Abstract
Introduction
Short bowel syndrome (SBS) is associated with a significant mental and physical burden for patients and caregivers. Standard of care (SOC) for SBS includes parenteral support (PS) to optimize intestinal function. Teduglutide, a recombinant human glucagon-like peptide 2 analogue, reduces the need for PS in patients with SBS. In this study, we assessed the cost-effectiveness of teduglutide in pediatric patients with SBS from multiple perspectives, considering the caregiver’s burden.
Methods
A Markov model was used to evaluate cost (Japanese yen, JPY) and effectiveness (quality-adjusted life years, QALYs) of teduglutide compared with SOC for pediatric patients with SBS in Japan. We conducted a base-case analysis and selected sensitivity and scenario analyses from three perspectives: (1) the public healthcare payer, (2) the public healthcare and long-term care payer, and (3) society.
Results
In the base-case analysis, the incremental cost-effectiveness ratio (ICER) was 9,533,412 JPY per QALY from the public healthcare payer perspective, 6,335,980 JPY per QALY from the public healthcare and long-term care payer perspective, and 3,510,371 JPY per QALY from the societal perspective. The probability that cost-effectiveness of teduglutide is favorable from a societal perspective was 59.3%. In all scenario analyses, consistent with the base-case analysis, ICERs for teduglutide compared with SOC were different depending on whether caregiver utility and productivity loss were considered.
Conclusions
Incorporating the caregiver’s burden in the cost-effectiveness analysis of teduglutide for pediatric patients with SBS provided a more comprehensive assessment of the value of teduglutide for patients, their families, and society. This approach enhances our understanding of the overall value of a treatment, especially for diseases with significant caregiver burden.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02995-7.
Keywords: Caregivers, Cost-effectiveness analysis, Short bowel syndrome
Key Summary Points
| Caring for pediatric patients with short bowel syndrome (SBS) imposes significant mental and physical burden on caregivers. Teduglutide has been shown in clinical trials to reduce the need for parenteral support of pediatric patients with SBS. |
| This study aimed to provide a comprehensive cost-effectiveness analysis of teduglutide for pediatric patients with SBS in Japan, including considering the caregiver’s burden, an aspect that has been missing from previous teduglutide cost-effectiveness analyses in Japan. |
| Teduglutide was estimated to cost more than standard of care but prolonged patient quality-adjusted life years (QALYs). The estimated incremental cost-effectiveness ratio (ICER) for teduglutide versus standard of care ranged from 9.5 million JPY/QALY from the public healthcare payer’s perspective to 3.5 million JPY/QALY from a societal perspective (i.e., considering caregiver’s quality of life and productivity loss in addition to the public healthcare payer’s perspective). |
| In our scenario analyses, which were consistent with the base-case analysis, different ICERs were observed for teduglutide versus standard of care depending on whether caregivers’ quality of life and productivity loss were considered. |
| This study demonstrated that incorporating the burden of caregivers in the cost-effectiveness analysis provided a more comprehensive assessment of the value of teduglutide for patients, their families, and society. This approach enhances our understanding of the overall value of a treatment, especially for diseases with significant caregiver burden. |
Introduction
Short bowel syndrome (SBS), the most common cause of intestinal failure (IF), is defined as gut function that is inadequate to maintain growth and development due to unsatisfactory absorption of macronutrients, water, or electrolytes [1]. The standard of care (SOC) for SBS is long-term administration of parenteral support (PS; parenteral nutrition and/or intravenous fluids), which is life-saving but associated with potentially life-threatening complications such as IF-associated liver disease (IFALD), central line-associated blood stream infections, and central venous thrombosis [2–5]. In a study that followed 268 adult patients with SBS receiving home parenteral nutrition for a median of 4.4 years, the 5-year survival rate was 70%, and the 10-year survival rate was 52% [6].
Moreover, PS often requires long-term support in the home setting, which significantly impacts the quality of life (QoL) of both the patient and their caregiver, who is usually a family member [7]. Impacts include a reduction in social activities, disrupted family relationships and friendships, depression, and financial constraints [8, 9]. Caregivers of children with IF spend a substantial amount of time providing direct medical care to their children [10].
As of 2011, there were 128 pediatric patients with SBS in Japan [11]. The universal healthcare system in Japan covers all medical services provided in hospitals and clinics. Most pediatric patients with SBS in Japan are treated in large hospitals with over 200 beds, necessitating travel between hospital and their homes, which may affect the family’s work productivity.
Teduglutide is a recombinant human glucagon-like peptide 2 analogue that has been shown in clinical trials to reduce the need for PS in heterogeneous populations of both adult and pediatric patients with SBS [10, 12–22]. In April 2019, a cost-effectiveness evaluation predominantly from the public healthcare payer’s perspective was introduced to the pricing system in Japan for the purpose of adjusting the price after a reimbursement decision. Teduglutide was officially designated as a targeted drug for evaluation in 2021, with cost-effectiveness evaluated in both adult and pediatric patients with SBS compared with the SOC [23]. The following three incremental cost-effectiveness ratio (ICER) thresholds were used for the cost-effectiveness evaluation of teduglutide for pediatric patients with SBS: 7.5 million Japanese yen (JPY), 11.25 million JPY, and 15 million JPY per quality-adjusted life year (QALY), with the price adjustment rate increasing stepwise [24]. As a result of the appraisal, additional benefits were recognized for pediatric patients with SBS compared with the SOC, and the ICER in the cost-effectiveness assessment was evaluated to be over 7.5 million JPY but less than 11.25 million JPY per QALY, from the public payer’s perspective. Consequently, the listed drug price was reduced by one level out of a maximum of three levels [25].
To our knowledge, there has not been a cost-effectiveness analysis for teduglutide for the treatment of SBS in Japan that considers the impact on the caregiver’s QoL and their work productivity. With this in mind, and to provide a comprehensive assessment of the value of teduglutide for pediatric patients with SBS in Japan, we carried out a cost-effectiveness analysis for teduglutide compared with SOC from the following three perspectives: (1) the public healthcare payer’s perspective, (2) the public healthcare and long-term care payer’s perspective, and (3) the societal perspective, with analyses also considering the caregiver’s burden.
Methods
This article is based on previously conducted studies; the authors did not conduct any new studies with animals or human participants.
Overview
The target population for this cost-effectiveness analysis was pediatric patients with SBS in Japan. An average starting age for treatment of 6 years was used in the model, based on results from a 24-week phase 3 trial of teduglutide in pediatric patients with SBS (TED-C14-006) [17, 26]. Teduglutide was compared with SOC for SBS, which aims to alleviate symptoms through optimization of residual intestinal function using PS and enteral nutrition. We conducted analyses from three perspectives: (1) the public healthcare payer’s perspective, (2) the public healthcare and long-term care payer’s perspective (i.e., the utility of caregivers was accounted for in addition to perspective 1), and (3) the societal perspective (i.e., productivity loss for caregivers was accounted for in addition to perspective 2). We used a Markov model with a lifetime analysis period and an analysis cycle of 1 month. The discount rate was 2% per annum for both costs and effects [27].
Model Structure
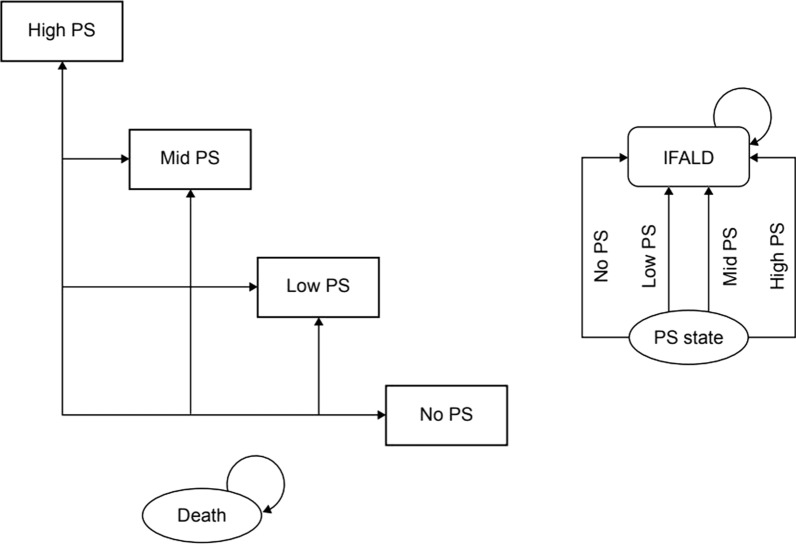
The model structure is shown in Fig. 1. The model was based on the following four health states, defined by the number of days that PS was required per week: “No PS” (without PS–PS withdrawal state); “Low PS” (PS required 1–3 days per week); “Mid PS” (PS required 4–5 days per week); “High PS” (PS required 6–7 days per week); and “Death” (total of five states). Patients entered the analysis model based on the PS distribution at baseline in the TED-C14-006 trial [17, 26]. They could transfer reversibly between PS states each cycle or irreversibly transition to “Death”.
Fig. 1.
Model structure. IFALD intestinal failure-associated liver disease, PS parenteral support
The analysis model also considered the occurrence of IFALD as a complication of PS. Patients who developed IFALD were assumed to progress irreversibly to liver fibrosis and cirrhosis [28]. In the model, teduglutide treatment was discontinued if the reduction in PS from baseline to 24 weeks was less than 20%. Of those patients who were still alive 2 years after the start of teduglutide treatment, 15% continued treatment for the rest of their lives and 85% discontinued treatment. After discontinuation of treatment, there was no transition between PS states and only mortality was considered.
Model Inputs
The main parameters used in the analysis are summarized in Supplementary Table 1. The patients’ health status at the start of the analysis and transition probabilities between PS states were set on the basis of the TED-C14-006 trial [17]. The probabilities of transitioning between states in the first 6 months of treatment (the duration of the TED-C14-006 trial) were calculated on the basis of the observed monthly PS administration days in patients from the teduglutide 0.05 mg/kg/day and SOC treatment groups. Between 7 and 24 months, teduglutide was assumed to repeat the average transition probabilities seen in the final 3 months of the TED-C14-006 trial [17]. From 25 months onwards, there was assumed to be no transition between PS states. SOC was assumed to revert to the baseline distribution at 6 months, with no transition between states from 7 months onwards.
The prevalence of IFALD in patients with SBS was based on results of a modified Delphi survey used in the cost-effectiveness evaluation of teduglutide by the National Institute for Health and Care Excellence (NICE) in the UK [28]. The progression of patients with IFALD to liver fibrosis and cirrhosis was cited from the report by Cavicchi et al. [29]. We considered adverse events (AEs) related to treatment with both teduglutide and SOC with occurrence based on AEs experienced by at least 20% of patients in either the teduglutide 0.05 mg/kg/day group or the SOC group in the TED-C14-006 trial [17]. The occurrence rate of AEs after discontinuation of teduglutide treatment was assumed to be the same as that seen in the SOC treatment group. Mortality was set according to the PS state. Mortality for patients not receiving PS was assumed to be the same as that of the general Japanese population [30]; mortality for patients in other PS states was estimated on the basis of the report by Fullerton et al. for pediatric patients with IF in the USA [31].
Costs
Costs related to teduglutide treatment include the medication cost of teduglutide and management cost associated with self-injection. On the basis of the average weight of the study population in a pediatric teduglutide clinical trial in Japan, the prescribed dosage of teduglutide was assumed to be one vial per day (at a reimbursement cost of 79,300 JPY) [26, 32, 33]. Colonoscopy costs were also included, as detailed in the teduglutide package insert [26, 32]. The cost for each PS state was calculated on the basis of results of a medical resource consumption survey (targeting physicians who treat patients with SBS) [34], and according to the medical service fee point summary and drug price standard [32, 35]. Treatment costs associated with AEs were estimated on the basis of the guidelines and the expected treatment, with costs set according to medical service fee point summary and drug price standard [32]. For AEs for which additional treatment is not necessary or that can be managed with over-the-counter drugs, no cost was assumed. The cost for treating IFALD was set on the basis of the study by Ikeda et al. [36].
Utilities
As there are no reported utilities in the literature for pediatric patients with SBS based on the number of days of PS required, we used the mean utility scores from a study of adult patients in the UK [37] to set the following utility values for each PS state: No PS, 0.820; Low PS, 0.717; Mid PS, 0.545; High PS, 0.385. We set the utility value for IFALD on the basis of a study by Hirao et al. [38] and used the weighted average value (0.830) of utilities for chronic hepatitis (inactive and active) and liver cirrhosis (compensated and decompensated) for both hepatitis B and hepatitis C. The utility reduction due to AEs was set on the basis of referenced literature [38].
The main parameters related to caregiver utility are summarized in Supplementary Table 2. We considered caregiver utility based on the PS state of the care recipient, with caregiver utility derived from the results of the EQ-5D survey completed by caregivers of patients with SBS in the UK [39]. The utility for caregivers in the “No PS”, “Low PS”, “Mid PS”, and “High PS” patient states was accumulated over the analysis period.
Productivity Loss
Productivity loss for pediatric patients with SBS was not considered but productivity loss for caregivers was included. The main parameters related to caregiver productivity loss are summarized in Supplementary Table 2 and are based on the results of a Work Productivity and Activity Impairment (WPAI) survey conducted on caregivers of patients with SBS in the UK (the same source as used for caregiver utilities), taking into account the PS state of the care recipient [39]. The gender ratio of caregivers, employment rate, percentage of caregivers in regular employment, percentage of full-time stay-at-home parents of either sex, average wages, and the value of unpaid labor were set by extrapolating from Japanese national statistics for the population [40–43]. On the basis of the opinions of Japanese and global medical specialists, the number of caregivers per patient was assumed to be 1.5 caregivers for patients under 18 years of age, and 0.8 caregivers for patients aged 18 years and over. The gender ratio of caregivers was based on the ratio published by the Ministry of Internal Affairs and Communications [42], and the age of caregivers was assumed to be 50 years throughout the analysis period.
Analysis Methods: Base-Case Analysis
The QALYs and ICERs were calculated to assess the cost-effectiveness of teduglutide compared with SOC. For the base-case analysis, we conducted the cost-effectiveness analyses from (1) the public healthcare payer perspective, (2) the public healthcare and long-term care payer perspective, and (3) the societal perspective using the analysis conditions and base value parameters described above and in Supplementary Tables 1 and 2.
Analysis Methods: Sensitivity Analyses
For the societal perspective (3), the most comprehensive analysis, we conducted a deterministic sensitivity analysis (DSA) and a probabilistic sensitivity analysis (PSA). The setting range and distribution of each variable are provided in Supplementary Tables 1 and 2.
For the DSA, the sensitivity analysis range was set on the basis of the 95% confidence interval (CI). For parameters without a 95% CI, a range of ± 20% was used. The discount rate was set within the range of 0–4% according to the cost-effectiveness evaluation guidelines [27]. Parameters of the survival function for mortality were assumed to follow a multivariate normal distribution; for PS state transition probabilities, the 2.5th and 97.5th percentiles of the Dirichlet distribution function were set as lower and upper limits, respectively. The analysis results were presented using tornado diagrams, highlighting the top 10 variables with the largest variation.
For the PSA, QoL values were assumed to follow a beta distribution for probabilities (excluding PS state transition probabilities), costs were assumed to follow a gamma distribution, PS state transition probabilities were assumed to follow a Dirichlet distribution, and survival curve parameters were assumed to follow a multivariate normal distribution. For parameters without reported or estimable standard errors (SE), a SE value of 10% of the set value was assumed. We generated random numbers following the specified probability distributions for each parameter, and the simulation was performed by conducting 10,000 data extractions.
Analysis Methods: Scenario Analyses
Two different scenario analyses were conducted for perspective (2), the public healthcare and long-term care payer perspective. In scenario 1, the decreased utility (negative value; taken from the UK study) [39] of the caregiver from “No PS” to “Low PS”, “Mid PS”, and “High PS” was cumulatively added to the utility of the patient over the analysis period; the utility of caregivers in the “No PS” state was assumed to be zero (the decrement method). In scenario 2, the state-specific utility of caregivers of patients with SBS was obtained using the Delphi method (utility values for this scenario are shown in Supplementary Table 2) [44]. Similarly, two different scenario analyses were also conducted for the societal perspective (3). In scenario 3, the analysis combined the conditions of scenario 1 with the productivity loss of the caregiver (decrement method and productivity loss). In scenario 4, the analysis combined the conditions of scenario 2 with the productivity loss of the caregiver (Delphi panel and productivity loss).
Results
Base-Case Analysis
The results of the base-case analysis are presented in Table 1. From the public healthcare payer perspective (1), the incremental effectiveness of teduglutide over SOC was 10.152 QALYs with incremental costs of 96,786,684 JPY. This gave an ICER of 9,533,412 JPY per QALY. From the public healthcare and long-term care payer’s perspective (2), the incremental effect of teduglutide over SOC was 15.276 QALYs with incremental costs of 96,786,684 JPY. This gave an ICER of 6,335,980 JPY per QALY. The incremental effect of teduglutide over SOC on caregivers was 5.123 QALYs. From the societal perspective (3), the incremental effect of teduglutide over SOC was 15.276 QALYs with incremental costs of 53,623,459 JPY. This gave an ICER of 3,510,371 JPY per QALY. The incremental effect of teduglutide over SOC on caregivers was 5.123 QALYs. The caregiver’s productivity gain was 43,163,225 JPY.
Table 1.
Results of the base-case analysis
| Strategy | Cost (JPY) | Incremental cost (JPY) | QALY | Incremental QALY | ICER (cost/QALY) |
|---|---|---|---|---|---|
| Perspective 1: public healthcare payer | |||||
| Teduglutide | 254,562,866 | 96,786,684 | 22.421 | 10.152 | 9,533,412 |
| SOC | 157,776,182 | – | 12.268 | – | – |
| Perspective 2: public healthcare and long-term care payer | |||||
| Teduglutide | 254,562,866 | 96,786,684 | 55.023 | 15.276 | 6,335,980 |
| SOC | 157,776,182 | – | 39.747 | – | – |
| Perspective 3: societal | |||||
| Teduglutide | 291,957,668 | 53,623,459 | 55.023 | 15.276 | 3,510,371 |
| SOC | 238,334,209 | – | 39.747 | – | – |
ICER incremental cost-effectiveness ratio, JPY Japanese yen, QALY quality-adjusted life year, SOC standard of care
Sensitivity Analyses
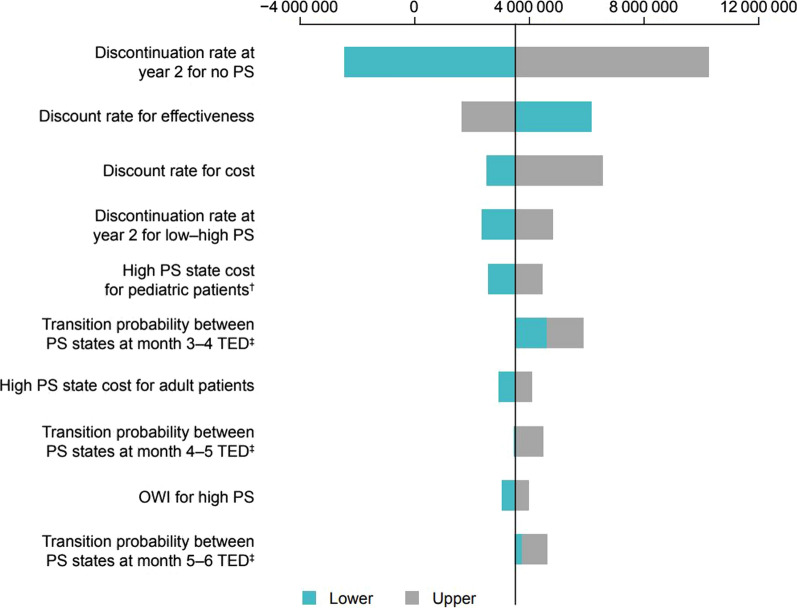
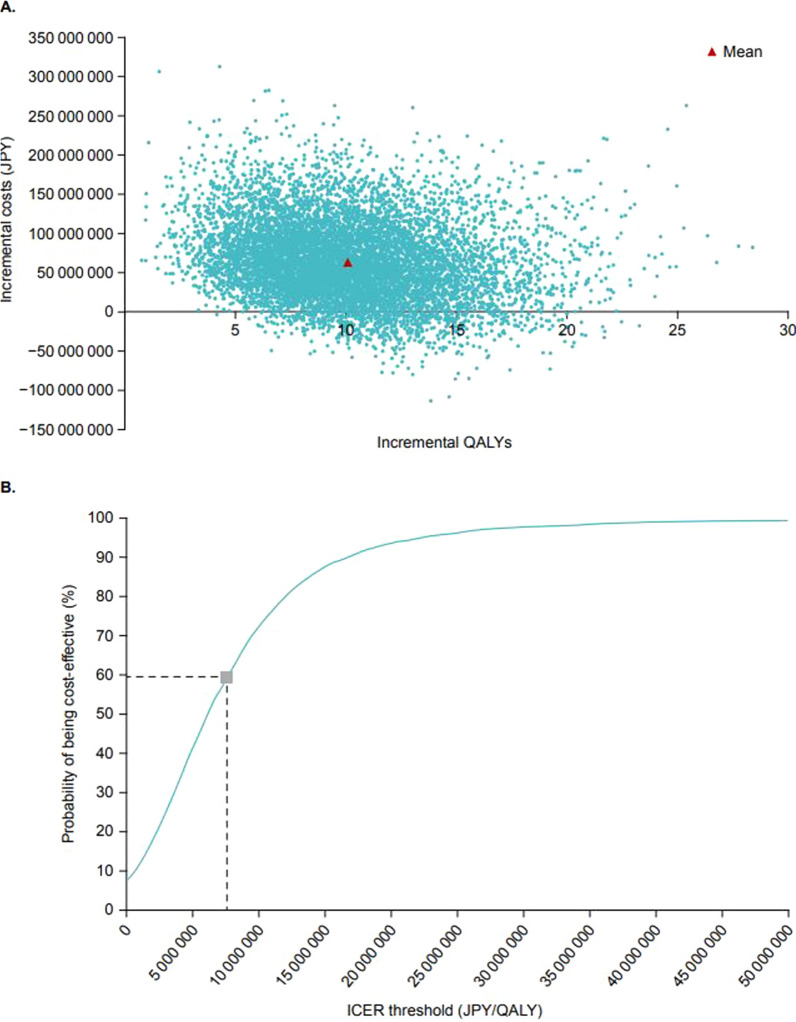
The results of the DSA for the societal perspective (3) are shown in Fig. 2. The top three parameters with the most impact on the ICER were “discontinuation rate at year 2 for No PS”, “discount rate for effectiveness”, and “discount rate for cost”. The results of the PSA for the societal perspective (3) are shown in Fig. 3. The average incremental effect over 10,000 simulations was 10.08 QALY and the average incremental cost was 63.57 million JPY (Fig. 3a). When an ICER value of 7.5 million JPY per QALY (the threshold value for price adjustments for pediatric patients with SBS in Japan) was used, the probability of teduglutide being cost-effective was 59.3% (Fig. 3b).
Fig. 2.
Results of deterministic sensitivity analysis (societal perspective). †The sensitivity analysis range was set on the basis of the 95% CI. ‡Values based on 1.5 caregivers for the patients under 18 years of age and 0.8 caregivers for patients 18 years of age and older. The 2.5% and 97.5% points of the Dirichlet distribution were set as the lower and upper limits, respectively. CI confidence interval, ICER incremental cost-effectiveness ratio, JPY Japanese yen, OWI overall work impairment, PS parenteral support, QALY quality-adjusted life year, TED teduglutide
Fig. 3.
Results of the probabilistic sensitivity analysis (societal perspective). Data show a scatter plots of incremental costs and incremental QALYs and b the cost-effectiveness acceptance curve. ICER incremental cost-effectiveness ratio, JPY Japanese yen, QALY quality-adjusted life year
Scenario Analyses
The results of the scenario analyses are presented in Table 2. From the public healthcare and long-term care payer’s perspective (2), the incremental effect of teduglutide over SOC for scenario 1 was 12.090 QALYs and the ICER was 8,005,541 JPY per QALY. From the same perspective for scenario 2, the incremental effect of teduglutide over SOC was 18.384 QALYs and the ICER was 5,264,812 JPY per QALY. From the societal perspective (3), the incremental cost of teduglutide over SOC for scenario 3 was 53,623,459 JPY and the ICER was 4,435,371 JPY per QALY. From the same perspective for scenario 4, the incremental cost of teduglutide over SOC was 53,623,459 JPY and the ICER was 2,916,903 JPY per QALY.
Table 2.
Results of the scenario analyses
| Strategy | Cost (JPY) | Incremental cost (JPY) | QALY | Incremental QALY | ICER (cost/QALY) |
|---|---|---|---|---|---|
| Perspective 2: public healthcare and long-term care payer | |||||
| Scenario 1. Decrement method | |||||
| Teduglutide | 254,562,866 | 96,786,684 | 20.806 | 12.090 | 8,005,541 |
| SOC | 157,776,182 | – | 8.716 | – | – |
| Scenario 2. Delphi panel | |||||
| Teduglutide | 254,562,866 | 96,786,684 | 52.205 | 18.384 | 5,264,812 |
| SOC | 157,776,182 | – | 33.821 | – | – |
| Perspective 3: societal | |||||
| Scenario 3. Decrement method and productivity loss | |||||
| Teduglutide | 291,957,668 | 53,623,459 | 20.806 | 12.090 | 4,435,371 |
| SOC | 238,334,209 | – | 8.716 | – | – |
| Scenario 4. Delphi panel and productivity loss | |||||
| Teduglutide | 291,957,668 | 53,623,459 | 52.205 | 18.384 | 2,916,903 |
| SOC | 238,334,209 | – | 33.821 | – | – |
ICER incremental cost-effectiveness ratio, JPY Japanese yen, QALY quality-adjusted life year, SOC standard of care
Discussion
Caring for patients with SBS imposes significant mental and physical burden on caregivers, especially in the case of pediatric patients. Therefore, incorporating the extent of alleviation of caregiver burden is crucial when evaluating the value of teduglutide treatment. To this end, we conducted a cost-effectiveness analysis of teduglutide versus SOC for pediatric patients with SBS in Japan from multiple perspectives, considering the caregiver’s burden. The ICER for teduglutide versus SOC was approximately 9.5 million JPY/QALY from the public healthcare payer’s perspective, 6.3 million JPY/QALY from the public healthcare and long-term care payer’s perspective, and 3.5 million JPY/QALY from the societal perspective. Based on the threshold for cost-effectiveness evaluation of teduglutide for pediatric patients with SBS in Japan (7.5 million JPY per QALY) [23, 24], the probability that the cost-effectiveness of teduglutide is favorable from a societal perspective was 59.3%.
In our scenario analyses from multiple perspectives, we applied both caregiver disutilities and utilities because there is debate among researchers about which method to use. When caregiver disutilities were applied, the ICER was approximately 8.0 million JPY per QALY from the public healthcare and long-term care payer’s perspective and 4.4 million JPY per QALY from the societal perspective. These ICERs were higher than when caregiver’s utilities were applied (approximately 5.3 million JPY per QALY from the public healthcare and long-term care payer’s perspective and 2.9 million JPY per QALY from the societal perspective). Applying caregiver utilities leads to a larger incremental effect compared with applying disutilities because an extension to a patient’s survival years adds QALYs for the caregiver. Conversely, subtracting caregiver disutilities from patient utilities during the extended survival years leads to a smaller effect [45]. The different results from the disutilities approach challenge the conventional approach of evaluating the value of treatment based on the extension of survival years. The Institute for Clinical and Economic Review in the USA has pointed out that there is the potential for “counter-intuitive” findings when using a caregiver disutility approach [45]. In another of our scenario analyses, caregiver utilities as assessed by a Delphi panel were lower in terms of PS state-specific utilities than those obtained from the EQ-5D survey. This resulted in lower ICER values in the scenario analyses compared with the base analysis. As the two surveys used different evaluation scales, results cannot be directly compared. The same would be true when comparing results obtained using the time trade-off method. In all scenario analyses, consistent with the base-case analysis, the ICERs for teduglutide compared with SOC were significantly different when the caregiver utility or caregiver productivity loss (depending on the analysis) were considered, highlighting the impact of including these considerations on the outcomes of cost-effectiveness analyses.
In the current cost-effectiveness evaluation system in Japan, it is possible to present analyses that consider the impact on the utilities of caregivers or nurses, including family members. However, the appraisal and price adjustments are generally based on ICER from the perspective of the public healthcare payer and, in reality, analyses from the perspective of the public healthcare and long-term care payer are not considered for final decision-making [27]. Conversely, in the NICE health technology assessment manual, it states that “evaluations should consider all health effects for patients, and, when relevant, caregivers. When presenting health effects for caregivers, evidence should be provided to show that the condition is associated with a substantial effect on the caregiver’s health-related quality of life and how the technology affects caregivers (4.3.17)” [46]. However, it should be noted that productivity costs are not included in reference cases in the NICE manual, in line with the evaluation system in Japan. Considering the difference between healthcare systems and policies between countries, there is no one correct way to carry out assessments. However, our analysis demonstrates that it is important to carry out evaluations from multiple perspectives to inform decision-making.
There were some limitations to our study. As SBS is a rare disease, there is a lack of long-term epidemiological data and limited clinical research against which our results can be validated. The phase 3 trial (TED-C14-006) [17] conducted in North American and European patients, which was the source of input data (e.g., patient background and transition probabilities), and the UK adult patient data that was used to estimate patient utilities, caregiver utilities, and caregivers’ WPAI scores [37, 44], may not accurately reflect the situation for SBS in Japan. However, it has been shown that there is no significant difference in the pathology of SBS between Japan and other countries, and the causes leading to SBS are similar [47, 48]. Furthermore, in the Japanese phase 3 trial (SHP633-302) [10], no differing trends were observed compared to the international phase 3 trial (TED-C14-006) [17], indicating no significant differences between Japan and other countries. The use of caregiver utilities derived from a survey of caregivers of adult (not pediatric) patients with SBS in the UK is nevertheless an unavoidable limitation of our study. Cost data or wage and employment rates used to estimate productivity reflect the current situation in Japan as accurately as possible and is a strength of our study.
Conclusion
We conducted cost-effectiveness analyses of teduglutide versus SOC for pediatric patients with SBS in Japan from multiple perspectives, considering the caregiver’s burden. Our analysis suggests that for diseases with a significant caregiver burden, such as SBS, incorporating this aspect could contribute to a more comprehensive assessment of the value that treatments can bring to patients, their families, and society. As a result, this approach could potentially affect the appraisal process for future cost-effectiveness evaluation and help guide more appropriate resource allocation within the medical insurance system in Japan.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing/Editorial Assistance
The authors would like to thank Dr Sachie Inoue and Hidetoshi Shibahara of CRECON Medical Assessment Inc. for the study’s analysis models and calculations, and contributors inside Market Access, Public Affairs & Patient Experience, Japan Pharma Business Unit, Takeda Company Limited for providing advice on the formulation of overarching research goals, aims, and plan. Medical writing support was provided by Emily Manktelow PhD of Oxford PharmaGenesis, Melbourne, Australia, and funded by Takeda Pharmaceutical Company Limited, Tokyo, Japan, in accordance with Good Publication Practice 2022 (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Author Contributions
Hisato Deguchi was responsible for formulating and clarifying the overarching research goals, aims, and plan from the caregiver perspective; searching and gathering information from previous studies to input into the outline; conducting the research, including evaluating the validity of the analytical model and parameters; and drafting the proposal of modifications to the overall discussion strategy. Masafumi Kato was responsible for formulating and clarifying the overarching research goals, aims, and plan from the HTA perspective; and reviewing the proposal of modifications to the overall discussion strategy. Both Hisato Deguchi and Masafumi Kato were responsible for the design and development of the methodology and the interpretation of the results. Both authors took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study, and payment of the journal’s Rapid Service and Open Access Fees, were funded by Takeda Pharmaceutical Company Limited, Tokyo, Japan.
Data Availability
All data generated or analyzed in this study are included in this published article or as supplementary materials.
Declarations
Conflict of Interest
Hisato Deguchi and Masafumi Kato are both employees of Takeda Pharmaceutical Company Limited, Tokyo, Japan.
Ethical Approval
This article is based on previously conducted studies; the authors did not conduct any new studies with animals or human participants.
References
- 1.Youssef NN, Mezoff AG, Carter BA, Cole CR. Medical update and potential advances in the treatment of pediatric intestinal failure. Curr Gastroenterol Rep. 2012;14(3):243–52. [DOI] [PubMed] [Google Scholar]
- 2.Bines JE. Intestinal failure: a new era in clinical management. J Gastroenterol Hepatol. 2009;24(Suppl 3):S86–92. [DOI] [PubMed] [Google Scholar]
- 3.Dreesen M, Foulon V, Spriet I, et al. Epidemiology of catheter-related infections in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr. 2013;32(1):16–26. [DOI] [PubMed] [Google Scholar]
- 4.Duggan CP, Jaksic T. Pediatric intestinal failure. N Engl J Med. 2017;377(7):666–75. [DOI] [PubMed] [Google Scholar]
- 5.Gosselin KB, Duggan C. Enteral nutrition in the management of pediatric intestinal failure. J Pediatr. 2014;165(6):1085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amiot A, Messing B, Corcos O, Panis Y, Joly F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin Nutr. 2013;32(3):368–74. [DOI] [PubMed] [Google Scholar]
- 7.Jeppesen PB, Shahraz S, Hopkins T, Worsfold A, Genestin E. Impact of intestinal failure and parenteral support on adult patients with short-bowel syndrome: a multinational, noninterventional, cross-sectional survey. JPEN J Parenter Enteral Nutr. 2022;46(7):1650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CE. Quality of life in long-term total parenteral nutrition patients and their family caregivers. JPEN J Parenter Enteral Nutr. 1993;17(6):501–6. [DOI] [PubMed] [Google Scholar]
- 9.Winkler MF, Smith CE. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38(1 Suppl):32S–S37. [DOI] [PubMed] [Google Scholar]
- 10.Chiba M, Masumoto K, Kaji T, et al. Efficacy and safety of teduglutide in infants and children with short bowel syndrome dependent on parenteral support. J Pediatr Gastroenterol Nutr. 2023;77(3):339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Information Center for Specific Pediatric Chronic Diseases, Japan. Short bowel. 2014. https://www.shouman.jp/disease/details/12_13_037/. Accessed 2 Sep 2024.
- 12.Carter BA, Cohran VC, Cole CR, et al. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. J Pediatr. 2017;181(102–11):e5. [DOI] [PubMed] [Google Scholar]
- 13.Jeppesen PB. Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome. Therap Adv Gastroenterol. 2012;5(3):159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeppesen PB, Gabe SM, Seidner DL, Lee HM, Olivier C. Factors associated with response to teduglutide in patients with short bowel syndrome and intestinal failure. Gastroenterology. 2018;154(4):874–85. [DOI] [PubMed] [Google Scholar]
- 15.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143(6):1473–81.e3. [DOI] [PubMed] [Google Scholar]
- 17.Kocoshis SA, Merritt RJ, Hill S, et al. Safety and efficacy of teduglutide in pediatric patients with intestinal failure due to short bowel syndrome: a 24-week, phase III study. JPEN J Parenter Enteral Nutr. 2020;44(4):621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura S, Wada M, Mizushima T, et al. Efficacy, safety, and pharmacokinetics of teduglutide in adult Japanese patients with short bowel syndrome and intestinal failure: two phase III studies with an extension. Surg Today. 2023;53(3):347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Keefe SJ, Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol. 2013;11(7):815–23.e1–3. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz LK, O’Keefe SJ, Fujioka K, et al. Long-term teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol. 2016;7(2):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidner DL, Fujioka K, Boullata JI, Iyer K, Lee HM, Ziegler TR. Reduction of parenteral nutrition and hydration support and safety with long-term teduglutide treatment in patients with short bowel syndrome-associated intestinal failure: STEPS-3 study. Nutr Clin Pract. 2018;33(4):520–7. [DOI] [PubMed] [Google Scholar]
- 22.Vipperla K, O’Keefe SJ. Study of teduglutide effectiveness in parenteral nutrition-dependent short-bowel syndrome subjects. Expert Rev Gastroenterol Hepatol. 2013;7(8):683–7. [DOI] [PubMed] [Google Scholar]
- 23.Center for Outcomes Research and Economic Evaluation for Health (C2H). Target items and results after full-scale introduction. 2019. https://c2h.niph.go.jp/results/item.html. Accessed 21 Feb 2023.
- 24.Hasegawa M, Komoto S, Shiroiwa T, Fukuda T. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51. [DOI] [PubMed] [Google Scholar]
- 25.The Central Social Insurance Medical Council. Regarding the cost-effectiveness evaluation proposal for pharmaceuticals and medical treatments. 2023. https://www.mhlw.go.jp/content/12404000/001039379.pdf. Accessed 21 Feb 2023.
- 26.Takeda Pharmaceutical Company Ltd. Summary of Product Characteristics, Revestive. Pharmaceuticals and Medical Devices Agency. 2017. https://www.pmda.go.jp/drugs/2021/P20210614001/index.html. Accessed 21 Feb 2023.
- 27.The Central Social Insurance Medical Council. Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council (version 3). 2022. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed 21 Feb 2023.
- 28.National Institute for Health and Care Excellence. Teduglutide for treating short bowel syndrome technology appraisal guidance [TA804]. 2022. https://www.nice.org.uk/guidance/ta804. Accessed 21 Feb 2023.
- 29.Cavicchi M, Beau P, Crenn P, Degott C, Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132(7):525–32. [DOI] [PubMed] [Google Scholar]
- 30.Ministry of Health, Labour and Welfare. Summary of the 2020 Life Table. 2020. https://www.mhlw.go.jp/toukei/saikin/hw/life/life20/index.html. Accessed 30 Mar 2022.
- 31.Fullerton BS, Sparks EA, Hall AM, Duggan C, Jaksic T, Modi BP. Enteral autonomy, cirrhosis, and long term transplant-free survival in pediatric intestinal failure patients. J Pediatr Surg. 2016;51(1):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medical Fee Point Summary April 2020/21 Revised Edition [Medical]: Igaku Tsushinsha. 2021.
- 33.Pharmaceuticals and Medical Devices Agency. Revestive for subcutaneous injection 3.8 mg. 2021. https://www.pmda.go.jp/drugs/2021/P20210614001/index.html. Accessed 15 Nov 2023.
- 34.Takeda Pharmaceutical Company Ltd. Cost-effectiveness evaluation of teduglutide, medical resource consumption survey - brief report (data on file). Accessed 4 Feb 2022.
- 35.Ministry of Health, Labour and Welfare. List of drug price standard covered items and information on generic drugs (until 31 Mar 2022). 2022. https://www.mhlw.go.jp/topics/2021/04/tp20210401-01.html. Accessed 21 Feb 2023.
- 36.Ikeda T, Kobayashi M, Shimoda S. Various measures related to viral liver disease: research on health economic evaluation of 2011–2015. Comprehensive report. 2013. https://mhlw-grants.niph.go.jp/system/files/2013/135013/201333004A/201333004A0007.pdf. Accessed 21 Feb 2023.
- 37.Ballinger R, Macey J, Lloyd A, et al. Measurement of utilities associated with parenteral support requirement in patients with short bowel syndrome and intestinal failure. Clin Ther. 2018;40(11):1878–93.e1. [DOI] [PubMed] [Google Scholar]
- 38.Hirao T, Yatsuhashi H, Ikeda T, Yoda K. Estimating the utility value of viral hepatitis-related diseases using EQ-5D. Research on medical economic evaluation of various countermeasures related to viral liver disease collaborative research report. https://mhlw-grants.niph.go.jp/system/files/2012/125013/201240004A/201240004A0006.pdf. Accessed 21 Feb 2023.
- 39.Shire. Impacts on carers of patients with short bowel syndrome-associated intestinal failure receiving parenteral support—study report (data on file). 22 June 2017.
- 40.Cabinet Office, National Institute of Economic and Social Research. Monetary evaluation of unpaid labor. 2018. https://www.esri.cao.go.jp/jp/sna/sonota/satellite/roudou/contents/kajikatsudou_181213.html. Accessed 21 Feb 2023.
- 41.Ministry of Health, Labour and Welfare. Basic survey on wage structure. https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450091&tstat=. Accessed 21 Feb 2023.
- 42.Ministry of Internal Affairs and Communications. Results of the 2021 basic survey on social life. https://www.stat.go.jp/data/shakai/2021/kekka.htm. Accessed 21 Feb 2023.
- 43.Ministry of Internal Affairs and Communications. Labour Force Survey. https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00200531&tstat=000000110001. Accessed 21 Feb 2023.
- 44.Shire. Teduglutide (Revestive®) for short bowel syndrome (SBS) in paediatric patients: cost-effectiveness model - technical report (data on file). 29 May 2019.
- 45.Pennington B, Eaton J, Hatswell AJ, Taylor H. Carers’ health-related quality of life in global health technology assessment: guidance, case studies and recommendations. Pharmacoeconomics. 2022;40(9):837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Institute for Health and Care Excellence. NICE health technology evaluations: the manual. 2022. https://www.nice.org.uk/process/pmg36/chapter/economic-evaluation. Accessed 21 Feb 2023.
- 47.Weber TR, Tracy T Jr, Connors RH. Short-bowel syndrome in children. Quality of life in an era of improved survival. Arch Surg. 1991;126(7):841–6. [DOI] [PubMed] [Google Scholar]
- 48.Vanderhoof JA, Langnas AN, Pinch LW, Thompson JS, Kaufman SS. Short bowel syndrome. J Pediatr Gastroenterol Nutr. 1992;14(4):359–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are included in this published article or as supplementary materials.