Abstract
Reduced sense of smell is a common symptom in patients with chronic rhinosinusitis (CRS). Although it is often under-diagnosed by healthcare providers, reduced sense of smell can have a substantial negative impact on patient’s quality of life as measured by health-related quality of life (HRQoL) assessments and patient-reported outcomes. This narrative review describes current smell loss diagnosis and management guidelines in CRS, and the relationship between smell loss and CRS. Reduced sense of smell can be an indication of CRS disease severity in patients with (CRSwNP) and without nasal polyps (CRSsNP), and recovery of smell can be an indicator of successful CRS treatment. The current first-line therapeutic options for smell loss are intranasal corticosteroids and nasal irrigation, and second-line therapeutic options include systemic steroids and surgery. Shared decision-making between patient, caregiver, and healthcare provider is important when choosing the most appropriate CRS treatment option. Emerging biologic therapies that target type 2 inflammation signaling pathways, such as dupilumab, omalizumab, and mepolizumab, have been shown to improve smell and taste in randomized controlled trials of patients with CRSwNP.
A graphical abstract and video abstract are available with this article.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02984-w.
Keywords: Adults, Anosmia, Benralizumab, Chronic rhinosinusitis, Dupilumab, Mepolizumab, Omalizumab, Patient-reported outcomes
Plain Language Summary
Chronic rhinosinusitis (CRS) is an inflammatory condition often associated with a loss of smell and taste. Patients with CRS and a loss of smell often rate their quality of life as poor and are more likely to also suffer from depression and anxiety than patients without smell loss. Patients with severe smell loss are also more likely to have increased severity of CRS disease by other measures. Standard treatments for smell loss include topical steroids, corticosteroids absorbed into the whole body system (systemic), and/or sinonasal surgery, but the effects may not last, and patients may experience side effects when they use repeated short bursts or long-term treatment with systemic corticosteroids. A newer treatment option for CRS is biologic therapy, which targets the immunologic pathways associated with inflammation. Biologic therapies have been shown to be effective in the treatment of CRS with nasal polyps including improvement in sense of smell. Here, we review the most common diagnostic tests and treatment options for CRS-associated smell loss and show how severity of smell loss is linked to severity of CRS.
Supplementary file1 (MP4 60193 kb)
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02984-w.
Key Summary Points
| Loss of smell strongly affects the quality of life in patients with chronic rhinosinusitis (CRS). |
| Despite a high prevalence in patients with CRS, loss of smell is often under-diagnosed by healthcare providers. |
| Second-line therapeutic options for smell loss in CRS include systemic corticosteroids and sinonasal surgery. |
| Given the risk of symptom recurrence and adverse events with second-line treatment options, shared decision-making is important in the management of smell loss. |
| Monoclonal antibodies targeting type 2 inflammatory signaling pathways have demonstrated effectiveness at improving loss of smell in patients with CRS with nasal polyps (CRSwNP). |
Digital Features
This article is published with digital features, including a graphical abstract and video abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.26862922.
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disease that affects up to 15% of the adult population in the US and Western Europe [1] and is characterized by four cardinal symptoms: facial pain or pressure, reduction or loss of smell, nasal blockage, and nasal discharge. Reduced sense of smell is a common symptom of CRS, affecting 38–83% of patients overall, with complete anosmia in approximately 25% [2]. CRS can be phenotypically categorized by the presence or absence of nasal polyps (CRSwNP and CRSsNP, respectively), and in CRSwNP the incidence of smell loss is much higher, with 77–91% of patients affected [3]. In comparison, the incidence of reduced sense of smell in the general population is estimated at 5%, with higher rates in persons over the age of 65 [4]. Common causes of reduced sense of smell other than CRS include COVID-19 and other post-viral smell loss, neurodegenerative disease, and trauma [4, 5]. This review highlights the importance of smell in the diagnosis and management of CRS, and the impact of treatment on reversing reduced sense of smell. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Disease Definition
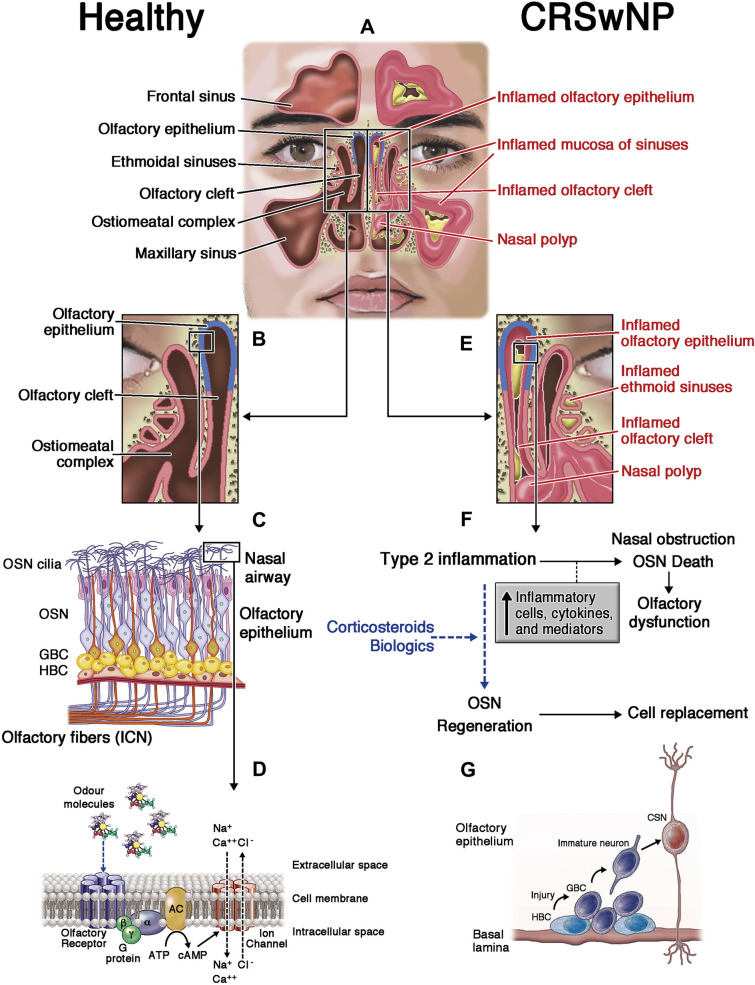
CRS is a long-term disease that involves chronic inflammation of the nasal and paranasal sinus mucosa [6, 7]. CRSwNP displays a predominantly type 2 inflammation signature (87% of cases), particularly in populations of European descent, with upregulation of interleukin (IL)-4, IL-5, IL-13, and local immunoglobulin E (Fig. 1) [8–10]. Type 2 inflammation is also reported in 30–55% of cases of CRSsNP, with the remaining cases mediated either by cytokines such as interferon gamma and interferon alpha (type 1 inflammation) or by IL-17, IL-22, and IL-23 (type 3 inflammation) [8, 9]. There appear to be geographic differences in the involvement of type 2 inflammation, with over half of CRSwNP cases in East Asia presenting with non-type 2 inflammation [11].
Fig. 1.
Inflammation in the nasal cavities in CRS, differentiating between normal and swollen tissue with nasal polyps. The peripheral olfactory system in health and disease. Healthy status: A and B The olfactory epithelium is located in the olfactory cleft, in the upper part of the nasal cavity. C This olfactory epithelium contains the cell bodies of mature and immature olfactory sensory neurons (OSNs) generated from horizontal (HBC) and globose (GBC) basal stem cells (BSCs). OSN axons exit the olfactory epithelium in fascicles of olfactory fibers projecting the first cranial nerve (ICN) through the cribriform plate to olfactory bulbs. C and D Odor transduction occurs in the cilia from OSN dendritic knobs where olfactory receptors (7-transmembrane domain G-protein-coupled receptors) recognize odor molecules through the activation of the adenylyl cyclase (AC) pathway and membrane ion channels. Inflammatory status in CRSwNP: A–E CRS inflammation, with or without nasal polyps, affects the mucosa of bilateral paranasal sinuses and nasal cavities, including the olfactory cleft and epithelium. F and G Type 2 inflammation (mainly eosinophilic) of the olfactory cleft mucosa leads to olfactory epithelium shedding and OSN degeneration as potential causes of the loss of smell. Anti-inflammatory therapy (corticosteroids, biologics, and others) potentially reduces olfactory cleft inflammation and induces BSC proliferation and OSN regeneration (dashed arrows), causing the partial or total recovery of the sense of smell. a-b-g G-protein subunits, ATP adenosine triphosphate, cAMP cyclic adenosine monophosphate, Ca++ calcium ion, Cl− chloride ion, CRS chronic rhinosinusitis, CRSwNP chronic rhinosinusitis with nasal polyps, Na+ sodium ion. Reprinted from [10] with permission from Elsevier
Different mechanisms may account for smell loss in CRS. One reduced sense of smell mechanism is conductive, caused by obstruction of airflow to the olfactory cleft due to inflammation, while another is sensorineural, caused by shedding and degeneration of the olfactory epithelium or nerves [4, 10]. A recent study in transgenic mice showed that chronic IL-13 exposure in the olfactory epithelium is associated with time-dependent neuronal loss [12]. Short-term intranasal administration of IL-4 is also associated with smell loss [13]. Ultimately, long-term inflammation is believed to cause neuroepithelial remodeling and interference with odorant binding [4]. While healthcare interventions can make a difference in CRS-associated smell loss, it is often under-diagnosed by healthcare providers.
Impact and Burden of Smell Loss
Reduced sense of smell can have a substantial negative impact on patients’ quality of life (QoL), as measured by the 26-item Short Form health survey and patient self-assessment [14]. Effects can include reduced enjoyment of food and difficulty or lack of interest in cooking. Loss of smell can also be a safety hazard (e.g., eating spoiled food or inability to detect a gas leak), and affected individuals may also worry about personal hygiene and body odor, which may affect their willingness to socialize. Loss of smell may also be associated with negative effects on mental health, correlating with both depression and anxiety in CRS and COVID-19-related loss of smell [15, 16]. Among patients attending an otorhinolaryngology outpatient clinic, those with CRS were found to be more likely than non-CRS controls to have consulted a family physician for depression and/or anxiety, although the difference was statistically significant only for CRSsNP [17]. Smell is also closely linked with taste, and, while the actual sensation of taste (sweet, bitter, salty, etc.) may be unaffected in CRS, patients are likely to experience reduced flavor perception [18, 19]. Importantly, patients may not appreciate the consequences of smell loss and its effect on mood and food preferences, or its relation to taste.
Diagnostic Guidelines
International guidelines define CRSwNP and CRSsNP as at least two cardinal symptoms (facial pain or pressure, reduction or loss of smell, nasal blockage, and nasal discharge) lasting for more than 12 weeks [8, 20]. The symptoms of CRS overlap partially with allergic rhinitis (AR), an immunoglobulin E-mediated inflammatory condition, but the two conditions can be distinguished by their location, with AR limited to the nasal passageway and CRS including inflammation in the paranasal sinuses [21]. Classification into CRSwNP and CRSsNP is generally based on the simple presence or absence of polyps on endoscopy or imaging [8], although it is now apparent that CRS represents a spectrum of disease variants, and some guidelines classify CRS on the basis of primary versus secondary disease, localization, and type 2 versus non-type 2 inflammation [20].
It is recommended that physicians ask patients about reduced sense of smell as part of the diagnostic work-up for CRS [22]. However, this may not be done as part of the standard evaluation, where the focus may be on the most apparent symptoms of nasal congestion, rhinorrhea/postnasal drip, and facial pain/pressure. A range of methods is available for the assessment of sense of smell in patients with CRS (summarized in Table 1) [23]. One of the most widely used instruments in clinical trials is the Smell Identification Test (formerly known as the University of Pennsylvania Smell Identification Test [UPSIT]), which comprises four booklets each containing 10 odorant strips. Patients’ responses are classified from normal to complete absence of smell perception. The UPSIT has high reliability and is validated against population norms [23, 24]. Another commonly used method in clinical trials is Sniffin Sticks [25]. This set of 12 or 16 felt-tipped ‘pens’ can be used for odor discrimination and identification tests, with reduced smell defined as a score below the 90th centile for the normal population. Unlike the UPSIT, Sniffin Sticks cannot be self-administered by the patient and requires the presence of a medical assistant who has undergone a short training period [25]. A simple alternative to UPSIT and Sniffin Sticks is to use a swab soaked in 70% isopropyl alcohol [23]. The pad is brought toward the patient’s nose until they can detect the smell, and the distance from pad to nostril is measured.
Table 1.
Tools for the evaluation and monitoring of reduced sense of smell, including response to treatment, in patients with CRS
| Evaluation tool | Type/format |
|---|---|
| SNOT-22 smell/taste item | Patient-completed questionnaire |
| QOD-NS | Patient-completed questionnaire |
| VAS for symptoms | Patient-completed questionnaire |
| UPSIT | 'Scratch and sniff’ booklets |
| Smell identification test | |
| Sniffin Sticks | Set of pen-like odor dispensing devices |
|
Odor discrimination test Odor identification test | |
| Alcohol swab smell test | 70% isopropyl alcohol pad |
QOD-NS Questionnaire of Olfactory Disorders Negative Statements, SNOT-22 22-item Sino-Nasal Outcome Test, UPSIT University of Pennsylvania Smell Identification Test, VAS visual analog scale
However, psychometric measures of smell loss do not always correlate with the subjective impact of smell loss on patients with CRS. More broadly, health-related quality of life (HRQoL) in CRS can be assessed using the 22-item Sinonasal Outcome Test (SNOT-22), which is a survey that includes an item assessing smell and taste [26]. The SNOT-22 is a practical tool for the routine assessment of the clinical impact of CRS on patients [27]. Another patient-reported outcome (PRO) instrument available in the management of CRS is the Questionnaire of Olfactory Disorders Negative Statements, which is becoming more widely used in outcome studies relating to smell loss in CRS [28]. For a simple assessment of PROs in CRS, patients can be asked to rate the impact of their symptoms on a 100-mm visual analog scale, providing a severity score of 1–100. Furthermore, a meta-analysis found nasal polyp scoring systems were not significantly associated with PROs, and that reduction in polyp size did not correlate to improved PROs [29]. It is important to note that all clinical smell testing at this point is subjective in nature; although we can more easily quantify psychophysical measures such as these listed above, the biases of subjective testing remain. Objective smell testing is currently being developed and may be more widely available in the future.
Reduced Sense of Smell as a Marker of CRS Disease Severity
Studies show that reduced sense of smell in patients with CRS is multifactorial [30]. The presence of polyps is a key factor: a recent prospective, multi-institutional case–control study found that patients with CRSwNP had impairments in olfactory threshold, discrimination, and identification, whereas those with CRSsNP had only an impaired threshold [30]. Multivariate modeling confirmed the impact of polyp status, also identifying older age, diabetes mellitus, and asthma as significant independent predictors of smell loss. An association between impaired sense of smell and sinus disease severity in patients with CRSwNP has been confirmed in post hoc analyses of phase drug III trials in patients with severe CRSwNP [31]. The degree of sinus opacification (particularly anterior ethmoid) on computed tomography (CT) scans was found to be greatest in patients with the most severe impairment of smell, assessed using UPSIT, the SNOT-22 smell/taste item, and a 4-point loss-of-smell scale. Furthermore, reduced sense of smell may be the first sign of disease recurrence following surgical or medical treatment [32].
Management of Smell Loss in CRS
Primary medical treatment of CRS involves intranasal corticosteroids (INCS) or nasal irrigation [20, 33]. Antihistamines should not be prescribed for CRS because evidence to support their use is very limited [20]. Similarly, the European Position Paper on Rhinosinusitis and Nasal Polyps steering committee expressed uncertainty about recommending a short course of antibiotics, citing a lack of evidence supporting their use and frequent gastrointestinal adverse events such as anorexia and diarrhea [20]. If primary treatment does not lead to improvement in symptoms within 6–12 weeks, it is recommended that patients be referred for an outpatient appointment at an ear, nose, and throat clinic.
Secondary treatment options include systemic corticosteroids (SCS), polypectomy, and endoscopic sinus surgery (ESS) [33]. Shared decision-making with the patient and caregivers, and presence of comorbidities such as asthma and AR are important in the selection of further treatment options [34, 35].
It is well known that short courses of SCS can significantly improve CRS symptoms and nasal polyps [20]. However, the effects are often temporary [36]. Short courses of oral corticosteroids are generally safe and well tolerated, although possible short-term side effects include insomnia, mood changes, and gastrointestinal events [20]. Importantly, repeated short-term oral corticosteroid use is associated with an increased risk of adverse events [37–39].
For patients without adequate medical response to treatment, ESS may provide effective reduction of CRS symptoms and improvement in sense of smell, particularly for patients with CRSwNP. A randomized prospective study comparing ESS with conventional medical management found that both groups experienced improvements from baseline in cardinal CRS symptoms [40]. However, significantly more patients who underwent surgery experienced complete resolution of thick nasal discharge and facial pain/pressure, and nasal congestion or blockage compared with medical management alone. The benefits of surgery seemed to be largely driven by the presence of nasal polyps; in patients with CRSwNP, resolution of all cardinal signs of CRS, including smell/taste, was reported in significantly more patients who underwent ESS compared with medical management. Surgery can provide disease control for extended periods, but nasal polyps often recur: a recent meta-analysis found a mean rate of revision surgery of 16% over 7 years of follow-up [40]. Topical high-volume steroid irrigations can prolong the benefit of surgery in CRS, although patient adherence can be variable [41]. Furthermore, there is evidence that patients whose olfaction does not improve after SCS are unlikely to recover smell loss following surgery [36].
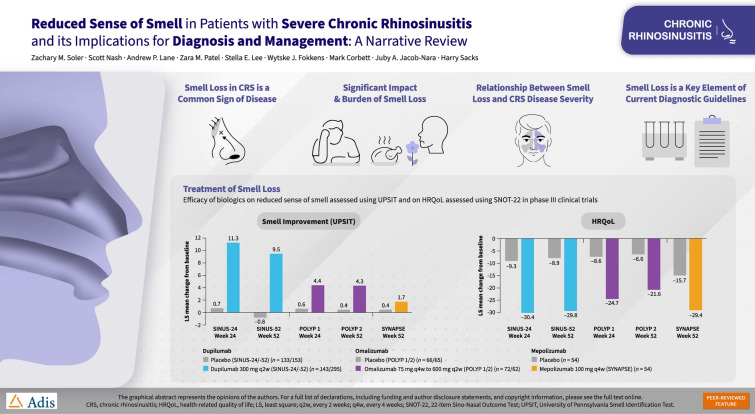
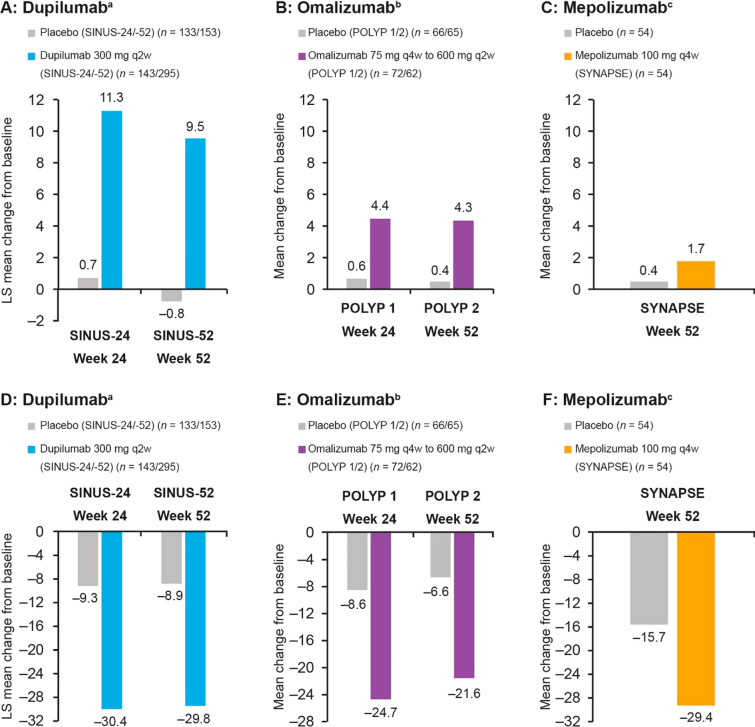
A number of biologic agents have been developed that target the inflammatory signaling pathways underlying CRSwNP [42]. Dupilumab is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, which are key and central drivers of type 2 inflammation. In phase III trials, dupilumab on background INCS significantly improved olfactory outcomes versus placebo as well as nasal polyp score (NPS), nasal congestion/obstruction, sinus Lund-Mackay CT score, and SNOT-22 score (Fig. 2A and D) [43]. Improvements were observed as early as 3 days for daily patient-reported loss of smell score, and at the first post-baseline assessment (2 weeks) for UPSIT, and the proportion of patients with anosmia declined from 78% at baseline to 45% by week 2 and 28% by week 24 [44]. Similarly, phase III trials of the IgE-targeted monoclonal antibody omalizumab showed significant benefits of treatment over placebo in terms of UPSIT as well as NPS, nasal congestion, and SNOT-22 score (Fig. 2B and E) in patients with CRSwNP and an inadequate response to INCSs [45]. Omalizumab effects on UPSIT score were observed at the first time point assessed (8 weeks). Mepolizumab is a monoclonal antibody that targets IL-5, another cytokine that is upregulated in type 2 autoimmunity. In a phase III trial in patients with severe CRSwNP refractory to prior therapy, mepolizumab was associated with significant improvements in UPSIT, NPS, nasal obstruction, and SNOT-22 score (Fig. 2C and F) compared with placebo. All three biologic agents were well tolerated in clinical trials. Common adverse events included headaches (7% vs. 9% dupilumab vs. placebo; 8% vs. 5% omalizumab vs. placebo; 18% vs. 22% mepolizumab vs. placebo), nasopharyngitis (13% vs. 15% dupilumab vs. placebo; 9% vs. 6% omalizumab vs. placebo; 25% vs. 22% mepolizumab vs. placebo), asthma (2% vs. 7% dupilumab vs. placebo; 4% vs. 12% omalizumab vs. placebo; 2% vs. 9% mepolizumab vs. placebo), and injection-site reactions (6% vs. 8% dupilumab vs. placebo; 5% vs. 2% omalizumab vs. placebo; 3% vs. 2% mepolizumab vs. placebo) [43, 45, 46].
Fig. 2.
Efficacy of biologics on reduced sense of smell assessed using UPSIT (A–C) and on HRQoL assessed using SNOT-22 (D–F) in phase III clinical trials. Data from aBachert et al. [43]; bGevaert et al. [45]; cHan et al. [46]. HRQoL health-related quality of life, LS least-squares, q2w every 2 weeks, q4w every 4 weeks, SNOT-22 22-item Sino-Nasal Outcome Test, UPSIT University of Pennsylvania Smell Identification Test
Although there are no direct head-to-head studies to date, an indirect treatment comparison of biologic therapy involving 1913 patients with CRSwNP found that compared with benralizumab, mepolizumab, and omalizumab, at 24 weeks dupilumab was associated with a greater improvement in loss of smell severity [standardized mean difference (95% confidence interval): – 1.01 (– 1.65, – 0.37), – 0.59 (– 1.14, – 0.33), – 0.66 (– 1.23, – 0.10), respectively]. At 24 weeks, dupilumab was associated with a greater improvement in SNOT-22 compared with benralizumab and mepolizumab, but not omalizumab [mean difference: – 16.91 (– 22.52, – 11.30), – 7.94 (– 13.91, – 1.97), –3.45 (– 8.57, 1.66), respectively] [47]. However, limitations of this indirect treatment comparison include the differing patient baseline demographics between trials, and the lack of mean change from baseline data in the phase II mepolizumab trial [48]. A retrospective cohort study comparing dupilumab with ESS in patients with CRSwNP showed effective symptom reduction with both treatments [49]. There were, however, differences between the treatments, with dupilumab associated with greater improvements in olfaction, cough, postnasal drainage, and thick nasal drainage than ESS, while ESS was associated with a greater reduction in polyp burden.
Conclusions
Reduced sense of smell is a cardinal symptom of CRS and can have a negative impact on patient QoL, but it may be under-diagnosed in the primary care setting. Furthermore, reduced sense of smell can be an indication of CRSwNP disease severity, and recovery of smell can be an indicator of successful treatment. Systemic steroids and ESS are the main second-line therapies and can be effective in restoring sense of smell, but the effects are often temporary, and systemic steroids come with an increased risk of side effects. Topical steroids delivered via high-volume irrigation after surgery can prolong the benefit of surgery, but patient adherence to these regimens can be variable. Biologics have been shown to improve the cardinal symptoms of CRSwNP including smell, and rhinolaryngoscopy by a specialist is essential before prescribing biologic agents to treat CRS-associated olfactory loss. Biologic agents are effective during treatment but are costly for the payer, and CRS symptoms often recur upon cessation [42]. Shared decision-making with the patient or caregiver is important to determine the optimal treatment approach.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Medical writing support provided by Claire L. Jarvis, PhD of Adelphi Group, Macclesfield, UK, funded by Sanofi and Regeneron Pharmaceuticals Inc. in accordance with Good Publication Practice. The authors thank Asif H. Khan (Sanofi) for insights and guidance.
Author Contributions
All authors (Zachary M. Soler, Scott Nash, Andrew P. Lane, Zara M. Patel, Stella E. Lee, Wytske J. Fokkens, Mark Corbett, Juby A. Jacob-Nara, and Harry Sacks) conceived the article, performed the literature search and data analysis, drafted and critically revised the work. All authors agree to be accountable for all aspects of the work and have read and approved the final manuscript.
Funding
Open access funding provided by the Carolinas Consortium. Funding for the medical writing support, the creation and publication of digital features, and the Rapid Service and Open Access fee was provided by Sanofi and Regeneron Pharmaceuticals Inc. in accordance with Good Publication Practice.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Zachary M. Soler: Lyra, Novartis, Olympus, and Optinose – advisory board and/or consultant; GlaxoSmithKline, Regeneron Pharmaceuticals Inc., and Sanofi – speakers’ fees; Healthy Humming – Medical Director. Scott Nash, Harry Sacks: Regeneron Pharmaceuticals Inc. – employees, may hold stock and/or stock options in the company. Andrew P. Lane: Regeneron Pharmaceuticals Inc. and Sanofi – advisory board member. Zara M. Patel: Dianosic, InfiniteMD, Mediflix, Medtronic, and Optinose – advisory board and/or consultant; Olfera Therapeutics – Chief Medical Officer. Stella E. Lee: AstraZeneca, Genentech, GlaxoSmithKline, Lyra Therapeutics, Optinose, Regeneron Pharmaceuticals Inc. and Sanofi – clinical trial funding and advisory boards. Wytske J. Fokkens: BioInspire Technologies, GlaxoSmithKline, Meda Pharmaceuticals, and Sanofi – research grants. Mark Corbett, Juby A. Jacob-Nara: Sanofi − employees, may hold stock and/or stock options in the company.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152:S1-s39. [DOI] [PubMed] [Google Scholar]
- 2.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macchi A, Giorli A, Cantone E, et al. Sense of smell in chronic rhinosinusitis: a multicentric study on 811 patients. Front Allergy. 2023;4:1083964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54:1–30. [DOI] [PubMed] [Google Scholar]
- 5.Hellings PW. SWOT analysis of chronic rhinosinusitis care anno 2022. J Allergy Clin Immunol Pract. 2022;10:1468–71. [DOI] [PubMed] [Google Scholar]
- 6.Breiteneder H, Peng YQ, Agache I, et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy. 2020;75:3039–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor-Clark T. Histamine in allergic rhinitis. Adv Exp Med Biol. 2010;709:33–41. [DOI] [PubMed] [Google Scholar]
- 8.Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11:213–739. [DOI] [PubMed] [Google Scholar]
- 9.Vlaminck S, Acke F, Scadding GK, Lambrecht BN, Gevaert P. Pathophysiological and clinical aspects of chronic rhinosinusitis: current concepts. Front Allergy. 2021;2: 741788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullol J, Marino-Sanchez F, Valls M, Alobid I, Marin C. The sense of smell in chronic rhinosinusitis. J Allergy Clin Immunol. 2020;145:773–6. [DOI] [PubMed] [Google Scholar]
- 11.Seah JJ, Thong M, Wang Y. The diagnostic and prognostic role of biomarkers in chronic rhinosinusitis. Diagnostics (Basel). 2023;13:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saraswathula A, Liu MM, Kulaga H, Lane AP. Chronic interleukin-13 expression in mouse olfactory mucosa results in regional aneuronal epithelium. Int Forum Allergy Rhinol. 2023;13:230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara Y, Jha MK, Mattoo H, et al. Interleukin 4 directly activates olfactory neurons and induces loss of smell in mice. J Allergy Clin Immunol. 2023;151:128.36154846 [Google Scholar]
- 14.Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life–an updated review. Chem Senses. 2014;39:185–94. [DOI] [PubMed] [Google Scholar]
- 15.Dudine L, Canaletti C, Giudici F, et al. Investigation on the loss of taste and smell and consequent psychological effects: a cross-sectional study on healthcare workers who contracted the COVID-19 infection. Front Public Health. 2021;9:666442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simopoulos E, Katotomichelakis M, Gouveris H, et al. Olfaction-associated quality of life in chronic rhinosinusitis: adaptation and validation of an olfaction-specific questionnaire. Laryngoscope. 2012;122:1450–4. [DOI] [PubMed] [Google Scholar]
- 17.Erskine SE, Hopkins C, Clark A, et al. Chronic rhinosinusitis and mood disturbance. Rhinology. 2017;55:113–9. [DOI] [PubMed] [Google Scholar]
- 18.Rombaux P, Huart C, Levie P, Cingi C, Hummel T. Olfaction in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2016;16:41. [DOI] [PubMed] [Google Scholar]
- 19.Kern RC, Quinn B, Rosseau G, Farbman AI. Post-traumatic olfactory dysfunction. Laryngoscope. 2000;110:2106–9. [DOI] [PubMed] [Google Scholar]
- 20.Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58:1–464. [DOI] [PubMed] [Google Scholar]
- 21.Helman SN, Barrow E, Edwards T, et al. The role of allergic rhinitis in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2020;40:201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fokkens WJ, Lund V, Bachert C, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019;74:2312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrobel BB, Leopold DA. Clinical assessment of patients with smell and taste disorders. Otolaryngol Clin North Am. 2004;37:1127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–8. [DOI] [PubMed] [Google Scholar]
- 25.Wolfensberger M, Schnieper I, Welge-Lussen A. Sniffin’Sticks: a new olfactory test battery. Acta Otolaryngol. 2000;120:303–6. [DOI] [PubMed] [Google Scholar]
- 26.Khan AH, Reaney M, Guillemin I, et al. Development of Sinonasal Outcome Test (SNOT-22) domains in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2022;132:933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morley AD, Sharp HR. A review of sinonasal outcome scoring systems - which is best? Clin Otolaryngol. 2006;31:103–9. [DOI] [PubMed] [Google Scholar]
- 28.Mattos JL, Schlosser RJ, Mace JC, Smith TL, Soler ZM. Establishing the minimal clinically important difference for the questionnaire of olfactory disorders. Int Forum Allergy Rhinol. 2018;8:1041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong SS, Chen T, Nguyen SA, Edwards TS, Schlosser RJ. Correlation of polyp grading scales with patient symptom scores and olfaction in chronic rhinosinusitis: a systematic review and meta-analysis. Rhinology. 2022. 10.4193/Rhin22.011. [DOI] [PubMed] [Google Scholar]
- 30.Schlosser RJ, Smith TL, Mace JC, et al. Factors driving olfactory loss in patients with chronic rhinosinusitis: a case control study. Int Forum Allergy Rhinol. 2020;10:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SE, Hopkins C, Mullol J, et al. Dupilumab improves health related quality of life: results from the phase 3 SINUS studies. Allergy. 2022;77:2211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakhshaee M, Sharifian MR, Ghazizadeh AH, Nahid K, Jalaeian Samani K. Smell decline as a good predictor of sinonasal polyposis recurrence after endoscopic surgery. Iran J Otorhinolaryngol. 2016;28:125–34. [PMC free article] [PubMed] [Google Scholar]
- 33.Deutsch PG, Lord S, Salamat S, Jolly K. The management of chronic rhinosinusitis in primary care: an evidence-based guide. Br J Gen Pract. 2019;69:44–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han JK, Bosso JV, Cho SH, et al. Multidisciplinary consensus on a stepwise treatment algorithm for management of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2021;11:1407–16. [DOI] [PubMed] [Google Scholar]
- 35.Ramkumar SP, Lal D, Miglani A. Considerations for shared decision-making in treatment of chronic rhinosinusitis with nasal polyps. Front Allergy. 2023;4:1137907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogdanov V, Walliczek-Dworschak U, Whitcroft KL, Landis BN, Hummel T. Response to glucocorticosteroids predicts olfactory outcome after ESS in chronic rhinosinusitis. Laryngoscope. 2020;130:1616–21. [DOI] [PubMed] [Google Scholar]
- 37.Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201:276–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeConde AS, Mace JC, Alt JA, et al. Investigation of change in cardinal symptoms of chronic rhinosinusitis after surgical or ongoing medical management. Int Forum Allergy Rhinol. 2015;5:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey RJ, Snidvongs K, Kalish LH, Oakley GM, Sacks R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double-blinded placebo-controlled trial for chronic rhinosinusitis after sinus surgery. Int Forum Allergy Rhinol. 2018;8:461–70. [DOI] [PubMed] [Google Scholar]
- 42.Hellings PW, Verhoeven E, Fokkens WJ. State-of-the-art overview on biological treatment for CRSwNP. Rhinology. 2021;59:151–63. [DOI] [PubMed] [Google Scholar]
- 43.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–50. [DOI] [PubMed] [Google Scholar]
- 44.Mullol J, Bachert C, Amin N, et al. Olfactory outcomes with dupilumab in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2022;10:1086-95.e5. [DOI] [PubMed] [Google Scholar]
- 45.Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146:595–605. [DOI] [PubMed] [Google Scholar]
- 46.Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:1141–53. [DOI] [PubMed] [Google Scholar]
- 47.Miglani A, Soler ZM, Smith TL, Mace JC, Schlosser RJ. A comparative analysis of endoscopic sinus surgery versus biologics for treatment of chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2023;13:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai S, Xu S, Lou H, Zhang L. Comparison of different biologics for treating chronic rhinosinusitis with nasal polyps: a network analysis. J Allergy Clin Immunol Pract. 2022;10:1876-86.e7. [DOI] [PubMed] [Google Scholar]
- 49.Dharmarajan H, Falade O, Lee SE, Wang EW. Outcomes of dupilumab treatment versus endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2022;12:986–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.