Abstract
Neurodegenerative diseases are central or peripheral nervous system disorders associated with progressive brain cell degeneration. Common neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis have been widely studied. However, current therapeutics only reduce the symptoms and do not ameliorate the pathogenesis of these diseases. Recent studies suggested the roles of neuroinflammation, apoptosis, and oxidative stress in neurodegenerative diseases. Mesenchymal stem cells (MSCs) exert anti-apoptotic, anti-inflammatory, and antioxidative effects. Therefore, investigating the effects of MSCs and their applications may lead to the discovery of more effective therapies for neurodegenerative diseases. In this study, we review different approaches used to identify therapies for neurodegenerative diseases using MSCs.
Keywords: Neurodegenerative diseases, Stem cell therapy, Mesenchymal stem cells, Circadian rhythm, Antibody drugs, Neuroinflammation
1. Introduction
Neurodegenerative diseases, characterized by substantial loss of neuronal cell populations or impairment of neuronal function, have become major health concerns worldwide. Common neurodegenerative diseases include Alzheimer's diseases (AD), Parkinson's disease (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS) [1].
The clinical symptoms of these diseases overlap [2]. For example, patients with PD, HD, or ALS share motor system deficits that make it difficult to control their movements [3,4]. Brain regions affected by neuronal loss vary and are specific to each disease. However, the mechanisms underlying neuronal loss are similar [2]. Accumulating evidence suggests that neurodegeneration is related to oxidative stress damage and neuroinflammation, which is characterized by the activation of microglial and astrocytic cells [[5], [6], [7], [8]]. Mitochondria are a major source of oxidative stress and cause of neuronal apoptosis [9]. Mitochondrial dysfunction has been reported in animal model of neurodegeneration diseases [[10], [11], [12]]. Disturbances in the redox balance due to neuroinflammation or mitochondrial dysfunction are implicated in the pathogenesis of several neurodegenerative diseases [13,14]. Therapeutic approaches targeting reactive oxygen species formation and neuroinflammation have yielded positive results [13].
Despite increases in the understanding of the pathogenesis of neurodegenerative diseases, development of treatments has lagged because of several factors, including a lack of methods to precisely deliver drugs into the lesion brain tissue [15,16], inhibition of specific inflammatory microglial/astrocytic phenotypes [17,18], and manipulation of specific messenger proteins that restore neuronal signaling pathways [19]. Recently, stem cell-based therapy has gained attention because of its ability to self-repair and regenerate the brain. The mechanisms underlying the effects of stem cells on the nervous system may surpass those of traditional medications for treating neurodegenerative diseases.
Neurodegeneration disturbs the circadian rhythm regulation in the brain [[20], [21], [22], [23]]. Disruption of the sleep-wake cycle and circadian homeostasis plays pivotal roles in the biological mechanisms underlying neurodegeneration [24]. Recent studies reported the importance of circadian rhythm regulation on stem cell homeostasis and function [25,26]. Controlling circadian rhythms is a promising strategy for neurodegenerative diseases using stem cell-based therapeutics.
This review summarizes the current understanding of stem-cell based therapies for the treatment of neurodegenerative diseases and highlights the involvement of circadian rhythm sleep-wake disorders and role of mesenchymal stem cells (MSCs) in the era of antibody drugs.
2. Stem cell-based therapy and neurodegenerative diseases
Stem cells can be categorized as embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs). Blastocysts are the most common sources of ESCs. ESCs derived from blastocysts are pluripotent and can differentiate into all three germ layers [27,28]. iPSCs are derived from somatic cells that have been reprogrammed into a pluripotent state [29]. ASCs are obtained from a wide range of adult tissues such as the bone marrow, peripheral blood, and umbilical cord blood. ASCs are multipotent, which limits their differentiation potential compared with that of ESCs [30]. Use of ESCs in stem cell-based therapy is limited because of ethical issues, a lack of knowledge on their differentiation regulation, and the risk of tumorigenicity [27,31,32]. Unlike ESCs, iPSCs are not associated with ethical issues. However, the direct use of iPSCs in patients with neurodegenerative diseases poses a high risk of tumorigenicity, limiting their clinical application [33]. The development of genome editing techniques has expanded the application of iPSCs in neurodegenerative modelling. These models provide tools for studying the etiology of neurological diseases [[34], [35], [36]]. Thus, the roles of iPSCs in neurodegenerative diseases have mainly been determined through their application in creating platforms to study ex vivo pathology, particularly neurodegenerative disorders [37]. The most common ASCs used for therapeutic application in neurodegenerative diseases are MSCs because of their simple collection and diverse strategies for therapeutic use. After transplantation, MSCs can directly migrate to brain lesions and differentiate into neurons and glial cells, replacing and reconstructing damaged neurons [38]. After reaching the target areas, MSCs secrete a variety of regulatory chemicals, such as growth factors, cytokines, chemokines, and various enzymes that strongly modulate immune reactions, angiogenesis, and apoptotic processes [[39], [40], [41], [42]].
The ability of MSCs to spontaneously migrate to inflamed tissues, also known as homing ability, has been widely studied. Recent studies suggested that MSC homing can be classified into non-systemic and systemic homing according to the administration route [43]. In non-systemic homing, MSCs are administered locally near the injured area and then translocated according to the chemokine gradient. In systemic homing, MSCs are administered into the bloodstream and undergo five additional steps–tethering and rolling, activation, arrest, transmigration, and migration–to reach the targeted site [44]. Since the primary advantage of MSC therapies is mainly their homing efficiency, intensive investigation is necessary to understand this process. Each neurodegenerative disease affects a specific brain region. Moreover, MSCs must cross the blood-brain barrier (BBB), which has distinct characteristics that prevent most cell types from entering the brain. Targeted administration via intracerebral injection is the most facile method for increasing the ability of MSCs to cross the BBB and reach the damaged brain regions. Numerous studies reported the development of medical technologies for intracerebral injections. Additionally, techniques focusing on optimizing systemic homing efficiency such as genetic modification, cell surface engineering, target tissue modification have been developed [44]. However, the appropriate route of MSC administration remains controversial [[45], [46], [47]].
Neuroinflammation is observed in the pathogenesis of almost all neurodegenerative diseases [48]. Inflammatory mediators such as cytokines and chemokines either increase or decrease the progression of neurodegeneration [49]. Elevated cytokine levels were reported to parallel the progression of neuroinflammation [50]; for example, IL-1 and TNFα significantly increase before neuronal cell death. In vitro and in vivo studies indicated that intracerebral administration of IL-1, TNFα, and IFNγ induces neuronal apoptosis [[51], [52], [53], [54]]. In contrast, TGFβ, IL-10 are considered to be neuroprotective [50]. Cytokines mainly interact with glial cells. TNFα and IL-1 activate microglia and astrocytes, after which reactive microglia and astrocytes release additional cytokines that exacerbate or suppress neuroinflammation. Microglia and astrocytes polarize into different phenotypes. The M1 and A1 phenotypes are implicated in more severe neuroinflammation, whereas the M2 and A2 phenotypes are thought to have neuroprotective effects [8,55]. Interestingly, MSCs exert immunosuppressive effects via paracrine functions [56]. MSCs response to high levels of cytokines such as IL-1, TNFα, and IFNγ as activating factors to initiate their immunosuppressive effects [57]. After activation, MSCs produce exosomes (MSC-exos) containing bioactive molecules. These exosomes can penetrate the BBB, enter brain cells via membrane fusion, and subsequently modulate their physiological functions [58]. MSC-exos can also be isolated in vitro and systemically administered to patients to deliver bioactive molecules to brain lesions. Thus, administration of MSC-exos can prevent immune rejection and reduce the risk of infection [59]. MSC-exos can release bioactive substrates not only extracellularly to interact with receptors, but also intracellularly in the case of microRNAs to alter gene expression at the transcriptional level. Wen et al. reported that exosomes derived from MSCs ameliorated neuroinflammation and neuronal apoptosis via inducing transformation of the M1 phenotype towards the M2 phenotype. Their results suggested that microglial transformation is related to the microRNA-181b mediated IL-10/STAT3 pathway [60]. Zhao et al. also reported that MSC-exos significantly inhibited M1 microglial polarization and increased M2 microglial cells by reversing CysLT2R-ERK1/2-mediated microglia M1 polarization [61]. MSC-based therapy inhibits the polarization of A1 astrocytes and promotes astrocytic polarization toward the A2 phenotype [62]. In addition to their roles in microglial and astrocyte polarization, MSCs show potential as cell-specific targeted therapy for the treatment of neurodegenerative diseases.
2.1. Alzheimer disease
AD is a progressive neurodegenerative disease that causes memory and cognitive impairments. The pathological characteristics of AD are distinct from those of neurofibrillary tangles and neuritic plaques [63]. The progression of AD can be classified into three stages: preclinical, mild cognitive impairment, and dementia stages [64]. In patients with AD, neurodegeneration progresses until death [65]. Because of their ability to improve memory and cognitive symptoms, symptomatic agents remain the gold standard for treating treatment patients with AD; however, disease-modifying therapies that reduce biomarkers of AD pathogenesis including amyloid β (Aβ) accumulation and neuronal degeneration or injury have been developed and may be commercially available in the near future [64]. Unlike symptomatic agents, disease-modifying therapies can be used to treat AD in the preclinical stages to delay the onset and progression of dementia. The therapeutic effects of MSCs on the pathogenesis of AD have been investigated in preclinical and clinical studies. Farahzadi et al. showed that MSCs protected against Aβ-induced decreases in telomere length as a biomarker of AD associated neurodegeneration and prevented decreases in telomerase activity. MSC administration also attenuated the mTOR, AMPK, GSK-3β, and Wnt/β-catenin signaling pathways, which are related to AD [66]. Human umbilical cord MSCs attenuate neuronal damage induced by hyperphosphorylated tau, stimulating synaptic plasticity in the hippocampus of AD mice [67]. Intravenous transplantation of human umbilical cord MSCs decreased oxidative stress markers and upregulated antioxidant enzymes such as superoxide dismutase (SOD) and neuronal nitric oxide synthase, thus recovering cognitive function in a Tg2576 mouse AD model [68]. Human amniotic MSCs attenuated Aβ deposition, microglia activation, and neuroinflammation in the hippocampus of APP/PS1 mice [69]. Transplantation of human umbilical cord MSC-derived cholinergic-like neurons decreased Aβ expression and neuronal apoptosis in Aβ-induced AD rat model [70]. Many substrates, including resveratrol and melatonin, were reported to increase the efficiency of MSCs in the treatment of neurodegenerative diseases [[71], [72], [73]]. MSC-exos were found to attenuate Aβ expression and modulate neuroinflammation. MSC-exos also decreased Aβ levels in an AD cell model [74]. MSC-exos upregulate anti-inflammatory factors, such as IL-10 and tissue inhibitor matrix metalloproteinase 1 in AD, resulting in the reduction of M1 microglial polarization [75]. Accumulating evidence has revealed the role of MSC-exo-associated microRNAs. MSC-derived exo miR-223 inhibited neuronal apoptosis via the PTEN-PI3K/Akt pathway in an Aβ1–40 induced AD cell model [76]. Exosomal miR-146a secreted from bone marrow MSCs transferred into astrocytes reduced astrocytic inflammation induced by the inflammatory transcription factor NF-κB in AD model mice [77]. Several clinical trials confirmed the safety of MSC transplantation in patients with AD [78]. The most common adverse side effects include headache, nausea, and vomiting, which are typically not serious and subside within 36 h [79,80]. Nonetheless, further clinical trials are required to demonstrate the safety of MSCs and their effects on the AD brain.
2.2. Parkinson's disease
PD is clinically diagnosed based on the presence of bradykinesia in combination with at least one motor disorder symptom, such as rigidity or tremor, after considering the absolute exclusion and supportive criteria. Beyond motor disorders, non-motor symptoms include cognitive impairment, autonomic dysfunction, sleep–wake cycle deficits, and depression. The neuropathology of PD is characterized by neuronal loss in the substantia nigra compacta (SNc) and accumulation of intracellular protein (α-synuclein). Neuroinflammation has been implicated as a salient feature of PD [81]. Currently, there is no available treatment for PD that completely terminates the progression of neurodegeneration. Thus, methods for decelerating PD advancement are needed. Neuronal dysfunction in PD occurs long before the onset of motor symptoms in prodromal PD [82]. Pharmacological management of PD restores dopaminergic function and ameliorates motor symptoms but has no effect on non-motor symptoms and is difficult to use in the prodromal stage because of intolerable side effects [83]. MSC-based therapies have been investigated to improve the efficacy of PD treatment, with numerous experimental MSC-based therapies developed for use in animal models of PD. Pharmacological PD mouse models induced by neurotoxic agents, including methamphetamine (MA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), are widely used for screening of PD therapies. These agents induce loss of dopaminergic neurons in the SNc and striatum, mimicking brain damage in patients with PD [84,85]. SB623 MSC transplantation protected against MPTP-induced impairment of DA levels and tyrosine hydroxylase immunoreactivity in the striatum of mice [86]. Additionally, MSCs differentiated into dopaminergic neurons and significantly recovered striatal dopamine and dopamine metabolites in an MPTP-induced PD animal model [87]. MSCs migrated into the SNc and released TGF-β1, which downregulate microglial activation and neuroinflammation induced by MPTP [88]. MSCs attenuated MA-induced rotation behavior and protected against MA-induced tyrosine hydroxylase-positive cell loss in an animal model of PD [89]. Exosomes derived from MSCs exert neuroprotective effects against PD by stimulating cell proliferation and inhibiting apoptosis in the striata of PD mice [90]. Intraperitoneal injection of MSC-exos decreased α-synuclein aggregation and reduced MPTP-induced decreases in tyrosine hydroxylase immunoreactivity in the SNc [91].
2.3. Amyotrophic lateral sclerosis
ALS is characterized by the degeneration of motor neurons that project from the cortex to the brainstem or spinal cord, and from the brainstem or spinal cord to the muscle. A neuropathological characteristic of ALS is high accumulation of proteinaceous proteins. Preclinical studies revealed that the pathophysiology of ALS is associated with impairment in protein homeostasis caused by the deterioration of both transcriptional and translational processes. In addition, mitochondrial dysfunction and the regulation of astrocytic and microglial differentiation contribute to neuronal loss in ALS disease [92]. MSCs were reported to attenuate ALS pathology in a transgenic SOD1G93A animal model, which is the most common animal model for studying ALS. Intravenous administration of MSCs delayed the onset of ALS and extended the lifespan of SOD1G93A mice. MSC-treated SOD1G93A mice showed less severe motor deficits in both behavioral and neuropathological assessments [93]. After transplantation, MSCs can survive for several weeks [93,94]. While inside the central nervous system (CNS), MSCs are reported to exert neuroprotective effects mainly by controlling apoptosis and inflammatory markers, rather than by replacing dead neurons [94]. Despite the promising results achieved in preclinical studies, few clinical studies have reported significant improvements in patients with ALS [95]. Therefore, further studies are required to clarify the role of MSCs in patients with ALS.
2.4. Huntington's disease
HD is an inherited neurodegenerative disease associated with mutations in the huntingtin gene, which plays a pivotal role in the regulation of brain cell transcription. Mutation in the huntingtin gene leads to the dysfunction of transcription factors such as p53 and CREB, causing neuronal death, mitochondrial dysfunction, and disruption of the protein synthesis machinery [96]. Increasing doses of 3-nitropropionic acid in rodents induce HD-like mitochondrial oxidative stress and neurodegeneration. MSC transplantation significantly increases striatal neurotrophic factors and protect transplanted mice from motor deficits and striatal neuronal loss [97]. MSCs showed protective effects similar to those in an HD transgenic mouse model [98,99]. These results suggest that MSCs can be used as therapeutic agents for patients with HD.
2.5. Circadian rhythm and sleep disorder
Neuronal loss in neurodegenerative diseases can affect brain regions that regulate sleep-wake cycles and circadian rhythms. Sleep disorders accelerate neuronal apoptosis [24]. Numerous neurodegenerative diseases including AD, HD, PD, and ALS are associated with circadian disruptions [[100], [101], [102]]. Circadian rhythms are regulated by groups of clock genes, and alterations in clock gene expression can result in neurodegeneration, oxidative stress injuries, and activation of neuroinflammatory cells [103,104]. Bmal1 deletion induces synaptic terminal degeneration, increases reactive oxygen species formation, and alters mitochondrial redox homeostasis. Treatment with the antioxidant N-acetyl-l-cysteine rescued oxidative stress damage in aging Bmal1 knockout mice [103]. Therefore, Bmal1 deletion may interfere with the glutathione system and reduce the expression of glutathione peroxidase-1 (GPx-1). Evidence suggests that glutathione peroxidase-1 protects against neurodegenerative disease [105,106]. MSC administration upregulates the expression of antioxidant enzymes and may restores oxidative defense enzyme systems [107]. Preclinical and clinical studies reported the involvement of sleep disturbances in HD [[108], [109], [110]]. Yu-Taeger et al. showed that MSC treatment attenuated sleep disturbances in R6/2 HD transgenic mice. Sleep deprivation resulted in an increase in Aβ within microglia and exacerbated microglial activation; these changes were associated with Bmal1 expression [111]. Recent evidence revealed that the relationship between circadian clock genes and neurodegenerative diseases involve not only neuron loss but also disease pathogenesis. A presenilin 1 or APP/PS1 transgenic mouse model of AD showed that the accumulation of amyloid plaques began at the same time as sleep-wake cycle impairment. As amyloid plaques spread, sleep disorders become more severe [112]. Interstitial fluid tau levels were significantly increased by sleep deprivation in both mice and humans [113]. Chronic short sleep causes sustained increases in tau oligomer spreading and aggregation [114]. Similarly, α-synuclein accumulation increases with increased neuronal activity during wakefulness caused by sleep deprivation [115,116]. These findings raise the possibility of developing therapeutic interventions targeting sleep disorders to treat neurodegenerative diseases. MSCs overexpressing Bmal1 can reverse aging-induced downregulation of Bmal1 [117]. Interestingly, in a 6-OHDA induced PD rat model, MSC-exo administration increased dopamine and serotonin levels by elevating the mRNA levels of CLOCK, BMAL1, and PER2 [118]. Peroxisome proliferation-activated receptor γ (PPARγ) inhibition caused neurodegeneration in animal models of several neurodegenerative diseases such as AD and HD [119,120]. Recovery of circadian rhythm-associated gene expression not only attenuated PD-associated sleep disorder but also ameliorated neurodegeneration and mitochondrial deficits by increasing PPARγ activities. These pilot studies provide a foundation for developing treatments for neurodegenerative diseases and clarifying the effects of MSCs on the circadian rhythm and sleep-wake disorders.
3. The role of MSC-based therapy in correspondence with antibody drug-based therapy for treating neurodegenerative diseases
The CNS is well-known for its immune privileges. Immune privilege in the brain is conferred via the BBB. The BBB strictly regulates molecular transport into the brain and restricts antibody entry from the peripheral blood. Therefore, the CNS is considered off-limits to monoclonal antibody (mAb) therapeutics [121]. However, recently developed mAbs that can penetrate the BBB were shown to exert therapeutic effects on the brain parenchyma [121]. Antibodies against Aβ [[122], [123], [124]] and tau protein [125,126] showed inhibitory effects against AD in both preclinical and clinical studies. Anti-α-synuclein immunotherapy for PD also showed preliminary preclinical successes [121,127]. mAb therapies for the treatment of neurodegenerative diseases have consistently shown promising results. Lecanemab and aducanumab are two new mAbs approved in the USA for treating AD [128]. Although mAbs are now considered a therapeutic approach for neurodegenerative diseases, studies are needed to overcome many major limitations, such as poor penetration and adverse side effects.
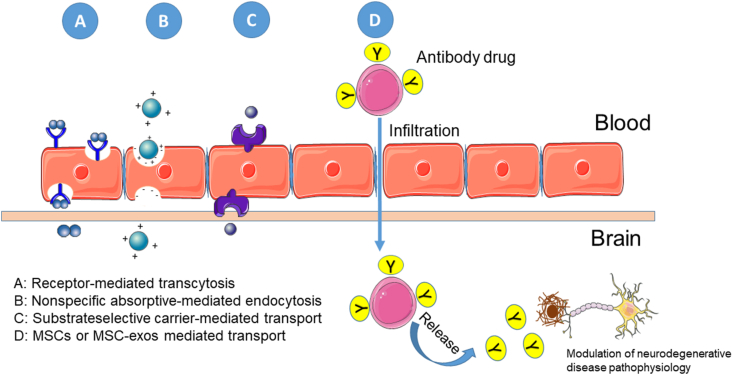
The poor penetration of mAbs through the BBB is closely associated with adverse drug reactions. The most common adverse drug reactions associated with mAb drugs for AD, such as aducanumab and lecanemab, is amyloid-related imaging abnormalities (ARIA). Only 0.1 % of circulating antibodies cross the BBB and penetrate the brain via three mechanisms: (1) nonspecific absorptive-mediated endocytosis, (2) substrate-selective carrier-mediated transport, and (3) receptor-mediated transcytosis [129]. The easiest strategy for penetrating more mAb into the CNS is increasing the dose. However, this can lead to an increase in adverse drug reactions. Scientists have taken advantage of routes that transport substrates in a more specific manner to develop a method for therapeutic antibodies to pass through the BBB at better-tolerated doses. A more specific route to the brain parenchyma, including carrier-mediated transport and receptor-mediated transcytosis, may enable transport of mAbs at lower doses than those used in absorptive-mediated endocytosis [121,130]. However, specificity must be optimized to avoid lysosomal degradation [[131], [132], [133]]. Thus, decreasing the mAb dose by increasing its specificity remains a limitation. Application of MSC-exos as a transport route for drugs to pass through the BBB has been frequently reported and reviewed [[134], [135], [136]]. Tashima suggested that loaded MSC-based drug delivery systems can infiltrate the BBB and release antibodies inside the brain tissue [137]. Thus, MSC-based delivery systems may greatly contribute to the development of new antibody drugs.
4. Limitations of MSC-based therapy for neurodegenerative diseases
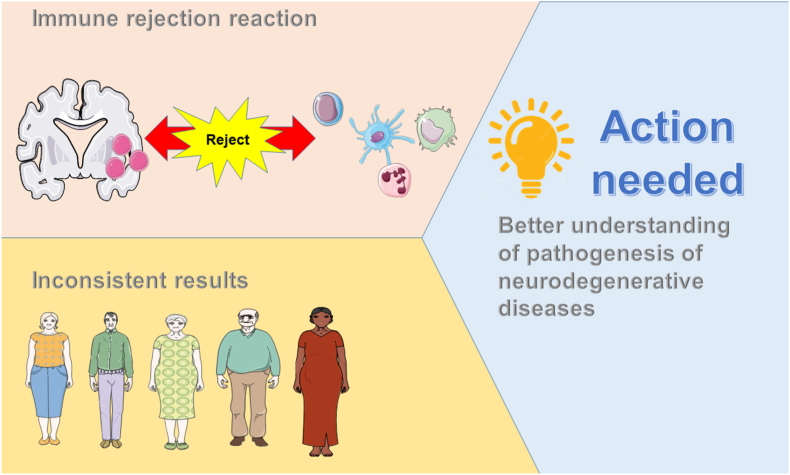
MSC-based therapies show potential for the treatment of neurodegenerative diseases. However, several limitations remain. MSCs can cause immune rejection. Methods for managing immune rejection should be developed along with the development of MSC-based therapies. Different approaches using mAbs have been attempted preclinically to replace immunosuppressive drugs but are associated with many adverse side effects [138]. Clinical studies of MSC-based therapy have provided inconsistent results because of the variability of patients, sources of MSCs, delivery routes, and outcomes [83]. The pathogenesis of neurodegenerative diseases should be extensively investigated to overcome these challenges (see Fig. 1, Fig. 2, Fig. 3).
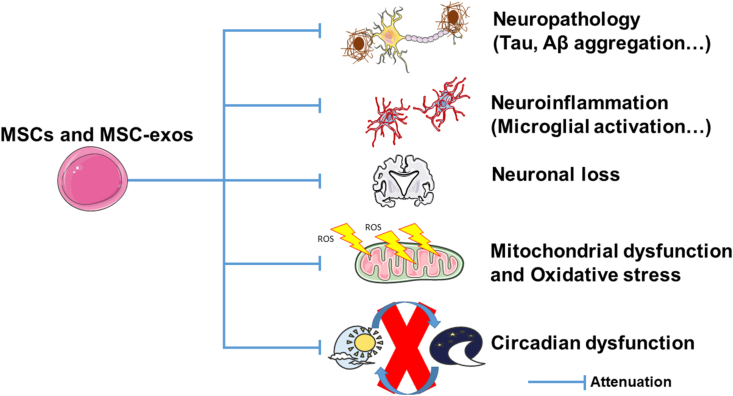
Fig. 1.
Mechanism of MSCs and MSC-exos therapy in treatment of neurodegenerative diseases.
Fig. 2.
MSCs application as antibody drug delivery system in treatment of neurodegenerative diseases.
Fig. 3.
Challenges of MSCs and MSC-exos therapy in treatment of neurodegenerative diseases in practice.
5. Conclusion
Regenerative medicine and stem cell transplantation have been developed and may serve as alternative treatments for neurodegenerative diseases. Numerous studies reported the positive effects of MSCs in the treatment of diverse neurodegenerative diseases, including AD, PD, ALS, and HD. In this study, we review several achievements in using MSCs in both preclinical and clinical studies. The protective effects of MSCs may be mainly exerted through their anti-inflammatory and anti-oxidative properties rather than their ability to replace lost neuronal cells. Therefore, the application of MSC-exos has been widely examined, and optimistic results indicate that progress is being made. In addition, circadian rhythm dysfunction linked to neurodegeneration was recently reported as a new target. Technology using MSC-exos may lead to new findings on the mechanism by which circadian clock genes mediate neurodegeneration and encourage new therapeutic approaches for better and safer treatments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Yao P., Zhou L., Zhu L., Zhou B., Yu Q. Mesenchymal stem cells: a potential therapeutic strategy for neurodegenerative diseases. Eur Neurol. Jul. 2020;83(3):235–241. doi: 10.1159/000509268. [DOI] [PubMed] [Google Scholar]
- 2.Gan L., Cookson M.R., Petrucelli L., La Spada A.R. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci. 2018;21(10):1300–1309. doi: 10.1038/s41593-018-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tröster A.I., Pulaski S.J., Woods S.P. In: Neuropsychology of movement disorders and motor neuron disease: Parkinson’s disease, progressive supranuclear palsy, essential tremor, Huntington’s disease, and amyotrophic lateral sclerosis BT - handbook of medical neuropsychology: applications of cogni. Armstrong C.L., Morrow L.A., editors. Springer International Publishing; Cham: 2019. pp. 415–440. [DOI] [Google Scholar]
- 4.Saljuqi M., Ghaderyan P. A novel method based on matching pursuit decomposition of gait signals for Parkinson's disease, Amyotrophic lateral sclerosis and Huntington's disease detection. Neurosci Lett. 2021;761 doi: 10.1016/j.neulet.2021.136107. [DOI] [PubMed] [Google Scholar]
- 5.Lee Mosley R., Benner Eric J., Kadiu Irena, Thomas Mark, Boska Michael D., Hasan Khader, et al. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson's disease. Clin Neurosci Res. Dec. 2006;6(5):261–281. doi: 10.1016/j.cnr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3(3):205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 7.Olufunmilayo E.O., Gerke-Duncan M.B., Holsinger R.M.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants. 2023;12(2) doi: 10.3390/antiox12020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon H.S., Koh S.-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9(1):42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganjam Goutham K., Bolte Kathrin, Matschke Lina A., Neitemeier Sandra, Dolga Amalia M., Höllerhage Matthias, et al. Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons. Cell Death Dis. 2019;10(11):865. doi: 10.1038/s41419-019-2091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin Eun-Joo, Tran Hai-Quyen, Nguyen Phuong-Tram, Jeong Ji Hoon, Nah Seung-Yeol, Jang Choon-Gon, et al. Role of mitochondria in methamphetamine-induced dopaminergic neurotoxicity: involvement in oxidative stress, neuroinflammation, and pro-apoptosis—a review. Neurochem Res. 2018;43(1):66–78. doi: 10.1007/s11064-017-2318-5. [DOI] [PubMed] [Google Scholar]
- 11.Angelova P.R., Esteras N., Abramov A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: finding ways for prevention. Med Res Rev. Mar. 2021;41(2):770–784. doi: 10.1002/med.21712. [DOI] [PubMed] [Google Scholar]
- 12.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rego A.C., Oliveira C.R. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28(10):1563–1574. doi: 10.1023/A:1025682611389. [DOI] [PubMed] [Google Scholar]
- 15.Kumar Asit, Zhou Lina, Zhi Kaining, Raji Babatunde, Pernell Shelby, Tadrous Erene, et al. Challenges in biomaterial-based drug delivery approach for the treatment of neurodegenerative diseases: opportunities for extracellular vesicles. Int J Mol Sci. 2020;22(1):138. doi: 10.3390/ijms22010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barchet T.M., Amiji M.M. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expet Opin Drug Deliv. Mar. 2009;6(3):211–225. doi: 10.1517/17425240902758188. [DOI] [PubMed] [Google Scholar]
- 17.Song G.J., Suk K. Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci. 2017;9 doi: 10.3389/fnagi.2017.00139. https://www.frontiersin.org/articles/10.3389/fnagi.2017.00139 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isik S., Yeman Kiyak B., Akbayir R., Seyhali R., Arpaci T. Microglia mediated neuroinflammation in Parkinson's disease. Cells. 2023;12(7) doi: 10.3390/cells12071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuny G.D. Neurodegenerative diseases: challenges and opportunities. Future Med Chem. Aug. 2012;4(13):1647–1649. doi: 10.4155/fmc.12.123. [DOI] [PubMed] [Google Scholar]
- 20.Coogan Andrew N., Schutová Barbora, Husung Susanne, Furczyk Karolina, Baune Bernhard T., Kropp Peter, et al. The circadian system in Alzheimer's disease: disturbances, mechanisms, and opportunities. Biol Psychiatr. 2013;74(5):333–339. doi: 10.1016/j.biopsych.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Cermakian N., Waddington Lamont E., Boudreau P., Boivin D.B. Circadian clock gene expression in brain regions of alzheimer ’s disease patients and control subjects. J Biol Rhythm. Mar. 2011;26(2):160–170. doi: 10.1177/0748730410395732. [DOI] [PubMed] [Google Scholar]
- 22.Videnovic A., Golombek D. Circadian dysregulation in Parkinson's disease. Neurobiol sleep circadian Rhythm. 2017;2:53–58. doi: 10.1016/j.nbscr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Videnovic A., Lazar A.S., Barker R.A., Overeem S. ’The clocks that time us’—circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10(12):683–693. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fifel K., Videnovic A. Circadian and sleep dysfunctions in neurodegenerative disorders—an update. Front Neurosci. 2021;14 doi: 10.3389/fnins.2020.627330. https://www.frontiersin.org/articles/10.3389/fnins.2020.627330 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Wenzhen, Li Rong, Ye Meilin, Zhang Lanxin, Zheng Jiawen, Yang Yuqing, et al. The circadian clock has roles in mesenchymal stem cell fate decision. Stem Cell Res Ther. 2022;13(1):200. doi: 10.1186/s13287-022-02878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benitah S.A., Welz P.-S. Circadian regulation of adult stem cell homeostasis and aging. Cell Stem Cell. 2020;26(6):817–831. doi: 10.1016/j.stem.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Salem H.K., Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cell. Mar. 2010;28(3):585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossant J. Stem cells from the mammalian blastocyst. Stem Cell. Nov. 2001;19(6):477–482. doi: 10.1634/stemcells.19-6-477. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. Aug. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Hansen D.K., Inselman A.L. In: Chapter 58 - applications of stem cells in developmental toxicology. R. C. B. T.-R., Gupta D.T., editors. Academic Press; San Diego: 2011. pp. 783–792. [DOI] [Google Scholar]
- 31.Blum B., N. B. T.-A. in C. R. Benvenisty . Academic Press; 2008. The tumorigenicity of human embryonic stem cells; pp. 133–158. [DOI] [PubMed] [Google Scholar]
- 32.Lo B., Parham L. Ethical issues in stem cell research. Endocr Rev. May 2009;30(3):204–213. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández-Susavila H., Bugallo-Casal A., Castillo J., Campos F. Adult stem cells and induced pluripotent stem cells for stroke treatment. Front Neurol. 2019;10 doi: 10.3389/fneur.2019.00908. https://www.frontiersin.org/articles/10.3389/fneur.2019.00908 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe R.G., Daley G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019;20(7):377–388. doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valadez-Barba Valeria, Cota-Coronado A., Hernández-Pérez O.R., Lugo-Fabres Pavel H., Padilla-Camberos Eduardo, Fabián DÃaz Néstor, et al. iPSC for modeling neurodegenerative disorders. Regen Ther. 2020;15:332–339. doi: 10.1016/j.reth.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scudellari M. How iPS cells changed the world. Nature. 2016;534(7607):310–312. doi: 10.1038/534310a. [DOI] [PubMed] [Google Scholar]
- 37.Pandey S., Jirásko M., Lochman J., Chvátal A., Chottova Dvorakova M., Kučera R. iPSCs in neurodegenerative disorders: a unique platform for clinical research and personalized medicine. J Personalized Med. 2022;12(9) doi: 10.3390/jpm12091485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chopp M., Zhang X.H., Li Y., Wang L., Chen J., Lu D., et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11(13) doi: 10.1097/00001756-200009110-00035. https://journals.lww.com/neuroreport/Fulltext/2000/09110/Spinal_cord_injury_in_rat__treatment_with_bone.35.aspx [Online]. Available: [DOI] [PubMed] [Google Scholar]
- 39.Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 40.Singer N.G., Caplan A.I. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. Jan. 2011;6(1):457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 41.Bronckaers Annelies, Hilkens Petra, Martens Wendy, Gervois Pascal, Ratajczak Jessica, Struys Tom, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181–196. doi: 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. Jun. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nitzsche F., Müller C., Lukomska B., Jolkkonen J., Deten A., Boltze J. Concise review: MSC adhesion cascade—insights into homing and transendothelial migration. Stem Cell. Jun. 2017;35(6):1446–1460. doi: 10.1002/stem.2614. [DOI] [PubMed] [Google Scholar]
- 44.Ullah M., Liu D.D., Thakor A.S. Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience. 2019;15:421–438. doi: 10.1016/j.isci.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunningham Miles G., Bolay Hayrunnisa, Scouten Charles W., Moore Christopher, Jacoby Douglas, Moskowitz Michael, et al. Preclinical evaluation of a novel intracerebral microinjection instrument permitting electrophysiologically guided delivery of therapeutics. Neurosurgery. 2004;54(6) doi: 10.1227/01.neu.0000125007.03145.00. https://journals.lww.com/neurosurgery/Fulltext/2004/06000/PRECLINICAL_EVALUATION_OF_A_NOVEL_INTRACEREBRAL.31.aspx [Online]. Available: [DOI] [PubMed] [Google Scholar]
- 46.Harting Matthew T., Jimenez Fernando, Xue Hasan, Fischer Uwe M., Baumgartner James, Dash Pramod K., et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury: laboratory investigation. J Neurosurg JNS. 2009;110(6):1189–1197. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kean T.J., Lin P., Caplan A.I., Dennis J.E. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cell Int. 2013;2013 doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kempuraj D., Thangavel R., Natteru P.A., Selvakumar G.P., Saeed D., Zahoor H., et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg spine. 2016;1(1) [PMC free article] [PubMed] [Google Scholar]
- 49.Wyss-Coray T., Mucke L. Inflammation in neurodegenerative disease—a double-edged sword. Neuron. Aug. 2002;35(3):419–432. doi: 10.1016/s0896-6273(02)00794-8. 10.1016/S0896-6273(02)00794-8 [DOI] [PubMed] [Google Scholar]
- 50.Allan S.M., Rothwell N.J. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2(10):734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 51.Hu S., Peterson P.K., Chao C.C. CYTOKINE-MEDIATED neuronal apoptosis. Neurochem Int. 1997;30(4):427–431. doi: 10.1016/S0197-0186(96)00078-2. [DOI] [PubMed] [Google Scholar]
- 52.Yamasaki Y., Matsuura N., Shozuhara H., Onodera H., Itoyama Y., Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. Apr. 1995;26(4):676–681. doi: 10.1161/01.STR.26.4.676. [DOI] [PubMed] [Google Scholar]
- 53.Lawrence C.B., Allan S.M., Rothwell N.J. Interleukin-1β and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur J Neurosci. Mar. 1998;10(3):1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 54.Barone F.C., Arvin B., White R.F., Miller A., Webb C.L., Willette R.N., et al. Tumor necrosis factor-α. Stroke. Jun. 1997;28(6):1233–1244. doi: 10.1161/01.STR.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 55.Hwang Y., Park J.H., Kim H.-C., Shin E.-J. GABAB receptor activation alters astrocyte phenotype changes induced by trimethyltin via ERK signaling in the dentate gyrus of mice. Life Sci. 2023;319 doi: 10.1016/j.lfs.2023.121529. [DOI] [PubMed] [Google Scholar]
- 56.Han Yuyi, Yang Jianxin, Fang Jiankai, Zhou Yipeng, Candi Eleonora, Wang Jihong, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Targeted Ther. 2022;7(1):92. doi: 10.1038/s41392-022-00932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren Guangwen, Zhao Xin, Zhang Liying, Zhang Jimin, L'Huillier Andrew, Ling Weifang, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. Mar. 2010;184(5):2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Do A.D., Kurniawati I., Hsieh C.-L., Wong T.-T., Lin Y.-L., Sung S.-Y. Application of mesenchymal stem cells in targeted delivery to the brain: potential and challenges of the extracellular vesicle-based approach for brain tumor treatment. Int J Mol Sci. 2021;22(20) doi: 10.3390/ijms222011187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Yu-Yan, Li Yun, Wang Lu, Zhao Yan, Yuan Rui, Yang Meng-Meng, et al. Mesenchymal stem cell-derived exosomes regulate microglia phenotypes: a promising treatment for acute central nervous system injury. Neural Regen Res. Aug. 2023;18(8):1657–1665. doi: 10.4103/1673-5374.363819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen Liang, Wang Ya-Dong, Shen Dong-Feng, Zheng Pei-Dong, Tu Meng-Di, You Wen-Dong, et al. Exosomes derived from bone marrow mesenchymal stem cells inhibit neuroinflammation after traumatic brain injury. Neural Regen Res. Dec. 2022;17(12):2717–2724. doi: 10.4103/1673-5374.339489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y., Gan Y., Xu G., Yin G., Liu D. MSCs-derived exosomes attenuate acute brain injury and inhibit microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization. Neurochem Res. 2020;45(5):1180–1190. doi: 10.1007/s11064-020-02998-0. [DOI] [PubMed] [Google Scholar]
- 62.Pang Qi-Ming, Chen Si-Yu, Fu Sheng-Ping, Zhou Hui, Zhang Qian, Ao Jun, et al. Regulatory role of mesenchymal stem cells on secondary inflammation in spinal cord injury. J Inflamm Res. Jan. 2022;15:573–593. doi: 10.2147/JIR.S349572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener. 2019;14(1):32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jack Clifford R., Jr., Albert Marilyn S., Knopman David S., McKhann Guy M., Sperling Reisa A., Carrillo Maria C., et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. May 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madnani R.S. Alzheimer's disease: a mini-review for the clinician. Front Neurol. 2023;14 doi: 10.3389/fneur.2023.1178588. https://www.frontiersin.org/articles/10.3389/fneur.2023.1178588 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farahzadi R., Fathi E., Vietor I. Mesenchymal stem cells could Be considered as a candidate for further studies in cell-based therapy of Alzheimer's disease via targeting the signaling pathways. ACS Chem Neurosci. May 2020;11(10):1424–1435. doi: 10.1021/acschemneuro.0c00052. [DOI] [PubMed] [Google Scholar]
- 67.Jia Yali, Cao Ning, Zhai Jinglei, Zeng Quan, Zheng Pei, Su Ruyu, et al. HGF mediates clinical-grade human umbilical cord-derived mesenchymal stem cells improved functional recovery in a senescence-accelerated mouse model of Alzheimer's disease. Adv Sci. Sep. 2020;7(17) doi: 10.1002/advs.201903809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui YuanBo, Ma ShanShan, Zhang ChunYan, Cao Wei, Liu Min, Li DongPeng, et al. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer's disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res. 2017;320:291–301. doi: 10.1016/j.bbr.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 69.Zheng Xiao-Yu, Wan Qian-Quan, Zheng Chuan-Yi, Zhou Hong-Long, Dong Xing-Yu, Deng Qing-Shan, et al. Amniotic mesenchymal stem cells decrease Aβ deposition and improve memory in APP/PS1 transgenic mice. Neurochem Res. 2017;42(8):2191–2207. doi: 10.1007/s11064-017-2226-8. [DOI] [PubMed] [Google Scholar]
- 70.Hu W., Feng Z., Xu J., Jiang Z., Feng M. Brain-derived neurotrophic factor modified human umbilical cord mesenchymal stem cells-derived cholinergic-like neurons improve spatial learning and memory ability in Alzheimer's disease rats. Brain Res. 2019;1710:61–73. doi: 10.1016/j.brainres.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 71.Wang Xinxin, Ma Shanshan, Yang Bo, Huang Tuanjie, Meng Nan, Xu Ling, et al. Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer's disease. Behav Brain Res. 2018;339:297–304. doi: 10.1016/j.bbr.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nasiri E., Alizadeh A., Roushandeh A.M., Gazor R., Hashemi-Firouzi N., Golipoor Z. Melatonin-pretreated adipose-derived mesenchymal stem cells efficeintly improved learning, memory, and cognition in an animal model of Alzheimer's disease. Metab Brain Dis. 2019;34(4):1131–1143. doi: 10.1007/s11011-019-00421-4. [DOI] [PubMed] [Google Scholar]
- 73.Ramezani M., Komaki A., Hashemi-Firouzi N., Mortezaee K., Faraji N., Golipoor Z. Therapeutic effects of melatonin-treated bone marrow mesenchymal stem cells (BMSC) in a rat model of Alzheimer's disease. J Chem Neuroanat. 2020;108 doi: 10.1016/j.jchemneu.2020.101804. [DOI] [PubMed] [Google Scholar]
- 74.Chen Yi-An, Lu Cheng-Hsiu, Ke Chien-Chih, Chiu Sain-Jhih, Jeng Fong-Shya, Chang Chi-Wei, et al. Mesenchymal stem cell-derived exosomes ameliorate Alzheimer's disease pathology and improve cognitive deficits. Biomedicines. 2021;9(6) doi: 10.3390/biomedicines9060594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Contreras M., Thakor A.S. Human adipose tissue-derived mesenchymal stem cells and their extracellular vesicles modulate lipopolysaccharide activated human microglia. Cell Death Dis. 2021;7(1):98. doi: 10.1038/s41420-021-00471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei H., Xu Y., Chen Q., Chen H., Zhu X., Li Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020;11(4):290. doi: 10.1038/s41419-020-2490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakano M., Kubota Kenta, Kobayashi Eiji, Chikenji Takako S., Saito Yuki, Konari Naoto, et al. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer's disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-67460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rahbaran Mohaddeseh, Zekiy Angelina Olegovna, Bahramali Mahta, Jahangir Mohammadsaleh, Mardasi Mahsa, Sakhaei Delaram, et al. Therapeutic utility of mesenchymal stromal cell (MSC)-based approaches in chronic neurodegeneration: a glimpse into underlying mechanisms, current status, and prospects. Cell Mol Biol Lett. 2022;27(1):56. doi: 10.1186/s11658-022-00359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim Hee Jin, Seo Sang Won, Chang Jong Wook, Lee Jung Il, Kim Chi Hun, Chin Juhee, et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: a phase 1 clinical trial. Alzheimer’s Dement Transl Res Clin Interv. Sep. 2015;1(2):95–102. doi: 10.1016/j.trci.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim Hee Jin, Cho Kyung Rae, Jang Hyemin, Lee Na Kyung, Jung Young Hee, Kim Jun Pyo, et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: a phase I clinical trial. Alzheimer's Res Ther. 2021;13(1):154. doi: 10.1186/s13195-021-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poewe Werner, Seppi Klaus, Tanner Caroline M., Halliday Glenda M., Brundin Patrik, Volkmann Jens, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3(1) doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 82.Stern M.B., Lang A., Poewe W. Toward a redefinition of Parkinson's disease. Mov Disord. Jan. 2012;27(1):54–60. doi: 10.1002/mds.24051. [DOI] [PubMed] [Google Scholar]
- 83.Fričová D., Korchak J.A., Zubair A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. npj Regen Med. 2020;5(1):20. doi: 10.1038/s41536-020-00106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shin Eun-Joo, Jeong Ji Hoon, Hwang Yeonggwang, Sharma Naveen, Dang Duy-Khanh, Nguyen Bao-Trong, et al. Methamphetamine-induced dopaminergic neurotoxicity as a model of Parkinson's disease. Arch Pharm Res (Seoul) 2021;44(7):668–688. doi: 10.1007/s12272-021-01341-7. [DOI] [PubMed] [Google Scholar]
- 85.Shin Eun-Joo, Jeong Ji Hoon, Sharma Garima, Sharma Naveen, Kim Dae-Joong, Pham Duc Toan, et al. Protein kinase Cδ mediates methamphetamine-induced dopaminergic neurotoxicity in mice via activation of microsomal epoxide hydrolase. Food Chem Toxicol. 2019;133 doi: 10.1016/j.fct.2019.110761. [DOI] [PubMed] [Google Scholar]
- 86.Tate Ciara C., Chou Vivian P., Campos Carla, Moalem Alimohammed S., Di Monte Donato A., McGrogan Michael, et al. Mesenchymal stromal SB623 cell implantation mitigates nigrostriatal dopaminergic damage in a mouse model of Parkinson's disease. J Tissue Eng Regen Med. Jun. 2017;11(6):1835–1843. doi: 10.1002/term.2081. [DOI] [PubMed] [Google Scholar]
- 87.Wolff Erin F., Gao Xiao-Bing, Yao Katherine V., Andrews Zane B., Du Hongling, Elsworth John D., et al. Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model. J Cell Mol Med. Apr. 2011;15(4):747–755. doi: 10.1111/j.1582-4934.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chao Y.X., He B.P., Tay S.S.W. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson's disease. J Neuroimmunol. Nov. 2009;216(1):39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki Syuuichirou, Kawamata Jun, Iwahara Naotoshi, Matsumura Akihiro, Hisahara Shin, Matsushita Takashi, et al. Intravenous mesenchymal stem cell administration exhibits therapeutic effects against 6-hydroxydopamine-induced dopaminergic neurodegeneration and glial activation in rats. Neurosci Lett. 2015;584:276–281. doi: 10.1016/j.neulet.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 90.Chen Hong-Xu, Liang Fu-Chao, Gu Ping, Xu Bian-Ling, Xu Hong-Jun, Wang Wen-Ting, et al. Exosomes derived from mesenchymal stem cells repair a Parkinson's disease model by inducing autophagy. Cell Death Dis. 2020;11(4):288. doi: 10.1038/s41419-020-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xue Chunling, Li Xuechun, Ba Li, Zhang Mingjia, Yang Ying, Gao Yang, et al. MSC-derived exosomes can enhance the angiogenesis of human brain MECs and show therapeutic potential in a mouse model of Parkinson's disease. Aging Dis. 2021;12(5):1211. doi: 10.14336/AD.2020.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hardiman Orla, Al-Chalabi Ammar, Chio Adriano, Corr Emma M., Logroscino Giancarlo, Wim Robberecht Wim, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Prim. 2017;3(1) doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 93.Zhao C.-P., Zhang C., Zhou S.N., Xie Y.-M., Wang Y.-H., Huang H., et al. Human mesenchymal stromal cells ameliorate the phenotype of SOD1-G93A ALS mice. Cytotherapy. Jan. 2007;9(5):414–426. doi: 10.1080/14653240701376413. [DOI] [PubMed] [Google Scholar]
- 94.Vercelli A., Mereuta O.M., Garbossa D., Muraca G., Mareschi K., Rustichelli D., et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31(3):395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 95.Gugliandolo A., Bramanti P., Mazzon E. Mesenchymal stem cells: a potential therapeutic approach for amyotrophic lateral sclerosis? Stem Cell Int. 2019;2019 doi: 10.1155/2019/3675627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Irfan Zainab, Khanam Sofia, Karmakar Varnita, Mohammed Firdous Sayeed, El Khier Bothaina Samih Ismail Abou, Khan Ilyas, et al. Pathogenesis of Huntington's disease: an emphasis on molecular pathways and prevention by natural remedies. Brain Sci. 2022;12(10) doi: 10.3390/brainsci12101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rossignol Julien, Boyer Cécile, Lévèque Xavier, Fink Kyle D., Thinard Reynald, Blanchard Frédéric, et al. Mesenchymal stem cell transplantation and DMEM administration in a 3NP rat model of Huntington's disease: morphological and behavioral outcomes. Behav Brain Res. 2011;217(2):369–378. doi: 10.1016/j.bbr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Snyder B.R., Chiu A.M., Prockop D.J., Chan A.W.S. Human multipotent stromal cells (MSCs) increase neurogenesis and decrease atrophy of the striatum in a transgenic mouse model for Huntington's disease. PLoS One. Feb. 2010;5(2):e9347. doi: 10.1371/journal.pone.0009347. [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pollock Kari, Dahlenburg Heather, Nelson Haley, Fink Kyle D., Cary Whitney, Hendrix Kyle, et al. Human mesenchymal stem cells genetically engineered to overexpress brain-derived neurotrophic factor improve outcomes in Huntington's disease mouse models. Mol Ther. May 2016;24(5):965–977. doi: 10.1038/mt.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leng Y., Musiek E.S., Hu K., Cappuccio F.P., Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. Mar. 2019;18(3):307–318. doi: 10.1016/S1474-4422(18)30461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voysey Z., V Fazal S., Lazar A.S., Barker R.A. The sleep and circadian problems of Huntington's disease: when, why and their importance. J Neurol. Jun. 2021;268(6):2275–2283. doi: 10.1007/s00415-020-10334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Zhilin, Liu Qiang, Peng Yu, Dai Jiaying, Xie Youna, Chen Weineng, et al. Circadian rhythm dysfunction accelerates disease progression in a mouse model with amyotrophic lateral sclerosis. Front Neurol. Apr. 2018;9:218. doi: 10.3389/fneur.2018.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Musiek Erik S., Lim Miranda M., Yang Guangrui, Bauer Adam Q., Qi Laura, Lee Yool, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. Dec. 2013;123(12):5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Musiek E.S., Holtzman D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. Nov. 2016;354(6315):1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharma Naveen, Shin Eun-Joo, Pham Duc Toan, Sharma Garima, Dang Duy-Khanh, Duong Chu Xuan, et al. GPx-1-encoded adenoviral vector attenuates dopaminergic impairments induced by methamphetamine in GPx-1 knockout mice through modulation of NF-κB transcription factor. Food Chem Toxicol. 2021;154 doi: 10.1016/j.fct.2021.112313. [DOI] [PubMed] [Google Scholar]
- 106.Sharma Garima, Shin Eun-Joo, Sharma Naveen, Nah Seung-Yeol, Mai Huynh Nhu, Nguyen Bao Trong, et al. Glutathione peroxidase-1 and neuromodulation: novel potentials of an old enzyme. Food Chem Toxicol. 2021;148 doi: 10.1016/j.fct.2020.111945. [DOI] [PubMed] [Google Scholar]
- 107.Stavely R., Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. Sep. 2020;9(9):985–1006. doi: 10.1002/sctm.19-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goodman A.O.G., Morton A.J., Barker R.A. Identifying sleep disturbances in Huntington's disease using a simple disease-focused questionnaire. PLoS Curr. Oct. 2010;2:RRN1189. doi: 10.1371/currents.RRN1189. RRN1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morton A.J., Wood N.I., Hastings M.H., Hurelbrink C., Barker R.A., Maywood E.S. Disintegration of the sleep-wake cycle and circadian timing in huntington's disease. J Neurosci. Jan. 2005;25(1):157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cuesta M., Aungier J., Morton A.J. The methamphetamine-sensitive circadian oscillator is dysfunctional in a transgenic mouse model of Huntington's disease. Neurobiol Dis. 2012;45(1):145–155. doi: 10.1016/j.nbd.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 111.Parhizkar Samira, Gent Grace, Chen Yun, Rensing Nicholas, Gratuze Maud, Strout Gregory, et al. Sleep deprivation exacerbates microglial reactivity and Aβ deposition in a TREM2-dependent manner in mice. Sci Transl Med. Aug. 2023;15(693):eade6285. doi: 10.1126/scitranslmed.ade6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roh Jee Hoon, Huang Yafei, Bero Adam W., Kasten Tom, Stewart Floy R., Bateman Randall J., et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. Sep. 2012;4(150):150ra122. doi: 10.1126/scitranslmed.3004291. 150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holth Jerrah K., Fritschi Sarah K., Wang Chanung, Pedersen Nigel P., Cirrito John R., Mahan Thomas E., et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. Feb. 2019;363(6429):880–884. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu Yan, Zhan Guanxia, Fenik Polina, Brandes Madison, Bell Patrick, Francois Noelle, et al. Chronic sleep disruption advances the temporal progression of tauopathy in P301S mutant mice. J Neurosci. Nov. 2018;38(48):10255 LP–10270. doi: 10.1523/JNEUROSCI.0275-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamada K., Iwatsubo T. Extracellular α-synuclein levels are regulated by neuronal activity. Mol Neurodegener. 2018;13(1):9. doi: 10.1186/s13024-018-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uemura N., Uemura M.T., Luk K.C., Lee V.M.-Y., Trojanowski J.Q. Cell-to-Cell transmission of tau and α-synuclein. Trends Mol Med. Oct. 2020;26(10):936–952. doi: 10.1016/j.molmed.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cha S., Wang J., Lee S.M., Tan Z., Zhao Q., Bai D. Clock-modified mesenchymal stromal cells therapy rescues molecular circadian oscillation and age-related bone loss via miR142-3p/Bmal1/YAP signaling axis. Cell Death Dis. 2022;8(1):111. doi: 10.1038/s41420-022-00908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Z., Li Y., Xu X., Gu J., Chen H., Gui Y. Exosomes rich in Wnt5 improved circadian rhythm dysfunction via enhanced PPARγ activity in the 6-hydroxydopamine model of Parkinson's disease. Neurosci Lett. 2023;802 doi: 10.1016/j.neulet.2023.137139. [DOI] [PubMed] [Google Scholar]
- 119.Li Zheng-Yi, Chung Yoon Hee, Shin Eun-Joo, Dang Duy-Khanh, Jeong Ji Hoon, Ko Sung Kwon, et al. YY-1224, a terpene trilactone-strengthened Ginkgo biloba, attenuates neurodegenerative changes induced by β-amyloid (1-42) or double transgenic overexpression of APP and PS1 via inhibition of cyclooxygenase-2. J Neuroinflammation. 2017;14(1):94. doi: 10.1186/s12974-017-0866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Corona J.C., Duchen M.R. PPARγ and PGC-1α as therapeutic targets in Parkinson's. Neurochem Res. 2015;40(2):308–316. doi: 10.1007/s11064-014-1377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu Y.J., Watts R.J. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. Jul. 2013;10(3):459–472. doi: 10.1007/s13311-013-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adolfsson Oskar, Pihlgren Maria, Toni Nicolas, Varisco Yvan, Buccarello Anna Lucia, Antoniello Katia, et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci. Jul. 2012;32(28):9677 LP–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Farlow Martin, Arnold Steven E., van Dyck Christopher H., Aisen Paul S., Snider B. Joy, Porsteinsson Anton P., et al. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimer's Dementia. Jul. 2012;8(4):261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 124.Ostrowitzki Susanne, Deptula Dennis, Thurfjell Lennart, Barkhof Frederik, Bohrmann Bernd, Brooks David J., et al. Mechanism of amyloid removal in patients with alzheimer disease treated with gantenerumab. Arch Neurol. Feb. 2012;69(2):198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 125.Chai Xiyun, Wu Su, Murray Tracey K., Kinley Robert, Cella Claire V., Sims Helen, et al. Passive immunization with anti-tau antibodies in two transgenic models: reduction of tau pathology and delay of disease progression. J Biol Chem. Sep. 2011;286(39):34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boutajangout A., Ingadottir J., Davies P., Sigurdsson E.M. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118(4):658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Masliah Eliezer, Rockenstein Edward, Adame Anthony, Alford Michael, Crews Leslie, Hashimoto Makoto, et al. Effects of α-synuclein immunization in a mouse model of Parkinson's disease. Neuron. Jun. 2005;46(6):857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 128.Cummings J. Anti-amyloid monoclonal antibodies are transformative treatments that redefine Alzheimer's disease therapeutics. Drugs. 2023;83(7):569–576. doi: 10.1007/s40265-023-01858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kouhi A., Pachipulusu V., Kapenstein T., Hu P., Epstein A.L., Khawli L.A. Brain disposition of antibody-based therapeutics: dogma, approaches and perspectives. Int J Mol Sci. 2021;22(12):6442. doi: 10.3390/ijms22126442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao P., Zhang N., An Z. Engineering antibody and protein therapeutics to cross the blood–brain barrier. Antib Ther. 2022;5(4):311–331. doi: 10.1093/abt/tbac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Niewoehner Jens, Bohrmann Bernd, Collin Ludovic, Urich Eduard, Sade Hadassah, Maier Peter, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. Jan. 2014;81(1):49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 132.Kariolis Mihalis S., Wells Robert C., Getz Jennifer A., Kwan Wanda, Mahon Cathal S., Tong Raymond, et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med. May 2020;12(545):eaay1359. doi: 10.1126/scitranslmed.aay1359. [DOI] [PubMed] [Google Scholar]
- 133.Yu Y. Joy, Zhang Yin, Kenrick Margaret, Hoyte Kwame, Luk Wilman, Lu Yanmei, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. May 2011;3(84):84ra44. doi: 10.1126/scitranslmed.3002230. 84ra44. [DOI] [PubMed] [Google Scholar]
- 134.Ghasempour E., Hesami S., Movahed E., keshel S.H., Doroudian M. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy in the brain tumors. Stem Cell Res Ther. 2022;13(1):527. doi: 10.1186/s13287-022-03212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang Z., Li Y., Wang Z. Recent advances in the application of mesenchymal stem cell-derived exosomes for cardiovascular and neurodegenerative disease therapies. Pharmaceutics. 2022;14(3) doi: 10.3390/pharmaceutics14030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rao D., Huang D., Sang C., Zhong T., Zhang Z., Tang Z. Advances in mesenchymal stem cell-derived exosomes as drug delivery vehicles. Front Bioeng Biotechnol. 2022;9 doi: 10.3389/fbioe.2021.797359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tashima T. Mesenchymal stem cell (MSC)-Based drug delivery into the brain across the blood–brain barrier. Pharmaceutics. 2024;16(2) doi: 10.3390/pharmaceutics16020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McGinley Lisa M., Chen Kevin S., Mason Shayna N., Rigan Diana M., Kwentus Jacquelin F., Hayes John M., et al. Monoclonal antibody-mediated immunosuppression enables long-term survival of transplanted human neural stem cells in mouse brain. Clin Transl Med. 2022;12(9):e1046. doi: 10.1002/ctm2.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]