Abstract
The goal of this study was to compare the advantages of conventional laparoscopic surgery (CLS) and the split-leg supine position single-port plus one laparoscopic surgery (SSP SILS + 1) in esophageal surgery. This study enrolled 73 patients who previously underwent radical esophagectomy for esophageal cancer from August 2021 to February 2023. Among them, 36 patients underwent SSP SILS + 1, whereas the remaining 37 patients underwentCLS. Surgical time, bleeding volume, number of dissected lymph nodes, incision length, and postoperative abdominal pain score between the two groups of patients were compared using either the Student’s t-test or chi-square test. Time of abdominal incision (1.4 ± 0.2 min vs. 5.2 ± 0.7 min, p < 0.001) was shorter in patients in the SSP SILS + 1 group compared with those in the CLS group. However, the average incision length was shorter in the SSP SILS + 1 group compared with that in the CLS group (35.4 ± 4.0 cm vs. 4.6 ± 4.1 cm, p < 0.001). Notably, the pain score on postoperative day (POD) 1 was lower in the SSP SILS + 1 group compared with that in the CLS group (5.7 ± 0.7 vs.6.3 ± 0.7, p = 0.001). The SCAR score was also lower in the SSP SILS + 1 group compared with that in the CLS group (3.5 ± 0.9 vs. 8.3 ± 1.4, p = 0.019). There was no significant difference in the number of dissected abdominal lymph nodes and positive lymph nodes (p > 0.01) between the two groups. The SSP SILS + 1 intervention offers multiple benefits over conventional surgical procedures, encompassing shorter incision length and pain scores on POD 1. In accelerated rehabilitation surgery for esophageal cancer, this surgical procedure demonstrated high safety, feasibility.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78837-x.
Keywords: Split-leg supine position, Single-port plus one laparoscopic surgery, Esophageal cancer
Subject terms: Cancer therapy, Gastrointestinal cancer
Introduction
Esophageal cancer is the sixth most prevalent malignant tumor globally. At present, China has the highest incidence rate and mortality attributed to esophageal cancer1. Esophagectomy is currently the gold standard for the treatment of resectable esophageal cancers, whilst minimally invasive surgery is the preferred surgical treatment. However, the minimally invasive McKeown esophagectomy involves a surgical incision approximately 6–8 cm long and four puncture holes.Is it possible to reduce the size of the incision for the abdominal surgery? A literature review reported an approach, namely single incision laparoscopic surgery plus one port (SILS + 1), that can be applied for various surgeries, especially gallbladder, colorectal, appendix, and gynecological surgeries2–5. Nevertheless, the use of the procedure for upper abdominal tumors remains in its infancy. As a minimally invasive alternative to traditional laparoscopic surgery, SILS + 1 is becoming increasingly popular among surgeons and patients, and relevant safety studies are ongoing6,7 to evaluate its safety, effectiveness, and long-term effects. There are currently relevant studies that have explored the application of SILS + 1 in gastric surgery8 as well as for esophagectomy9. Nonetheless, this study revealed that the position and number of surgeons were similar to conventional laparoscopic surgery (CLS), with the sole improvement being in the incision length. Can this surgical procedure be optimized so that only two surgeons can perform the surgical intervention? On the premise of minimally invasive esophageal surgery, the split-leg supine position single-port plus one laparoscopic surgery (SSP SILS + 1) procedure was pioneered in this study. More specifically, our goal was to compare the advantages of CLS and SSP SILS + 1 in esophageal surgery.
Methods
This study included a total of 73 patients who underwent radical resection of esophageal cancer at the Affiliated Cancer Hospital of Zhengzhou University from August 2021 to February 2023, comprising 40 males and 33 females. The baseline demographics, preoperative chest, and upper abdominal contrast-enhanced CT, as well as results of gastroscopy, pathological examination, and laboratory examination, were collected to confirm the diagnosis of esophageal squamous cell carcinoma. Prior to treatment, multidisciplinary consultations were initiated. Among the enrolled patients, 36 cases underwent SSP SILS + 1, whereas 37 cases underwent CLS. The surgical procedure of both groups of patients was executed by the same surgical team. This study was conducted in accordance with the Declaration of Helsinki (2013 Edition) and was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University (ethical number: 2023 − 404). Informed consent was obtained from all subjects, and the consent of patients was also obtained when obtaining photos of patients, and the photos did not disclose their personal information.
Surgical procedure: All patients underwent McKeown minimally invasive esophagectomy (MIE) combined with lymphadenectomy. We didn’t have a specific choice method to decide which procedure to perform. Sometimes we talk to patients about which surgery to perform, and sometimes patients propose what kind of procedure to perform based on what they see and hear.
Chest procedure
The patient was first positioned in the left lateral decubitus position. Then, an incision was made at the seventh intercostal space along the right anterior axillary line. The incision made at the 4th intercostal space along the right anterior axillary line served as the primary operative site, whilst the incision made at the 4th intercostal space along the posterior axillary line served as an auxiliary operating field. A second auxiliary field was established by making an incision at the 9th intercostal space along the posterior axillary line. Thereafter, the thoracic esophagus was mobilized, and mediastinal lymph nodes (LNs), including left/right laryngeal recurrent nerve lymph nodes, were dissected.
Abdominal procedure
SSP SILS + 1
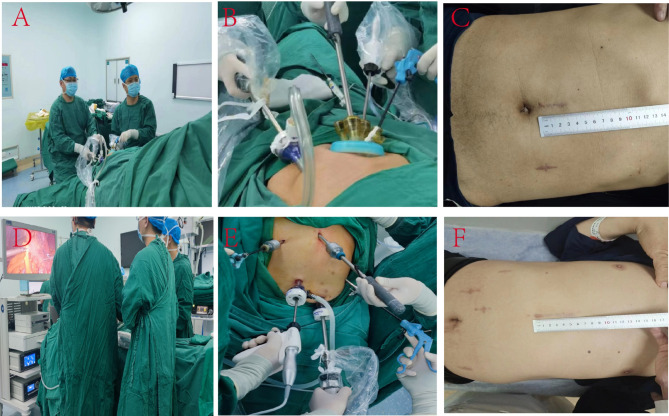
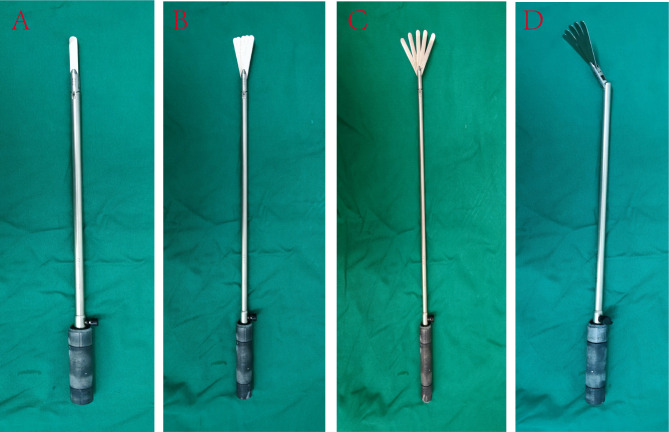
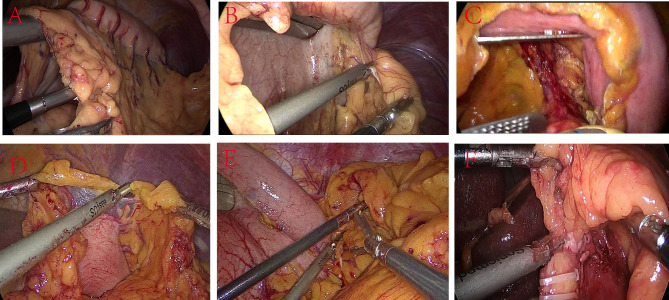
The patient assumed a supine position with split legs, with the head and feet elevated at 35° and a rightward tilt of 25°. The chief surgeon was positioned on the patient’s right side while the assistant stood between the patient’s legs (Fig. 1A). Next, a multi-channel single-hole puncture device, with an opening of approximately 3–4 cm above the umbilicus, was introduced to induce pneumoperitoneum (two 12 mm and two 5 mm operating holes), and a puncture device with a diameter of 12 mm was placed at the level of the umbilicus along the left mid-clavicular line to serve as the endoscopic observation hole. The multi-channel single-hole puncture device was then adjusted to puncture a 12 mm operating hole at the bottom left and top right sides (Fig. 1B), with the 12 mm operating hole located at the bottom right designated as the main operating site for the primary instruments and the upper right hole as an auxiliary hole for the chief surgeon (Fig. 1B and C). Afterward, five-leaf fan-shaped forceps (FLFSF) (Fig. 2) was inserted into the abdominal cavity through the 12 mm operating hole on the upper left side, and the greater curvature was retracted to allow the chief surgeon to expose the greater omentum while avoiding contact with the free-hanging greater omentum surrounding the gastric artery. After the gastric omentum was exposed, the FLFSF were gently introduced into the gastric omental gap, and the greater curvature was lifted to fully expose the gastric omentum gap. After disconnecting the greater omentum (Fig. 3A), the splenic area was dissected, the short gastric artery was clamped with a Hemlock clamp and subsequently ligated, and the left diaphragm hiatus was exposed (Fig. 3B). The FLFSF was then placed on the gastric fundus to lift the stomach and fully expose the left gastric artery (Fig. 3C). Then, the left gastric lymph nodes were dissected, and the left gastric vessels were excised, followed by the ligation of the left gastric artery (Fig. 3C) (Video 1). Subsequently, FLFSF was inserted under the liver, which was elevated to expose the lesser sac until reaching the diaphragm hiatus. Then, the diaphragm hiatus was fully exposed while avoiding diaphragmatic rupture. Afterward, the lesser sac was exposed to the pylorus. Then, FLFSF was used to place the greater curvature through the fundus to elevate the greater curvature. The chief surgeon avoided opening the gastric omentum artery arch between the omentum and detached the greater omentum towards the pylorus. Bleeding sites were cauterized, and then the stomach was extracted from the body for tubular stomach making. We use 3–4 staplers to make the gastric tube, starting from the lesser curvature of gastric antrum to the gastric fundus, with the gastric tube width was approximately 3 cm. Then, we use barbed suture to continuously suture the cutting edge.
Fig. 1.
SSP SILS + 1 compared to CLS: position of the physicians and surgical instruments, and wound healing at the 1-month postoperative follow-up. (A–C) SSP SILS + 1; D-F: CLS. A: The patient was placed in the SSP, with the surgeon positioned on the right side of the patient, the assistant positioned between the two legs. This procedure could be performed with only 2 surgeons; (B) The arrangement of surgical instruments and incision sites during the operation, with the primary operating area situated above the umbilicus and the entry hole located at the level of the left mid-clavicular line; (C) At the one-month post-operative follow-up, two scars at the position of the hole and incision can be observed, and the size of the main operating hole is roughly 3 cm; (D) The standing position of the three operators during the CLS intervention; (E) The relative position of various instruments during CLS intervention; (F) A follow-up examination was conducted one month post-operatively, and the healing rate of the wounds was evaluated. The main operating hole was approximately 6 cm long, with four additional hole incisions.
Fig. 2.
The different deformable situations of the FLFSF, the shape could be changed according to the needs of different surgical situations.
Fig. 3.
Comparison of view and instrument placement between SSP SILS + 1 and CLS. A-C: SSP SILS + 1; D-F: CLS. (A) Upon exposing the greater omentum, the FLFSF was placed posteriorly to the stomach to lift the stomach through the omental opening, while also pulling the omentum to expose the surgical field; this maneuver facilitated the use of the ultrasonic scalpel to detach the omentum; (B) FLFSF were used to lift the stomach in the upper right direction, thereby exposing the short gastric vessels; (C) After ligating the left gastric artery, FLFSF was used to lift the stomach, stretch and expose the left gastric artery, which was excised with a linear cutting stapler; (D) The surgeon and assistant collaborated to disconnect the omentum with the assistance of the first assistant; this procedure involved the coordinated effort of three surgons; (E) Routine five hole exposure of severed gastric short blood vessels requiring an assistant to use FLFSF to individually lift the liver to expose the surgical field; (F) The CLS procedure exposing the severed left gastric blood vessel, while an assistant using FLFSF to individually lift the liver to expose the surgical field.
CLS
The patient was positioned in the supine position, with the chief surgeon and the first assistant standing on the right side of the patient and the other assistant standing on the left side of the patient (Fig. 1D). Next, a 12 mm incision was made adjacent to the umbilicus, followed by the insertion of a pneumoperitoneum needle to establish the pneumoperitoneum. Then, a 12 mm puncture device was placed in the observation hole to visualize signs of fluid accumulation or macroscopic metastasis in the abdominal cavity. A 5 mm puncture device was placed at the right mid-clavicular line along the costal margin. Meanwhile, a 12 mm puncture device was inserted at the umbilical plane of the left mid-clavicular line at the lower border of the xiphoid process and at the umbilical plane of the right mid-clavicular line (Fig. 1E and F). Afterward, disconnecting the greater omentum (Fig. 3D). The greater omentum was detached from the diaphragmatic hiatus (Fig. 3E), and lymph nodes in the left gastric cardia were dissected, the chief surgeon uses forceps to press the pancreas, which can expose and remove lymph nodes adjacent to the common hepatic artery. Then, the left gastric vessels were ligated (Fig. 3F), and FLFSF was placed through the trocar under the xiphoid process to elevate the liver to expose the lesser sac. Then an incision was extended at the lower edge of the xiphoid process by approximately 5–6 cm. The stomach was extracted from the body for tubular stomach making. The method of making gastric tubes is the same as that of SSP SILS + 1.
Neck procedure
An incision was made on the inner edge of the left side of the sternocleidomastoid muscle, the cervical esophagus was exposed, and the tubular stomach was moved from the body to the neck through the esophageal bed. Lastly, a double-layer anastomosis was created between the esophagus and the tubular stomach.
The time of abdominal incision referred to the duration from the skin incision to the initiation of abdominal surgery when the instrument could be inserted. The abdominal surgery time referred to the duration from the skin incision to the formation of the tubular stomach. Abdominal incision length was the cumulative length of all incisions in the abdominal area. Abdominal bleeding volume denoted the amount of blood in the negative pressure drainage device throughout the abdominal surgery, as well as the amount of blood-stained gauze during surgery (calculated as 5 mL of blood per gauze, we conducted preliminary experiments and found that when the gauze was soaked and unable to squeeze out water, the water absorption was 5 ml). The postoperative abdominal pain score was based on the visual analysis scale (VAS) pain score standard, and the pain score was recorded at intervals of 6 h on the first-, third-, and fifth-days post-surgery, with the highest pain score recorded.
Abdominal incision time, abdominal surgery time, abdominal bleeding volume, number of abdominal lymph node dissection, postoperative pain score, postoperative mortality rate, and length of hospital stay between the two groups were compared.
Statistical analysis
Regarding statistical analysis, the Pearson χ2 test, Fisher’s exact test, or Kruskal Wallis test was used for inter-group comparison. Quantitative data were expressed as mean ± standard deviation, whereas categorical variables were presented as numbers (percentages). A two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using R software (version 4.3.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
The baseline data of the two groups of patients are listed in Table 1. Notably, gender, age, BMI, tumor location, and tumor stage were comparable between the two groups (p > 0.05). On the other hand, a significant difference was identified in the pain score on postoperative day (POD) 1 between the two groups (5.7 ± 0.7 vs. 6.3 ± 0.7, p = 0.001, Table 2). However, there were no statistically significant differences in the pain score between the two groups on the remaining days. As anticipated, time of abdominal incision (1.4 ± 0.2 min vs. 5.2 ± 0.7 min, p < 0.001), abdominal incision length (35.4 ± 4.0 vs. 74.6 ± 4.1, p < 0.001), the SCAR score (3.5 ± 0.9 vs. 8.3 ± 1.4, p = 0.019) were lower in patients in the SSP SILS + 1 group compared with those in the CLS group, as summarized in Table 2. Contrastingly, there was no significant difference between the two groups in the number of dissected abdominal lymph nodes and the number of positive lymph nodes. Likewise, there was no statistical difference between the two groups in the incidence of postoperative complications (52.8% vs. 43.2, p = 0.415), anastomotic fistula (8.3% vs. 10.8%, p = 0.719), and abdominal complications (0% vs. 5.4%, p = 0.157). As summarized in Table 3, there was no statistical difference in postoperative hospitalization time, the incidence of hiatal hernia of esophagus, abdominal incisional hernia and mortality rate between the two groups.
Table 1.
Comparison of baseline data between the SSP SILS + 1 and CLS groups.
| Characteristics | SSP SILS + 1 | CLS | P value |
|---|---|---|---|
| Gender | |||
| Male | 20 | 22 | 0.736 |
| Female | 16 | 15 | |
| Age (year) | |||
| ≤ 65 | 13 | 20 | 0.124 |
| > 65 | 23 | 17 | |
| Hypertension | 0.530 | ||
| Yes | 9 | 7 | |
| No | 27 | 30 | |
| Diabetes | 0.689 | ||
| Yes | 5 | 4 | |
| No | 31 | 33 | |
| Drink | 0.360 | ||
| Yes | 10 | 14 | |
| No | 26 | 23 | |
| Smoking | 0.223 | ||
| Yes | 8 | 13 | |
| No | 28 | 24 | |
| BMI | 22.9 ± 2.8 | 23.6 ± 3.3 | 0.172 |
| Tumor location: upper/middle/lower | 5/23/8 | 10/14/13 | 0.081 |
| T: 0/1/2/3/4 | 7/6/10/13/0 | 8/9/5/15/0 | 0.482 |
| N: 0/1/2/3 | 24/8/3/1 | 19/11/5/2 | 0.599 |
| TNM stage: 0/1/2/3/4A/X | 6/9/10/10/1/0 | 5/7/8/13/2/2 | 0.548 |
Table 2.
Comparison of surgical-related indicators between the SSP SILS + 1 and CLS groups.
| Characteristics | SSP SILS + 1 | CLS | P value |
|---|---|---|---|
| Time of abdominal incision (min) | 1.4 ± 0.2 | 5.2 ± 0.7 | < 0.001 |
| Abdominal surgery time (min) | 41.9 ± 8.4 | 41.1 ± 4.5 | 0.614 |
| Abdominal incision length (mm) | 35.4 ± 4.0 | 74.6 ± 4.1 | < 0.001 |
| Abdominal bleeding volume (mL) | 7.0 ± 3.0 | 8.0 ± 4.0 | 0.532 |
| Number of dissected abdominal lymph nodes | 10.0 ± 4.5 | 9.2 ± 5.3 | 0.502 |
| Station number of abdominal lymph node dissection | 4.4 ± 0.7 | 4.4 ± 0.9 | 0.936 |
| Number of positive lymph nodes | 0.6 ± 1.1 | 0.6 ± 1.3 | 0.888 |
| Maximum pain score (POD 1) | 5.7 ± 0.7 | 6.3 ± 0.7 | 0.001 |
| Maximum pain score (POD 3) | 4.3 ± 0.6 | 4.4 ± 0.7 | 0.386 |
| Maximum pain score (POD 5) | 2.7 ± 0.8 | 3.0 ± 0.9 | 0.193 |
| SCAR score | 3.5 ± 0.9 | 8.3 ± 1.4 | 0.019 |
Table 3.
Comparison of postoperative complications and mortality rate between the SSP SILS + 1 and CLS groups.
| Characters | SSP SILS + 1 | CLS | P value |
|---|---|---|---|
| Total postoperative complications | 19 (52.8%) | 16 (43.2%) | 0.415 |
| severe complications (Clavien-Dindo ≥ 3) | 3 (8.3%) | 4 (10.8%) | 0.719 |
| Postoperative pulmonary complications | 1 (2.8%) | 4 (10.8%) | 0.174 |
| Pleural effusion | 5 (13.9%) | 8 (21.6%) | 1.000 |
| Anastomotic fistula | 3 (8.3%) | 4 (10.8%) | 0.719 |
| Total abdominal complications | 0 (0%) | 2 (5.4%) | 0.157 |
| Abdominal incision-related complications | 0 (0%) | 1 (2.7%) | 0.321 |
| Abdominal infection | 0 (0%) | 0 (0%) | NA |
| Ascites | 0 (0%) | 0 (0%) | NA |
| Abdominal bleeding | 0 (0%) | 0 (0%) | NA |
| Abdominal incision fat liquefaction | 0 (0%) | 1 (2.7%) | 0.321 |
| Abdominal wound dehiscence | 0 (0%) | 0 (0%) | NA |
| Bleeding at the abdominal incision site | 0 (0%) | 0 (0%) | NA |
| Intestinal obstruction | 0 (0%) | 0 (0%) | NA |
| Total postoperative complications (Clavien-Dindo grade) | |||
| I | 11 (30.6%) | 5 (13.5%) | 0.078 |
| II | 5 (13.9%) | 8 (21.6%) | 0.388 |
| III | 3 (8.3%) | 1 (2.7%) | 0.291 |
| IV | 0 (0%) | 3 (8.1%) | 0.081 |
| V | 0 (0%) | 0 (0%) | NA |
| Hospital stay | 18.33 ± 10.41 | 15.76 ± 8.53 | 0.25 |
| Hiatal hernia of esophagus | 0 (0%) | 0 (0%) | NA |
| Abdominal incisional hernia | 0 (0%) | 0 (0%) | NA |
| In-hospital mortality rate | 0% | 0% | NA |
| 30 days mortality rate | 0% | 0% | NA |
| 90 days mortality rate | 0% | 0% | NA |
Discussion
This study compared the efficacy and safety of SSP SILS + 1 with CLS, and the results revealed that SSP SILS + 1 was superior to CLS in terms of pain score on POD1, time of abdominal incision, and abdominal bleeding. Nevertheless, there was no statistical difference in the number of dissected lymph nodes, postoperative hospital stays, postoperative complications, and mortality rate between the two groups.
Single-port plus one laparoscopic surgery (SILS + 1) could be applied for various surgeries, especially for gallbladder, appendix, colorectal tumor, and gynecological-related surgeries2–5. Earlier research has reported that SILS + 1 laparoscopic surgery yields similar outcomes to traditional laparoscopic surgery, including short hospital stays, low postoperative pain, and fast recovery time5–7,10−13. Despite some challenges, SILS + 1, as a minimally invasive alternative to traditional laparoscopic, is becoming increasingly popular among surgeons and patients. It has already been performed for esophageal cancer9. Of note, this surgical procedure has been established as a safe and feasible approach for radical esophagectomy. However, this study demonstrated that SILS + 1 only resulted in improvements in the total incision length. By changing the patient’s position, the position of the first assistant could be changed, thereby minimizing mutual interference between the surgeon and the first assistant throughout the surgical modality. In the SSP, the surgeon and the first assistant could sit and perform operations, consequently alleviating the surgeon’s workload. Moreover, the use of FLFSF was optimized. By adjusting the size of the FLFSF, the stomach could be completely removed or lifted to expose the surgical field. More importantly, this maneuver facilitated manual operations, thereby eliminating the necessity for a second assistant, and subsequently limiting additional staff. In the CLS group, the second assistant is required to hold the gripper with one hand and the FLFSF with the other hand. Both upper limbs, especially the right upper limb, remain suspended, posing challenges in maintaining a consistent position for a prolonged time, resulting in displacement of the FLFSF and poor exposure to the surgical field. In the SSP SILS + 1 group, the patient adopted the SSP, and the assistant positioned between the patient’s legs with one hand holding the FLFSF. Using the SILS + 1 approach, the body’s center of gravity remains stable, allowing for a seated and cooperative posture; this mitigated fatigue experienced by the assistant. Additionally, the assistant holds the FLFSF homeopathically, and the patient’s head was elevated while the lower limbs were positioned lower; consequently, the upper limbs were no longer suspended, leading to decreased levels of fatigue. Besides, the CLS intervention typically requires the participation of three surgeons. However, in the SSP SILS + 1 group, only two surgeons are required to perform the abdominal surgery, and the degree of fatigue of the assistant is significantly reduced.
Postoperative pain management plays a vital role in ERAS for esophageal cancer. Reducing postoperative pain in patients could enhance postoperative pulmonary rehabilitation, such as coughing and expectoration, and concurrently lower the incidence of pulmonary complications14–17. Our results exposed that the pain score of patients undergoing SSP SILS + 1 was significantly lower than that of patients undergoing CLS on POD1, possibly attributed to the smaller number and length of incisions. SSP SILS + 1 appears to possess certain advantages in ERAS for esophageal cancer. However, this pain score result may be controversial or compromised owing to the potential influence of postoperative painkillers, which could lead to inaccurate pain assessment for patients. Future studies should aim to address this shortcoming to obtain more credible results.
The SSP SILS + 1 approach was associated with a shorter abdominal incision time compared to CLS. The main incision size of the two group is similar, but CLS has 3 more holes than the SSP SILS + 1, approximately 2–3 cm more, this is the reason why CLS has longer incision than SSP SILS + 1. Of note, the exposed surgical field of SSP SILS + 1 was minimally traumatic to both the stomach and surrounding tissues. Thoracolaparoscopic esophagectomy primarily involves the separation of the stomach and the creation of a tubular stomach via the main operating hole. Besides, the incidence of digestive tract reconstruction-related complications in laparoscopic surgery was relatively low. The SSP SILS + 1 procedure may be indicated for esophageal cancer. During the operation, the assistant chiefly exposed the surgical field by FLFSF, thereby facilitating the operation and reducing the use of other instruments. It is worthwhile emphasizing that the use of FLFSF, which have a large contact area with the stomach, minimized trauma during abdominal surgery and consequently increased surgical safety.
The results uncovered no statistically significant difference in the abdominal surgical duration between the two groups. Ascribed to the smaller incisions, it was more challenging to insert the nasogastric tube, which extended the surgical time. Nevertheless, the CLS approach outperformed the SILS + 1 procedure in terms of simply dissecting the stomach and dissecting lymph nodes surrounding the left gastric artery. During the initial stage of SSP SILS + 1, a nasogastric tube was systematically introduced into every patient to achieve postoperative nasal feeding. In the future, the insertion of the nasogastric tube will be discontinued to allow patients to promptly transition to oral intake to not only decrease the duration of abdominal surgery but also expedite patient recovery.
In addition, abdominal lymph nodes, encompassing those adjacent to the left gastric artery, the paracardial nodes, the gastric lesser curvature lymph nodes, common hepatic artery and splenic nodes, constitute regional lymph nodes of esophageal cancer and can metastasize. Therefore, it is critical to thoroughly dissect these lymph nodes during the intervention. Notwithstanding, our results indicated that there was no significant difference between the two groups in terms of the number of dissected lymph nodes. In other words, the number of dissected lymph nodes was comparable between both approaches.
Furthermore, the length of abdominal incisions is recommended to exceed 3 cm. More specifically, this limitation arises from the need to extract the stomach through the abdominal incision during the creation of the tubular stomach, with the width of the tubular stomach generally measuring about 3 cm. Hence, the minimum length of the abdominal incision should be no less than 3 cm. Moreover, a prevalent approach involves suturing the incision edge following the creation of the tubular stomach. Thus, the width of the incision is typically limited to approximately 3 cm.
The mean SCAR score of patients in the SILS + 1 group was lower than that in the conventional laparoscopic group (p = 0.019).
Limitations
(1) This was a retrospective study with a restricted sample size. (2) It was more challenging to retain the nasogastric tube with a single hole and a small incision. The low opening position, in conjunction with the small incision, complicated nasogastric tube retention. At the beginning of the surgical modality, the incision was extended to retain the nasogastric tube in patients with a high risk of anastomotic leakage; this alteration did not significantly prolong the abdominal surgery time. On the contrary, the time required for dissecting the stomach via the SSP SILS + 1 procedure was significantly lower than that using the CLS method. Post-operatively, the nasogastric tube was removed from most patients, leading to a shorter surgical duration without the need for increasing the incision length.
Conclusion
The SSP SILS + 1 intervention offers multiple benefits over conventional surgical procedures, encompassing shorter surgical time and incision length, and pain scores on POD 1. In accelerated rehabilitation surgery for esophageal cancer, this surgical procedure demonstrated high safety, feasibility.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thanks HOME for Researchers for linguistic advice.
Author contributions
Wang Xin, Yu Yongkui and Xing Wenqun designed the study, Wang Xin, Yu Yongkui, Qin Zimin, Wang Haojie, Xie Jinyi, Wu Yiju, Xu Zihou, Chen Peinan, Liu Qi, Li Haomiao, Wang Zongfei and Xing Wenqun collected the data, Xu Lei, Yu Yongkui, Chen Yongfeng analyzed the data, Wang Xin and Yu Yongkui prepared the Figs. 1, 2, 3, Xu Lei, Yu Yongkui, Chen Yongfeng and Chen Peinan prepared the Tables 1, 2 and 3. Yu Yongkui, Wang Xin, Xu Lei, and Xingwenqun wrote the manuscript. All authors reviewed the manuscript.
Funding
This study was funded by the Henan Province Medical Science and Technology Research Program Joint Construction Project (LHGJ20230082).
Data availability
Data is provided within the supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249. 10.3322/caac.21660 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Keller, D. S. et al. SILS v SILS + 1: a case-matched comparison for colorectal surgery. J. Gastrointest. Surg.19, 1875–1879. 10.1007/s11605-015-2921-1 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Portenkirchner, C., Turina, M. & Rickenbacher, A. Single incision laparoscopic surgery (SILS) versus conventional laparoscopic technique for ileostomy: a retrospective cohort study. Langenbecks Arch. Surg.407, 1757–1763. 10.1007/s00423-022-02473-0 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Zhou, H., Ying, J., Wang, A., Bian, C. & Xiang, H. Laparoscopic intersphincteric resection with a single-incision plus one port for very low rectal cancer - A video vignette. Colorectal Dis.10.1111/codi.16415 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Wang, Y. et al. Short-term outcomes of single-incision plus one-port laparoscopic versus conventional laparoscopic surgery for rectosigmoid cancer: a randomized controlled trial. Surg. Endosc.33, 840–848. 10.1007/s00464-018-6350-6 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zhou, W. et al. Initial experience of single-incision plus one port left-side approach totally laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction. World J. Gastroenterol.26, 4669–4679. 10.3748/wjg.v26.i31.4669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou, H., Bian, C., Wang, A. & Xiang, H. Single-incision plus one port laparoscopic right hemicolectomy with complete mesocolic excision and intracorporeal anastomosis. Tech. Coloproctol. 27, 237–238. 10.1007/s10151-022-02646-5 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Yin, J. H. et al. Feasibility and preliminary experience of single-incision plus one-port laparoscopic total gastrectomy with overlap esophagojejunostomy for gastric cancer: a study of 10 cases. Front. Surg.9, 1071363. 10.3389/fsurg.2022.1071363 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin, N. et al. Comparative study on short-term efficacy of single incision plus one (SI + 1) port and multiportal 3D laparoscopic minimally invasive esophagectomy. J. Gastrointest. Oncol.12, 1277–1284. 10.21037/jgo-21-441 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du, G. S. et al. Single-incision plus one-port laparoscopic gastrectomy versus conventional multi-port laparoscopy-assisted gastrectomy for gastric cancer: a retrospective study. Surg. Endosc. 36, 3298–3307. 10.1007/s00464-021-08643-3 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Katagiri, H. et al. Standardized single-incision plus one-port laparoscopic left lateral sectionectomy: a safe alternative to the conventional procedure. Langenbecks Arch. Surg.407, 1277–1284. 10.1007/s00423-021-02340-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, A., Bian, C. & Zhou, H. Single-incision plus one port laparoscopic low anterior resection for mid-low rectal cancer-a video vignette. Colorectal Dis.24, 674–675. 10.1111/codi.16071 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Zhou, H., Bian, C. & Wang, A. Single incision plus one port laparoscopic left hemicolectomy with intracorporeal anastomosis-a video vignette. Colorectal Dis.10.1111/codi.16355 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Booka, E. et al. The impact of epidural catheter insertion level on pain control after esophagectomy for esophageal cancer. Esophagus17, 175–182. 10.1007/s10388-019-00682-z (2020). [DOI] [PubMed] [Google Scholar]
- 15.Ohkura, Y. et al. A new postoperative pain management (intravenous acetaminophen: Acelio(R)) leads to enhanced recovery after esophagectomy: a propensity score-matched analysis. Surg. Today48, 502–509. 10.1007/s00595-017-1616-5 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Nimmo, S. M., Foo, I. T. H. & Paterson, H. M. Enhanced recovery after surgery: Pain management. J. Surg. Oncol.116, 583–591. 10.1002/jso.24814 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Wang, W. et al. Hand-assisted sputum excretion can effectively reduce postoperative pulmonary complications of esophageal cancer. Ann. Palliat. Med.9, 3721–3730. 10.21037/apm-20-1267 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the supplementary information files.