Abstract
To determine the prevalence of functional, respiratory and renal impairments and of post-intensive-care-syndrome (PICS) among patients who had attended a post-ICU multidisciplinary consultation (post-ICU-MC) around 3 months after ICU discharge, we performed a retrospective, monocentric observational study, at Clermont Ferrand University hospital, France. We included patients who had attended a post-ICU-MC. Their characteristics during ICU stay and at the post-ICU-MC were collected. Functional status was assessed by the 6-min-walking test, handgrip test and peak inspiratory pressure, respiratory function by exploratory functional outcomes, mental status by SF-36 score, and quality of life by SF-36 score and European Quality of Life 5 Dimensions questionnaire. Overall, we enrolled 67 patients, of whom 70%, 74%, and 68% had functional, respiratory, and renal impairments, respectively, at the post-ICU-MC. Additionally, 40%, 28%, 19%, and 2.5% had three, two, one, and none of these impairments, respectively. All patients experienced mental disorders and a decline in quality of life. Functional impairment correlated with frailty score and sex, and respiratory function with age. To conclude, the prevalence of PICS in our cohort was high, as was that of functional, respiratory and renal failure.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78686-8.
Keywords: Long term outcomes, Multidisciplinary consultations, Intensive care
Subject terms: Health care, Medical research
Introduction
Mortality after an ICU stay is tending to decrease owing to advances in critical care medicine despite patients getting older and having more comorbidities and less autonomy1. One of the unfortunate consequences of this better survival rate is an increased number of sequelae among survivors, such as cognitive disorders (impaired memory, executive function, language skills, and spatial and visual attention), physical disorders (muscle weakness, chronic pain) and psychological disorders (depression, anxiety and post-traumatic stress symptoms)2. These post-ICU sequelae collectively constitute what is called the post-intensive care syndrome or PICS. Several tests, scores, scales and questionaries can be used to explore all the component of the PICS (Table S1). The term PICS was coined in 2010 by the Society of Critical Care Medicine, whose aims were to raise awareness among clinicians, patients and the general population of the various impairments that occur during ICU stay and after discharge, increase evaluation of the syndrome and create a new field of research3. Since 2010, several studies have reported data on the prevalence of PICS (Table S2). For instance, in the study of Weidman et al.4, involving a cohort of COVID-19 patients discharged alive from the ICU, the prevalence of PICS reached 90%. The authors defined PICS by the percentage of patients with at least one impairment. Cognition was assessed by the Montreal Cognitive Assessment (MoCA) test, and the psychological status of patients by the Hospital Anxiety and Depression (HAD) scale, the Posttraumatic Stress Syndrome (PTSS-10) questionnaire, or the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5). In another study5 involving 60 patients, the prevalence of PICS was 80%: 47% of the patients were impaired in at least two domains and 20% in all three domains of the PICS spectrum. In this study cognition was assessed by the MoCA test, and the psychological components of PICS by the HAD scale and the Impact of Event Scale-6. Physical function was assessed by the European Quality of Life 5 Dimensions (EuroQoL-5D) survey. Several studies have also shown that PICS is associated with increased risk of death as, for instance, in that of Yanagi et al.6, who found a significant association up to 1 year after ICU discharge.
Since 2018, the Medical Intensive Care Unit of the Clermont-Ferrand University Hospital, France, has referred eligible patients for post-ICU multidisciplinary follow-up consultations (post-ICU-MC) that all took place in a day hospital around 3 months after ICU discharge. Patients had several consultations with different specialists including nephrologists, pulmonologists, nutritionists and intensivists. Different components of PICS were then assessed such as motricity, mental impairment, and health care-related quality of life (HRQoL).
Against this background, the main aim of our study, called the PICS-MIR study, was to assess the prevalence of long-term alteration of the functional status among patients who had attended a post-ICU-MC. We also documented the prevalence of respiratory and renal impairment, deterioration of HRQoL, and PICS in the cohort.
Material and method
Type of study
This study was a monocentric, retrospective, observational study, involving patients who had attended a post-ICU-MC of Clermont-Ferrand University Hospital, from April 2018 to January 2024.
Population
The criteria to propose attendance at a post-ICU-MC were an ICU length of stay (LOS) of at least 4 days, and one of the following criteria: body mass index (BMI) < 19 or > 35 kg/m2 on admission, and/or administration for at least 48 h of a vasopressor, and/or invasive mechanical ventilation (IMV), and/or renal replacement therapy (RRT), and/or age over 80 years, or an ICU LOS of at least 7 days. Only patients who attended the post-ICU-MC were eligible. Patients who refused use of their data were excluded.
Ethical considerations
The PICS-MIR study was approved by the local ethics committee (IRB13412, CHU de Clermont Ferrand IRB #1, IRB number 2023-CF229). Each participant was informed of the possible use of anonymized data for research. The families were given a written informed consent form on ICU admission, and the same written information was displayed in the waiting room of the ward. None of the participants were opposed to use of their data. All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation, whether institutional or regional, and in agreement with the Helsinki Declaration of 1975.
Description of the post-ICU-MC
More details of the post-ICU-MC are given in the supplemental file. Briefly, the post-ICU -MC included nephrology, pneumology, nutrition, physiotherapy and intensivist consultations. Health status was assessed by the Knaus score, the activity daily living (ADL) and instrumental ADL (IADL) scores, and the Medical Outcomes Study Short Form 36 (SF-36). CT-scans, respiratory function tests (RFT), hand grip test (HGT) and 6-minute walking test (6mWT) were performed.
Data collection
We collected data on ICU admission, during ICU stay, and between ICU stay and attendance at the post-ICU-MC. Data were (1) on ICU admission: age, BMI, main comorbidities, autonomy as assessed by the Knauss score, clinical frailty score and place of residence before ICU admission, and baseline severity measured by the Simplified Acute Physiology Score II (SAPS II); (2) during ICU stay, requirement for and duration of oxygen therapy, IMV, vasopressors and RRT, occurrence of nosocomial infections, and ICU LOS; and (3) during the post-ICU-MC, autonomy, health care-related quality of life (HRQoL) evaluated by SF-36 and EuroQoL-5D questionnaires, conclusions of the consultations, and results of the explorations. All the data were extracted from current ICU and hospital patient electronic files.
Outcome measures and definitions
The primary outcome of the study was deterioration of motor function as assessed during the post-ICU-MC by the distance covered during the 6mWT. Age- and sex-normative values were obtained from the reference equations of Enright et al.7, and the distance was defined as low if less than 80% of the expected distance8.
Secondary outcomes were impairment of functional status, respiratory, renal, and mental functions, autonomy, and HRQoL9,10.
Impairment of functional status was defined by a low HGT and/or 6mWT score, and/or a peak inspiratory pressure (PIP) of less than 80% of the theoretical value. A Jamar hydraulic hand dynamometer was used to perform the HGT. The three measurements were made at 30-s intervals between contractions. The best performance was considered for analysis. HGT was considered pathological if < 25 kg in men aged ≤ 60 years and < 23 kg in men between 61 and 79 years, and < 14 kg in women aged ≤ 60 years and < 13 kg in women between 61 and 79 years11.
The SF-36 explored eight items of physical and mental disorders. The physical component summary (PCS) and mental component summary (MCS) scales combine information from all eight domains of SF-36 9,10. An MCS < 50, a PCS < 50, and any of the subcomponents of the SF-36 - physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role-motional (RE), and mental health (MH) - < 50 were considered as pathological. The EQ-5D-5 L was pathological if an item achieved a score of more than or equal to 2.
Impairment of mental function was defined by an MCS score of the SF36 < 50.
Impairment of respiratory function was defined by a decrease in one of the indices obtained by respiratory functional tests (< 80% theoretical value), including full expiratory volume (FEV) per second, and/or forced vital capacity (FVC), and/or diffusion coefficient of CO (DLCO).
Impairment of renal function was defined by a glomerular filtration rate (GFR) < 45 mL/min/1.73m2 and/or GFR decline > 5 mL/min/1.73m2.
Patients had PICS if they presented mental and/or functional impairments.
Nutritional status was assessed by BMI measurement, and weight loss between ICU admission and post-ICU-MC.
Statistical analysis
Patient characteristics were expressed in numbers (percentage) for categorical variables and median (interquartile range [IQR]) for continuous variables. Comparisons were made with exact Fisher tests for categorical variables and Wilcoxon tests for continuous variables.
To identify factors associated with HGT, distance and PIP, these variables were first dichotomized using a median. Correlations between measurements and these outcomes were then assessed by Spearman correlation coefficient. For every test, a two-sided α of 0.05 was considered as significant. Missing variables were handled by the median. All statistical analyses were performed with SAS software, Version 9.4 (SAS Institute, Cary, NC) and R (version 3.6.3).
Results
Characteristics of the cohort during hospitalization
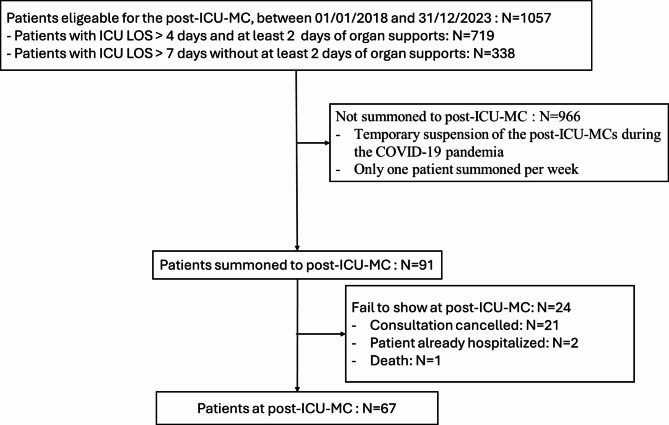
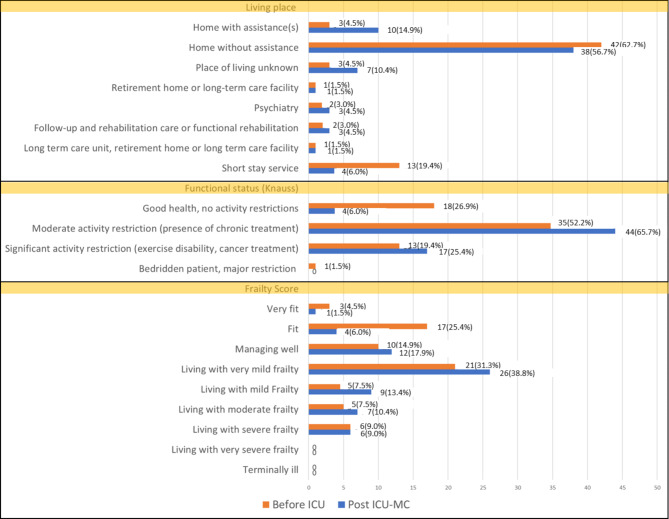
Between April 2018 and December 2023, 67 patients were enrolled in the PICS-MIR study (Fig. 1). Most of the patients hospitalized in the ICU came either directly from home without (42 patients) or with assistance (3 patients), or from a short-stay facility (13 patients) (Fig. 2). The characteristics of the patients are given in Table 1. Their median age was 64 years [55.3; 71.8], and 62.7% were male. The median BMI was 27.4 kg/m² [22.6–34.6]. Their main comorbidities were chronic cardiovascular disease (38.8%) and diabetes (23.9%). Their median SAPS II was 40 [30–57]. Overall, 42 patients (62.7%) were mechanically ventilated, and 43 (64.2%) and 21 (31.3%), respectively, required vasopressors and RRT. The median ICU LOS was 12 days [7–25].
Fig. 1.
Flow chart. Post ICU MC: post intensive care multidisciplinary consultations; ICU LOS: intensive care unit length of stay.
Fig. 2.
Distribution of the patients depending on their place of residence, functional status and frailty score before ICU and at the post-ICU multidisciplinary consultations. ICU: Intensive care unit; Post-ICU-MC: post intensive care multidisciplinary consultations.
Table 1.
Patients’ demographics and characteristics at ICU admission and characteristics of their ICU stay.
| Variables | N (%) or median (IQR) |
|---|---|
| Number of patients | 67 |
| Characteristics on admission | |
| Age | 63.91 [55.3; 71.8] |
| Gender (male) | 42 (62.7) |
| BMI (kg/m²) | 27.38 [22.6; 34.6] |
| Main comorbidities | |
| Chronic Cardiovascular Disease | 26 (38.8) |
| Chronic Respiratory Failure | 6 (9.0) |
| Long-term oxygen therapy | 3/6 (50) |
| NIV or CPAP | 3/6 (50) |
| Chronic Kidney Disease | 7 (10.5) |
| Hemodialysis | 3/7 (42.9) |
| Chronic Liver Failure | 1 (1.5) |
| Immunosuppression | 5 (7.5) |
| Diabetes | 16 (23.9) |
| Characteristics of ICU stay | |
| Medical motif of admission | 64 (95.5) |
| SAPS II score | 40 [30; 57] |
| IMV | 42 (62.7) |
| Duration of IMV (days) | 3 [0; 16] |
| Oxygen administration | 67(100) |
| Duration of oxygen administration (days) | 10 [5; 18] |
| Administration of blocking agent | 24 (35.8) |
| RRT | 21 (31.3) |
| Duration of RRT | 9 [3; 17] |
| Vasopressors | 43 (64.2) |
| Duration of vasopressors | 6 [3; 12] |
| Steroids | 34 (50.8) |
| Duration of steroids (days) | 7 [5; 14] |
| Nosocomial infections | 15 (22.4) |
| Surgery | 6 (9.0) |
| Limitation of care | 9 (13.4) |
| ICU LOS (days) | 12 [7; 25] |
| Hospital LOS (days) | 27 [12; 43] |
| Main motif of inclusion | |
| ICU LOS ≥ 4 days and | |
| BMI < 19 or > 35 (kg/m2) or | 24 (35.8) |
| Vasopressors ≥ 2 days or | 36 (53.7) |
| IMV for ≥ 2 days | 39 (58.2) |
| RRT ≥ 2 days | 16 (23.9) |
| ICU LOS ≥ 7 days | 15 (22.4) |
| ICU LOS > 2 days and age > 80 years | 1 (1.5) |
BMI = body mass index; NIV = Non-invasive ventilation; CPAP = Continuous Positive Airway Pressure ; ICU = intensive care unit ; SAPS II: IMV = invasive mechanical ventilation; RRT = Renal replacement therapy; LOS = Length of stay.
Characteristics of the cohort at the post-ICU-MC
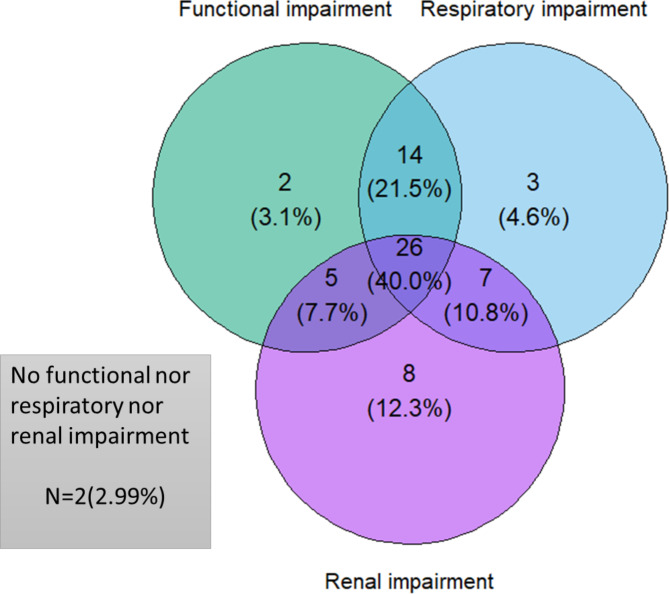
The median time from ICU discharge to post-ICU-MC was 164 days [117–235] (Table 2). At post-ICU-MC, only two patients had no functional, no respiratory and no renal impairments (Fig. 3).
Table 2.
Characteristics of the patients at the post-ICU-multidisciplinary consultations.
| Variables | N (%) or median [IQR] |
|---|---|
| Time from ICU discharge to post-ICU-MC (days) | 164 [117; 235] |
| Time from hospital discharge to post-ICU-MC (days) | 146 [90; 210] |
| Nephrological assessment | 58 (86.6) |
| Renal replacement therapy | 2/58 (3.5) |
| Indication for nephrology consultation | |
| GFR < 45 mL/min/1.73m2 | 12/57 (21.1) |
| GFR decline > 5 mL/min/1.73m2 | 42/57 (73.7) |
| Respiratory assessment | 53 (79.1) |
| Long-term oxygen therapy | 3/53 (5.7) |
| NIV or CPAP | 4/53 (6.0) |
| Respiratory Functional Investigation (Raw value - value < 80% th) | 62 (92.5) |
| Th FEV1 (%) | 83 [72; 100] − 28/62 (45.2) |
| Th FVC (%) | 98 [82; 113] − 12/62 (19.4) |
| Th TLC (%) | 106.5 [92; 114] − 7/60 (11.7) |
| Th DLCO (%) (missing data = 3) | 63 [47; 74] − 47/55 (85.5) |
| Th KCO (%) (missing data = 3) | 80 [65; 91] − 27/55 (49.1) |
| Th PIP (%) | 80.2 [57.6; 87.8] − 31/62 (50) |
| Obstructive syndrome (FEV/CPT < 80%) | 6/62 (9.7) |
| Desaturation during the 6-MWT | 17/56 (30.4) |
| Nutritional assessment | |
| Weight loss (%) - > 5% | 1.47 [-3.3; 6.1] − 25 (37.3) |
| BMI (kg/m²) | 27.1 [23.0; 31.1] |
| Albumin (g/L) - Albumin < (30 g/L) (missing data = 3) | 40 [37.4; 43] − 2/62 (3.2) |
| Pre-albumin (g/L) - < 0.2 (g/L) (missing data = 7) | 0.26 [0.22; 0.32] − 8/57 (14.0) |
| CRP (mg/L) - > 15 (mg/L) (missing data = 7) | 3.1 [1.7; 8.4] − 7/59 (11.9) |
| Functional assessment | |
| Handgrip score (kg) (missing data = 5) | 24.5 [20.3; 31.4] |
| Low hand grip test* (missing data = 5) | 7/61 (11.5) |
| Distance at the 6mWT (m) (missing data = 6) | 398.5 [268.3; 494.5] |
| Th distance at the 6mWT (%) - < 80% th (missing data = 6) | 78.15 [55.9; 93.7] − 31/56 (55.4) |
| Short Form 36 (Raw value - Value < 50) | 36 (53.7) |
| PF | 30 [15; 45] − 32/36 (82.1) |
| RP | 0 [0; 25] − 29/36 (80.6) |
| BP | 32 [22; 54] − 21/36 (58.3) |
| GH | 52 [40; 62] − 13/36 (36.1) |
| VT | 52 [40; 62] − 28/36 (77.8) |
| SF | 75 [37.5; 100] − 9/36 (25) |
| RE | 0 [0; 33.33] − 33/36(91.7) |
| MH | 64 [56; 72] − 6/36(16.7) |
| HT | 25 [0; 50] − 24/36 (66.7) |
| PCS | 26.02 [25.4; 26.7] − 36/36 (100) |
| MCS | 23.47 [22.7; 24.0] − 36/36 (100) |
| EQ-5D-5 L | 26 (38.8) |
| Impairment of mobility | 18/26 (69.2) |
| Impairment of autonomy | 11/26 (42.3) |
| Impairment of daily activity | 19/26 (73.1) |
| Pain discomfort | 23/26 (88.5) |
| Anxiety depression | 19/26 (73.1) |
GFR = Glomerular Filtration Rate; CKD-Epi = Chronic Kidney Disease-Epidemiology; NIV = non-invasive ventilation; CPAP: Continuous Positive Airway Pressure; Th = Theoretical; FEV1 = Full Expiratory Volume per Second; TLC = Total Lung Capacity; FVC = Forced Vital Capacity; DLCO = Diffusion Capacity of Carbon Monoxide, KCO = diffusion coefficient of CO; PIP = Peak Inspiratory Pressure; BMI = Body Mass Index; CRP = C-Reactive Protein; PF = Physical Functioning; RP = role-Physical; BP = Bodily Pain; GH = General Health; VT = Vitality; SF = Social Functioning; RE = role-Emotional; MH = Mental Health; HT = Health change; PCS = Physical Component Summary; MCS = Mental Component Summary.
* HGT was considered pathological if < 25 kg in men aged ≤ 60 years and < 23 kg in men between 61 and 79 years, and < 14 kg in women aged ≤ 60 years and < 13 kg in women between 61 and 79 years.
Fig. 3.
Venn diagram of the population according to functional, respiratory and renal impairment.
Nephrological assessment
Most of the 58 patients (86.6%) who had a nephrological evaluation experienced a decline in their GFR > 5mL/min/1.73m2 (73.7%), and 12 of the 58 (21.1%) had a GFR < 45 mL/min/1.73m2, including 2 dependent on RRT.
Respiratory assessment
Three patients were still being treated with long-term oxygen therapy and four with non-invasive mechanical ventilation. Most of the patients presented a decrease in their DLCO 47/55 (85%), and half of the patients had a decreased FEV (28/62 (45)) (Table 2).
Nutritional assessment
From ICU admission to post-ICU-MC, 25 patients (37.3%) lost more than 5% of their body weight. The median BMI was about 27.1 kg/m2. Albumin, pre-albumin and CRP dosages were abnormal for, respectively, 2/62 (3.2%), 8/57 (14%) and 7/59 (11.9%) patients.
Functional assessment and autonomy
The HGT, 6mWT and PIP were low in 7/61 (11.5%), 31/56 (55.4%) and 31/62 (50%) patients, respectively. At the time of post-ICU-MC, more patients were dependent on home care assistance than at the time of ICU admission, and the functional status of most of the patients decreased from ICU admission to the time of the post-ICU-MC. However, the proportion of patients living with severe frailty was unchanged (Fig. 2).
Health-related quality of life
The SF-36 questionnaire was completed by 39 patients (58.21%). The PCS was 25.47 [24.75–26.49] and MCS was 23.69 [22.8–24.24]. After ICU stay the main issues were physical limitations (100% under a score of 50) and mental limitations (92.31% under a score of 50). The main outcomes were a lack of physical activity, a loss of vitality, a change in health status, and physical pain (Table 2).
Correlations between parameters
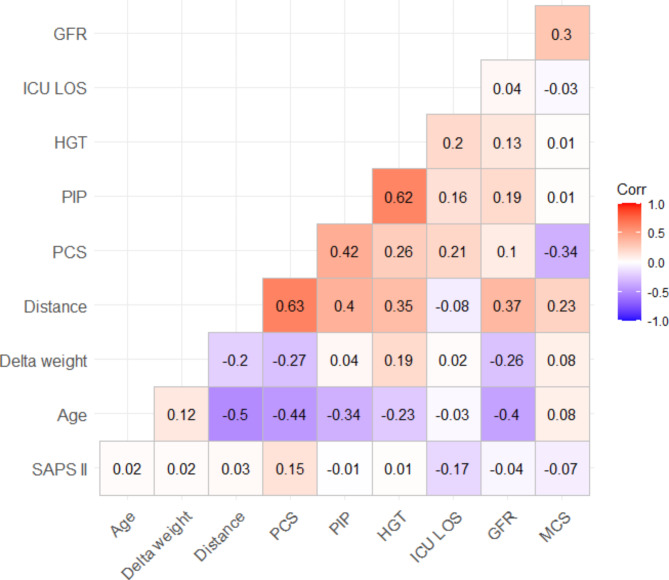
Age was significantly associated with 6mWT (-0.5), PCS (-0.4), PIP (-0.34) and GFR (-0.4) assessed during post-ICU-MC. At the post-ICU-MC, the variables assessing patient functionality (PIP, 6mWT and HGT) were significantly correlated. GFR was also correlated with 6mWT (0.37) (Fig. 4).
Fig. 4.
Correlation between parameters during ICU stay and during post-ICU-Multidisciplinary consultations. PCS = Physical summary score of the Short Form 36; PIP = Peak inspiratory pressure; HGT = handgrip test; ICU = intensive care unit; LOS = length of stay; GFR = glomerular filtration rate; MCS = Psychological summary score of the Short Form 36; SAPS II = Simplified acute physiology score.
Comparisons depending on the distance at the 6mWT (Table S3)
The patients who covered a short distance were more often diabetic (35.5% vs. 8%, p = 0.02), more often under IMV, and had a longer ICU and hospital LOS. At the time of post-ICU-MC, these patients had lower DLCO.
Comparisons depending on physical impairment (Table S3)
Women were more likely to have physical impairment than men, and frailty score on ICU admission was higher for patients with a functional impairment. At the post-ICU-MC, DLCO was lower for patients with physical impairment.
Comparisons depending on respiratory impairment (Table S4)
Older patients had a higher risk of respiratory impairment.
Comparisons depending on renal impairment (Table S4)
The SAPS II of patients with renal impairment was lower. The psychological components of SF-36 (General Health, Mental Health, Psychological Summary Score) were significantly associated with renal impairment. These patients also had a higher bodily pain score as assessed by SF-36.
Comparisons depending on ICU LOS and SAPS II score on ICU admission (Table S5)
No significant differences were observed at post-ICU-MC in relation to ICU LOS. Patients who were more severely affected on ICU admission, i.e. those with a higher SAPS II score, lost more weight and had several impairments in components of the SF-36. No other differences were observed.
Discussion
We finally enrolled 67 patients, of whom 70%, 74%, and 68% exhibited functional, respiratory, and renal impairments, respectively, at the time of the post-ICU-MCs. Additionally, 40%, 28%, 19%, and 2.5% had three, two, one, and none of these impairments, respectively. All patients experienced mental disorders and a decline in quality of life, which together warrant consideration for a diagnosis of PICS. Functional impairment correlated with frailty score and sex, while respiratory function correlated with age.
These findings prompt several observations
First, we reported that in our cohort, all the patients had a decrease in their MCS score < 50, and their mental health seemed more severely affected than usual. In the literature, mental health problems occur in about 10–40% of ICU survivors 3–12 months after critical illness12–14. For instance, Estrup et al. reported an MCS of 55 in patients who survived an ICU stay of more than 24 h [43–63]. However, in their cohort, only 50% of the patients were ventilated, and their median LOS in the ICU was 4 [2; 7 ] days15. Some of these patients could have had post-traumatic stress disorder (PTSD), which like anxiety and depression is considered as a mental health problem, but we were unable to identify them. In PICS, the prevalence of PTSD is generally less frequent than that of anxiety and depression16. Our results underline the need to assess PTSD during our multidisciplinary post-ICU-MC and for patients to consult with a psychologist particularly since multidisciplinary consultations have promising results for mental outcomes17,18. However, published data in this field are still scarce and sometimes conflicting, with two randomized controlled trials (RCT) showing no beneficial effect on selected outcomes of the mode of follow-up tested19,20. Additionally, in the latest published RCT, involving 547 patients, post-ICU-MCs were associated with a worse outcome at 12 months as defined by death or severe-to-extreme impairment of at least one EuroQoL-5D-5 L dimension21.
The functional outcomes of our patients were assessed by different tests, i.e.: the 6mWT, the HGT and the PIP. The 6mWT and HGT are widely used and now recommended for the assessment of the functional components of PICS22,23 unlike the PIP, which requires respiratory functional exploration. PIP can, however, be used to assess functional status because it reflects the functionality and strength of respiratory muscles24. Prolonged physical impairments occur in about 30% of ICU survivors 3–6 months after critical illness12,13 and often lead to persistent disabilities in activities of daily living (ADL). In one study, for instance, 33% of patients had partial dependence in at least one aspect of ADL 12 months after critical illness25. A reduction in HGT and 6mWT scores can indicate the persistence of ICU-acquired weakness26, and is also related to increased risk of long-term mortality27.
We observed that frailty score was correlated with long-term functional outcome, which clearly suggests that frailty should be used more often to select patients who would benefit the most from assessment at the post-ICU-MC. Evidence shows that in today’s aging society, frailty is one of the important risk factors for PICS28.
In our post-ICU-MCs, most of the patients underwent respiratory functional tests. Such evaluations have been widely performed for survivors of the COVID-19 pandemia. For instance, Laveneziana et al. assessed 485 survivors of COVID-19 3 months after their ICU discharge, and reported that 34%, 70% and 56% of the participants, respectively, had a restrictive lung defect, impaired DLCO, and significant radiological sequelae. They also reported that some of these patients improved their pulmonary function and also their radiological abnormalities, but the most severely affected still had significant lung sequelae and residual symptoms justifying prolonged follow-up29. Patients surviving ARDS underwent similar explorations [23, 29]. Complete assessment of respiratory function is not systematically mentioned in documented reports of PICS30. However, Dusart et al.31 recently described assessment of post-ICU pulmonary function.
We also assessed renal function during the post-ICU-MC. Other studies also document long-term renal function after ICU stay32. Acute kidney injury (AKI) affects about half of patients admitted to the ICU. Certain cases of AKI last more than 7 days and can also persist after 3 months to become chronic kidney disease. Some studies also reported that persistent AKI can modify long-term quality of life and that more deficits in physical than mental health domains are found in survivors of AKI in critical care33.
Contrary to other studies such as that of Lakenman et al.34, patients in our cohort had a non-significant variation in weight. In addition, their nutritional status was generally good at the post-ICU-MC.
Finally, we observed a prevalence of PICS of 100% in our preselected cohort of patients. The criteria used in our hospital to offer a post-ICU-MC seemed therefore appropriate. The prevalence of PICS varies widely from one study to another. For example, individual PICS symptoms have been reported to develop in 50–70% of critically ill patients, and their co-occurrence has been evidenced in about 20% of patients12,13. In addition, patient quality of life decreases with the onset of PICS, and around two-thirds, two-fifths, and one-third of previously employed ICU survivors are jobless up to 3, 12, and 60 months, respectively, after hospital discharge35,36.
Although PICS is recognized as a public health issue37 and post-ICU-MCs are routinely recommended23,38, the modalities of the consultations, which are not clearly established, require further investigation, and evidence of their effectiveness is lacking39.
The study of Sharshar et al.21, involving a very large panel of ICU survivors, showed no positive effect of the post-ICU-MCs. It is a good reminder that the criteria for selecting patients, monitoring frequency, and determining the tests to perform and the measures to adopt in case of impairments must be improved to render post-ICU-MCs useful.
The finding that all our patients, enrolled on the basis of preselected criteria, had PICS, is interesting as it shows that the criteria used in our hospital to refer patients for post-ICU-MC were appropriate. The other modalities of our post-ICU MCs, i.e.: the caregivers involved, the screening tools used, the frequency of follow-up, the measures adopted in case of deterioration, the coordination of the follow-up or interface of the consultations and the trajectory of the patients in the health care system could however be improved.
This study has both strengths and limitations. Its main strength was to report our own experience of post-ICU-MCs, to determine the incidence of PICS in our cohort, and to provide a full evaluation of the patients, showing the respiratory, renal and nutritional impairments.
The study has certain limitations. It was a monocentric retrospective study based on a quite small sample size, which limits the external value of the results. The sample size could be in part explained by the temporary suspension of the post-ICU-MCs during the COVID-19 pandemia and by the initial limited time dedicated to the consultations, which allowed only a few patients to be assessed per week. Consequently, we acknowledge our population is in part self-selected. There were also considerable differences in follow-up time, ranging from 3 months to 7 months. These discrepancies can be explained by the way we organized the post-ICU-MCs. All the patients were initially invited to attend a consultation 3 months after ICU discharge but, owing to the limited time dedicated per week for post-ICU-MCs and because of the availability of patients, some of the consultations had to be postponed and were not performed until 7 months after ICU discharge. We did not account for these discrepancies in the analyses. Not all components of PICS were explored, notably cognition. In the light of recent reports indicating cognitive alterations in around 40% of patients after prolonged ICU stays, this limitation warrants attention4,5,11,30,40,41. With regard to their mental function, not all of our patients completed the SF-3615,42,43 and/or the EuroQoL-5D [4, 5, 43, 44] questionnaires. There are now fewer missing assessments since, if the questionnaires are not fully completed before the post-ICU-MC, the intensivist fills them out in the presence of the patient. PTSD was not assessed4,40,44, and nor were scores of widely used questionnaires such as the HAD scale [37, 39, 40, 44–47]. In addition, the SF-36 did not seem to be the best measure to assess mental disorder and recent guidelines propose other assessment tools for the mental components of PICS. For instance, Nakanishi et al.45, in their study based on a Delphi method, recommended 20 PICS assessment instruments for mental health including the HAD scale, the IES-R (impact of event scale), and the PHQ-9 (Patient Health Questionnaire). Of note, SF-36 and EQ-5D-5 L, 3 L were not recommended for the assessment of mental health but rather for the assessment of the quality of life. In France, the HAS recommends using PHQ-8, GAD-7 (general anxiety disorder), and PCL-5 to assess psychological symptoms, the MoCA for cognition, and EuroQol-5D-5 L for quality of life. Finally, we only assessed a part of the ICU survivors. However, the patients who attended a post-ICU-MC were selected on the basis of criteria that are now recommended in France23.
Conclusion
Although our study did not assess all components of PICS, it nevertheless showed that almost all our selected patients experienced the syndrome and that more than half of them had respiratory, functional and/or renal impairments. Our criteria for offering patients a post-ICU-MC seemed to be appropriate and attendance at the consultation was useful to diagnose persistent organ failures. Finally, a clinical review of the patient’s status after an ICU stay should be recommended to optimize the trajectory of survivors. An intensivist would probably be best-suited to supervise and summarize the results of all the consultations and explorations. Our results suggest that systematic prescription of physiotherapy and psychological follow-up for patients should be considered after an ICU stay.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Jeffrey Watts for help in preparing the manuscript.
Abbreviations
- 6mWT
6-metre walking test
- ADL
Activity daily living
- BMI
Body mass index
- DLCO
The diffusion coefficient of CO
- EuroQoL-5D
The European Quality of Life 5 Dimensions survey
- FEV
The full expiratory volume per second
- FVC
The forced vital capacity
- GFR
The glomerular filtration rate
- HAD
The Hospital Anxiety and Depression scale
- HGT
Hand grip test
- HRQoL
Health care related quality of life
- IALD
Instrumental ADL scores
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- IQR
Interquartile range
- IRB
Institutional review board
- LOS
Length of stay
- MCS
The mental component summary scales
- MoCA
The Montreal Cognitive Assessment test
- PCL-5
The Posttraumatic Stress Disorder Checklist for DSM-5
- PCS
The physical component summary scales
- PICS
Post intensive care syndrome
- PIP
Peak inspiratory pressure
- Post-ICU-MC
Post ICU multidisciplinary consultations
- PTSS-10
The Posttraumatic Stress Syndrome questionnaire
- RFT
Respiratory function tests
- RRT
Renal replacement therapy
- SAPS II
The Simplified Acute Physiology Score II
- SF-36
Study Short Form 36
Author contributions
“Conceptualization, CD, BS; methodology, BS, CD.; software, CD.; validation, BS., CD, RB; LC formal analysis, CD; investigation, LM, SB, CD; resources, BS.; data curation, CD, writing—original draft preparation, CD.; LM, SB writing—review and editing, RB, NF, LM, SB, CP, GL, LC, FC, BS, CD; supervision, BS, CD.; project administration, BS. All authors have read and agreed to the published version of the manuscript.”
Data availability
Data are available under request at the correspondant author.
Declarations
Ethics approval and consent to participate
The PICS-MIR study was approved by the local ethics committee (IRB13412, CHU de Clermont Ferrand IRB #1, IRB number 2023-CF229). Each participant was informed of the possible use of anonymized data for research: The families were given a written consent form on ICU admission and the same written information was displayed in the waiting room of the ward. None of the participants were opposed to use of their data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iapichino, G. et al. Determinants of post-intensive care mortality in high-level treated critically ill patients. Intensive Care Med.29, 1751–1756 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Inoue, S. et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med. Surg.6, 233–246 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiser, S. L., Fatima, A., Ali, M. & Needham, D. M. Post-intensive care syndrome (PICS): recent updates. J. Intensive Care. 11, 23 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weidman, K. et al. Post–Intensive Care Unit Syndrome in a cohort of COVID-19 survivors in New York City. Ann. Am. Thorac. Soc.19, 1158–1168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maley, J. H. et al. Six-Month Impairment in Cognition, Mental Health, and physical function following COVID-19–Associated Respiratory failure. Crit. Care Explor.4, e0673 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanagi, N. et al. Post-intensive care syndrome as a predictor of mortality in patients with critical illness: a cohort study. PLOS ONE. 16, e0244564 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enright, P. L. & Sherrill, D. L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir Crit. Care Med.158, 1384–1387 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Mikkelsen, M. et al. (ed, E.) Society of critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term impairments after critical illness. Crit. Care Med.48 1670 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Ware, J. E. et al. SF-36 Health Survey: Manual and Interpretation Guide (The Health Institute, New England Medical Center, 1997).
- 10.Elliott, D. et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit. Care. 15, R142 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rousseau, A. F. et al. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann. Intensive Care. 11, 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra, A. et al. Co-occurrence of Post-intensive Care syndrome problems among 406 survivors of critical Illness*. Crit. Care Med.46, 1393–1401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakami, D. et al. Prevalence of post-intensive care syndrome among Japanese intensive care unit patients: a prospective, multicenter, observational J-PICS study. Crit. Care Lond. Engl.25, 69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson, J. C. et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med.2, 369–379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estrup, S. et al. Health-related quality of life, anxiety and depression and physical recovery after critical illness - A prospective cohort study. Acta Anaesthesiol. Scand.66, 85–93 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Righy, C. et al. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Crit. Care. 23, 213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rg, R. et al. Effects of post-ICU follow-up on subject outcomes: a systematic review and meta-analysis. J. Crit. Care52, 115–125 (2019). [DOI] [PubMed]
- 18.Khan, S. et al. Mobile critical care recovery program (m-CCRP) for acute respiratory failure survivors: study protocol for a randomized controlled trial. Trials. 19, 94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuthbertson, B. H. et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 339, b3723 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh, T. S. et al. Increased hospital-based Physical Rehabilitation and Information Provision after Intensive Care Unit Discharge: the RECOVER Randomized Clinical Trial. JAMA Intern. Med.175, 901–910 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Sharshar, T. et al. A randomized clinical trial to evaluate the effect of post-intensive care multidisciplinary consultations on mortality and the quality of life at 1 year. Intensive Care Med.50, 665–677 (2024). [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi, N. et al. Instruments to assess post-intensive care syndrome assessment: a scoping review and modified Delphi method study. Crit. Care. 27, 430 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HAS. Diagnostic et prise en charge des patients adultes avec un syndrome post-réanimation (PICS) et de leur entourage. (2023).
- 24.Laveneziana, P. et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir J.53(6), 1801214 (2019). [DOI] [PubMed]
- 25.Brummel, N. E. et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit. Care Med.42, 369–377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appleton, R. T., Kinsella, J. & Quasim, T. The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J. Intensive Care Soc.16, 126–136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ait Hssain, A. et al. Body composition and muscle strength at the end of ICU stay are associated with 1-year mortality, a prospective multicenter observational study. Clin. Nutr. Edinb. Scotl.42, 2070–2079 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Riegel, B. et al. Early Post-intensive Care Syndrome among older adult Sepsis survivors receiving Home Care. J. Am. Geriatr. Soc.67, 520–526 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlemmer, F. et al. Respiratory recovery trajectories after severe-to-critical COVID-19: a 1-year prospective multicentre study. Eur. Respir J.61, 2201532 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanwani-Nanwani, K. et al. Prevalence of post-intensive care syndrome in mechanically ventilated patients with COVID-19. Sci. Rep.12, 7977 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dusart, C. et al. Pulmonary functional outcomes at 3 months in critical COVID-19 survivors hospitalized during the First, Second, and third pandemic waves. J. Clin. Med.12, 3712 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chand, S. et al. Long-term follow up of renal and other Acute Organ failure in survivors of critical illness due to Covid-19. J. Intensive Care Med.37, 736–742 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forni, L. G. et al. Renal recovery after acute kidney injury. Intensive Care Med.43, 855–866 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakenman, P. L. et al. Nutritional status of patients with COVID-19 1-y post-ICU stay: a prospective observational study. Nutr. Burbank Los Angel Cty. Calif.111, 112025 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamdar, B. B. et al. Return to work after critical illness: a systematic review and meta-analysis. Thorax. 75, 17–27 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prescott, H. C. & Angus, D. C. Enhancing recovery from Sepsis: a review. JAMA. 319, 62–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rousseau, A. F. et al. Long-term outcomes after critical illness: recent insights. Crit. Care Lond. Engl.25, 108 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med.47, 1181–1247 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakanishi, N. et al. Post-intensive care syndrome follow-up system after hospital discharge: a narrative review. J. Intensive Care. 12, 2 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martillo, M. A. et al. Postintensive Care Syndrome in survivors of critical illness related to Coronavirus Disease 2019: Cohort Study from a New York City critical care recovery Clinic*. Crit. Care Med.49, 1427–1438 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Zhou, M. et al. Incidence of and risk factors for post–intensive care syndrome among Chinese respiratory intensive care unit patients: a cross-sectional, prospective study. Aust Crit. Care. 36, 464–469 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Sidiras, G. et al. Long term follow-up of quality of life and functional ability in patients with ICU acquired weakness – a post hoc analysis. J. Crit. Care. 53, 223–230 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Gil, D. et al. Health-related quality of life and stress-related disorders in COVID-19 ICU survivors: are they worse than with other causes of ARDS? J. Intensive Med.2, 103–109 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatch, R. et al. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: a UK-wide prospective cohort study. Crit. Care. 22, 310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakanishi, N. et al. Post-intensive Care Syndrome and its New challenges in Coronavirus Disease 2019 (COVID-19) pandemic: a review of recent advances and perspectives. J. Clin. Med.10(17), 3870 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available under request at the correspondant author.