Abstract
A new invasive Klebsiella pneumoniae liver abscess syndrome (IKPLAS) has been described. It is often described as primary liver abscess and metastatic infection in multiple systems. Patients often develop endogenous endophthalmitis (EE), when the infection affects the eyes. Isolated optic neuritis (ON) is an unusual manifestation of Klebsiella pneumoniae (K. pneumoniae) infection associated ocular complications. We report a rare case of isolated retrobulbar optic neuritis in a 33-year-old young man on the 4th day of admission with severe pneumonia as the first symptom. Cerebrospinal fluid (CSF) analysis showed higher protein levels (1.12 g/l) but aquaporin-4 (AQP4) & myelin oligodendrocyte glycoprotein (MOG) antibodies were negative. Pulse therapy with intravenous methylprednisolone (1 g daily for 3 days, followed by tapering for a total of 2 months) and immunoglobulin (37.5 g daily for 5 days) was effective on his ON. We suggest that this was a form of para-infectious optic neuritis (PON) triggered by K. pneumoniae infection. K. pneumoniae antigens induced para-infectious demyelination of the optic nerve may be involved in visual impairment.
Keywords: Invasive Klebsiella pneumoniae liver abscess syndrome, Optic neuritis, Klebsiella pneumoniae, Demyelination
1. Introduction
Invasive Klebsiella pneumoniae liver abscess syndrome (IKPLAS) is a serious multi-system infection caused by Klebsiella pneumoniae (K. pneumoniae) strains, especially in patients with immune compromise or metastatic abscesses [1]. It is characterized by primary liver abscess and metastatic extrahepatic infection [1]. The hypervirulent K. pneumoniae (hvKp) strains with serotype K1/K2 significantly increased the phagocytic activity against immune cells [2]. A number of patients have experienced ocular complications such as EE, orbital cellulitis, and optic neuritis (ON) simultaneously or within a few days. ON is a group of inflammatory diseases that affect the optic nerve for various causes [3]. Patients with ON often shows poor visual outcomes worldwide, especially in young people [4]. It's a tricky clinical problem yet. Etiology analysis of ON is not always easy in clinical practice. Patients with endogenous Klebsiella endophthalmitis may experience extremely severe visual impairment when infection migrates to the eye [2].
We report a case of ON that occurred on the 4th day of K. pneumoniae infection, antibiotic treatment failed, but high-dose glucocorticoid therapy improved his visual prognosis. We have not found direct evidence of K. pneumoniae infection. Therefore, we suggest that this was a form of para-infectious optic neuritis (PON).
2. Case presentation
A 33-year-old young man was admitted to the intensive care unit (ICU) with a 1-week history of weakness followed by 2-hour history of coma. His past medical history was not available from any of the family members. The patient exhibited persistent fever of 38.2 °C, tachypnoea (36 breaths/min), rapid heart rate (154 beats/min) and hypotension (83/47 mmHg). He presented with signs of coma, clammy skin, and wet rales in both lungs. Chest computed tomography (CT) showed irregular alveolar infiltration and consolidation in both lungs with a small amount of pleural effusion, suggesting a high likelihood of bacterial pneumonia. There were no cavities, lumps or abscesses in either lung. Cranial CT revealed no significant abnormalities. A high level of K. pneumoniae DNA was found in the bronchoalveolar lavage fluid (BLF) using two different molecular biology techniques, namely polymerase chain reaction (PCR) and next-generation sequencing (NGS). K. pneumoniae was also identified in the sputum culture. The results of PCR, NGS, and sputum culture provided substantial evidence for K. pneumoniae infection in the patient. Laboratory results showed high glucose (>30 mmol/L), metabolic acidosis (pH 6.926), high lactate level (3.77 mmol/L), white blood cell (WBC) count (21170 cells/mm3), high C-reactive protein (CRP) (98.22 mg/L) and high procalcitonin (PCT) (10.87 ng/ml). His sequential organ failure assessment (SOFA) score was 12 points. Initial management included emergency tracheal intubation and mechanical ventilation, early fluid resuscitation, blood sugar control with insulin, intravenous cefepime (2 g, every 8 h, for 3 days). The patient has relief from pneumonia and acidosis.
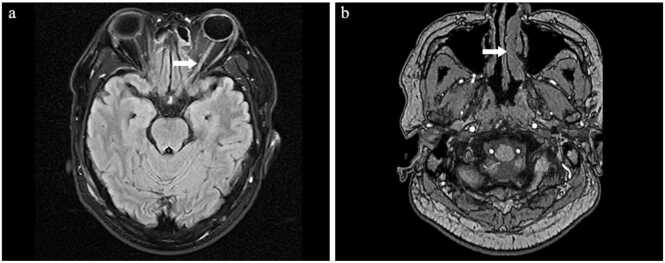
On the 4th day of admission, the patient developed visual impairment. Ophthalmic examination revealed light perception (LP) vision and relative afferent pupillary defect (RAPD) in both of his eyes, without evidence of endophthalmitis. Cornea, iris and conjunctiva were transparent without opacity. Orbital magnetic resonance imaging (MRI) showed enhanced signal in the orbital segment of the left optic nerve, suggesting isolated retrobulbar optic neuritis (Fig. 1). MRI showed hypertrophy of the left nasal turbinate, but there were no signs of acute inflammation (Fig. 1). The results of the slit lamp, ophthalmoscopy and ultrasound examination were negative. There was no evidence of retinitis, optic disk edema or vasculitis. In addition, no liver abscess or endophthalmitis was found in the patient.
Fig. 1.
Orbital MRI results: the arrow highlights the abnormal findings. Key: Orbital MRI showed enhanced signal in the orbital segment of the left optic nerve, indicating acute retrobulbar optic neuritis (a) Orbital MRI showed hypertrophy of the left nasal turbinate (b), but no signs of acute inflammation were observed.
We reviewed his medical history. The patient was a cook without history of alcohol-drinking or drug-taking. He had not received any vaccinations during the two months before admission. No serum-specific antibodies were detected, including antibodies against human immunodeficiency virus (HIV), mycoplasma, herpesvirus, tuberculosis, cytomegalovirus, and coronavirus. The rapid plasma reagin (RPR), treponema pallidum particle agglutination (TPPA), (13)-β-D-glucan, and galactomannan tests were negative. Blood cultures showed no growth of pathogenic microorganisms. The blood test results do not support the diagnosis of ION. Serum immune tests including immunoglobulins, complement components, lymphocyte subpopulations, nuclear antibody panel, anti-nuclear antigen, anti-neutrophilic cytoplasmic antigen, aquaporin-4 (AQP4) antibodies, and myelin oligodendrocyte glycoprotein (MOG) antibodies were all negative. Serum immune tests do not support the diagnosis of autoimmune diseases. Lumbar puncture revealed normal opening pressure and CSF analysis displayed normal cell count, glucose level but higher protein levels (1.12 g/l). Autoimmune antibodies against AQP4 & MOG were negative in both serum and CSF. There was no evidence of aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder (AQP4 +NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) or multiple sclerosis (MS). We also ruled out acute sinusitis or other potential causes (vascular, compressive, traumatic, vitamin B12 deficiencies or Leber’s hereditary optic neuropathy).
Currently, there is no exact clinical guideline for the treatment of ON combined with acute pneumonia. High-dose corticosteroid therapy may cause further spread of the infection. Initially, the patient received intravenous methylprednisolone (80 mg daily for 3 days) but showed unsatisfied visual outcomes, so we had no choice but to increase the dose of the glucocorticoids. Continuous intravenous infusion of methylprednisolone (1 g daily for 3days) and intravenous immunoglobulin (37.5 g daily for 5 days) was useful. The patient made a partial recovery in the right eye but LP vision in the left eye. Serum levels of inflammatory markers, including WBC count, CRP, and PCT, were within normal ranges. Follow-up of 2 years showed that the patient had not developed to any inflammatory demyelinating diseases in central nervous system (CNS). Therefore, we thought it was a form of PON.
3. Discussion
We report a rare case of isolated retrobulbar optic neuritis that occurred on the 4th day of K. pneumoniae infection. There was no evidence of ocular invasion by K. pneumoniae strains. Antibiotic treatment failed, but high-dose glucocorticoid therapy improved his visual prognosis.
In a review of the literature, we found three typical cases of ON in the context of K. pneumoniae infection [5], [6], [7] (Table 1). All three patients were diagnosed with ON, confirmed by neuroimaging. Only one case presented with isolated retrobulbar optic neuritis among them. Besides that, one case manifested as ON with endophthalmitis and the other with pyogenic ventriculitis. All three patients were middle-aged women. One of the three patients had a history of type 2 diabetes. Two of the three patients developed optic nerve inflammation on the right side, while the remaining one was on the left side. Despite intensified treatment with anti-infection and corticosteroids, all three patients could not regain their vision yet. Tatsuya Chiba et al. found K. pneumoniae strain on excised optic nerve stumps, confirming that ON was the result of direct invasion by K. pneumoniae [6]. Interestingly, Lee et al. reported a patient who developed isolated optic neuritis in the setting of K. pneumoniae liver abscess without endophthalmitis [7]. Lee et al. also reported K. pneumoniae septicemia with metastatic brain abscess in their case. They speculated on the possibility of hematogenous transmission of ON, although they could not provide direct evidence of ocular invasion by K. pneumoniae strains. Our patient was diagnosed with Klebsiella pneumonia accompanied by isolated retrobulbar optic neuritis. Unlike the case reported by Lee et al., our patient did not present with primary liver abscess or metastatic tissue abscess, which is inconsistent with the characteristics of IKPLAS.
Table 1.
Typical cases of ON in the context of K. pneumoniae infection: review of the literature.
| Study | Age/Gender | Pre- history | Location | Concomitant symptoms |
Visual prognosis | Final diagnosis | Medical management |
|---|---|---|---|---|---|---|---|
| Bouhout et al. | 59/ female |
T2DM | Right | Endophthalmitis & orbital cellulitis | Eye removed | EE | IV meropenem (2 g TID) IV ceftriaxone (2 g BID) PO ciprofloxacin (500 mg BID) PO prednisone PO amoxicillin-clavulanic (875 mg BID) IV ceftriaxone (2 g DIE) PO prednisone (30 mg daily as determined by clinical improvement) |
| Chiba et al. |
79/ female |
NA | Right | Ventriculitis | Eye removed | EE | NA |
| Lee et al. |
56/ female |
NA | Left | RON | LP | RON | IV ceftriaxone and moxifloxacin (for 20 days) IV dexamethasone (20 mg daily followed by oral prednisolone) |
Key: ON: optic neuritis; K. pneumoniae: Klebsiella pneumoniae; T2DM: Type 2 diabetes mellitus; EE: Endogenous endophthalmitis; IV: intravenous; TID: three times per day; BID: two times per day; PO: per os (by mouth); DIE: daily; RON: Para-infectious optic neuritis; LP: Light perception; NA: Not available.
Etiology analysis of ON is not always easy in clinical practice. Previous studies have demonstrated that ON can be the result of direct invasion of the optic nerve by a pathogen [8]. PON is an abnormal immune-inflammatory response that occurs after infection by a pathogen, without evidence of direct attack on the optic nerve [3]. ON can also be an ocular manifestation of inflammatory demyelinating diseases in CNS. Isolated retrobulbar optic neuritis is often an initial symptom of MS [9]. According to the site of onset, ON can be divided into four categories: retrobulbar neuritis, papillitis, perineuritis and neuroretinitis. A portion of patients with negative AQP4-IgG and MOG-IgG would experience recurrence or develop to new demyelinating lesions [10]. Follow-up of 2 years showed that the patient had not developed to any inflammatory demyelinating diseases in the CNS. The presence of oligoclonal IgG bands in CSF is supportive evidence of inflammatory demyelination in the CNS. We could not discover any new evidence in the follow-up study. Therefore, after ruling out other potential causes, we thought para-infectious demyelination of the optic nerve may be involved in visual impairment.
PON is typically associated with subacute progression, ocular pain and severe visual loss, despite normal funduscopic examination [11]. PON is strongly associated with viral etiology [12]. Typical cases of PON have been reported in previous studies, especially those occurring shortly after viral infections such as HIV, herpes virus, rubella virus, and mumps virus [8]. Additionally, PON may occur after infection with mycoplasma pneumoniae, orientia tsutsugamushi, tubercle bacilli, schistosoma or some bacteria [13]. PON is not the result of direct infection by a pathogen. An exaggerated self-immune response is involved in the pathogenesis of PON [14]. In children, ON is usually associated with para-infectious demyelination in previous studies [3]. Patients with PON usually have a rapid response to steroids, although some may experience a relapse after steroid withdrawal [11]. In a 5-year follow-up cohort study of the Optic Neuritis Treatment Trial, most patients maintained good vision with appropriate choice of therapy [12]. Intravenous immunoglobulin or plasma exchange have been considered for the treatment of acute ON. Infection-caused ON can be effectively treated with specific antibiotics but corticosteroid therapy is not always necessary [10]. Immune-mediated ON may respond well to glucocorticoids therapy [11]. Interferon β or Copaxone may be considered if a second clinical episode occurs within 2 years in patients with isolated ON [15]. Patients with diabetes are more susceptible to attack as they suffer from reduced immune function [16]. Diabetes optic neuropathy may occur in patients with poor glycemic control [17]. The specific ocular and increased blood-ocular barrier of diabetes may lead to ocular complications [18]. Strict control of blood glucose and ketoacidosis is also beneficial for the recovery of optic nerve function.
4. Conclusion
Isolated optic neuritis is a rare ocular complication of Klebsiella pneumoniae (K. pneumoniae) infection. Early identification of the etiology and precision therapy may improve visual outcomes in patients with ON. We suggest visual assessment in patients with severe K. pneumoniae infection to identify occult lesions.
Ethics approval and consent to participate
Our case report was approved by the ethics committee of Chengdu First People’s Hospital and conducted according to the Helsinki Declaration. The patient has signed informed consent.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. No funding was received from any organization or individuals.
CRediT authorship contribution statement
Shijie Duan: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Qing Wang: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. shenghui yu: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Shikun Deng: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Author Agreement
We the undersigned declare that this manuscript entitled “Isolated retrobulbar optic neuritis after Klebsiella pneumoniae infection: a rare case report and literature review” is original, has not been published before and is not currently being considered for publication elsewhere.
We would like to draw the attention of the Editor to the following publications of one or more of us that refer to aspects of the manuscript presently being submitted. Where relevant copies of such publications are attached.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Signed by all authors as follows: Qing Wang, Shijie Duan, Shikun Deng, Shenghui Yu.
Consent for publication
Informed written consent was obtained from the patient for publication.
Ethics approval and consent to participate
Our research was reviewed and approved by the Ethics Review Committee of Chengdu First People's Hospital, Chengdu, Sichuan, China.
Consent for publication
No applicable.
Authors' contributions
QW, SJD,SKD, SHY were responsible for the concept and design of the study. QW, SJD were responsible for drafting the manuscript. QW, SJD, SKD were participated in review and revision of the manuscript. All authors read and approval the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Contributor Information
Qing Wang, Email: 5746312236@qq.com.
Shijie Duan, Email: duanshijie91@163.com.
Shikun Deng, Email: dengsk@163.com.
Shenghui Yu, Email: yushenghui886@163.com.
References
- 1.Siu L.K., Yeh K.M., Lin J.C., Fung C.P., Chang F.Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi: 10.1016/s1473-3099(12)70205-0). [DOI] [PubMed] [Google Scholar]
- 2.Yang C.S., Tsai H.Y., Sung C.S., Lin K.H., Lee F.L., Hsu W.M. Endogenous Klebsiella endophthalmitis associated with pyogenic liver abscess. Ophthalmology. 2007;114(5):876–880. doi: 10.1016/j.ophtha.2006.12.035). [DOI] [PubMed] [Google Scholar]
- 3.Pau D., Al Zubidi N., Yalamanchili S., Plant G.T., Lee A.G. Optic neuritis. Eye (Lond, Engl) 2011;25(7):833–842. doi: 10.1038/eye.2011.81). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheremet N.L., Eliseeva D.D., Kalashnikova A.K., Zakharova M.N. Typical and atypical optic neuritis. Vestn Oftalmol. 2023;139(6):175–182. doi: 10.17116/oftalma2023139061175). [DOI] [PubMed] [Google Scholar]
- 5.Bouhout S., Lacourse M., Labbé A.C., Aubin M.J. A rare presentation of Klebsiella pneumoniae endogenous panophthalmitis with optic neuritis and orbital cellulitis from a urinary tract infection. IDCases. 2021;26 doi: 10.1016/j.idcr.2021.e01289). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiba T., Yoneyama S., Nakagomi T., Takahashi H., Iijima H. A Case of Metastatic Endophthalmitis Resulting from Liver Abscess Complicated with Pyogenic Ventriculitis via Optic Nerve. Nippon Ganka Gakkai zasshi. 2015;119(10):686–692. [PubMed] [Google Scholar]
- 7.Lee H.S., Choi K.D., Lee J.E., Park H.K. Optic neuritis after Klebsiella pneumonitis and liver abscess. J Neuro-Ophthalmol: J North Am Neuro-Ophthalmol Soc. 2009;29(2):134–135. doi: 10.1097/WNO.0b013e3181a59028). [DOI] [PubMed] [Google Scholar]
- 8.Suparmaniam S., Wan Hitam W.H., Thilagaraj S. Bilateral Parainfectious Optic Neuritis in Young Patient. Cureus. 2022;14(9) doi: 10.7759/cureus.29220). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jendretzky K.F., Bajor A., Lezius L.M., Hümmert M.W., Konen F.F., Grosse G.M., Schwenkenbecher P., Sühs K.W., Trebst C., Framme C., Wattjes M.P., Meuth S.G., Gingele S., Skripuletz T. Clinical and paraclinical characteristics of optic neuritis in the context of the McDonald criteria 2017. Sci Rep. 2024;14(1):7293. doi: 10.1038/s41598-024-57199-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshina Y., Seay M., Vegunta S., Stulberg E.L., Wright M.A., Wong K.H., Smith T.L., Shimura D., Clardy S.L. Isolated Optic Neuritis: Etiology, Characteristics, and Outcomes in a US Mountain West Cohort. J Neuro-Ophthalmol: J North Am Neuro-Ophthalmol Soc. 2024 doi: 10.1097/wno.0000000000002157). [DOI] [PubMed] [Google Scholar]
- 11.Gluckstein J.A., Chwalisz B.K., Gilbert A.L., Bouffard M.A. SARS-CoV-2 Parainfectious Optic Neuropathy: 3 Case Reports and a Review of the Literature. J Neuro-Ophthalmol: J North Am Neuro-Ophthalmol Soc. 2023;43(4):491–498. doi: 10.1097/wno.0000000000001822). [DOI] [PubMed] [Google Scholar]
- 12.Hipolito-Fernandes D., Elisa-Luís M., Trigo M., Tavares-Ferreira J. Parainfectious optic neuritis followed by microcystic macular oedema. BMJ case Rep. 2019;12(9) doi: 10.1136/bcr-2019-231442). ((http://) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun C.B., Ma Z., Liu Z. Case Report: Optic neuritis as the initial presentation of Orientia tsutsugamushi infection detected by metagenomic next-generation sequencing. Front Immunol. 2023;14:1129246. doi: 10.3389/fimmu.2023.1129246). ((http://) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shabani Z. Demyelination as a result of an immune response in patients with COVID-19. Acta Neurol Belg. 2021;121(4):859–866. doi: 10.1007/s13760-021-01691-5). ((http://) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comi G., Filippi M., Barkhof F., Durelli L., Edan G., Fernández O., Hartung H., Seeldrayers P., Sørensen P.S., Rovaris M., Martinelli V., Hommes O.R. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet (Lond, Engl) 2001;357(9268):1576–1582. doi: 10.1016/s0140-6736(00)04725-5). ((http://) [DOI] [PubMed] [Google Scholar]
- 16.Turk Wensveen T., Gašparini D., Rahelić D., Wensveen F.M. Type 2 diabetes and viral infection; cause and effect of disease. Diabetes Res Clin Pract. 2021;172 doi: 10.1016/j.diabres.2020.108637). ((http://) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee G.I., Han K., Park K.A., Oh S.Y. Risk of optic neuritis in type 2 diabetes mellitus: A nationwide cohort study. J Neurol Sci. 2023;450 doi: 10.1016/j.jns.2023.120673). ((http://) [DOI] [PubMed] [Google Scholar]
- 18.Chung C.Y., Wong E.S., Liu C.C.H., Wong M.O.M., Li K.K.W. Clinical features and prognostic factors of Klebsiella endophthalmitis-10-year experience in an endemic region. Eye (Lond, Engl) 2017;31(11):1569–1575. doi: 10.1038/eye.2017.92). ((http://) [DOI] [PMC free article] [PubMed] [Google Scholar]