcus haemolyticus 535,and multi-resistant S. aureus extracts were examined.
The methanol extract was inactive against the tested gram-negative bacterial and yeast strains. Still, it demonstrated the highest activity against the gram-positive strains M. flavus, S. aureus, and B. subtilis. Methanol extract effectively inhibited multi-resistant Staphylococcus species.
It showed ZOIs of 16 mm for S. epidermidis and S. aureus and 10 mm for S. haemolyticus. The hot aqueous extract only inhibited S. epidermidis and S. aureus (ZOI = 12 mm for each). S. haemolyticus showed a ZOI of 10 mm. Ansari et al. (2020) found MIC values of 550–625 µg/mL for ethanolic leaf extract against MRSA, P. aeruginosa, S. aureus, and K. pneumoniae.
6.2. Cytotoxic activity
The cytotoxicity of essential oil extracted from the leaves of O. fruticosa was evaluated against MCF-7 and MDA-MB-231 cell lines. The study’s results showed that the essential oil did not exhibit remarkable cytotoxicity against MCF-7 (IC50 = 55.1 ± 3.7 μg/mL) or MDA-MB- 231 (IC50 = 70.3 ± 1.9 μg/mL) cell lines (Ali, et al., 2017b). The air-dried plant's methanol and hot aqueous extract were also screened for cytotoxicity against two cancer cell lines (5637 and MCF-7). The IC50 value for methanol extract against 5637 and MCF-7 was > 250 μg/mL whereas for the hot aqueous extract, the IC50 value was > 500 μg/mL (Mothana et al., 2011). Methanol extract showed no cytotoxicity against MRC-5 cells (IC50 > 64.0 μg/mL) (Mothana, et al., 2014). Al-Madhagy et al. (2023) investigated the cytotoxic effects of methanol extract from O. fruticosa leaves using in vitro and in vivo models. In vitro testing against breast cancer cells (MCF-7) demonstrated moderate potency of the methanol extract (IC50 = 51 ± 9.8 µg/mL).
6.3. Antioxidant activity
Al-Madhagy et al. (2022) assessed the 2,2-Diphenyl-l-picrylhydrazyl (DPPH) radical scavenging experiment to assess the antioxidant activity of the leaves’ methanolic extract. Compared to normal ascorbic acid, which had an IC50 value of 18.3 ± 1.41 mg/mL, the extract's IC50 value was a remarkably reduced value of 3.64 ± 1.22 mg/mL. Furthermore, the oxygen radical absorbance capacity assay was utilized to evaluate the antioxidant activity of methanolic extract. The results indicated a further drop in the IC50 value to 3.48 ± 1.16 mg/mL compared to the tested standard Trolox, which revealed an IC50 value of 27.0 ± 13.4 mg/mL. Additionally, Mothana et al. (2011) assessed the antioxidant activity of the methanol and hot aqueous extract of the air-dried plant using the DPPH free radical scavenging test. The concentration range evaluated for each extract was 10 µg/mL to 1000 µg/mL. The findings are expressed as a percentage of radical scavenging activity. For methanol extract, the percentage of free radical scavenging activity was 10.2 % at 10 µg/mL, 15.0 % at 50 µg/mL, 38.8 % at 100 µg/mL, 92.6 % at 500 µg/mL, and 91.5 % at 10,000 µg/mL. At 500 µg/mL, the maximum activity was noted. A concentration-dependent pattern was observed in the hot aqueous extracts, meaning that the percentage of radical scavenging activity increased along with extract concentration: 12.1 % at 10 µg/mL, 22.8 % at 50 µg/mL, 25.1 % at 100 µg/mL, 30.2 % at 500 µg/mL, and 38.0 % at 1000 µg/mL. This is a negligible amount of activity. The study's findings unambiguously showed that methanol extract outperformed heated aqueous extracts in terms of activity.
6.4. Anti-inflammatory activity
Researchers evaluated the anti-inflammatory activity of O. fruticosa’s 70 % ethanol extract and its four fractions (hexane, chloroform, ethyl acetate, and n-butanol) using an in vivo carrageenan-induced paw edema model. The 400-mg/kg dose of the 70 % ethanol extract and the standard drug had a statistically significant inhibitory effect at the 3rd and 4th hours on mean increase in paw volume compared to the negative control group. The 200-mg/kg dose of the extract showed a statistically significant inhibitory effect at the 4th hour. The inhibition percentage at the peak of activity (4th hour) for 100-, 200-, and 400-mg/kg doses were 24.2 %, 50.4 %, and 75.7 %, respectively. The standard drug showed 80.6 % inhibition at the peak of activity, comparable to the 400-mg/kg dose of the extract. The chloroform fraction exhibited the most significant inhibition (67.9 %) among these fractions at the 4th hour, and the n-butanol fraction also showed considerable inhibition (51.8 %). The in vitro hyaluronidase inhibitory assay showed that the activity depends on concentration. The chloroform fraction exhibited the highest inhibition (85.75 %), followed by the butanol fraction (74.48 %) and crude extract (79.20 %), all at 100 µg/mL. The ethyl acetate and hydroalcoholic extract also showed significant inhibition. The chloroform fraction's effect (95.52 %) was similar to that of indomethacin. (Bahta et al., 2020). Ansari et al. (2021) assessed the anti-inflammatory properties of O. fruticosa's 70 % ethanolic extract in inflammatory bowel ulcerative colitis. In Wistar albino rats, intrarectal injection of 5 % acetic acid resulted in ulcerative colitis. The concentration levels, oxidative stress, macroscopic and microscopic characteristics of proinflammatory cytokines TNFα and IL-6 were measured to evaluate their anti-inflammatory activity. The study's results demonstrated that at doses of 200 and 400 mg/kg body weight, crude extract considerably (p < 0.001) reduced the concentration levels of TNFα and IL-6. Through the measurement of tissue total glutathione (GSH) and catalase enzyme activity (CAT), the antioxidant content in the colon homogenate of experimental animals was evaluated in the post-mitochondrial supernatant.
The animals' GSH and CAT decreased dramatically during acetic acid-induced colitis. Animals treated with a crude extract at 200 and 400 mg/kg body weight showed a significant increase in GSH levels.
Additionally, animals treated with crude extract at 200 and 400 mg/kg body weight showed a significant increase in CAT activity. Macroscopic anatomy revealed that the spleen and colon had a more favorable weight-to-length ratio. Compared to animals with colitis, the animals treated with crude extract plus prednisolone pretreatment had a relatively lower ratio, indicating reduced inflammation. Animals treated with microscopically coarse extract recover their histological parameters.
6.5. Analgesic activity
The analgesic efficacy of hydroalcoholic extract and its fractions—hexane, chloroform, and ethyl acetate—obtained by partitioning the extract with organic solvents was studied in vivo in the central and peripheral regions. The extract analgesic activity was evaluated using writhing and tail immersion tests at doses of 100, 200, and 400 mg/kg body weight. The highest dose (400 mg/kg) showed significant effects at 150 min, with a latency of 12.35 ± 0.41. The 200-mg/kg dose also demonstrated significant effects at 90, 120, 150, and 180 min whereasthe 100-mg/kg dose showed significant effects at 150 min. The higher dose exhibited a more substantial effect than the conventional medication pethidine (5 mg/kg). The peak analgesic action was reported at 150 min for the lowest dosage (100 mg/kg body weight) and intermediate dose (200 mg/kg body weight), respectively, with 7.27 ± 0.48 and 9.50 ± 0.98 s. However, the fractions were only evaluated at a single 400-mg/kg body weight dosage. The chloroform fraction had the most significant activity (12.00 ± 0.73 latency) among the fractions at 120 min, followed by butanol fractions with 11.67 ± 0.56 latency, which is more than the standard medication pethidine (10.20 ± 0.68 latency) (5 mg/kg body weight). The study showed that the extract at 100, 200, and 400 mg/kg body weight showed significant protection. The highest dose (400 mg/kg body weight) resulted in 90.9 % inhibition, similar to acetylsalicylic acid. The chloroform and butanol fractions showed 85.8 % and 80.9 % inhibition at 400 mg/kg body weight. No significant inhibition was observed in the hexane or ethyl acetate fractions (Bahta et al., 2020).
6.6. Nephroprotective activity
Nephrotoxicity, kidney damage that impairs kidney function, may result from oxygen-free radicals generated by chemicals, pharmaceuticals, and noninfectious substances. To determine whether the essential oil of O. fruticosa can safeguard rats from gentamicin-induced kidney damage, it was tested at dosages of 50, 100, and 200 mg/kg of body weight. The effectiveness of essential oils on antioxidant enzymes such as GPx, SOD, and CAT in groups of rats with renal injury was determined. According to the data, the positive control group's antioxidant enzyme activity was much lower. However, oral essential oil administration progressively raised antioxidant enzyme levels and enhanced renal function. A kidney histology study verified these findings (Al-Jumayi, 2020).
6.7. MAO-B inhibitory activity
The MAO-B enzyme is known to have a stronger correlation with Parkinson's disease-related neuronal loss and neurotoxic production. It is a well-known therapeutic target for many illnesses affecting the central nervous system. A fluorometric in vitro assay was used to evaluate the degree of inhibition of the O. fruticosa leaf methanolic extract against the MAO-B enzyme. Al-Madhagy et al. (2022) reported that the extract showed intriguing MAO-B inhibiting activity (IC50: 2.24 ± 0.08 mg/mL), in contrast to the reference substance, selegiline (0.55 ± 0.02 mg/mL).
6.8. Bronchodilatory activity
A hydroalcoholic (70 % ethanol in water) extract of O. fruticosa was examined for bronchodilatory effects to study the potential bronchodilators of natural origin. Experiments were conducted on guinea pig trachea to assess the bronchodilator effects. According to Ansari et al. (2020), the study's findings demonstrated that O. fruticosa's hydroalcoholic extract could induce bronchodilation through a variety of mechanisms, including voltage-gated Ca++ channel blockades and receptor-operated (antimuscarinic) cAMP elevations. These findings support the traditional use of O. fruticosa in lung disorders.
6.9. Larvicidal/repellent activity
The plant’s larvicidal and repellent properties against Culex pipiens were assessed using n-hexane, methanol, ethyl acetate, and chlorobenzene extract. The pigeon's ventral surface was used for the investigation after the feathers had been removed from its abdomen, and various extract concentrations were directly administered to it to assess its repellency against the mosquito under testing. O. fruticosa hexane extract showed a minimum concentration of 300 ppm to achieve the maximum larval mortality of 100 %. Chlorobenzene, ethyl acetate, and methanolic extracts showed the same larval death rate, with 600 ppm, 1000 ppm, and 1400 ppm, respectively. Hexane, chlorobenzene, ethyl acetate, and methanol extract had estimated LC50 (LC90) values of 129.92 (259.57) ppm, 261.13 (508.12) ppm, 585.48 (866.50) ppm, and 683.28 (1212.68) ppm, respectively. Additionally, the four extracts’ repellent properties were examined at three dosages: 0.83, 1.67, and 3.33 mg/cm2.. Methanol and ethyl acetate extracts displayed repellency of 64.13 % and 75.09 % at the highest dose (3.33 mg/cm2), but hexane and chlorobenzene extracts had greater repellency of 88.15 % and 80.15 %, at the maximum dose (3.33 mg/cm2). According to Shahat et al. (2020), the results again showed that the hexane extract was more effective than the other extracts tested.
6.10. Miscellaneous activities
Mothana et al. 2014 also tested several medicinal plants distributed in Saudi Arabia and Yemen for their in vitro antiplasmodial, antileishmanial, and antitrypanosomal activity. They reported themethanol extract of O. fruticosa showed antiplasmodial activity against P. falciparum and antileishmanial activity against L. infantum, with IC50 values of 34 ± 5.2 and > 64.0 μg/mL, respectively. Meanwhile, antitrypanosomal activity was evaluated against Trypanosoma cruzi and Trypanosoma. brucei. The methanol extract of O. fruticosa displayed antitrypanosomal activity, with IC50 values of 36.6 ± 4.9 and 35.2 ± 3.7 μg/mL, respectively.
7. Conclusion
This review paper summaries the most recent information on O. fruticosa, focusing on its traditional use in many cultures and its scientific evaluation, which includes its antibacterial, antioxidant, anti-inflammatory, nephroprotective, bronchodilator, cytotoxic, larvicidal, analgesic Miscellaneous activities. This review study also considers the volatile, non-volatile, and specific metabolites of O. fruticosa. The information from this review article will support future research activities for developing novel medicinal plant-based medication based on the evidence-based review of the usage of O. fruticosa. The discovery and identification of the phytochemicals responsible for the bioactivities will require further investigation. The body of research has demonstrated its crucial medical significance.
CRediT authorship contribution statement
Mohammed F. Hawwal: Writing – review & editing, Visualization, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Sarfaraz Ahmed: Writing – original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Perwez Alam: Validation, Supervision, Resources, Project administration, Formal analysis. Omer I. Fantoukh: Validation, Supervision, Resources, Project administration, Conceptualization. Gadah A. AlHamoud: Writing – review & editing, Validation, Resources, Project administration, Conceptualization. Hattan A. Alharbi: Writing – review & editing, Validation, Resources, Project administration, Formal analysis. Waleed A. Alobaid: Writing – review & editing, Resources, Formal analysis. Hanan Khojah: Writing – review & editing, Validation, Supervision, Resources, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to express our gratitude to Amin Ayed Al-Ahmadi, an environmental activist, for generously sharing images of O. fruticosa from Al-Madinah region, Bader governorate, wadi Alanig. Additionally, we extend our appreciation to the Medicinal Plant Garden at the College of Pharmacy, King Saud University, for their valuable collaboration.
References
- Abdelshafeek K.A., et al. Isolation and identification of some chemical constituents and antimicrobial activity of two Lamiaceae plants growing in Saini. Egypt. J. Chem. 2016;59:21–31. [Google Scholar]
- Abdelshafeek K.A., et al. Structure elucidation of phenolic acids, flavonoids and hypocholesterolemic activity of Nepeta septemcrenata and Otostegia fruticosa. Der Pharma. Chem. 2016;8:357–362. [Google Scholar]
- Aboutabl E.A., Sokkar N.M., Megid R.M.A. Composition and antimicrobial activity of Otostegia fruticosa Forssk. Oil. J. Essent. Oil Res. 1995;7:299–303. doi: 10.1080/10412905.1995.9698522. [DOI] [Google Scholar]
- Aboutabl E.A., Sokkar N.M., Awaad A.S. Phytochemical study of Otostegia fruticosa (Forssk.) and biological activity. Bull. Fac. Pharm. Cairo Univ. 2000;38:115–118. [Google Scholar]
- Ahmad V.U., et al. Two new trans-clerodane diterpenoids from Otostegia limbate. J. Asian Nat. Prod. Res. 2007;9:91–95. doi: 10.1080/10286020500478740. [DOI] [PubMed] [Google Scholar]
- Ahmad F., et al. Ethnopharmacological and phytochemical study of major species from labiatae family in Saudi Arabia: a systematic review. Int. J. Appl. Pharm. 2022;44–57 doi: 10.22159/ijap.2022.v14ti.40. [DOI] [Google Scholar]
- Ali N.A.A., et al. Ethnopharmacological Survey of Medicinal Plants in Albaha Region, Saudi Arabia. Phcog. Res. 2017;9:401–417. doi: 10.4103/pr.pr_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, N.A.A., et al., 2017b. Chemical composition, antimicrobial, and cytotoxic activities of the essential oil of Otostegia fruticosa subsp. schimperi from Yemen. Nat. Prod. Commun. 12, 969.
- Al-Jumayi H.A.R.O. Amelioration of gentamicin-induced nephrotoxicity using essential oil extract from Otostegia fruticosa leaves in albino rats. Plant Cell Biotechnol. Mol. Biol. 2020;21:90–106. [Google Scholar]
- Al-Madhagy S.A., et al. A new arsenal of polyphenols to make Parkinson's disease extinct: HPLC-MS/MS profiling, very interesting MAO-B inhibitory activity and antioxidant activity of Otostegia fruticose. Nat. Prod. Res. 2022;36:6075–6080. doi: 10.1080/14786419.2022.2044811. [DOI] [PubMed] [Google Scholar]
- Al-Madhagy S.A., et al. A new firewall in the fight against breast cancer: in-vitro and in-silico studies correlating chemistry to apoptotic activity of Otostegia fruticose. Nat. Prod. Res. 2023;37:2770–2775. doi: 10.1080/14786419.2022.2130306. [DOI] [PubMed] [Google Scholar]
- Al-Musayeib N.M., et al. Labdane diterpenes from Otostegia fruticosa. Phytochemistry. 2000;54:771–775. doi: 10.1016/s0031-9422(00)00185-0. [DOI] [PubMed] [Google Scholar]
- Andemariam W.S. Legislative regulation of traditional medicinal knowledge in Eritrea vis-à-vis Eritrea’s commitments under the convention on biological diversity: issues and alternatives. Law Environ. Dev. J. 2010;6:130–162. [Google Scholar]
- Ansari M.N., et al. Evaluation of bronchodialatory and antimicrobial activities of Otostegia fruticosa: A multi-mechanistic approach. Saudi Pharm. J. 2020;28:281–289. doi: 10.1016/j.jsps.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M.N., et al. Role of oxidative stress and inflammatory cytokines (TNF-α and IL-6) in acetic acid-induced ulcerative colitis in rats: Ameliorated by Otostegia fruticose. Life. 2021;11:195. doi: 10.3390/life11030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar K.J., et al. Constituents from O. Aucheri. J Bangladesh Chem. Soc. 2004;17:18–20. [Google Scholar]
- Bahta T., et al. Analgesic, anti-inflammatory and in-vitro hyaluronidase inhibitory properties of the leaf extract and solvent fractions of Otostegia Fruticosa (Forssk.) Schweinf. ex Penzig. Iran. J. Pharm. Res. 2020;19:218–230. doi: 10.22037/ijpr.2019.14657.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekut M., et al. Potential of selected Lamiaceae plants in anti(retro) viral therapy. Pharmacol. Res. 2018;133:301–314. doi: 10.1016/j.phrs.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borris R.P. Natural products research: perspectives from a major pharmaceutical company. J, Ethnopharmacol. 1996;51:29–38. doi: 10.1016/0378-8741(95)01347-4. [DOI] [PubMed] [Google Scholar]
- Briskin D.P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000;124:507–514. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collenette S. Riyadh; Saudi Arabia: 1999. National Commission for Wildlife Conservation and Development (NCWCD) [Google Scholar]
- Davigdor E., et al. The current status of knowledge of herbal medicine and medicinal plants in Fiche. Ethiopia. J Ethnobiol Ethnomed. 2014;10:38. doi: 10.1186/1746-4269-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyew M.A., et al. Status of medico-cultural commercial plants at Fiche town market Ethiopia. Int. J. Pharma. Health Care Res. 2013;1:227–236. [Google Scholar]
- Getaneh S., Girma Z. An ethnobotanical study of medicinal plants in Debre Libanos Wereda. Central Ethiopia. Afr. J. Plant Sci. 2014;8:366–379. doi: 10.5897/AJPS2013.1041. [DOI] [Google Scholar]
- Hostettmann K., Marston A., Maillard M., Hamburger M. Clarendon Press; Oxford: 1995. Phytochemistry of Plants Used in Traditional Medicine. [Google Scholar]
- Kaufman P.B., Csake L.J., Warber S., Duke J.A., Brielmann H.L. CRC Press; Boca Raton: 1999. Natural products from plants. [Google Scholar]
- Khan A., et al. Two New Flavonol Glycosides from Otostegia limbata BENTH. Chem Pharm Bull. 2009;57:276–279. doi: 10.1248/cpb.57.276. [DOI] [PubMed] [Google Scholar]
- Khan S., Syed F. Bioactive constituents from genus Otostegia. SARJ of Phys. Sci. 2013;1:15–25. [Google Scholar]
- Khoury M., et al. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial Potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. Based Complement. Alternat. Med. 2016;2547169 doi: 10.1155/2016/2547169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane D., et al. Community knowledge of traditional mosquito repellent plants in Kolla Temben District, Tigray, Northern Ethiopia. Sci. Res. Essay. 2013;8:1139–1144. doi: 10.5897/SRE11.1216. [DOI] [Google Scholar]
- Meragiaw M., et al. The status of ehnobotanical knowledge of medicinal plants and the impacts of resettlement in delanta, northwestern wello, Northern Ethiopia. Evid. Based Complement. Alternat. Med. 2016;5060247 doi: 10.1155/2016/5060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman D.E. An analysis of the food plants and drug plants of native North America. J. Ethnopharmacol. 1996;52:1–22. doi: 10.1016/0378-8741(96)01393-1. [DOI] [PubMed] [Google Scholar]
- Mothana R.A., et al. Assessment of selected Yemeni medicinal plants for their in vitro antimicrobial, anticancer, and antioxidant activities. Pharm. Biol. 2011;49(2):200–210. doi: 10.3109/13880209.2010.512295. [DOI] [PubMed] [Google Scholar]
- Mothana, R.A., et al., 2014. Evaluation of the In Vitro Antiplasmodial, Antileishmanial, and Antitrypanosomal Activity of Medicinal Plants Used in Saudi and Yemeni Traditional Medicine Evidence-Based Complementary and Alternative Medicine. ID 905639. http://dx.doi.org/10.1155/2014/905639. [DOI] [PMC free article] [PubMed]
- Nunez D.R., de Castro C.O. R.M. Harley and T; Reynolds, Royal Botanic Gardens, Kew, Richmond, UK: 1992. “The ethnobotany of the old world labiatae”, in Advances in Labiatae Science. [Google Scholar]
- Rahman M.A., et al. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 2004;75:149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Ramasubramania R.R. Medicinally potential plants of Labiatae (Lamiaceae) family: An Overview. J. Med. Plant Res. 2012;6:203–213. doi: 10.3923/rjmp.2012.203.213. [DOI] [Google Scholar]
- Rosselli S., Fontana G., Bruno M. A Review of the Phytochemistry, Traditional Uses, and Biological Activities of the Genus Ballota and Otostegia. Planta Med. 2019;85:869–910. doi: 10.1055/a-0953-6165. [DOI] [PubMed] [Google Scholar]
- Sadeghi Z., Akaberi M., Valizadeh J. Otostegia persica (Lamiaceae): A review on its ethnopharmacology, phytochemistry, and pharmacology. Avicenna J Phytomed. 2014;4:79–88. [PMC free article] [PubMed] [Google Scholar]
- Shahat M.A.M., et al. Activity of Otostegia fruticosa (Lamiaceae) leaves extracts against lymphatic filariasis vector, Culex pipiens L. (diptera: Culicidae). Egypt. Acad. J. Biolog. Sci. 2020;13(4):175–186. [Google Scholar]
- Tamokou J.D.D., Mbaveng T.A., Kuete V. Medicinal Spices and Vegetables from Africa: Therapeutic Potential against Metabolic Inflammatory Infectious and Systemic Diseases. Academic Press; Cambridge: 2017. Antimicrobial Activities of African Medicinal Spices and Vegetables; pp. 207–237. [Google Scholar]
- Tesso H., Konig W.A. Terpenes from Otostegia integrifolia. Phytochemistry. 2004;65:2057–2062. doi: 10.1016/j.phytochem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Uritu C.M., et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Research and Management. 2018;7801543 doi: 10.1155/2018/7801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural K., et al. Anxiolytic and antidepressant activities of some Ballota species. J. Fac. Pharm. Gazi. Univ. 1996;13:29–32. [Google Scholar]
- Yuan Y.W., et al. Further disintegration and redefinition of Clerodendrum (Lamiaceae): Implications for the understanding of the evolution of an intriguing breeding strategy. Taxon. 2010;59:125–133. doi: 10.1002/tax.591013. [DOI] [Google Scholar]
- Zinicovscaia I., et al. Elemental analysis of Lamiaceae medicinal and aromatic plants growing in the Republic of Moldova using neutron activation analysis Phytochem. Lett.ebruary. 2020;35:119–127. doi: 10.1016/j.phytol.2019.10.009. [DOI] [Google Scholar]