Abstract
Zinc ions are required by all known organisms. Maintaining zinc homeostasis by preventing toxic overload while ensuring sufficient acquisition for cellular functions is crucial for survival and growth of bacteria. Bacteria, however, frequently encounter and must survive in various environments. During infection in host animals, for example, bacteria are exposed to acidic conditions in the stomach and anaerobic conditions in the intestines, but the effects of oxygen on zinc homeostasis in Escherichia coli have not been well-studied. Previously, we reported a flavin-binding fluorescent protein-based zinc sensor, CreiLOVN41C, which can respond to changes in labile Zn2+ levels in bacteria under both aerobic and anaerobic conditions. Here, we combined the use of CreiLOVN41C with established oxygen-dependent fluorescent protein-based sensors, inductively coupled plasma-mass spectrometry, and growth curves to evaluate how oxygen levels affect zinc uptake in E. coli. Inductively coupled plasma-mass spectrometry results showed that cells grown aerobically with added zinc acquired more zinc, but no additional zinc was accumulated when cells were grown anaerobically. Using oxygen-independent CreiLOVN41C and the oxygen-dependent ZapCY series of sensors, intracellular labile zinc was detected in E. coli grown with varied zinc under varied conditions. Although little to no endogenous zinc was detected by any sensor in E. coli cells grown with up to 2 mM added zinc, CreiLOVN41C revealed that when Zn2+ was added and detected by cells in real-time, anaerobic cells required more Zn2+ to similarly saturate the sensor. Overall, this work reveals that zinc uptake in E. coli is impacted by oxygen levels during cell growth.
Keywords: zinc, metal, Escherichia coli, biosensor, fluorescence, FRET, microscopic imaging, protein chemistry
Zinc is an essential trace element for all living organisms and plays crucial roles in numerous cellular processes. Zinc ions serve as biological signaling molecules, catalytic cofactors, and are important for structural stabilization of many proteins (1, 2, 3). The total cellular zinc content, or zinc quota, varies from ∼108 atoms/mammalian cell to ∼107 atoms/yeast cell and ∼105 atoms/bacterial cell, but when adjusted for the different cell volumes, the total cellular zinc concentration is in the range of 0.1 to 0.5 mM (4, 5, 6, 7, 8). Intracellular zinc can be divided into a large pool of inaccessible zinc ions which are usually tightly bound to proteins and a smaller pool of labile zinc ions that are accessible or loosely bound to small molecules or proteins. To maintain cellular zinc ions at adequate levels to fulfill the nutrient requirement while excluding toxic excess, cells use a variety of transport, storage, and chaperone proteins (9, 10, 11). Although zinc is required for many cellular functions, protein mismetallation can occur in the presence of excess Zn2+ ions, thereby disrupting cellular functions and inhibiting bacterial cell growth (12, 13). Bacteria in the gastrointestinal tract of a host organism must compete for Zn2+ ions. As an important component of nutritional immunity, host organisms can restrict zinc bioavailability or impose a toxic overload of zinc on bacterial pathogens (14, 15, 16, 17). The mechanisms involved in maintaining zinc homeostasis have been relatively well-studied for model organisms like Escherichia coli and various pathogens, but many studies have focused on changes directly imposed by varied metal availabilities (17, 18, 19). Bacteria in different growth environments can encounter numerous environmental changes, including in oxygen levels, which are low in the gastrointestinal tract (20). E. coli is known to undergo various metabolic changes in the transition from aerobic to anaerobic growth, and changes in copper and iron uptake have been examined under these conditions, but the impact of oxygen levels on zinc homeostasis is not understood (21, 22, 23, 24, 25).
One approach to studying the mechanisms involved in cellular metal uptake, transport, and storage is to use fluorescent metal ion sensors. Many fluorescent Zn2+ sensors have been developed over the last few decades, ranging from synthetic small molecule probes to genetically encoded sensors and chemigenetic probes (26, 27, 28). Genetically encoded fluorescent protein-based sensors are particularly attractive for detecting biological zinc because they can be selectively expressed in specific cell types, tissues, or organisms. For bacteria, which are typically found in multispecies ecosystems, this feature is especially beneficial. Fluorescent protein-based sensors have been broadly used to detect, quantify, and track labile pools of zinc ions, but have thus far been applied primarily to mammalian systems (28). The small size and crowded intracellular environment of bacterial cells requires sensors to be expressed at low enough levels to avoid aggregation while maintaining sufficient brightness and sensitivity for metal detection. Additionally, many bacteria, including a large fraction of gut bacteria, are anaerobes and cannot grow in the presence of oxygen. Most fluorescent protein-based zinc sensors to date are based on the green fluorescent protein (GFP), which requires oxygen for maturation of the fluorescent chromophore (29, 30).
Our group recently reported the first oxygen-independent fluorescent protein-based zinc ion sensor using a mutant of a flavin-binding fluorescent protein, CreiLOV (31). CreiLOV noncovalently binds the ubiquitous flavin mononucleotide cofactor and does not require oxygen for fluorescence (32). CreiLOV has an inherent sensitivity to copper(I) and copper(II) ions and can be quenched with dissociation constants of 12 μM and 0.1 nM, respectively (31, 33). By introducing an additional cysteine in the hypothesized copper binding site, we generated CreiLOVN41C, which simultaneously exhibited an improved copper affinity and a new zinc sensitivity (31). Zinc ions quench the fluorescence of CreiLOVN41C reversibly and with Kd = 1 nM. CreiLOVN41C exhibits strong binding affinity to Cu2+ (Kd = 0.066 fM). Using E. coli BL21(DE3) cells, we showed that CreiLOVN41C could respond reversibly to exogenously added zinc ions and could also be used to monitor changes in endogenous zinc when cells were grown in media with varied concentrations of zinc. Since E. coli BL21(DE3) cells do not grow well anaerobically, we tested the function of the sensor under anaerobic conditions using E. coli MG1655 cells. We found that different concentrations of zinc in the growth medium were required to elicit a sensor response in aerobic versus anaerobic conditions. CreiLOVN41C was noticeably quenched by endogenous zinc in aerobically grown E. coli when the medium contained as little as ∼0.5 to 1.0 mM ZnCl2. On the other hand, at least 2.0 mM ZnCl2 was required to elicit a response in anaerobically grown E. coli MG1655. At the time, it was not clear if this difference was due to the different oxygen levels or the different E. coli strains.
In this work, we aimed to use CreiLOVN41C to monitor zinc dynamics using the same strain of E. coli (MG1655). We also evaluated the effects of varied zinc concentrations on E. coli growth under aerobic and anaerobic conditions by analyzing the growth kinetics, comparing the effects of different zinc salts, and quantifying total metal ion uptake using inductively coupled plasma-mass spectrometry (ICP-MS). At the same time, we sought to compare the performance of CreiLOVN41C in aerobically grown E. coli with a well-established GFP-based zinc sensor, ZapCY2 (34). ZapCY2 is a Förster resonance energy transfer (FRET)-based sensor where the donor protein, cyan fluorescent protein, is connected to the acceptor, yellow fluorescent protein, by a zinc-finger based zinc binding domain. Zinc binding modulates the FRET response and with sensor calibration and prior knowledge of the zinc dissociation constant, ZapCY2 can be used to quantify Zn2+ ions. Given that CreiLOVN41C and ZapCY2 have similar affinities for Zn2+ ions (Kd = 0.8–1.0 nM), we reasoned that the sensors should yield similar results when monitoring zinc in aerobically grown E. coli (31, 34). Collectively, we found that the total zinc content of aerobically grown E. coli increased when Zn2+ was added to the medium but stayed the same for anaerobically grown E. coli. The growth kinetics of anaerobically grown E. coli were impaired with excess Zn2+ regardless of the ion source (organic gluconate or inorganic chloride), but Zn2+-gluconate was less toxic to aerobically grown E. coli at high levels. All fluorescent protein-based sensors tested, oxygen-independent CreiLOVN41C and oxygen-dependent ZapCY1-2, responded to exogenously added zinc in aerobically grown E. coli. Neither CreiLOVN41C nor ZapCY2 detected endogenous zinc in E. coli grown aerobically in 2 mM ZnCl2, but the higher affinity ZapCY1 sensor (Kd = 2.53 pM) did (34). When Zn2+ influx was measured in real-time, both CreiLOVN41C and ZapCY2 responded to Zn2+ in aerobically grown E. coli. We found that higher concentrations of Zn2+ were required to detect real-time Zn2+ influx in anaerobically grown cells, confirming that the oxygen level directly impacts zinc uptake. Overall, by using complementary zinc detection methods, ICP-MS for total cellular zinc and fluorescent sensors for labile zinc, we demonstrated that the oxygen level in the growth environment alters zinc homeostasis in E. coli. Moreover, CreiLOVN41C was shown to be a powerful tool for tracking zinc in aerobic and anaerobic live E. coli cells.
Results
Zn2+ effects on growth of E. coli under aerobic and anaerobic conditions
We investigated the effects of Zn2+ on the growth kinetics of E. coli cultured under aerobic and anaerobic conditions. For both aerobic and anaerobic conditions, E. coli was grown in LB, a rich medium, supplemented with ZnCl2 (0–2 mM), and the OD600 was recorded until the cells reached the stationary phase (Fig. S1, A and B). The resulting growth curves were each fitted for the growth parameters, lag time, growth rate, and the maximum optical density at 600 nm (max OD, Fig. 1). As the concentration of added Zn2+ increased, the lag time for E. coli grown aerobically increased and the growth rate decreased (Fig. 1, A and B). The presence of higher Zn2+ concentrations in the growth medium, however, did not affect the maximum OD600 (Fig. 1C). Previously, we found no effects of Zn2+ on another strain of E. coli, BW25113, when grown aerobically with Zn2+ up to 1 mM in minimal medium (35). When E. coli was grown anaerobically, the lag time and growth rate followed the same trends as observed for aerobic growth conditions; however, the maximum OD600 of anaerobic E. coli was negatively affected by increased Zn2+ concentrations (Fig. 1, D–F). These results suggest that high Zn2+ concentrations (>∼1 mM) are detrimental to both aerobically and anaerobically grown E. coli. Additionally, anaerobically grown E. coli is more susceptible to Zn2+ toxicity since the max OD level decreased with increasing Zn2+ concentrations, whereas aerobic E. coli grew slower with added Zn2+ but was able to recover and reach a similar max OD for all Zn2+ concentrations.
Figure 1.
Effects of varied ZnCl2concentrations on growth kinetics parameters for Escherichia coli grown under aerobic (labeled with +O2) and anaerobic (labeled with –O2) conditions. A–C, growth parameters for aerobically grown E. coli, (D–F) growth parameters for anaerobically grown E. coli. Lag time, growth rate, and max OD600 represent the mean ± SEM of three biological replicates for each condition and Zn2+ concentration. Growth parameters were calculated from growth curves (Fig. S1, A and B) using nonlinear regression curve fitting to a four-parameter Logistic equation with GraphPad Prism 9 software. NS, not significant; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗∗p ≤ 0.0001 as determined by one-way ANOVA with Tukey multiple comparison test.
Given that organic zinc sources may have higher zinc bioavailability and different impacts on bacterial growth than inorganic zinc sources (36, 37), we also investigated whether zinc gluconate, an organic zinc source, would have similar effects on the growth of E. coli as ZnCl2, an inorganic zinc source. When E. coli was grown aerobically in LB medium containing varied concentrations of zinc gluconate, high Zn2+ concentrations had minor effects on lag time and growth rate and no effect on the maximum OD600 (Figs. S1C and S2, A–C). This result suggests that aerobically grown E. coli is more tolerant to high concentrations of zinc gluconate compared to ZnCl2. When E. coli was grown anaerobically in LB with 1.5 to 2 mM Zn gluconate, the lag time increased while the growth rate and maximum OD600 decreased (Figs. S1D and S2, D–F). Regardless of the zinc source, the growth of E. coli under anaerobic conditions was significantly reduced by high Zn2+ concentrations. We also grew E. coli under aerobic conditions with varied concentrations of ZnSO4, another inorganic zinc source, and compared the results to E. coli grown with ZnCl2 and zinc gluconate. The trend for E. coli grown with added ZnSO4 was the same as for E. coli grown with added ZnCl2 (Fig. S3). Collectively, the inorganic zinc sources have a greater impact on the aerobic growth of E. coli compared to the organic zinc source, but the inorganic and organic zinc sources have similar effects on the anaerobic growth of E. coli. Given that similar max OD600 values of E. coli were reached for both added ZnCl2 and Zn-gluconate sources, particularly when grown aerobically, ZnCl2 was used for the remaining experiments.
Quantification of the total zinc content of E. coli grown aerobically and anaerobically
Because E. coli grew more slowly with high concentrations of Zn2+ under aerobic and anaerobic conditions and reached a lower culture density (max OD600) under anaerobic conditions, we used ICP-MS to determine whether the total cellular zinc content would similarly vary with the growth conditions. Here, we grew E. coli with 0.5 mM ZnCl2 because this is the highest concentration tested that had minimal effects on the growth kinetics of E. coli (Fig. 1). E. coli was grown in LB and LB supplemented with 0.5 mM ZnCl2 under aerobic and anaerobic conditions until the culture densities reached the exponential phase (OD600 = 0.6–0.8). The cells were harvested, washed, and digested with trace metal grade concentrated HNO3 prior to ICP-MS measurements. The metal concentrations in the media were analyzed by inductively coupled plasma-optical emission spectrometry (ICP-OES). For LB and LB containing 0.5 mM ZnCl2, the measured zinc concentrations are 10 and ∼490 μM, respectively (Table S1 and Fig. S4). For cells grown under aerobic conditions, the total zinc content measured was increased by over 4-fold with 0.5 mM added ZnCl2, suggesting that E. coli can acquire more zinc when the zinc concentration increases (Fig. 2). Surprisingly, when E. coli was grown anaerobically in medium containing 0.5 mM ZnCl2, the cells did not accumulate additional zinc compared to when cells were grown anaerobically in medium without added zinc (Fig. 2). Based on the growth kinetics of E. coli and this ICP-MS analysis, we can suggest that the lag time and growth rate of E. coli grown aerobically in 0.5 mM ZnCl2 were moderately impacted due to increased zinc accumulation, whereas the lag time and growth rate of anaerobic E. coli were unaffected at least in part because the cells did not take up the excess zinc from the medium.
Figure 2.
ICP-MS quantification of metal contents of Escherichia coli grown aerobically (+O2) and anaerobically (–O2) in LB and LB containing 0.5 mM added ZnCl2. Data are shown as individual data points overlaid on the bar representation, with error bars as SD from three biological replicates. NS, not significant; ∗p ≤ 0.05; ∗∗∗p ≤ 0.001 as determined by one-way ANOVA with Tukey multiple comparison test.
We also measured the total metal contents in E. coli for several other metals (Figs. 2 and S5). For most biologically relevant metals, there were no significant changes in accumulation of the metal with supplementation of zinc, especially under aerobic conditions (Figs. 2, S5A, and Table S2). Under anaerobic conditions, there were some effects of oxygen and zinc supplementation on metal uptake (Figs. 2 and S5B, and Table S3). Among the abundant main group elements, calcium and potassium accumulation decreased by ∼2.2-fold with added zinc when E. coli was grown under anaerobic conditions. By comparison, the accumulation of these metals with added zinc under aerobic conditions did not change significantly. Selenium and molybdenum contents also decreased by a similar amount under anaerobic conditions, although these metals are significantly less abundant. Among the first-row transition metals, accumulations of iron and nickel were also reduced under anaerobic growth conditions, by ∼1.5-fold for iron and almost 3-fold for nickel. Pairwise comparisons of metal accumulation for cells grown in the same medium conditions but aerobically or anaerobically reveal additional differences in accumulation (Fig. S5, C and D). For example, the supplementation of zinc does not affect manganese contents, but when comparing anaerobically grown cells to aerobically grown cells, ∼7-17-fold less manganese was detected in anaerobic cells. On the other hand, several metals showed significant increases in accumulation under anaerobic conditions, including magnesium, iron, and molybdenum. E. coli has been previously reported to acquire more copper under anaerobic conditions, and although we did not detect significant changes in this work, there is a slight increase in copper for cells grown in LB and an even smaller decrease for cells grown in LB with supplemented ZnCl2 (21). Overall, these results demonstrate distinct patterns for the effects of supplemented zinc and aerobic versus anaerobic growth on cellular metal uptake.
Detecting intracellular labile Zn2+ in E. coli
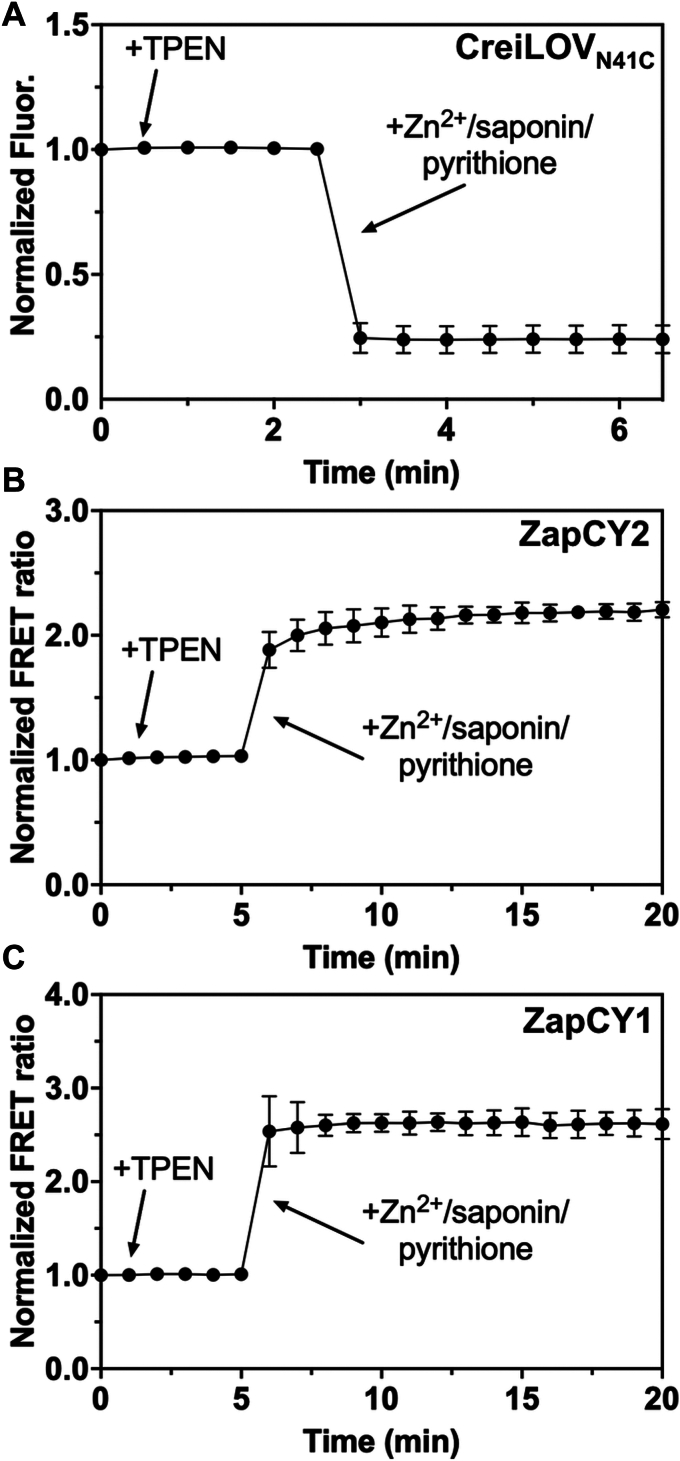
The zinc quota is distributed into a large pool of zinc ions that are tightly bound to proteins and a smaller pool of labile zinc ions. To better understand the changes in zinc uptake in E. coli grown aerobically and anaerobically, we measured the intracellular labile zinc using fluorescent protein-based zinc sensors. Previously, we showed that CreiLOVN41C is an excellent oxygen-independent zinc reporter for bacteria grown under either aerobic or anaerobic conditions (31). Here, we applied CreiLOVN41C, which has a nanomolar dissociation constant for Zn2+, in E. coli to detect changes in the labile zinc pool. For comparison, we used two GFP-based Zn2+ sensors, ZapCY2 (Kd = 811 pM) and ZapCY1 (Kd = 2.5 pM), which were previously applied to detect zinc in mammalian cells, but not in E. coli (34, 38). Initially, CreiLOVN41C was aerobically expressed using the same growth and protein expression conditions (0.5 mM IPTG, 37 °C, 3 h) as previously reported, but fluorescence microscopy analyses revealed an uneven distribution of fluorescence in the cells, possibly due to the formation of inclusion bodies (Fig. S6A) (31). After optimizing the protein expression conditions (0.1 mM IPTG, 18 °C, overnight), no inclusion bodies were observed (Fig. S6B). The ZapCY series sensors were also expressed in E. coli grown at 18 °C overnight. Using a live cell suspension expressing each of the sensors, labile Zn2+ was detected by first measuring the initial fluorescence intensity, then measuring the fluorescence after the addition of N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) and, subsequently, with the addition of ZnCl2/saponin/pyrithione (Figs. 3 and S7). For CreiLOVN41C, the results shown are based on changes in the fluorescence intensity whereas for the ZapCY series, the changes in the FRET ratio are shown. The FRET ratio is calculated by dividing the fluorescence intensity of the acceptor (yellow fluorescent protein) by the fluorescence intensity of the donor (cyan fluorescent protein). There was no fluorescence enhancement of CreiLOVN41C after the addition of TPEN, indicating no detectable endogenous zinc in E. coli (Figs. 3A and S7A). Subsequent addition of ZnCl2/saponin/pyrithione quenched the fluorescence of CreiLOVN41C, suggesting that E. coli took up a high amount of exogenous zinc. This is consistent with previously reported results using CreiLOVN41C in E. coli MG1655 cells grown anaerobically (31). Similarly, ZapCY2 was used to detect zinc in live aerobically grown E. coli cells, and there was no change in the FRET ratio after the treatment of TPEN and an increase in FRET ratio upon the addition of ZnCl2/saponin/pyrithione (Figs. 3B and S7B). The intracellular physiological concentration of zinc in E. coli is thought to be lower (∼10−15–10−12 M) than in many mammalian cells (∼10−10–10−9 M) (27, 39, 40, 41). Given that CreiLOVN41C and ZapCY2 have dissociation constants in the nanomolar range (1 nM and 0.811 nM, respectively), we hypothesized that any endogenous Zn2+ would likely not be within the detection limit range of the probes (31, 34). We repeated the above experiment using ZapCY1, which has a stronger binding affinity to zinc (Kd ≈ pM) (34, 38). When treated with TPEN, the FRET ratio of ZapCY1 did not decrease (Figs. 3C and S7C). ZapCY1 also detected no endogenous Zn2+ in E. coli, consistent with a physiological zinc concentration closer to femtomolar than picomolar levels in these bacteria. The FRET ratio of ZapCY1 increased upon saturation with exogenous Zn2+. These data show that E. coli accumulated no detectable endogenous labile Zn2+ under these growth conditions but confirm that these sensors can be used to reversibly monitor exogenously added Zn2+ in bacteria. These experiments are reproducible in bacteria that do not form inclusion bodies during protein expression, but in samples where inclusion bodies were formed the sensors can yield inconsistent results.
Figure 3.
Intracellular zinc in aerobic live Escherichia coli cells detected by fluorescent protein based Zn2+sensors. (A) CreiLOVN41C, (B) ZapCY2, and (C) ZapCY1. The fluorescence emission of CreiLOVN41C was recorded initially and every 30 s after addition of TPEN (50 μM) and ZnCl2/saponin/pyrithione (100 μM/0.002%/1.5 μM). The fluorescence emission of ZapCY2 and ZapCY1 was recorded initially and every 1 min after addition of TPEN (5 μM) and ZnCl2/saponin/pyrithione (10 μM/0.002%/1.5 μM). Buffer: 50 mM HEPES, 100 mM NaCl, pH 7.1. λex = 450 nm (CreiLOVN41C) and 433 nm (ZapCY2 and ZapCY1). All error bars represent the standard deviation for three biological replicates. TPEN, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine. HEPES, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid.
Next, we used fluorescence microscopy to visualize intracellular labile zinc in E. coli grown under aerobic and anaerobic conditions (Fig. 4). Aerobically grown E. coli cells expressing CreiLOVN41C were imaged by first collecting the initial fluorescence in the green channel, followed by the fluorescence after treatment with TPEN. As for the live cell suspension experiment, the addition of TPEN had no effect on the fluorescence signal of CreiLOVN41C, confirming that there is no detectable endogenous zinc in these cells (Fig. 4, A and C). Addition of ZnCl2/saponin/pyrithione quenched the fluorescence, and excess TPEN mostly reversed the fluorescence signal of CreiLOVN41C, which is also consistent with the live cell suspension experiment results described above. When grown under anaerobic conditions, E. coli cells expressing CreiLOVN41C were also imaged using fluorescence microscopy before and after the addition of TPEN and then after the addition of ZnCl2/saponin/pyrithione and subsequently, more TPEN. For anaerobic imaging, cells were grown and handled under anaerobic conditions throughout the whole experiment. Microscope slides were sealed with epoxy to maintain anaerobic conditions (details in Experimental procedures) (42). As for the previously reported live cell suspension (31), no endogenous zinc was detected since the fluorescence signal after TPEN addition did not change (Fig. 4, B and D). The fluorescence signal was quenched with the addition of ZnCl2/saponin/pyrithione and subsequently restored with excess TPEN, further confirming that CreiLOVN41C can be used to monitor exogenous zinc in E. coli grown and analyzed in both oxygen and oxygen-free environments.
Figure 4.
Fluorescence microscopy of Escherichia coli expressing CreiLOVN41Cunder aerobic and anaerobic conditions.A, aerobically grown and imaged E. coli. B, anaerobically grown and imaged E. coli. From left to right in A and B: initial fluorescence signal from CreiLOVN41C, fluorescence signal 5 min after the addition of 50 μM TPEN, 15 min after the addition of 100 μM ZnCl2/0.002% saponin/1.5 μM pyrithione, and 20 min after the addition of 200 μM TPEN. The scale bar represents 10 μm. C and D, quantification of change in fluorescence signals shown in parts A and B, respectively. Mean cell intensities were normalized to the initial fluorescence intensity. Individual data points representing individual cells are overlaid on the bar chart representation and were collected from three biological replicates, each with two or more technical replicates. Data were analyzed by means of one-way ANOVA with Tukey multiple comparison test. ∗∗∗∗p < 0.0001. Error bars represent the standard deviation. TPEN, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine.
Detecting changes in endogenous Zn2+ in E. coli grown aerobically and anaerobically with varied zinc concentrations
Our prior work revealed that CreiLOVN41C could detect increased endogenous zinc in E. coli MG1655 grown with 2 mM added ZnCl2 and slightly increased endogenous zinc in E. coli grown with 1.5 mM added ZnCl2 under anaerobic conditions (31). Less zinc was required to increase the endogenous Zn2+ levels when cells were grown under aerobic conditions in that work, but a different strain of E. coli was used, BL21(DE3). Here, we measured the endogenous zinc in aerobically grown E. coli MG1655 with varied concentrations of ZnCl2 to allow a direct comparison of the effects of oxygen on endogenous Zn2+ levels. For these live cell suspension experiments, starter cultures were inoculated in LB medium supplemented with ZnCl2 (0–2 mM), and expression of the sensor protein was induced by 0.5 mM IPTG at 37 °C for 3 h. Cells were harvested and transferred to a cuvette for fluorescence analysis. After measuring the initial fluorescence signal, cells were treated with TPEN and the fluorescence was recorded before adding exogenous zinc for signal calibration (ZnCl2/saponin/pyrithione). The addition of TPEN after recording the initial signal did not affect the fluorescence of CreiLOVN41C in E. coli grown in 0.1 to 1.5 mM ZnCl2, but the chelator increased the fluorescence in E. coli grown in 2 mM ZnCl2, indicating an increased level of endogenous Zn2+ in these cells after growth (Fig. 5A). This result is different from what we previously observed for a different strain of E. coli, BL21(DE3). When grown aerobically, E. coli BL21(DE3) cells grown with only ∼0.5 to 1.0 mM added ZnCl2 exhibited increased fluorescence upon addition of chelator to the cell suspension, consistent with increased endogenous Zn2+. We also repeated the experiment from our prior work for E. coli MG1655 grown anaerobically (31), and found increased levels of endogenous Zn2+ when cells were grown in medium supplemented with 2 mM ZnCl2, but not for cells grown with 1.5 mM supplemented ZnCl2 (Fig. 5B). This difference could be due to subtle changes in growth of the bacteria or possible formation of inclusion bodies when the protein is expressed at 37 °C with 0.5 mM IPTG (Fig. S6A). Therefore, when zinc is included in the growth medium under these conditions, E. coli MG1655 cells appear to respond similarly regardless of the presence of oxygen, and endogenous Zn2+ is only detected by CreiLOVN41C at the highest added ZnCl2 concentration (Fig. 5, A and B).
Figure 5.
Endogenous zinc as detected by fluorescent protein-based Zn2+sensors in live cell suspensions of Escherichia coli grown aerobically and anaerobically under different protein expression conditions. Detection of endogenous Zn2+ by CreiLOVN41C when protein expression was induced with 0.5 mM IPTG for 3 h at 37 °C and cells were grown (A) aerobically or (B) anaerobically. Detection of endogenous Zn2+ by (C) CreiLOVN41C, (D) ZapCY2, and (E) ZapCY1 when protein expression was induced by 0.1 mM IPTG at 18 °C overnight and cells were grown aerobically. Under all conditions, E. coli cells were grown in the presence of ZnCl2 (0–2 mM). For each sample, the fluorescence emission was recorded initially and every 30 s or 1 min after addition of TPEN (50 μM) and ZnCl2/saponin/pyrithione (100 μM/0.002%/1.5 μM). Buffer: 50 mM HEPES, 100 mM NaCl, pH 7.1. λex = 450 nm (CreiLOVN41C) and 433 nm (ZapCY2 and ZapCY1). All error bars represent the standard deviation for 3 biological replicates, each with ≥2 technical replicates. TPEN, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine. HEPES, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid.
Since we observed a slightly different zinc effect on the growth of aerobic E. coli when using zinc gluconate as zinc source, we decided to use CreiLOVN41C to monitor endogenous zinc in E. coli grown aerobically and anaerobically with zinc gluconate (0–2 mM). The expression of CreiLOVN41C was induced by 0.5 mM IPTG at 37 °C for 3 h. Under both aerobic and anaerobic conditions, there was a minor effect of TPEN on the fluorescence of CreiLOVN41C when E. coli was grown in the presence of 2 mM zinc gluconate, whereas no effect was observed when E. coli was grown in the presence of 0.1 mM–1.5 mM Zn2+ (Fig. S8). These results are similar to those observed for cells grown with varied ZnCl2.
As noted above, the initial protein expression conditions (0.5 mM IPTG, 37 °C, 3 h) that we used for expressing CreiLOVN41C for these live cell suspension experiments could result in inclusion bodies (Fig. S6A) and impact the reproducibility of these experiments. We therefore optimized the expression conditions to minimize the inclusion bodies (as evaluated by fluorescence microscopy, Fig. S6B). The optimized conditions (0.1 mM IPTG, 18 °C, overnight) were used to express CreiLOVN41C under aerobic conditions and analyze the effects of zinc in the growth medium. After TPEN treatment, the fluorescence of CreiLOVN41C expressed in the presence of varied Zn2+ concentrations did not increase, signifying that there was no detectable increase in endogenous zinc in E. coli grown with ZnCl2 (0–2 mM) (Fig. 5C). For comparison, we also used the well-established ZapCY2 sensor to monitor endogenous zinc in E. coli grown with varied Zn2+ concentrations. For all samples, ZapCY2 did not exhibit any changes in the FRET ratio when treated with metal chelator (Fig. 5D). No endogenous zinc was detected by ZapCY2 or CreiLOVN41C when E. coli was grown in the presence of low to high concentrations of zinc with optimal protein expression conditions. CreiLOVN41C and ZapCY2 both have dissociation constants around 1 nM and could not detect endogenous zinc, suggesting that the zinc concentration is below the nanomolar range. Therefore, we also evaluated endogenous zinc levels in aerobically grown E. coli using ZapCY1, which is a higher affinity Zn2+ sensor with a Kd of 2.5 pM (34). ZapCY1 did not detect any increase in endogenous zinc for cells grown with 0.1 mM–1.5 mM added Zn2+ (Fig. 5E). For cells grown with 2 mM Zn2+, however, a small increase in endogenous zinc was evidenced by a reduced FRET ratio with the addition of a chelator. Based on these results, we conclude that E. coli accumulates little labile Zn2+, up to only ∼3 pM with the inclusion of high ZnCl2 (2 mM) when grown under aerobic conditions overnight.
Sensing Zn2+ influx in aerobically and anaerobically grown E. coli
Since we found that endogenous labile Zn2+ levels were too low for detection by CreiLOVN41C and ZapCY2 when E. coli cells were grown with varied ZnCl2 under optimal protein expression conditions prior to cell harvesting and analysis, we next evaluated whether the sensors could detect Zn2+ influx in real time in E. coli cell suspensions. E. coli was grown in LB and the optimized protein expression conditions (0.1 mM IPTG, 18 °C, overnight) were used for both CreiLOVN41C and ZapCY2. Cells were harvested and transferred to a cuvette containing metal-free 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer. After measuring the initial fluorescence, ZnCl2 (0–75 μM) was added to each cell suspension, and the fluorescence was recorded every 10 min for 2 h. For subsequent calibration, TPEN was added, then ZnCl2/saponin/pyrithione, and the fluorescence was recorded for each addition. As the concentration of ZnCl2 increased from 0 to 75 μM, the quenching of CreiLOVN41C correspondingly increased from 30% for 10 μM ZnCl2 up to a maximum of 84% and 87% quenching for 50 μM and 75 μM ZnCl2 (Fig. 6A). For each addition, the maximum quenching was achieved after 30 min. We note that there was a modest increase in quenching even when no ZnCl2 was added to the cells, possibly indicating accumulation of zinc from the buffer for CreiLOVN41C-expressing cells (this was not observed for ZapCY2 below). CreiLOVN41C fluorescence was fully restored by excess TPEN for all samples and quenched by excess ZnCl2/saponin/pyrithione. Similarly, ZapCY2 detected Zn2+ influx with the addition of 10 to 75 μM ZnCl2. Normalized FRET ratios of 1.7 and 2.1 were reached with addition of 10 and 25 μM ZnCl2, and maximal FRET ratios of 2.3 were achieved with addition of 50 and 75 μM ZnCl2 (Fig. 6B). Excess TPEN dropped the FRET ratio back to 1 and ZnCl2/saponin/pyrithione raised the FRET ratio up to 2.3. Both CreiLOVN41C and ZapCY2 can monitor Zn2+ influx in real time in live cell suspensions of E. coli grown under aerobic conditions.
Figure 6.
Zn2+ ion influx in live cell suspensions of Escherichia coli grown aerobically and anaerobically as monitored by fluorescent protein-based Zn2+ sensors. Zn2+ ion influx for E. coli grown aerobically (A and B) and anaerobically (C), detected by CreiLOVN41C (A and C) and ZapCY2 (B). For each sample, the fluorescence emission was recorded initially and every 10 min after addition of ZnCl2 (0–75 μM), TPEN (100 μM for CreiLOVN41C and 150 μM for ZapCY2) and ZnCl2/saponin/pyrithione (200 μM/0.002%/1.5 μM). Buffer: 50 mM HEPES, 100 mM NaCl, pH 7.1. λex = 450 nm (CreiLOVN41C) and 433 nm (ZapCY2). All error bars represent the standard deviation for three biological replicates, each with ≥2 technical replicates. TPEN, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine. HEPES, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid.
Next, we aimed to monitor real time uptake of zinc ions in cells under anaerobic conditions. Here, CreiLOVN41C was expressed at low temperature overnight, and the live cell suspension assay was conducted as described above while maintaining anaerobic conditions. Without added ZnCl2, the fluorescence intensity of CreiLOVN41C increased up to 1.7-fold after 80 min and then gradually dropped after 2.7 h (Fig. 6C). It is possible that anaerobically grown E. coli initially exported excess labile zinc accumulated from the growth medium when transferred to HEPES buffer then later acquired more zinc. ICP-OES analysis reveals that HEPES buffer treated with Chelex-100 contains 0.17 μM Zn2+ (Table S4). Although this amount of Zn2+ is significantly reduced compared to the amount we found in LB medium (10.5 μM), E. coli could acquire some of the zinc remaining in chelexed HEPES buffer. No quenching effect was seen after 10 μM ZnCl2 was added to the cell suspension. In the presence of higher concentrations of added ZnCl2, zinc influx in anaerobic E. coli was observed. We measured 47%, 60%, and 65% quenching of CreiLOVN41C with addition of 25 μM, 50 μM, and 75 μM ZnCl2, respectively. The fluorescence intensity quenched by Zn2+ could be fully restored with TPEN and excess ZnCl2/saponin/pyrithione quenched the fluorescence intensity in all cell suspensions. Overall, CreiLOVN41C can be effectively used to detect zinc influx in E. coli under anaerobic conditions.
Discussion
Fluorescent Zn2+ sensors have provided significant insight to the roles of zinc across biology, but few fluorescent protein-based sensors have been applied to study bacterial metal homeostasis and there are few that also function under anaerobic conditions. Here, we demonstrate the utility of a previously reported sensor from our group, CreiLOVN41C, which functions independently of oxygen, alongside well-established oxygen-dependent zinc sensors, ZapCY2 and ZapCY1 (31, 34). By combining the use of sensors with bacterial growth curves and ICP-MS analyses to quantify total metal uptake, we show that anaerobically grown E. coli MG1655 cells take up less zinc and are more susceptible to toxicity from excess Zn2+ in the growth medium.
In our prior work, we showed that CreiLOVN41C could detect exogenous and endogenous zinc when E. coli cells were grown aerobically or anaerobically but found that more Zn2+ was required during growth to elicit a sensor response in the anaerobic cells (31). We also noticed that the reverse kinetics of the sensor response were slower under anaerobic conditions. Given that two different strains of E. coli were used in that work (BL21(DE3) for aerobic analyses and MG1655 for anaerobic analyses), here, we aimed to evaluate the impact of oxygen levels on zinc homeostasis in a single strain of E. coli, MG1655. Although the kinetics of the sensor response were similar in this strain regardless of aerobic or anaerobic growth conditions, there were clear differences in zinc uptake, as evidenced by application of the sensors and other methods. Growth kinetics curves revealed similar impacts of varied ZnCl2 on aerobic and anaerobic growth parameters of E. coli, with the exception of a reduction in the max OD600 for anaerobically grown cells as the zinc concentration increased (Figs. 1 and S1, A and B). Both conditions showed reduced growth rates and increased lag times, but aerobically grown cells recovered to similar max OD600 levels up to the highest Zn2+ concentration tested, 2 mM. Together, these growth curve analyses suggest that while the growth of E. coli is delayed under both aerobic and anaerobic conditions with excess Zn2+, aerobically grown E. coli were more tolerant to excess Zn2+ than anaerobically grown E. coli. Notably, anaerobically grown cells grew faster than those grown in the presence of oxygen, even at the highest Zn2+ concentration, although they never reached the same max OD600 levels as aerobically grown cells. With a moderate amount of added Zn2+ (0.5 mM), E. coli cells grown aerobically with added Zn2+ acquired >4-fold more zinc than when grown in LB with no added Zn2+ whereas anaerobically grown cells did not (Fig. 2, Tables S2, and S3). The effects of oxygen on zinc uptake in E. coli have not been previously investigated although a previous study showed that E. coli grown aerobically accumulated increased iron and copper with zinc stress (0.1 mM Zn2+) (43). Although we did not detect an increase in iron with zinc stress (0.5 mM Zn2+) here, the previous work used a minimal medium for the zinc stress analyses rather than LB as reported here. A limitation of using ICP-MS for quantifying cellular metal content, however, is that it cannot discriminate between the labile and tightly bound metal pools and it cannot measure dynamic metal uptake in living cells.
Fluorescent protein-based Zn2+ sensors were used to gain further insight to the effects of oxygen on zinc homeostasis in living cells. After confirming reversible and reproducible responses from CreiLOVN41C in aerobic and anaerobic E. coli MG1655 (Figs. 3A and 4) and ZapCY1 and ZapCY2 in aerobic E. coli (Fig. 3, B and C), sensors were applied to monitor changes in endogenous zinc levels with added zinc. Two approaches were taken. In the first approach, cells were grown, and protein was expressed in the presence of added Zn2+, then cells were harvested and transferred to metal-free medium for evaluation. In the second approach, cells were grown, and protein was expressed with no added Zn2+ prior to harvesting cells and then Zn2+ was added to cell suspensions in metal-free medium during the experiment. In the former approach, we found that the growth and protein expression conditions impacted the results. Many previous applications of fluorescent protein-based sensors have focused on mammalian cells rather than bacteria, which have much smaller cell sizes that necessitate careful control of protein expression to both avoid aggregation and maintain sufficient brightness and sensitivity for metal detection. When protein was quickly overexpressed (37 °C, 3 h), inclusion bodies could form in the cells and lead to inconsistent sensor response and high error bars (Figs. 5 and S6). Under these conditions, endogenous Zn2+ was detected only when cells were grown with 2 mM ZnCl2 added to LB medium, although with high error. More consistent results were obtained under lower temperature expression conditions (18 °C, overnight), where endogenous zinc was no longer detected even with 2 mM added ZnCl2. Both the more consistent sensor response and longer expression time, which could allow the cells time to respond to the added Zn2+, likely led to the lack of a sensor response. For comparison, we applied the zinc sensors ZapCY2 and ZapCY1. Only ZapCY1, which has significantly stronger affinity than CreiLOVN41C and ZapCY2, detected ∼3 pM endogenous Zn2+ when cells were grown with 2 mM ZnCl2. The ZapCY sensors can only be applied under aerobic conditions, however, so we also evaluated zinc uptake in real-time under aerobic and anaerobic conditions using CreiLOVN41C by adding Zn2+ directly to the cell suspensions in a 96-well plate during fluorescence monitoring. In these experiments, there were clear differences in the extent of CreiLOVN41C fluorescence quenching by Zn2+ between aerobic and anaerobic conditions. Higher concentrations of Zn2+ were required to fully quench CreiLOVN41C, suggesting that anaerobically grown cells take up less Zn2+. Intriguingly, even with no added Zn2+, aerobic cells appeared to be scavenging Zn2+ from the buffer, as evidenced by moderate and reversible quenching over the time of analysis (∼2 h). On the other hand, CreiLOVN41C in the anaerobic cells was dequenching over the first hour (possibly indicating Zn2+ ion efflux), before reequilibrating to the initial fluorescence level over the second hour.
Collectively, these results suggest that oxygen levels affect zinc homeostasis in E. coli. Future work will be needed to understand how the molecular responses of anaerobically grown E. coli to varied zinc differ from aerobically grown E. coli, in terms of both homeostasis mechanisms (e.g. regulators and transporters) and zinc utilization in the cells. E. coli is well-known to adapt to various external conditions, including the transition from aerobic to anaerobic respiration, by modulating the expression pattern of its genome (44, 45, 46, 47, 48). Although iron and copper homeostasis are known to be impacted by oxygen during the growth of E. coli, the effects of aerobic versus anaerobic respiration on zinc homeostasis have not been reported (21, 22, 23, 24, 25).
Overall, this work establishes CreiLOVN41C as an oxygen-independent zinc sensor that can be used alongside other protein-based zinc sensors and complementary methods for studying zinc homeostasis in bacteria. Here, we found that E. coli grown aerobically and anaerobically have distinct responses to varied zinc. These results have important implications for understanding the effects of metal toxicity in different environments, particularly in host organisms where bacteria are exposed to variations in acidity and oxygen levels. Furthermore, by using complementary methods and sensors, as well as comparing the effects of protein expression conditions on sensor performance in E. coli, this work lays a foundation for studying zinc homeostasis in other anaerobic bacteria using CreiLOVN41C.
Experimental procedures
General information
All reagents were purchased from commercial sources and used as received. Primers were purchased from Sigma-Aldrich. E. coli strain MG1655 was from American Type Culture Collection and made chemically competent by CaCl2. E. coli strain DH5α for cloning was obtained from New England Biolabs. Restriction enzymes, T4 DNA ligase, Q5 DNA polymerase, the Gibson assembly kit, and PCR cleanup kit were from New England Biolabs. Plasmid DNA miniprep kit was from Zymo Research. Fluorescence spectra were obtained on an Agilent Eclipse spectrofluorometer using quartz cuvettes (Starna cells) with 1 cm path lengths or on a Tecan Spark plate reader using 96-well black flat-bottom plates (Greiner Bio-One). All measurements were conducted at 25 °C and maintained by a circulating water bath. To remove metal ions, HEPES buffer was treated with Chelex-100 resin (Bio-Rad) according to the manufacturer’s batch protocol. Fluorescence data were obtained by exciting at 450 nm for CreiLOVN41C and 433 nm for ZapCY1 and ZapCY2. For CreiLOVN41C, excitation and emission slit widths were set to 10 nm, and emission acquired from 470 to 570 nm and from 475 to 580 nm on the spectrofluorometer and plate reader, respectively. For ZapCY1 and ZapCY2, excitation and emission slit widths were set to 5 and 10 nm on spectrofluorometer and plate reader, respectively. The emission was acquired from 443 to 570 nm and from 458 to 580 nm on spectrofluorometer and plate reader, respectively. Growth curve experiments were monitored using Tecan Spark plate reader with 96-well clear round-bottom plates (Greiner Bio-One). Experiments involving CreiLOVN41C were done anaerobically using buffer that was freshly degassed with argon. Anaerobic manipulations were done in an anaerobic chamber, using cuvettes with screw caps and septa and a gas-tight Hamilton syringe, adding mineral oil on top of the culture and using gas-sealed tape around the 96-well plate, or using a BD GasPak EZ anaerobe pouch and a gas generating sachet with anaerobic indicator. We note that higher error bars observed for some of the anaerobic measurements likely arise from the additional time required for reagent additions compared to aerobic conditions.
Growth curves of E. coli in the presence of varied concentrations of Zn2+
Single colonies of E. coli MG1655 were selected and grown in LB medium overnight at 37 °C and 250 rpm. The starter culture was inoculated at 1/100 dilution into fresh LB medium with either ZnCl2 or zinc gluconate (0–2 mM) and grown in a 96-well plate for 65 h at 37 °C with shaking. For anaerobic conditions, the starter culture was inoculated at 1/100 dilution into LB medium containing glucose (20 mM) as a carbon source, KNO3 (20 mM) as an electron source, and either ZnCl2 or zinc gluconate (0–2 mM) and grown anaerobically for 65 h at 37 °C. For both conditions, the OD600 was recorded every 30 min on a Tecan Spark plate reader. The growth parameters (lag time, growth rate, and max OD600) were calculated using nonlinear regression curve fitting to a four-parameter logistic equation with GraphPad Prism 9 software (GraphPad software, CA; https://www.graphpad.com) (49, 50, 51). Anaerobic manipulations were done in an anaerobic chamber or by adding mineral oil on top of the culture and using gas-sealed tape around the 96-well plates.
ICP-OES analysis of metals in the growth media
LB (2 ml) and LB supplemented with 0.5 mM ZnCl2 (2 ml) media were boiled in metal-free plastic centrifuge tubes (Eppendorf) until the media were dry. Dried media were digested with 560 μl Milli-Q water and 60 μl trace metal concentrated HNO3 at 80 °C overnight. Digested media were diluted with Milli-Q water to bring to a total volume of 2.5 ml. The samples were analyzed by an Agilent 725 Simultaneous ICP-OES equipped with VistaChip II charge coupled device detector and image mapping technology to provide complete wavelength coverage from 167 to 785 nm. Trace metal HNO3 (2%) was used as the blank. Metal concentrations in media were measured by ICP-OES for Ca, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Pb, Se, V, Zn, and Mo. ICP-OES calibration standards contained 0.2, 0.5, 1.0, 2.0, and 5.0 ppm for each metal and were all prepared by diluting commercially available Inorganic Ventures' ICP-MS Complete Standard 71A (10 ppm) and Inorganic Ventures' ICP-MS Refractory Elements Standard 71B (10 ppm) with 2% trace metal HNO3.
ICP-MS analysis of E. coli cells
The starter culture was inoculated at 1/100 dilution into fresh LB medium supplemented with ZnCl2 (0 or 0.5 mM) and grown at 37 °C and 250 rpm until OD600 reached 0.6 to 0.8. For anaerobic conditions, the starter culture was inoculated at 1/100 dilution into LB medium containing glucose (20 mM) as a carbon source, KNO3 (20 mM) as an electron source, and ZnCl2 (0 or 0.5 mM) and grown anaerobically at 37 °C until OD600 reached 0.6 to 0.8. Immediately after growth and prior to harvesting cells, live culture from each sample was collected to perform serial dilution from 10−1 to 10−5 in 0.85% saline solution for determining the colony-forming unit (CFU) count. For each dilution from 10−3 to 10−5, 10 μl of diluted culture was plated on an LB agar plate and incubated at 37 °C overnight. CFU/ml were both manually counted and automatically counted by ImageJ software. Cell pellets were harvested (4 °C, 5000g) and washed with Milli-Q water, 1 mM EDTA (3x), and then Milli-Q water. Cell pellets were dried overnight at 80 °C, weighed, and digested with 100 μl trace metal concentrated HNO3 at 65 °C for 30 min, then increased to 100 °C for 5 h. Digested cells were diluted with 1.4 ml Milli-Q water. All centrifuge tubes, containers, Teflon tubes, and pipet tips are plastic and were acid-washed before use. Cell samples were analyzed with an Agilent 8800 triple-quadrupole ICP-MS instrument (Agilent Technologies) equipped with an SPS 4 autosampler and using 2% trace metal HNO3 as the blank. The following settings were fixed for analysis: cell entrance, −50 V; cell exit, −70 V; plate bias, −70 V; octP bias, −18 V; collision cell helium flow, 4.3 ml/min. Optimal voltages for Extract 2, Omega Bias, Omega Lens, OctP RF, and Deflect were determined via auto tune with 1 ppb instrument tuning solution before each sample set was analyzed. Samples were introduced by a peristaltic pump with 0.5-mm-internal-diameter tubing through a MicroMist borosilicate glass nebulizer (Agilent Technologies). Samples were initially taken up at 0.3 rps for 60 s then stabilized for 15 s at 0.1 rps. Samples were analyzed in spectrum mode at 0.1 rps, and three replicates of 100 sweeps were performed for each element analyzed. Sampling probe and tubing were rinsed for 90 s at 0.3 rps with 2% trace metal HNO3 after every sample. Agilent MassHunter Workstation (https://www.agilent.com/en/product/software-informatics/mass-spectrometry-software/data-acquisition) was used for data acquisition and analysis. ICP-MS calibration standards were prepared in the same manner as ICP-OES standards, and contained 0.5, 1.0, 2.5, 5, 10, 16, 25, 40, 50, 100, 200, 500, 750, and 1000 ppb for each metal. Metal content was quantified by converting metal concentrations in ppb to atoms/CFU using CFU values calculated from serial dilutions corresponding to each sample (described above). Metal contents of cells were measured by ICP-MS for Na, Mg, K, Ca, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Se, Mo, and Pb.
Molecular cloning
The plasmid pQE80L-CreiLOVN41C was reported previously (31). The plasmid pUC57-Zap1 was from GenScript. The plasmid pBAD-ZapCY2 (unpublished) was previously prepared using pcDNA3-ZapCY2 (Addgene #36320) and pBAD-His-miRFP709 (Addgene #79986) (34, 52). All plasmids were verified by DNA sequencing.
pQE80L-ZapCY2
ZapCY2 was PCR amplified from pBAD-ZapCY2 using the primers listed below. The pQE80L vector from pQE80L-CreiLOVN41C was digested with BamHI and HindIII restriction enzymes. ZapCY2 was inserted between BamHI and HindIII restriction sites in the pQE80L vector. The primers are as follows: sense: 5′–CAGATCTGAGCTCGGATCC–3′ and antisense: 5′–GCCAAAACAGCCAAGCTTCG–3′
pQE80L-ZapCY1
Zap1 from pUC57-Zap1 and the pQE80L vector from pQE80L-ZapCY2 were PCR amplified and assembled by Gibson Assembly. Primers for each amplification are listed as follows: Zap1 sense: 5′–CGCCCGCATGCATAAAAACAATGACTTAAAATGCAAATGG–3′; Zap1 antisense: 5′–CTCACCATGAGCTCGATACCATGTTGACAATTAATATGG–3′; pQE80L sense: 5′–TCGAGCTCATGGTGAGCAAGGGCGAG–3′; pQE80L antisense: 5′–TTATGCATGCGGGCGGCGGT–3.
Live cell suspension assays for detecting intracellular labile Zn2+
The pQE80L-CreiLOVN41C, pQE80L-ZapCY1, and pQE80L-ZapCY2 plasmids were transformed into E. coli MG1655 and grown in LB agar plates containing ampicillin (100 μg/ml). Single colonies were inoculated into LB medium containing ampicillin (100 μg/ml) and grown overnight at 37 °C and 250 rpm. Starter cultures were inoculated at 1/100 dilution into fresh LB media containing ampicillin (100 μg/ml) and incubated at 37 °C with shaking until the cultures reached OD600 of 0.3 to 0.6. Protein expression was then induced with IPTG (0.1 mM), and cells were incubated overnight at 18 °C. Bacterial cells were harvested by centrifugation (4500 rpm, 4 °C, 10 min) and washed twice with metal-free HEPES buffer (50 mM HEPES and 100 mM NaCl at pH 7). The cells were resuspended in fresh HEPES buffer to a final OD600 of ∼1 for CreiLOVN41C samples and ∼0.5 for ZapCY1 and ZapCY2 samples. The cell suspension (3 ml) was transferred to a clean quartz cuvette. The initial fluorescence was recorded, followed by the fluorescence upon the addition of TPEN. The TPEN concentration was 50 μM for CreiLOVN41C samples and 5 μM for ZapCY1 and ZapCY2 samples. ZnCl2, saponin, and pyrithione were then added, and the fluorescence was recorded. ZnCl2/saponin/pyrithione concentrations were 100 μM/0.002%/1.5 μM for CreiLOVN41C samples and 10 μM/0.002%/1.5 μM for ZapCY1 and ZapCY2 samples. Lastly, the fluorescence was recorded after the addition of TPEN. TPEN concentrations were 200 μM for CreiLOVN41C samples and 20 μM for ZapCY1 and ZapCY2 samples. We note that although 50 μM TPEN and 100 μM ZnCl2 were initially used to treat cells with ZapCY series sensors, the ZapCY1 response to zinc was inconsistent under these conditions. ZapCY1 and ZapCY2 were ultimately evaluated using concentrations of 5 μM for TPEN and 10 μM ZnCl2 because the response of ZapCY1 to zinc was consistent under these treatment conditions.
Fluorescence microscopy imaging of live E. coli cells expressing CreiLOVN41C
E. coli MG1655 cells transformed with pQE80L-CreiLOVN41C were grown, and protein expression was induced as described for the live cell suspension assays for detecting intracellular labile Zn2+. Cell pellets were then harvested, washed, and resuspended in metal-free HEPES buffer as described above. Cell suspension (1 μl) was pipetted onto a thin layer of 3% agarose on a general microscope slide and the cover glass was placed on top. For anaerobic conditions, the starter cultures were inoculated at 1/100 dilution into LB medium containing glucose (20 mM) as a carbon source, KNO3 (20 mM) as an electron source, and ampicillin (100 μg/ml). The cultures were grown anaerobically and statically and protein expression was induced as described for the live cell suspension assays for detecting intracellular labile Zn2+. Cell pellets were then harvested, washed, and resuspended in metal-free HEPES buffer as described above. A BD GasPak EZ anaerobe pouch was prepared with a 3.5 × 1.5 cm hole on one of the faces of the pouch. The hole was covered by a cover glass and sealed with epoxy as previously described (42). The microscope slide with the sample was placed inside the pouch on the bottom of the cover glass. A gas generating sachet with anaerobic indicator was placed inside the pouch to keep the slide under anaerobic conditions during imaging. Imaging experiments were performed by using an ECHO Revolve microscope equipped with a 5MP CMOS Monochrome Camera (Fluorescence) and 12 MP Color Camera. The light source was a LED light cube-FITC(M). Fluorescence images were obtained with an oil-immersion objective at 100 × magnification. The exposure time, sensitivity, and contrast for acquisition of fluorescence images were kept constant for each series of images for each channel. Images were obtained by exciting at 470/40 nm. Both emission and brightfield images were obtained. The following procedure was carried out for aerobic and anaerobic conditions: images were collected initially and 5 min after the addition of TPEN (50 μM). ZnCl2/saponin/pyrithione (100 μM/0.002%/1.5 μM) was added to the cells, and images were recorded 15 min after the addition of ZnCl2. Lastly, TPEN (200 μM) was added to the cells and images were recorded 20 min after the treatment of TPEN. Anaerobic manipulations were done in an anaerobic chamber or using a BD GasPak EZ anaerobe pouch and a gas generating sachet with anaerobic indicator, and epoxy. Images were analyzed with ImageJ (http://imagej.nih.gov/ij/). Using an intensity threshold, each bacterial cell was selected, and the mean intensity measured by ImageJ. The background intensity was measured by selecting regions of the image without any cells and subtracted from the cellular intensities. The mean intensity per cell was used to calculate the sensor intensity initially, with TPEN, with Zn2+/saponin/pyrithione, and with subsequent TPEN.
Live cell suspension assays for detecting endogenous Zn2+ in E. coli grown with varied zinc concentrations
Starter cultures were inoculated at 1/100 dilution into fresh LB medium with either ZnCl2 or zinc gluconate (0–1.5 mM) and ampicillin (100 μg/ml), and starter cultures were inoculated at 1/25 dilution into LB medium containing either ZnCl2 or zinc gluconate (2 mM) and ampicillin (100 μg/ml). Cultures were grown at 37 °C with shaking to an OD600 of 0.3 to 0.6. Protein expression was then induced with IPTG (0.5 mM) for 3 h at 37 °C or with IPTG (0.1 mM) overnight at 18 °C. For anaerobic conditions, starters cultures were inoculated at 1/100 dilution into fresh LB medium supplemented with glucose (20 mM) as carbon source, KNO3 (20 mM) as electron source, ZnCl2 or zinc gluconate (0–1.5 mM), and ampicillin (100 μg/ml), and starter cultures were inoculated at 1/25 dilution into the LB with the same ingredients and high concentration of ZnCl2 or zinc gluconate (2 mM). Cultures were grown in an anaerobic chamber at 37 °C to an OD600 of 0.3 to 0.6. Protein expression was induced with IPTG (0.5 mM) for 3 h at 37 °C. The following procedure was carried out for aerobic and anaerobic conditions: bacterial cells were centrifuged at 4500 rpm, 4 °C, and 10 min. The cell pellets were washed twice and resuspended in metal-free HEPES buffer to a final OD600 of ∼1 for CreiLOVN41C samples and ∼0.5 for ZapCY1 and ZapCY2 samples. The cell suspension (3 ml) was transferred to a clean quartz cuvette or a cuvette with screw cap and septum inside an anaerobic chamber. The initial fluorescence was recorded, followed by the fluorescence upon the treatment of TPEN (50 μM) and after addition of ZnCl2/saponin/pyrithione (100 μM/0.002%/1.5 μM). Anaerobic manipulations were performed in anaerobic chamber or using cuvettes with screw caps and septa and a gas-tight Hamilton syringe.
Sensing zinc influx in E. coli using CreiLOVN41C and ZapCY2
E. coli MG1655 cells transformed with pQE80L-CreiLOVN41C and pQE80L-ZapCY2 were grown aerobically and, in the case of CreiLOVN41C also anaerobically, and protein expression was induced as described for the live cell suspension assays for detecting intracellular labile Zn2+. Cell pellets were then harvested, washed, and resuspended in metal-free HEPES buffer as described above. The cell suspension (200 μl) was transferred to a 96-well plate. The initial fluorescence was recorded, followed by the fluorescence upon the addition of ZnCl2 (0–75 μM). Fluorescence was recorded every 10 min up to 1.5 h for aerobic conditions or up to 2.5 h for anaerobic conditions. TPEN (100 μM for CreiLOVN41C and 150 μM for ZapCY2) was added, and the fluorescence was recorded every 10 min up to 1.5 h for aerobic conditions or up to 2.5 h for anaerobic conditions. Lastly, ZnCl2/saponin/pyrithione (200 μM/0.002%/1.5 μM) were added, and the fluorescence was recorded every 10 min up to 1.5 h for aerobic conditions or up to 2.5 h for anaerobic conditions. Anaerobic manipulations were done in an anaerobic chamber or using BD GasPak EZ anaerobe pouch and a gas generating sachet with anaerobic indicator.
Data availability
All data are contained within the manuscript and in the supporting information.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We would like to thank Dr Yongjun Gao for assistance with ICP-OES and ICP-MS. We also thank all members of the Zastrow group for valuable feedback and discussions.
Author contributions
H. N. N., U. H., and M. L. Z. methodology; H. N. N., U. H., and M. L. Z. formal analysis; H. N. N., U. H., and M. L. Z. writing–review and editing; H. N. N., U. H., and M. L. Z. validation; H. N. N. and U. H., investigation; H. N. N. and M. L. Z. visualization; H. N. N. and M. L. Z. writing–original draft; H. N. N. and M. L. Z. conceptualization; H. N. N. data curation; M. L. Z. supervision; M. L. Z. resources; M. L. Z. funding acquisition; M. L. Z. project administration.
Funding and additional information
This work was supported by NIH-NIGMS grant R35 GM138223 (M. L. Z.) and the Welch Foundation grant E-2176 (M. L. Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Joan B. Broderick
Supporting information
References
- 1.Maywald M., Wessels I., Rink L. Zinc signals and immunity. Int. J. Mol. Sci. 2017;18:2222. doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCall K.A., Huang C., Fierke C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000;130:1437S–1446S. doi: 10.1093/jn/130.5.1437S. [DOI] [PubMed] [Google Scholar]
- 3.Alberts I.L., Nadassy K., Wodak S.J. Analysis of zinc binding sites in protein crystal structures. Protein Sci. 1998;7:1700–1716. doi: 10.1002/pro.5560070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Outten C.E., O’Halloran T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 5.MacDiarmid C.W., Gaither L.A., Eide D. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000;19:2845–2855. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmiter R.D., Findley S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suhy D.A., Simon K.D., Linzer D.I.H., O’Halloran T.V. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J. Biol. Chem. 1999;274:9183–9192. doi: 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- 8.Eide D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Capdevila D.A., Giedroc D.P. Comprehensive Coordination Chemistry III. Elsevier; 2021. 8.37 Metal ion homeostasis; pp. 929–953. [DOI] [Google Scholar]
- 10.Mikhaylina A., Ksibe A.Z., Scanlan D.J., Blindauer C.A. Bacterial zinc uptake regulator proteins and their regulons. Biochem. Soc. Trans. 2018;46:983–1001. doi: 10.1042/BST20170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blencowe D.K., Morby A.P. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 2003;27:291–311. doi: 10.1016/S0168-6445(03)00041-X. [DOI] [PubMed] [Google Scholar]
- 12.Chandrangsu P., Helmann J.D. Intracellular Zn(II) intoxication leads to dysregulation of the PerR regulon resulting in heme toxicity in Bacillus subtilis. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Ren X., Fan B., Huang Z., Wang W., Zhou H., et al. Zinc toxicity and iron-sulfur cluster biogenesis in Escherichia coli. Appl. Environ. Microbiol. 2019;85:e01967-18. doi: 10.1128/AEM.01967-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehl-Fie T.E., Skaar E.P. Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botella H., Peyron P., Levillain F., Poincloux R., Poquet Y., Brandli I., et al. Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong C.L.Y., Gillen C.M., Barnett T.C., Walker M.J., McEwan A.G. An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J. Infect. Dis. 2014;209:1500–1508. doi: 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch C.C., Skaar E.P. Nutritional immunity: the battle for nutrient metals at the host–pathogen interface. Nat. Rev. Microbiol. 2022;20:657–670. doi: 10.1038/s41579-022-00745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capdevila D.A., Wang J., Giedroc D.P. Bacterial strategies to maintain zinc metallostasis at the host-pathogen interface. J. Biol. Chem. 2016;291:20858–20868. doi: 10.1074/jbc.R116.742023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh U., Nguyen H.N., Trinh B.K., Elhaj J., Zastrow M.L. A bioinformatic analysis of zinc transporters in intestinal Lactobacillaceae. Metallomics. 2023;15 doi: 10.1093/mtomcs/mfad044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singhal R., Shah Y.M. Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 2020;295:10493–10505. doi: 10.1074/jbc.REV120.011188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Outten F.W., Huffman D.L., Hale J.A., O’Halloran T.V. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 2001;276:30670–30677. doi: 10.1074/jbc.M104122200. [DOI] [PubMed] [Google Scholar]
- 22.Tan G., Cheng Z., Pang Y., Landry A.P., Li J., Lu J., et al. Copper binding in IscA inhibits iron-sulphur cluster assembly in Escherichia coli. Mol. Microbiol. 2014;93:629–644. doi: 10.1111/mmi.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan G., Yang J., Li T., Zhao J., Sun S., Li X., et al. Anaerobic copper toxicity and iron-sulfur cluster biogenesis in Escherichia coli. Appl. Environ. Microbiol. 2017;83:e00867-17. doi: 10.1128/AEM.00867-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beauchene N.A., Myers K.S., Chung D., Park D.M., Weisnicht A.M., Keleş S., et al. Impact of anaerobiosis on expression of the iron-responsive fur and ryhb regulons. mBio. 2015;6:e01947-15. doi: 10.1128/mBio.01947-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauchene N.A., Mettert E.L., Moore L.J., Keleş S., Willey E.R., Kiley P.J. O2 availability impacts iron homeostasis in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2017;114:12261–12266. doi: 10.1073/pnas.1707189114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter K.P., Young A.M., Palmer A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014;114:4564–4601. doi: 10.1021/cr400546e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt E.P.S., Damon L.J., Anson K.J., Palmer A.E. Tools and techniques for illuminating the cell biology of zinc. Biochem. Biophys. Acta Mol. Cell Res. 2021;1868 doi: 10.1016/j.bbamcr.2020.118865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen G.C., Janis M.K., Nguyen H.N., David O.W., Zastrow M.L. Fluorescent protein-based sensors for detecting essential metal ions across the tree of life. ACS Sens. 2024;9:1622–1643. doi: 10.1021/acssensors.3c02695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsien R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 30.Remington S.J. Fluorescent proteins: maturation, photochemistry and photophysics. Curr. Opin. Struct. Biol. 2006;16:714–721. doi: 10.1016/j.sbi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Zou W., Nguyen H.N., Zastrow M.L. Mutant flavin-based fluorescent protein sensors for detecting intracellular zinc and copper in Escherichia coli. ACS Sens. 2022;7:3369–3378. doi: 10.1021/acssensors.2c01376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee A., Weyant K.B., Agrawal U., Walker J., Cann I.K.O., Schroeder C.M. Engineering and characterization of new LOV-based fluorescent proteins from Chlamydomonas reinhardtii and Vaucheria frigida. ACS Synth. Biol. 2015;4:371–377. doi: 10.1021/sb500237x. [DOI] [PubMed] [Google Scholar]
- 33.Zou W., Le K., Zastrow M.L. Live-cell copper-induced fluorescence quenching of the flavin-binding fluorescent protein CreiLOV. ChemBioChem. 2020;21:1356–1363. doi: 10.1002/cbic.201900669. [DOI] [PubMed] [Google Scholar]
- 34.Qin Y., Dittmer P.J., Park J.G., Jansen K.B., Palmer A.E. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huynh U., Qiao M., King J., Trinh B., Valdez J., Haq M., et al. Differential effects of transition metals on growth and metal uptake for two distinct lactobacillus species. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.01006-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lule V.K., Tomar S.K., Chawla P., Pophaly S., Kapila S., Arora S. Bioavailability assessment of zinc enriched lactobacillus biomass in a human colon carcinoma cell line (Caco-2) Food Chem. 2020;309 doi: 10.1016/j.foodchem.2019.125583. [DOI] [PubMed] [Google Scholar]
- 37.Zhang N., Lu C., Zhang Z., Zhang H., Liu L., Jiang D., et al. Enhancing photo-fermentative biohydrogen production using different zinc salt additives. Bioresour. Technol. 2022;345 doi: 10.1016/j.biortech.2021.126561. [DOI] [PubMed] [Google Scholar]
- 38.Park J.G., Qin Y., Galati D.F., Palmer A.E. New sensors for quantitative measurement of mitochondrial Zn2+ ACS Chem. Biol. 2012;7:1636–1640. doi: 10.1021/cb300171p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes-Caballero H., Campanello G.C., Giedroc D.P. Metalloregulatory proteins: metal selectivity and allosteric switching. Biophys. Chem. 2011;156:103–114. doi: 10.1016/j.bpc.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D., Hosteen O., Fierke C.A. ZntR-mediated transcription of zntA responds to nanomolar intracellular free zinc. J. Inorg. Biochem. 2012;111:173–181. doi: 10.1016/j.jinorgbio.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hara T., Takeda Ta, Takagishi T., Fukue K., Kambe T., Fukada T. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017;67:283–301. doi: 10.1007/s12576-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karunatilaka K.S., Coupland B.R., Cameron E.A., Martens E.C., Koropatkin N.K., Biteen J.S. Single-molecule imaging can be achieved in live obligate anaerobic bacteria. Proc. SPIE. 2013;8590:85900K. [Google Scholar]
- 43.Xu Z., Wang P., Wang H., HangYu Z., Au-Yeung H.Y., Hirayama T., et al. Zinc excess increases cellular demand for iron and decreases tolerance to copper in Escherichia coli. J. Biol. Chem. 2019;294:16978–16991. doi: 10.1074/jbc.RA119.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salmon K., Hung S., Mekjian K., Baldi P., Hatfield G.W., Gunsalus R.P. Global gene expression profiling in Escherichia coli K12. J. Biol. Chem. 2003;278:29837–29855. doi: 10.1074/jbc.M213060200. [DOI] [PubMed] [Google Scholar]
- 45.Covert M.W., Knight E.M., Reed J.L., Herrgard M.J., Palsson B.O. Integrating high-throughput and computational data elucidates bacterial networks. Nature. 2004;429:92–96. doi: 10.1038/nature02456. [DOI] [PubMed] [Google Scholar]
- 46.Kang Y., Weber K.D., Qiu Y., Kiley P.J., Blattner F.R. Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function. J. Bacteriol. 2005;187:1135–1160. doi: 10.1128/JB.187.3.1135-1160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Constantinidou C., Hobman J.L., Griffiths L., Patel M.D., Penn C.W., Cole J.A., et al. A reassessment of the FNR regulon and transcriptomic analysis of the effects of nitrate, nitrite, NarXL, and NarQP as Escherichia coli K12 adapts from aerobic to anaerobic growth. J. Biol. Chem. 2006;281:4802–4815. doi: 10.1074/jbc.M512312200. [DOI] [PubMed] [Google Scholar]
- 48.Partridge J.D., Scott C., Tang Y., Poole R.K., Green J. Escherichia coli transcriptome dynamics during the transition from anaerobic to aerobic conditions. J. Biol. Chem. 2006;281:27806–27815. doi: 10.1074/jbc.M603450200. [DOI] [PubMed] [Google Scholar]
- 49.Zwietering M.H., Jongenburger I., Rombouts F.M., van’t Riet K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Motulsky H., Christopoulos A. Oxford University Press USA-OSO; New York, NY: 2004. Fitting Models to Biological Data Using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting. [Google Scholar]
- 51.Pla M.L., Oltra S., Esteban M.D., Andreu S., Palop A. Comparison of primary models to predict microbial growth by the plate count and absorbance methods. Biomed. Res. Int. 2015;2015:365025. doi: 10.1155/2015/365025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shcherbakova D.M., Baloban M., Emelyanov A.V., Brenowitz M., Guo P., Verkhusha V.V. Bright monomeric near-infrared fluorescent proteins as tags and biosensors for multiscale imaging. Nat. Commun. 2016;7:12405. doi: 10.1038/ncomms12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript and in the supporting information.