Abstract
Background
Complete preoperative comprehension of the adjacent structures of the kidney and location of renal vessels is essential for robot-assisted partial nephrectomy (RAPN). The effectiveness of three-dimensional (3D) visualization techniques in improving perioperative outcomes of RAPN has been inconsistent and has not been reported in Northeastern China.
Methods
In this cohort study, we reviewed patients with renal tumours who underwent RAPN between April 2019 and April 2024. Three-dimensional visualization models were reconstructed to evaluate resectability parameters, including vascular variations, collection system infiltration, and lymphatic involvement. Subsequently, a meta-analysis combining previous studies utilising 3D visualization techniques for partial nephrectomy was conducted.
Results
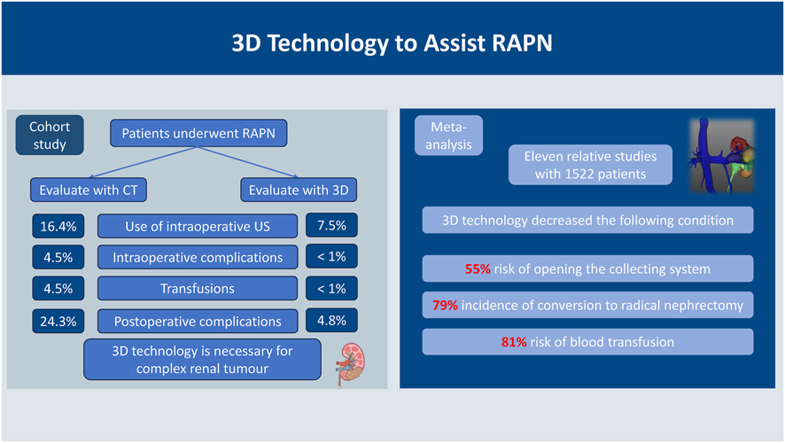
Of the 324 patients in the cohort, 147 were preoperatively evaluated using the 3D technology. Group 3D had significantly less estimated blood loss (P < 0.001) and a shorter operative time (P = 0.016) than in group No 3D. We also found that the rates of intraoperative ultrasound use (P = 0.015), intraoperative complications (P = 0.007), intraoperative transfusions (P = 0.007), and postoperative Clavien complications (P < 0.001) in group 3D were significantly lower than in group No 3D. The above findings were consistent in the subgroup with R.E.N.A.L. ≥ 8 points partly. Furthermore, a meta-analysis identified 11 studies that included 1522 patients who underwent RAPN. Use of 3D visualization technology resulted in decreased 55 % risk of opening the collecting system (Risk Ratio [RR] = 0.45[0.22–0.92], P = 0.030) and 79 % incidence of conversion to radical nephrectomy (RR = 0.21[0.08–0.57], P = 0.002). The RAPN group assisted by 3D visualization techniques showed an 81 % reduction in the risk of blood transfusion than in the control group (RR = 0.19[0.08–0.44], P < 0.001).
Conclusions
The application of 3D technology in RAPN appears to be superior for improving precise tumour removal and reducing adverse perioperative outcomes and should be considered for wide use in clinical practice.
Keywords: Nephrectomy, Kidney neoplasms, Three-dimensional, Cohort studies, Meta-analysis
Graphical abstract
Highlights
-
•
RAPN has become the preferred treatment for small renal tumours.
-
•
3D visualization can improve precise tumour removal and reduce adverse perioperative outcomes.
-
•
This study bridges the research gap in northeastern China for perioperative outcomes in RAPN.
1. Introduction
Small renal masses have been increasingly detected over the past two decades owing to the advent of cross-sectional imaging. Partial nephrectomy (PN) has been broadly embraced as the standard of care in the treatment of T1 renal parenchymal tumours [[1], [2], [3], [4], [5], [6], [7]]. Nephron-sparing surgery has been proven to significantly lower the potential risk of chronic renal failure compared with radical nephrectomy [8,9]. Protecting normal renal parenchyma from ischaemic injury can effectively prevent poor clinical outcomes such as cardiovascular events and chronic kidney disease, as well as improve overall survival and mortality [[10], [11], [12], [13]].
Generally, blood flow is controlled to attain a sufficient resection margin by clamping the main hilar vessels during surgery [2]. However, a prolonged warm ischaemia time may hinder renal function rehabilitation, especially in patients with risk factors such as diabetes and hypertension [2,14]. Therefore, the duration of warm ischaemia and surgery as well as estimated blood loss (EBL) must be mitigated to alleviate renal function injury. Off-clamp robot-assisted partial nephrectomy (RAPN) has also been proposed to reduce renal ischaemic injury and better preserve postoperative renal function. While there have been reports of avoiding renal artery clamping may lead to a higher risk of intraoperative complications, estimated blood loss and positive surgical margin [15,16]. However, a recent meta-analysis of 10 studies with 2307 patients indicated that the perioperative complication rate of off-clamp RAPN group was similar to that of the on-clamp RAPN group and may be better protected against renal function and reduce the rate of positive margins [17]. This new finding suggests that the off-clamp technique is becoming more established and is improving renal function in patients as RAPN becomes more widespread and urologists enhance their surgical skills. Given the current “precision surgery” era, it is an indispensable preoperative step to comprehensively understand the detailed surgical anatomy of the kidney. Identifying the intrarenal vessel anatomy using only two-dimensional computed scan slices is challenging [5]. Three-dimensional (3D) reconstruction images generated using a software-aided technique to analyse enhanced computed tomography (CT) scan images may be considered a superior and promising choice [10,18]. Preoperative strategies and intraoperative real-time navigation can be achieved using 3D reconstruction [4,9,19,20]. Preoperative 3D reconstruction based on enhanced computed tomography (CT) has emerged as a potential technique for improving the quality of surgical resection. This rapidly evolving technology has greatly improved doctors' understanding of tumours and surrounding anatomical structures [[21], [22], [23]].
For more complex endophytic renal tumours (R.E.N.A.L. ≥ 8 score), intraoperative bleeding is higher than exophytic tumour due to the deeper location of the tumour and proximity to the collecting system; moreover, it is often not feasible to differentiate tumour and the renal parenchyma due to their closed relationship. Therefore, for patients with complex renal tumours, the rational application of preoperative 3D visualization can significantly control intraoperative bleeding, reduce operative time, and achieve the goal of trifecta achievement [3,[24], [25], [26]]. The flexibility of the robotic arm allows RAPN to be a more precise approach for complex clinical stage T1 renal tumours [27]. Minimally invasive laparoscopic and robotic approaches are less traumatic to patients and maintain oncologic efficacy compared to open nephron-sparing surgery, resulting in reduced convalescence time and fewer complications [2,9,28,29].
Currently, studies on RAPN are limited, especially in China, and the effectiveness of 3D-related technologies in this field remains inconclusive. Thus, this study employed a retrospective cohort design to evaluate the perioperative effects of 3D reconstruction in patients who underwent RAPN. Given the limitations of this single-centre study, we conducted a Meta-analysis of 3D technology in RAPN to provide a comprehensive assessment of its current status within the field.
2. Materials and methods
2.1. Retrospective cohort study
2.1.1. Patient selection and criteria
This retrospective study was conducted at our center and included 324 consecutive patients with renal tumours who underwent RAPN between April 2019 and April 2024. All patients were informed about the treatment, including surgery, risks, and complications, and were then divided into two groups according to whether they volunteered to undergo preoperative CT-based 3D reconstruction: 147 patients underwent 3D reconstruction based on enhanced CT, whereas the remaining 177 patients had their tumours assessed using conventional enhanced CT. The inclusion criteria were as follows: (1) clinical stage determined as T1N0M0 or carefully selected T2N0M0 by enhanced CT, isolated tumour; (2) normal contralateral kidney; and (3) no history of kidney surgery. Ethical approval for the study was granted by the Ethics Review Committee of the Second Affiliated Hospital of Dalian Medical University, and due to the retrospective nature of the study, the requirement for informed consent from the patients was waive (Ethical Approval Number: 202124).
2.1.2. CT and three-dimensional reconstruction
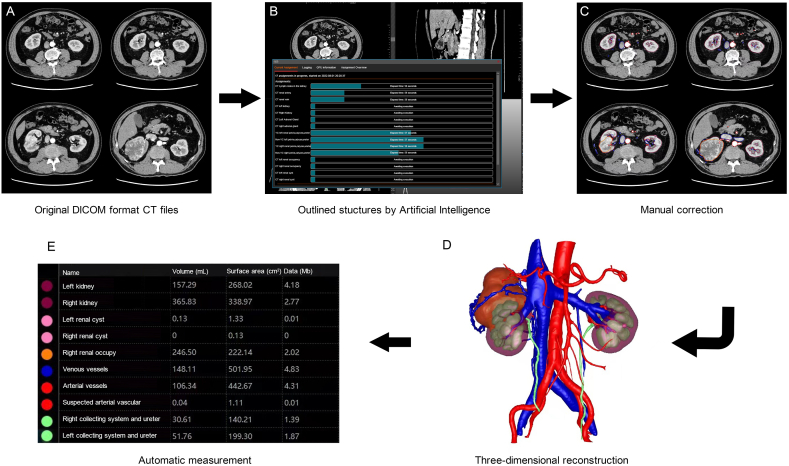
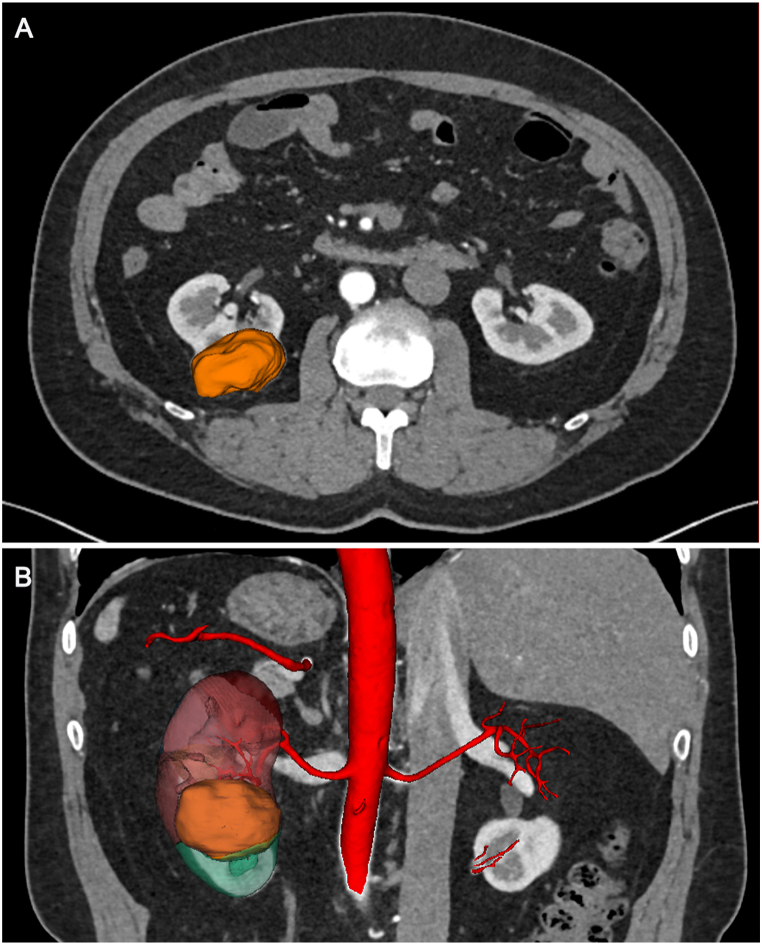
Preoperatively, a 64-multidetector row CT scanner with a 0.5 mm step interval was used to perform contrast-enhanced CT scans on all patients. Imaging data from the arterial, portal vein, and delayed phases were obtained by intravenous injection of a contrast medium into the patient's peripheral vein. Raw contrast-enhanced CT in the DICOM format forms the basis of three-dimensional reconstruction, which involves extracting related anatomical parameters, such as arteries, veins, renal tumours, abdominal aorta, renal parenchyma, and the renal collecting system, through artificial intelligence (Fig. 1A and B). These parameters are manually analysed and integrated by IPS 3D reconstruction software (YORKTAL) into a retroperitoneal 3D image (Fig. 1C and D). Volumetric measurements of the individual structures are automatically performed (Fig. 1E). The 3D reconstruction technique allows surgeons to recognise the size and location of tumours and the anatomical relationship between the tumours and surrounding structures more clearly before surgery (Fig. 2A and B). The surgical simulation software YORKTAL includes tools for measuring the tumour volume and diameter. In addition, the platform allows doctors to observe tumours from any angle and determine the spatial distribution of retroperitoneal structures. Otherwise, individual anatomical structures can be zoomed in, out, transparentized, or hidden by the surgeon so that doctors can further prepare for and understand the surgery. The 3D reconstruction technique provides surgeons with a comprehensive assessment of the patient's condition and a complete awareness of the complexity of the operation.
Fig. 1.
Flow diagram of three-dimensional reconstruction: (A): original DICOM format computed tomography files; (B): structures outlined by artificial intelligence; (C): manual correction; (D): three-dimensional reconstruction; (E): automatic measurement of structures.
Fig. 2.
Combination of two-dimensional and three-dimensional images in axial (A) and coronal (B) views.
2.1.3. Combination of three-dimensional renal reconstruction and operation
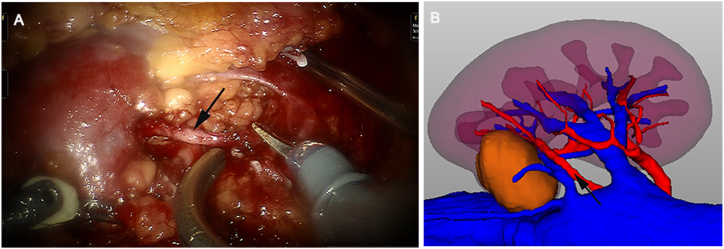
All RAPNs were performed by an experienced urologist and achieved with da Vinci ® Si surgical systems. Ultrasound was used appropriately during surgery. All patients underwent RAPN under general anaesthesia. The robotic arm was placed into three trocar holes in the lumbar region. After dilation of the retroperitoneal space, retroperitoneal fat was dissociated, and Gerota's fascia was sharply dissected. After the hilum was located and perirenal fat was removed, the locations of the tumour and renal artery could be clearly identified. A surgical assistant stood beside the surgeon, who provided 3D imaging of the tumour on a touchscreen device for intraoperative guidance and reference. The imaging data of anatomical structures can vary in size and direction with changes in the surgical field. Furthermore, the assistant can demonstrate relevant anatomy to be isolated based on the preoperative discussion to facilitate surgeon's effort in locating the tumours and surrounding structures and improve success rate of complete tumour resection. Moreover, the position of bulldog clamps of renal hilar vessels was consistent with the preoperative preparation (Fig. 3A and B). Tumour resection was performed, and the renal pelvis, collecting system, renal medulla, and cortex were sutured using 2-0 V-Loc and 3-0 V-Loc sutures to avoid possible postoperative bleeding and urine leakage. Bulldog clamps were removed after ensuring that all possible entryways were closed. No active bleeding was detected in the surgical area, and haemostatic gauze was placed on the surgical wound and renal artery. Pathological specimens were removed using a specimen bag, and an indwelling retroperitoneal drainage catheter was placed and appropriately secured.
Fig. 3.
The renal artery (black arrows) was identified during robot-assisted partial nephrectomy (A), and its anatomical morphology and surrounding adjacencies were consistent with the preoperative three-dimensional visualization reconstruction (B).
2.1.4. Statistical methods and data
Demographic data, preoperative characteristics, perioperative variables, and pathological results were recorded. Details of the perioperative and Clavien complications are shown in Supplementary Table 1 and Supplementary Table 2. Various statistical methods have been applied to diverse types of statistical data. Measurement data were tested by Student's t-test and Mann‒Whitney U test in accordance with whether the data met the normal distribution and then reported as the mean ± standard deviation (SD) or median and interquartile range (IQR). For counting data, statistical tests such as Pearson's chi-square test, Fisher's exact test, and continuity correction were chosen based on the total number of cases and the expected frequency. A two-sided P value < 0.05 was considered statistically significant in this study. All statistical calculations in this study were conducted using the IBM SPSS Statistical Software (IBM SPSS Statistics 26.0, IBM Corporation, USA).
2.2. Meta-analysis
2.2.1. Search strategy
This meta-analysis adhered to the PRISMA guidelines. We searched MEDLINE, Cochrane Library, EMBASE, and Scopus databases for relevant articles. The search string was built as follows: ((Robot assisted partial nephrectomy) OR (RAPN) OR (robot-assisted laparoscopic partial nephrectomy) OR (RALPN) OR (RPN)) AND ((Three-dimensional) OR (3D) OR (3-dimensional)). Searches were restricted to the period from the inception of the database to May 13, 2023 and there were no restrictions on country or article type. Two independent reviewers assessed articles that matched the study by title and abstract, as well as the original full text, including as many eligible articles as possible. This meta-analysis was approved by the PROSPERO review protocol (CRD42023426266).
2.2.2. PICOS criteria
P (Population): Patients diagnosed with renal parenchymal tumour and undergoing RAPN.
I (Intervention): Using 3D technology to assist urologists in perioperative operations, including 3D reconstruction, 3D real-time navigation, 3D virtual reality, 3D augmented reality, and other related 3D technologies.
C (Comparator): Patients who did not use 3D technology to assist with RAPN.
O (Outcome):
Intraoperative outcomes: global ischaemia, global ischaemia time, partial ischaemia time, EBL, operative time, console time, opening of the collecting system, intraoperative complications, intraoperative ultrasound use, and conversion to radical nephrectomy.
Postoperative outcomes: postoperative complication, transfusion, surgical margins, and length of stay.
S (Study design): All comparative studies, including randomised clinical studies, case-control studies, and cohort studies, were included.
2.2.3. Eligibility criteria
Articles included in this study were required to meet the following inclusion and exclusion criteria:
Inclusion criteria.
-
1.
Surgery in patients with renal cancer performed by RAPN;
-
2.
Surgery in patients in the experimental group utilized 3D technology to assist in the RAPN;
-
3.
Contains the required outcomes information.
Exclusion criteria.
-
1.
Animal experiments, reviews, case reports, commentaries;
-
2.
Missing key information or data that cannot be converted;
-
3.
Single-arm study.
2.2.4. Data extraction and quality assessment
We extracted the following basic information from the 11 final trials, including this cohort study: first author, year of publication, country or region, study type, and mode of use of 3D technology. In addition, we extracted the study sample size, participant age, clinical or pathological stage, and perioperative outcomes to be analysed. Two reviewers independently extracted and cross-checked the data, and any disagreements were resolved after arbitration by a third reviewer.
The Newcastle-Ottawa Scale (NOS) was used for cohort studies. A total score of 9 on the NOS scale was obtained: the selection section accounted for 4 points, the comparability section contained 2 points, and the outcome section contained 3 points. Higher scores indicated higher quality studies. Quality assessment of the RCT was performed according to the Cochrane Collaboration risk-of-bias tool, which assesses article bias in seven aspects.
2.2.5. Statistical analysis
For all dichotomous variables, outcomes were compared using risk ratio (RR). For continuous variables, effect size was defined as the mean difference (MD). It is worth noting that for continuous variables, we extracted the mean and SD; if only the median and IQR were provided in the text, we transformed the data using the method for Unknown Non-Normal Distributions [30]. When heterogeneity was high, I squared >50%, a random-effects model was applied; otherwise, a fixed-effects model was adopted for the analysis. All meta-analyses were performed using Review Manager 5.3 (RevMan, Version 5.3) and P < 0.05 was considered significant.
3. Results
3.1. Retrospective cohort study
3.1.1. Overall preoperative baseline demographics
All 147 patients in the 3D model group underwent successful preoperative reconstruction. The average age of the patients in group 3D was 58.6 years, while that in group No 3D was 58.1 years. The proportion of males in group 3D was 59.9 % compared to 64.4 % in group No 3D. Analysis of demographics and preoperative characteristics revealed that both groups were comparable in terms of sex distribution (P = 0.401), age (P = 0.654), BMI (P = 0.227), Eastern Cooperative Oncology Group (ECOG) performance (P = 0.889), American Society of Anaesthesiologists (ASA) score (P = 0.095), tumour size on CT scan (P = 0.908), and clinical stage (P = 0.319). In assessing the morphology and location of the renal tumour, there were no significant differences in the R.E.N.A.L. score (P = 0.069), PADUA score (P = 0.733), or tumour location in different orientations between the two groups (Table 1).
Table 1.
Overall patient demographics and preoperative characteristics.
| No 3D | 3D | P value | |
|---|---|---|---|
| No. of patients | 177 | 147 | |
| Malesa | 114 (64.4) | 88 (59.9) | 0.401 |
| Ageb (yr) | 58.1 (11.7) | 58.6 (12.0) | 0.654 |
| BMIc (kg/m2) | 25.7, 25.0 (23.5; 27.5) | 25.1, 25.1 (23.2; 26.7) | 0.227 |
| ECOGc | 0.21, 0 (0; 0) | 0.24, 0 (0; 0) | 0.889 |
| ASA scorec | 1.39, 1 (1; 2) | 1.29, 1 (1; 2) | 0.095 |
| Preoperative hemoglobinc (g/L) | 141.7, 143.0 (130.0; 156.0) | 139.7, 141.0 (129.0; 151.0) | 0.281 |
| Tumor size at CT scanc (mm) | 34.4, 32.0 (26.0; 41.0) | 35.5, 33.0 (25.0; 43.0) | 0.908 |
| Clinical stagea | 0.319 | ||
| cT1a | 130 (73.4) | 104 (70.7) | |
| cT1b | 43 (24.3) | 35 (23.8) | |
| ≥cT2 | 4 (2.3) | 8 (5.4) | |
| R.E.N.A.L. scorec | 7.57, 8.0 (6.0; 9.0) | 7.29, 7.0 (6.0; 8.0) | 0.069 |
| PADUA scorec | 8.5, 8.0 (7.0; 9.0) | 8.5, 8.0 (7.0; 10.0) | 0.733 |
| Tumor location (up/low)a | 0.480 | ||
| Upper pole | 72 (40.7) | 62 (42.2) | |
| Mediorenal | 49 (27.7) | 47 (32.0) | |
| Lower pole | 56 (31.6) | 38 (25.9) | |
| Tumor location (anterior/posterior)a | 0.549 | ||
| Anterior | 89 (50.3) | 69 (46.9) | |
| Posterior | 88 (49.7) | 78 (53.1) | |
| Tumor location (rim)a | 0.573 | ||
| Medial rim | 68 (38.4) | 61 (41.5) | |
| Lateral rim | 109 (61.6) | 86 (58.5) |
ASA = American Society of Anesthesiologists; BMI = body mass index; CT = computed tomography; ECOG = Eastern Cooperative Oncology Group; IQR = interquartile range, a = no. (%); b = mean (standard deviation); c = mean, median (interquartile range).
3.1.2. Overall perioperative outcomes
Among the perioperative parameters, the estimated blood loss (P < 0.001) and operative time (P = 0.016) were significantly lower in group 3D. Moreover, group 3D also had a significantly lower rate of intraoperative ultrasound (US) use (P = 0.015) and postoperative Clavien complications (P < 0.001). In addition, it is worth noting that there was a significantly higher incidence of intraoperative complications (P = 0.007) and intraoperative transfusion rates (P = 0.007) in the No 3D group, whereas these conditions did not occur in any of the patients in the 3D group. No significant differences were found in length of stay (P = 0.077), ischaemia time (P = 0.878), the incidence of conversion to radical nephrectomy (P = 0.298), or the change between preoperative serum creatine (SCr) and postoperative SCr (ΔSCr) (P = 0.840) (Table 2).
Table 2.
Overall perioperative variables.
| No 3D | 3D | P value | |
|---|---|---|---|
| No. of patients | 177 | 147 | |
| Ischaemia timec (min) | 22.0, 20.0 (17.0; 27.0) | 22.2 21.0 (17.0; 27.0) | 0.878 |
| EBLc (ml) | 113.7, 50.0 (50.0; 100.0) | 59.6, 50.0 (30.0; 50.0) | <0.001 |
| Operative timec (min) | 144.0, 130.0 (103.5; 173.0) | 128.9, 120.0 (100.0; 150.0) | 0.016 |
| Use of intraoperative USa | 29 (16.4) | 11 (7.5) | 0.015 |
| Intraoperative complicationsa | 8 (4.5) | 0 (0.0) | 0.007 |
| Transfusionsa | 8 (4.5) | 0 (0) | 0.007 |
| Conversion to radical nephrectomya | 2 (1.1) | 0 (0) | 0.298 |
| Postoperative complications, Claviena | <0.001 | ||
| 1 | 27 (15.3) | 5 (3.4) | |
| ≥2 | 16 (9.0) | 2 (1.4) | |
| Preoperative stage of renal insufficiencya | 0.350 | ||
| 1 | 154 (87.0) | 135 (91.8) | |
| 2 | 20 (11.3) | 11 (7.5) | |
| 3 | 3 (1.7) | 1 (0.7) | |
| Postoperative stage of renal insufficiencya | 0.264 | ||
| 1 | 143 (80.8) | 128 (87.1) | |
| 2 | 32 (18.1) | 17 11.6) | |
| 3 | 2 (1.1) | 2 (1.4) | |
| ΔSCrc (μmol/L) | 3.2, 2.0 (−3.6; 9.4) | 2.8, 2.5 (−3.4; 7.6) | 0.840 |
| Length of stayc (day) | 10.9, 10 (8; 12) | 10.3, 10 (8; 11) | 0.077 |
EBL = estimated blood loss; SCr = serum creatine; US = ultrasonography; a = no. (%); c = mean, median (interquartile range).
3.1.3. Overall pathological outcomes
Analysis of the pathological analysis revealed statistically significant differences in the distribution of pathological stages (P < 0.001) and pathological histology (P = 0.030), as determined by the pathological sections in the two groups. No significant differences were observed in malignancy (P = 0.069), tumour size at final pathology (P = 0.893), ISUP grade (P = 0.168), or positive surgical margins (P = 0.433) (Table 3).
Table 3.
Pathological results.
| No 3D | 3D | P value | |
|---|---|---|---|
| No. of patients | 177 | 147 | |
| Malignanta | 161(91.0) | 124 (84.4) | 0.069 |
| Pathological stagea | <0.001 | ||
| pT1a | 114 (64.4) | 93 (63.3) | |
| pT1b | 46 (26.0) | 20 (13.6) | |
| pT2 | 0 (0) | 7 (4.8) | |
| pT3 |
1 (0.8) |
6 (4.1) |
|
| NR | 16 (9.0) | 21 (14.3) | |
| Tumor size at final pathologyc (mm) | 34.5, 30.0 (25.0; 40.0) | 35.4, 32.0 (24.0; 40.0) | 0.893 |
| Positive surgical margina | 4 (2.3) | 2 (1.4) | 0.433 |
| Histologya | 0.003 | ||
| Clear cell carcinoma |
154 (87.0) |
113 (76.9) |
|
| Angiomyolipoma | 8 (4.5) | 11 (7.5) | |
| Papillary | 7 (4.0) | 6 (4.1) | |
| Oncocytoma | 1 (0.6) | 7 (4.8) | |
| Cromophobe | 0 (0.0) | 4 (2.7) | |
| Unclassified | 5 (2.8) | 0 (0.0) | |
| Other | 2 (1.1) | 6 (4.1) | |
| ISUP gradea | 0.168 | ||
| 1 | 12 (7.5) | 16 (13.1) | |
| 2 | 115 (71.4) | 87 (71.3) | |
| 3 |
31 (19.3) |
19 (15.6) |
|
| 4 | 3 (1.9) | 0 (0.0) |
ISUP = International Society of Urological Pathology; a = no. (%); c = mean, median (interquartile range).
3.1.4. Perioperative outcomes by subgroup analysis of R.E.N.A.L. Scores
To more deeply dissect the reasons for the differences in perioperative parameters, we classified all eligible patients into two groups according to a cutoff value of 8 in the classical R.E.N.A.L. score for renal tumours. In the R.E.N.A.L. score <8 subgroup, EBL was significantly lesser in the group 3D than in the control group (P = 0.002) (Table 4). In the R.E.N.A.L. score ≥8 subgroup, the EBL (P = 0.046) and length of hospital stay (P = 0.027) in group 3D were markedly lesser than in group No 3D. In addition, patients in group 3D were significantly less likely to have intraoperative complications (P = 0.030), intraoperative transfusions (P = 0.030), and postoperative Clavien complications (P < 0.001) than in group No 3D (Table 4). Additionally, in the subgroups of simple tumours (R.E.N.A.L. score <8) and complex tumours (R.E.N.A.L. score ≥8), there was no significant difference between preoperative and postoperative stage of renal insufficiency, and three-dimensional reconstruction did not significantly affect the change between preoperative serum creatinine (SCr) and postoperative SCr (ΔSCr).
Table 4.
Perioperative variables in different subgroups by R.E.N.A.L. score.
| R.E.N.A.L. score <8 | R.E.N.A.L. score ≥8 | |||||
|---|---|---|---|---|---|---|
| No 3D | 3D | P value | No 3D | 3D | P value | |
| No. of patients | 88 | 91 | 89 | 56 | ||
| Ischaemia timec (min) | 19.4, 19.0 (15.0; 22.5) | 19.6, 20.0 (15.0; 22.0) | 0.783 | 24.7, 23.5 (20.0; 29.5) | 26.4, 26.0 (20.0; 28.5) | 0.256 |
| EBLc (ml) | 79.6, 50.0 (50.0; 100.0) | 49.5, 50.0 (20.0; 50.0) | 0.002 | 147.9, 50 (50; 100) | 75.9, 50 (50; 100) | 0.046 |
| Operative timec (min) | 131.6, 122.5 (100.0; 155.0) | 118.5, 117.0 (95.0; 135.0) | 0.027 | 156.0, 145.0 (110.0; 182.5) | 145.7, 132.5 (120.0; 166.0) | 0.521 |
| Use of intraoperative USa | 8 (9.1) | 2 (2.2) | 0.093 | 21 (23.6) | 9 (16.1) | 0.191 |
| Intraoperative complicationsa | 1 (1.1) | 0 (0) | 0.492 | 7 (7.9) | 0 (0) | 0.030 |
| Transfusionsa | 1 (1.1) | 0 (0) | 0.492 | 7 (7.9) | 0 (0) | 0.030 |
| Conversion to radical nephrectomya | 1 (1.1) | 0 (0) | 0.492 | 1 (1.1) | 0 (0) | 0.618 |
| Postoperative complications, Claviena | 0.092 | <0.001 | ||||
| 1 | 8 (9.1) | 3 (3.3) | 19 (21.3) | 2 (3.6) | ||
| ≥2 | 4 (4.5) | 1 (1.1) | 12 (13.5) | 1 (1.8) | ||
| Preoperative stage of renal insufficiencya | 0.147 | 0.488 | ||||
| 1 | 76 (86.4)76 (86.4) | 86 (94.5) | 78 (87.6) | 49 (87.5) | ||
| 2 | 11 (12.5) | 4 (4.4) | 9 (10.1) | 7 (12.5) | ||
| 3 | 1 (1.1) | 1 (1.1) | 2 (2.2) | 0 (0.0) | ||
| Postoperative stage of renal insufficiencya | 0.085 | 0.728 | ||||
| 1 | 72 (81.8) | 83 (91.2) | 71 (79.8) | 45 (80.4) | ||
| 2 | 15 (17.0) | 6 (6.6) | 17 (19.1) | 11 (19.6) | ||
| 3 | 1 (1.1) | 2 (2.2) | 1 (1.1) | 0 (0.0) | ||
| ΔSCrc (μmol/L) | 0.2, 0.3 (−5.8; 5.8) | 1.4, 1.9 (−3.6; 5.9) | 0.321 | 6.0, 3.6 (−2.0; 3.5) | 5.2, 5.1 (−3.1; 11.3) | 0.845 |
| Length of stayc (day) | 10.1, 10 (8; 11) | 10.2, 10 (8; 11) | 0.944 | 11.7, 11 (9; 14) | 10.5, 10 (8; 12) | 0.027 |
EBL = estimated blood loss; SCr = serum creatine; US = ultrasonography; a = no. (%); c = mean, median (interquartile range).
3.2. Meta-analysis
3.2.1. Study selection and characteristics
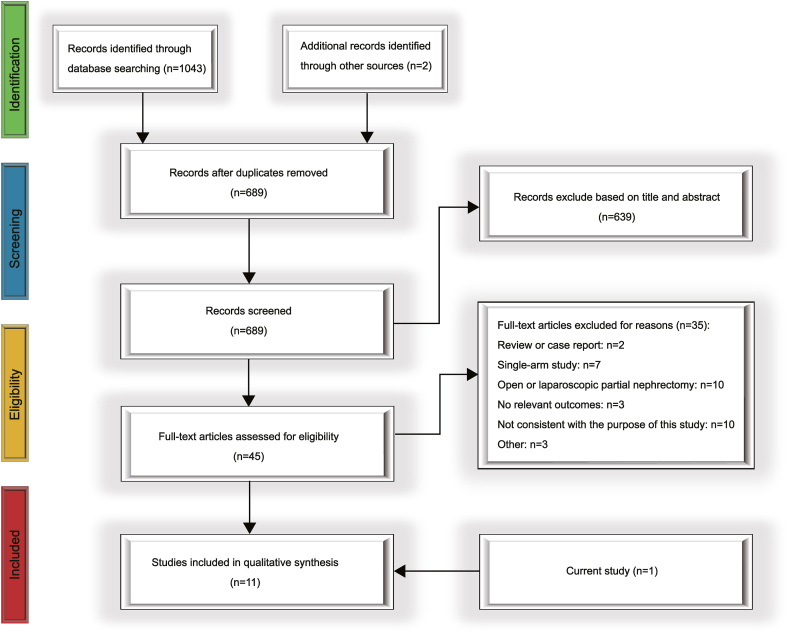
Fig. 4 illustrates the flow of study selection in detail. We initially searched for 1045 articles. After removing duplicates and filtering for study purposes by abstract and title, we read 45 full-text articles. We excluded 35 articles for the following reasons: two articles were reviews and case reports; seven articles were single-arm studies; 10 studies included patients’ surgical approach which did not fit the study; three articles did not have appropriate outcome indicators; 10 studies were inconsistent with the purpose of the study; and three articles accounted for other reasons. Eleven articles, including those included in our study, were included. The included studies were published from 2018 to 2023, four of which were from Asia, and the rest were from Europe or the Americas. Ten articles were non-randomised controlled studies, and one was a randomised controlled study. Five of these articles investigated the use of 3D technology in intraoperative navigation. Additionally, there were three studies each on 3D virtual reality, 3D printing, and reconstruction technologies. Table 5 shows the detailed underlying information of the included articles. The total NOS scores for the cohort studies ranged from 4 to 9, with six trials scoring greater than or equal to 7(Supplementary Table 3). This randomised controlled study demonstrated good control of the risk of bias (Supplementary Fig. 1).
Fig. 4.
PRISMA flow diagram of study selection for this meta-analysis.
Table 5.
The main characteristics of the eleven articles included in the meta-analysis.
| Reference | Country or Region | Study Type | Model | Paitents |
Age [Mean (SD)] |
Stage (T1a/T1b/ ≥ T2a) |
NOS score | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl group | 3D group | Ctrl group | 3D group | Ctrl group | 3D group | |||||
| Wu C 2023 [31] | China | Prospective study | Intraoperative guiding | 19 | 63 | 50 [46–58]a | 50 [41–60]a | 11/4/1c | 38/16/3c | ★★★★★★★ |
| Berger L 2021 [32] | London | Prospective study (retrospective control group) | Three-dimensional virtual reality | 22 | 22 | 56.6 (11.7) | 56.6 (11.1) | NR | 12/10/0b | ★★★★★★ |
| Li L 2021 [33] | China | Prospective study (retrospective control group) | Navigated by 3D augmented model rendering | 25 | 16 | 56.1 (12.4) | 45.8 (13.0) | 12/11/2c | 4/9/3c | ★★★★★★ |
| Michiels C 2021 [34] | France | Retrospective study | Three-dimensional image-guided RAPN | 415 | 230 | 61.2 [52–69]a | 62 [50–69]a | −/−/55c | −/−/73c | ★★★★★★★★ |
| Kobayashi S 2020 [35] | Japan | Retrospective study | Real-time surgical navigation | 42 | 42 | 58 [42–67]a | 58 [49–68]a | 25/10/7c | 35/7/0c | ★★★★★★★★★ |
| Porpiglia F 2020 [26] | Italy | Retrospective study | Three-dimensional static and elastic augmented reality systems | 43 | 48 | 58 (9.8) | 62 (15) | 19/22/2b 10/20/8c |
21/22/5b 14/19/7c |
★★★★★★★ |
| Shirk JD 2019 (1) [36] | America | Prospective study | Three-dimensional virtual reality | 48 | 44 | 57.6 (12.3) | 64.6 (9.7) | NR | NR | NR |
| Shirk JD 2019 (2) [37] | America | Prospective (retrospective control group) | Three-dimensional virtual reality | 30 | 30 | 60.9 (15.0) | 63.4 (13.0) | NR | NR | ★★★★★★ |
| Maddox MM 2018 [38] | America | Prospective study | Three-dimensional -printed soft-tissue physical models | Database | 7 | 60.9 | 61.6 (7.7) | NR | 2/4/-c | ★★★★ |
| Porpiglia F 2018 [39] | Italy | Prospective study | Hyper-accuracy 3D reconstruction | 31 | 21 | 59.5 (10.6) | 60.8 (12.3) | 10/16/5b 6/11/11c |
4/11/6b 3/8/9c |
★★★★★★★★ |
| This study 2024 | China | Retrospective study | Three-dimensional reconstruction | 177 | 147 | 58.1 (11.7) | 58.6 (12.0) | 130/43/4b | 104/35/8b | ★★★★★★★ |
3D: Three-dimensional; NOS: Newcastle–Ottawa Scale; NR: Not reported; a:median (IQR); b:Clinical staging; c Pathology staging.
3.2.2. Intraoperative outcomes
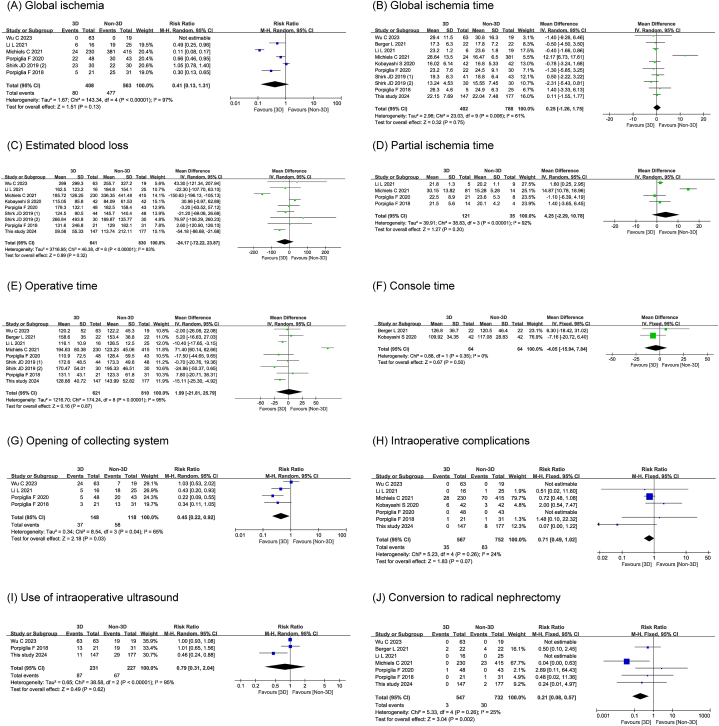
Four studies, including 266 patients, provided information on the opening of the collecting system and found that the incidence of opening of the collecting system (RR 0.45, 95 % CI 0.22,0.92; P = 0.030) was significantly lower in the 3D technology-assisted RAPN group than in the conventional RAPN group. Seven studies comparing intraoperative surgical conversions suggested that the 3D technology-assisted RAPN group was significantly less likely to undergo conversion to radical nephrectomy (RR 0.21, 95 % CI 0.08,0.57; P = 0.002) than the conventional RAPN group. There were no differences in global ischaemia (RR 0.41, 95 % CI 0.13,1.31; P = 0.130), global ischaemia time (MD 0.25, 95 % CI -1.26,1.75; P = 0.750), partial ischaemia time (MD 4.25, 95 % CI -2.29,10.78; P = 0.200), EBL (MD -24.17, 95 % CI -72.77,23.87; P = 0.320), operative time (MD 1.99, 95 % CI -21.87, 25.79; P = 0.870), console time (MD -4.05, 95 % CI -15.94,7.84; P = 0.500), incidence of intraoperative complications (RR 0.71, 95 % CI 0.49,1.02; P = 0.070), and rate of intraoperative ultrasound use (RR 0.79, 95 % CI 0.31, 2.04; P = 0.620) between the two groups. The detailed intraoperative results of the forest plot are shown in Fig. 5A–J.
Fig. 5.
The forest plots of intraoperative outcomes.
3.2.3. Postoperative outcomes
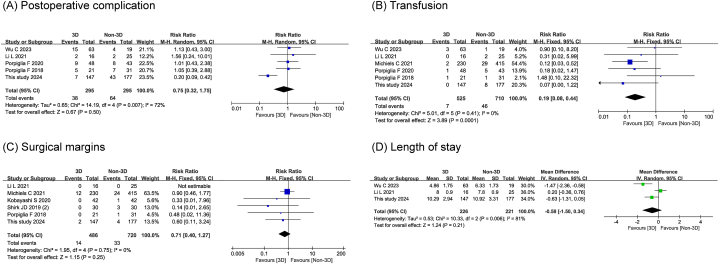
Six studies enrolling 1138 individuals showed a statistically lower incidence of transfusion (RR 0.19, 95 % CI 0.08,0.44; P < 0.001) in the 3D technology-assisted RAPN group than in the control group. We did not find any statistically significant changes in postoperative complication (RR 0.75, 95 % CI 0.32, 1.75; P = 0.500), surgical margins (RR 0.71, 95 % CI 0.40,1.27; P = 0.250), and length of stay (MD -0.58, 95 % CI -1.50,0.34; P = 0.210) in either group. The detailed postoperative results of the forest plot are shown in Fig. 6A–D.
Fig. 6.
The forest plots of postoperative outcomes.
4. Discussion
For small renal tumours, the treatment outcome of PN is not inferior to that of radical nephrectomy [40,41]. However, despite the extensive use of PN over the past decade, surgery-associated renal ischaemia may lead to impaired renal function, which cannot be overlooked. Evidence indicates that preserving the renal parenchyma is essential for mitigating postoperative renal insufficiency and decreasing adverse outcomes such as cardiovascular events, acute renal failure, prolonged hospital stay, and death [12,42,43]. Therefore, PN was recommended as the standard approach for T1a tumours (≤4 cm) by the American Urological Association and European Association of Urology guidelines [6]. Guidelines recommend using PN for patients with larger tumours (T1b) and emphasise the importance of preserving the renal parenchyma.
Minimally invasive surgical approaches have been widely adopted in PN to minimise patient trauma when treating renal tumours. Laparoscopic partial nephrectomy is a challenging technique, with a 13.6 % conversion rate to laparoscopic radical nephrectomy [44]. However, da Vinci robotic surgical systems have dramatically advanced renal surgery by providing excellent 3D visualization of the surgical field and six-degree manipulation of surgical instruments, resulting in high-precision surgeries. Some studies have demonstrated that RAPN offers comparable or even better clinical outcomes than with other surgical methods [43,45,46]. These advances have expanded the benefits of PN for renal function reconstruction [47,48]. Despite the feasibility, minimal invasiveness, and efficacy of RAPN, some urologists still avoid its use for complex tumours. Renal tumour complexity can be measured using the R.E.N.A.L. and PADUA scoring systems [49,50]. Regardless of the complexity of the tumour, the ultimate objective is complete resection of the tumour while preserving the healthy renal parenchyma. Accordingly, accurate and comprehensive preoperative assessment is necessary to formulate the simplest and most efficient operative strategies.
The essential aspects of preoperative assessment in partial nephrectomy involve assessing neighbouring visceral relationships, kidney vascular anatomy, tumour complexity, and tumour location in relation to the blood vessels and collecting system. Conventional two-dimensional (2D) CT is widely used for routine preoperative examinations. However, it is difficult for surgeons to accurately define anatomical relationships in the surgical field because of the inherent limitations of relatively weak efficiency in revealing the internal renal structure and spatial resolution. Consequently, preoperative simulation of lesion orientation and vascular dissection using 3D imaging technology from a stereoscopic perspective has become possible. This technology reduces the intraoperative time required for renal artery dissection and tumour localisation, thereby significantly reducing renal parenchymal damage [51,52]. Furthermore, visualization of 3D reconstruction structures generated using software-aided techniques to analyse enhanced CT scan images can also help patients become more aware of their condition, which is beneficial for promoting doctor–patient communication.
Both the evidence-based analysis and the results of this single-centre clinical trial show good potential for the use of 3D technology in RAPN. However, the clinical outcomes that may arise will be less consistent owing to the different ways in which 3D technology is employed. By analysing the overall perioperative variables of the included patients, we found that patients in group 3D had markedly lower EBL (P < 0.001) and operative time (P = 0.016) than those in group No 3D; in addition, it was also demonstrated that the incidence rates of intraoperative US use (P = 0.015), intraoperative complications (P = 0.007), transfusions (P = 0.007), and postoperative Clavien complications (P < 0.001) were all significantly different. These results suggest that 3D visualization reconstruction is essential for preoperative planning and intraoperative surgical guidance. Moreover, to analyse the effectiveness of 3D reconstruction in different degrees of complexity tumours, all the patients were further classified into a simple tumour subgroup (R.E.N.A.L. score <8) and complex tumour subgroup (R.E.N.A.L. score ≥8) according to the cutoff value of 8 in the R.E.N.A.L. score. Compared to patients in the R.E.N.A.L. score <8 subgroup, patients with complex renal tumours who underwent 3D visualization had a lower risk of intraoperative complications, intraoperative transfusions, and postoperative Clavien complications. Notably, in the complex tumour subgroup, patients who underwent 3D reconstruction had significantly shorter hospital stays than those who did not; this difference was a direct factor influencing the patients' experience of the benefits of the technique. In addition, we found less operative time using 3D reconstruction in the R.E.N.A.L score <8 group, which also indicates that the advantage of 3D technology in shortening surgical time is more pronounced in tumours with lower complexity. In addition, as a major concern in renal surgery, we analysed perioperative changes in serum creatine levels for renal function evaluation. We found that no obvious differences were observed in serum creatine changes between two groups, although in the No 3D group, there were more patients with postoperative stage of renal insufficiency ≥2 than in the 3D group. This result suggests that preoperative 3D reconstruction has little effect on renal function preservation, regardless of tumour complexity.
The off-clamp technique in RAPN has been demonstrated to effectively preserve renal function in patients [17,[53], [54], [55]]. The first meta-analysis in the field was from 2014, which suggested that the off-clamp technique group increases the risk of blood transfusion compared to the on-clamp technique group [53]. However, through years of exploration and practice by urologists, a review from 2023 indicates that there are no longer clinically significant differences between the off-clamp and on-clamp groups in terms of perioperative and postoperative outcomes [15]. More notably, a recent meta-analysis from 2024 showed that patients who underwent the off-clamp technique demonstrated better renal function preservation and a lower rate of positive surgical margins, while perioperative complications were comparable to the on-clamp RAPN group [17]. Traditionally, the on-clamp technique was considered suitable for most partial nephrectomy, particularly for complex or large renal tumours, as it reduces bleeding, providing a clearer surgical field and allowing urologists to more precisely resect tumours and repair renal tissue. However, two recent studies focusing on larger and Complicated renal tumours revealed that patients in the off-clamp group had significantly shorter operative times, a lower rate of significant renal function deterioration, and a significantly higher likelihood of achieving trifecta outcomes compared to the on-clamp group [56,57]. These findings suggest that the off-clamp technique is not limited to superficial or small renal tumours but can also be applied to complex renal tumours and beneficial to patient prognosis. Given the lower rates of trifecta achievement and renal function impairment associated with prolonged warm ischaemia, the off-clamp technique in RAPN is expected to become more widespread in the future, which means that adequate preoperative knowledge of renal tumor characteristics and surrounding structures is more crucial [56].
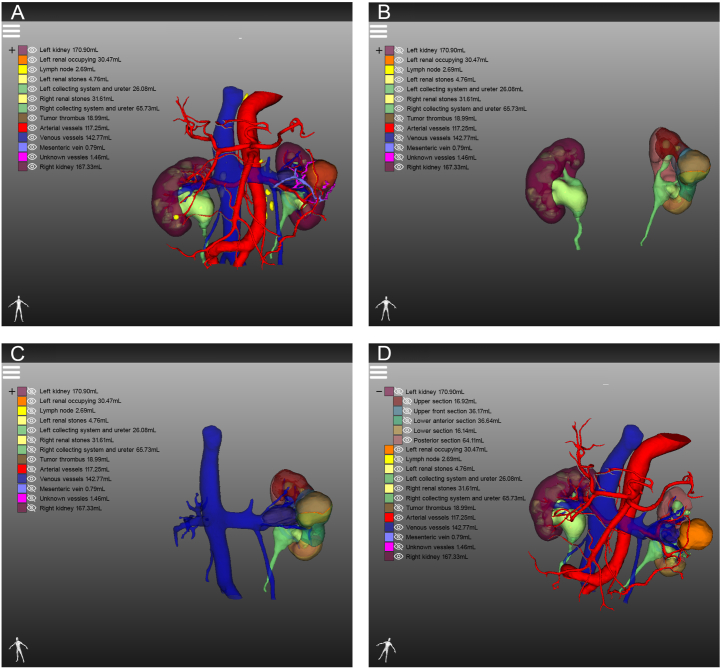
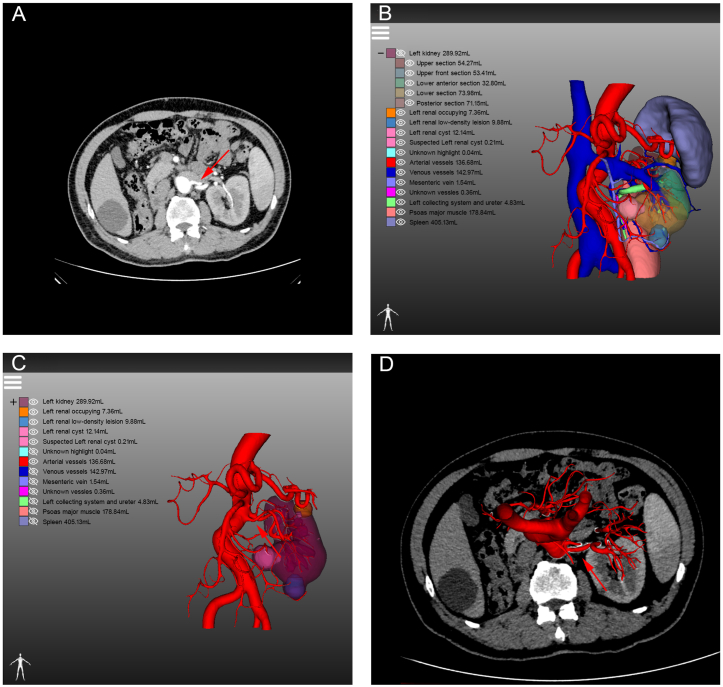
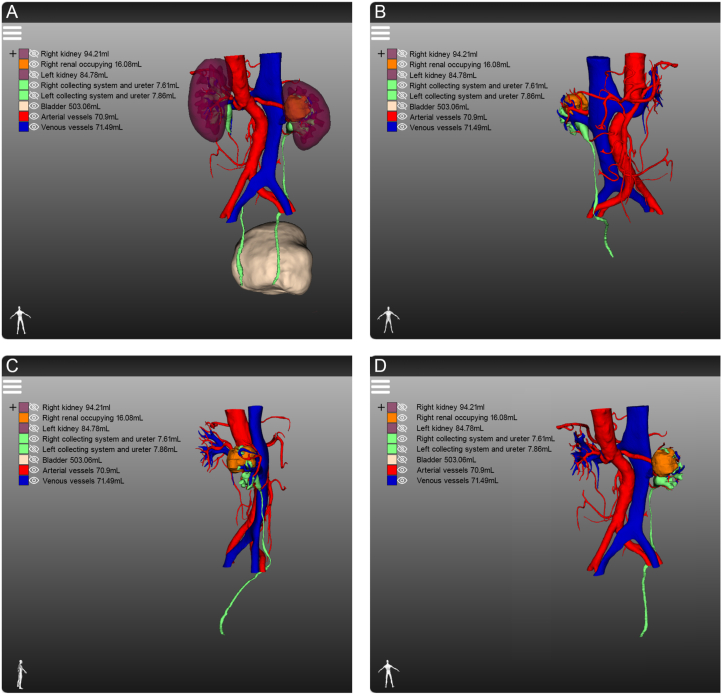
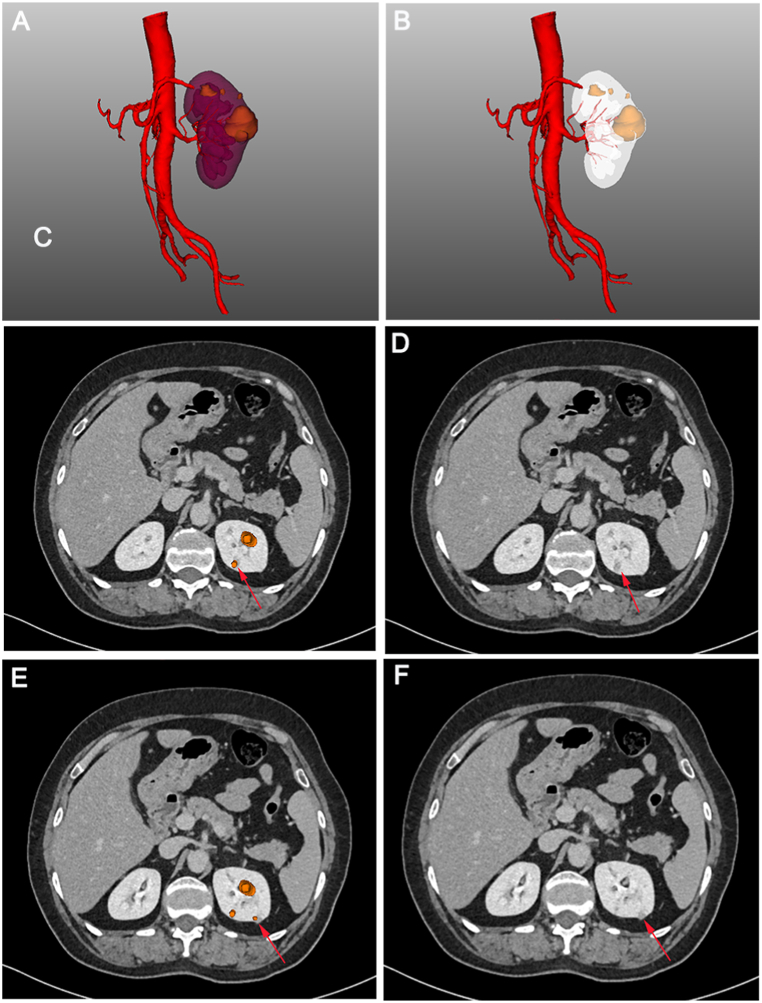
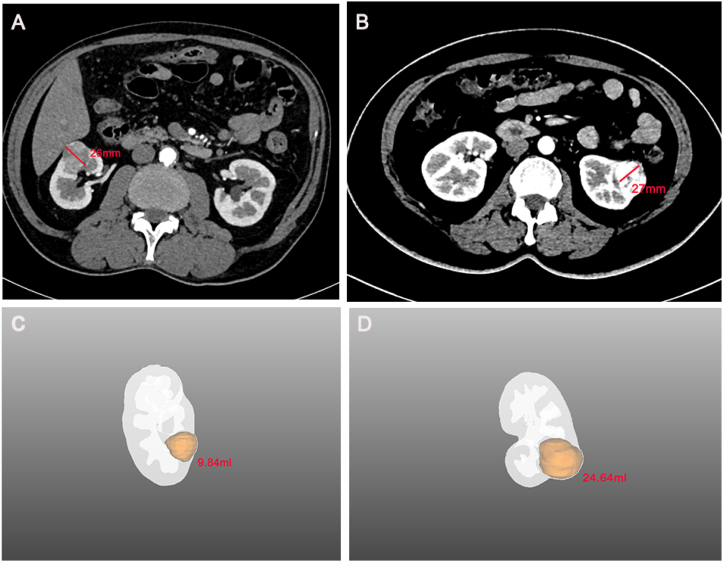
With the application of 3D visualization technology in RAPN, urologists can preoperatively identify complex renal tumours with enhanced precision, allowing them to obtain crucial information that is often difficult to capture using conventional 2D imaging techniques. For instance, the first patient had a complicated condition caused by a left-sided tumour, bilateral renal stones, and a left renal venous tumour thrombus (Fig. 7A). The 3D reconstruction software displayed the right renal stone, collecting system, and ureters independently, indicating that the left renal stone filled almost the entire pelvis and calyces, necessitating immediate removal to prevent right kidney failure (Fig. 7B). Furthermore, urologists can determine the size and location of the venous tumour thrombus from multiple angles in a view that only displays the venous system, tumour thrombus, and kidney (Fig. 7C). Additionally, surgeons can hide the partial partitioning of the kidney to accurately determine the relative positions of the tumour, renal pelvis, and calyces (Fig. 7D). This advanced visualization technology provides a clear and intuitive demonstration of useful structures to the surgical team, contributing to the development of a better preoperative surgical plan. In another case, the patient had a history of right radical nephrectomy and presented with several tumours in the left kidney at admission (Fig. 8A). Owing to the specific medical history of the second patient, intraoperative blocking of the renal artery became the key to ensuring good postoperative renal function. While conventional enhanced CT imaging reveals renal arterial variants, limited observation angles necessitate preoperative three-dimensional (3D) reconstruction for accurate visualization. This technique provides a clear combination of 2D images with 3D structures, enabling visualization of the complete structure of the renal artery (Fig. 8B–D). Simultaneously, 3D reconstruction illustrates the morphology, nature, location, size of each mass, and distance to the renal pelvis, allowing urologists to recognise the relationship between each tumour and its supplying arteries, thereby estimating the size of the tumour to be resected during surgery. The third patient was diagnosed with renal cancer of the T1a stage. We originally planned to perform RAPN; however, we modified the surgical approach to robotic radical nephrectomy after reconstructing the kidney using 3D technology. The 3D imaging revealed that the tumour, located near the right renal hilum, was too large to be resected entirely (Fig. 9A–D). Furthermore, 3D reconstruction technology plays an unexpected role in the identification of tumours and the determination of tumour volume. The patient was initially diagnosed with renal carcinoma, which included two lesions. However, after performing 3D reconstruction, we serendipitously discovered two additional minute tumours that would have been easily overlooked using traditional 2D imaging. (Fig. 10A–F). The patient subsequently underwent RAPN and was discharged from the hospital without recurrence to date. In the subsequent two patients, the 3D images revealed that a similar diameter in a CT scan did not necessarily represent a parallel 3D volume (Fig. 11A–D), which verified the superior ability of 3D images to measure tumour size for the preoperative evaluation of surgical difficulty.
Fig. 7.
Demonstrating the different structures of patients with complex kidney cancer from different perspectives by three-dimensional reconstruction: (A) overall view; (B) renal stones, collecting system, ureter and surrounding structures; (C) venous system, venous tumour thrombus, tumour and the kidney; (D) partial section of left kidney was hidden.
Fig. 8.
Evaluation of renal cancer patient with renal arterial variant (red arrows) by different imaging approaches: (A) traditional enhanced computed tomography; (B) overall view of the three-dimensional (3D) reconstruction; (C) demonstration of the arterial system, kidney and occupying lesion independently by 3D reconstruction; (D) combination of enhanced computed tomography imaging and 3D reconstruction in axial view.
Fig. 9.
Different views of the relationship between the tumour and the collecting system of the kidney cancer patient in three-dimensional visualization: (A) overall view; (B) frontal view; (C) lateral view; (D) back view.
Fig. 10.
Locating masses (red arrows) easily ignored by traditional computed tomography through three-dimensional visualization reconstruction.
Fig. 11.
Excellent ability to measure tumor size preoperatively with three-dimensional (3D) visualization technique: (A) patient A demonstrates a tumor diameter of 26 mm in computed tomography images; (B) patient B demonstrates a tumor diameter of 27 mm in computed tomography images; (C) the volume of tumor in patient A was 9.84 mL through 3D reconstruction technique; (D) the volume of tumor in patient B was 24.64 mL through 3D reconstruction technique.
In the field of RAPN, the efficacy of related 3D technology applications has been evaluated with some relevant investigations. Porpiglia et al. prospectively enrolled 52 patients who underwent RAPN, of whom 21 underwent hyperaccurate 3D (HA3D) reconstruction and found that more patients underwent selective clamping during surgery in the HA3D group [39]. Notably, intraoperative planning of the renal pedicle was conducted as preoperatively managed in 90 % of the HA3D group cases. However, in 39 % of the group no HA3D, management was changed intraoperatively (P = 0.04). Schiavina et al. prospectively described and analysed the clinical information of 20 consecutive patients with clinical diagnoses of renal masses using the 3D digital reconstruction of renal models. After assessment of the 3D virtual model, the clamping plan based on conventional imaging of eight patients was modified for the selective clamping method (P = 0.01) [18]. Although the patients in our study were treated with on-clamping RAPN, intraoperative US and transfusion were performed as preoperatively managed in 92 % (P = 0.015) and 100 % (P = 0.007) of the 3D group, respectively. Few studies have been conducted on the use of 3D printing technology in the urological field, particularly in RAPN. Nonetheless, a study has shown that preoperative "cognitive" RAPN rehearsal was feasible using a 3D printed model. Despite these technical advances, concerns remain regarding the cost, fabrication process, and potential limitations of this technology, leading to its lack of large-scale adoption [[58], [59], [60], [61]].
Despite the important insights and implications of our findings, we acknowledge that some limitations must be considered. First, given the initiation of new technology, our study was designed as a single-centre, retrospective study with a small sample size. Nonetheless, in comparison with other studies that focused on preoperative 3D reconstruction of RAPN, our sample size was relatively large. Second, selective clamping of arterial branches in RAPN has not been applied at our institution; therefore, it was not possible to investigate the effects of 3D reconstruction using this method. Third, the 3D technology used in our study could not be integrated into a robotic console to provide real-time navigational guidance to the surgeons while performing the procedure. More importantly, long-term follow-up is essential to meticulously assess the oncological outcomes of our study. In light of these limitations, there is a need for a larger-scale, prospective, multi-centre study to enhance the accuracy of the investigation.
China, one of the world's largest developing countries, faces considerable economic disparities across regions. Currently, 3D visualization and reconstruction techniques are not covered by medical insurance and fall under the category of patient out-of-pocket expenses, imposing a substantial financial burden on many poor patients. In line with the findings of Wang Z et al., the use of the 3D reconstruction technique was observed to be more important in patients with relatively complex renal cancer (R.E.N.A.L. ≥ 8 score) than in patients with simpler tumours (R.E.N.A.L. < 8 score) [62]. These results provide a strong basis for recommending 3D visualization before surgery. For complex tumours, we believe that using 3D visualization as a tool can greatly improve the understanding of individual cases, accelerate recovery time, and enhance patient prognosis. For relatively simpler tumours, we will consider whether 3D visualization reconstruction is necessary given the ability of 2D conventional imaging to identify tumours and surrounding tissues, considering the patient's financial situation and personal preferences. As the use of 3D visualization technology has become more widespread in developing countries, an increasing number of doctors and patients have recognised its value throughout the diagnosis and treatment process. Preoperative 3D visualization reconstruction has emerged as a new trend in this field. By combining 3D visualization with precision medicine, we offer a novel strategy for clinicians to independently evaluate whether each patient requires preoperative 3D visualization reconstruction.
5. Conclusion
The 3D reconstruction technique was effective in reducing EBL, operative time, and length of stay in patients who underwent RAPN. The rate of intraoperative US, transfusions, intraoperative complications, and postoperative Clavien complications also showed significant improvement with 3D technology. Especially in complex tumours, 3D reconstruction technology has demonstrated even more value in the perioperative period, reducing the risk of intraoperative complications, intraoperative transfusions, and postoperative Clavien complications and markedly reducing the EBL and length of hospital stay.
CRediT authorship contribution statement
Yuchao Wang: Writing – review & editing, Writing – original draft, Methodology, Investigation. Qiliang Teng: Writing – original draft, Methodology, Investigation. Zhihong Dai: Methodology, Investigation. Chunyu Chen: Writing – original draft, Methodology, Investigation. Liren Zhang: Investigation. Jiaxin Xie: Supervision, Software. Hao Wang: Validation. Zihan Xin: Investigation. Sishan Chen: Investigation. Yu Tai: Validation. Liang Wang: Writing – review & editing, Project administration, Conceptualization. Bo Fan: Writing – review & editing, Project administration, Funding acquisition, Conceptualization. Zhiyu Liu: Writing – review & editing, Project administration, Conceptualization.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
This study was reviewed and approved by the Ethics Review Committee of the Second Affiliated Hospital of Dalian Medical University with the approval number: 202124, dated July 3, 2021. Due to the retrospective nature of the study, the requirement for informed consent from the patients was waive.
Funding sources
The present study was supported by the Joint Fund Project of Liaoning Provincial Science and Technology Programme(2023-MSLH-021), the Interdisciplinary Research Cooperation Project Team Funding of Dalian Medical University Youth-specific category of free exploration (JCHZ2023020), the United Foundation for Medico-engineering Cooperation from Dalian Neusoft University of Information and the Second Hospital of Dalian Medical University (LH-JSRZ-202201), “1+X”Program for Clinical Competency Enhancemen–interdisciplinary Innovation Project, the Second Hospital of Dalian Medical University (2022JCXKYB15), the Young Reserve Talent Project of the Second Hospital of Dalian Medical University (Grant no. dy2yhbrc202010) and the Scientific Research Project of Ministry of Education of Liaoning Province (LJKQZ20222382).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38806.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Kim S.P., Murad M.H., Thompson R.H., Boorjian S.A., Weight C.J., Han L.C., Erwin P.J., Costello B.A., Chow G.K., Leibovich B.C. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J. Urol. 2012;S0022–5347(12) doi: 10.1016/j.juro.2012.10.026. 05254–8. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa J., Miyake H., Tanaka K., Sugimoto M., Fujisawa M. Console-integrated real-time three-dimensional image overlay navigation for robot-assisted partial nephrectomy with selective arterial clamping: early single-centre experience with 17 cases. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS. 2014;10:385–390. doi: 10.1002/rcs.1574. [DOI] [PubMed] [Google Scholar]
- 3.Shao P., Li P., Xu Y., Cao Q., Ju X., Qin C., Yin C., Tang L. Application of combined computed tomography arteriography, venography, and urography in laparoscopic partial nephrectomy with segmental artery clamping. Urology. 2014;84:1361–1365. doi: 10.1016/j.urology.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 4.Wang D., Zhang B., Yuan X., Zhang X., Liu C. Preoperative planning and real-time assisted navigation by three-dimensional individual digital model in partial nephrectomy with three-dimensional laparoscopic system. Int. J. Comput. Assist. Radiol. Surg. 2015;10:1461–1468. doi: 10.1007/s11548-015-1148-7. [DOI] [PubMed] [Google Scholar]
- 5.Ljungberg B., Albiges L., Abu-Ghanem Y., Bensalah K., Dabestani S., Fernández-Pello S., Giles R.H., Hofmann F., Hora M., Kuczyk M.A., Kuusk T., Lam T.B., Marconi L., Merseburger A.S., Powles T., Staehler M., Tahbaz R., Volpe A., Bex A. European association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur. Urol. 2019;75:799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Liu J., Liu J., Wang S., Zhao H., Tian C., Shi B., Jiang X. Three-dimensional nephrometry scoring system: a precise scoring system to evaluate complexity of renal tumors suitable for partial nephrectomy. PeerJ. 2020;8 doi: 10.7717/peerj.8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer R.J., Jonasch E., Agarwal N., Alva A., Baine M., Beckermann K., Carlo M.I., Choueiri T.K., Costello B.A., Derweesh I.H., Desai A., Ged Y., George S., Gore J.L., Haas N., Hancock S.L., Kapur P., Kyriakopoulos C., Lam E.T., Lara P.N., Lau C., Lewis B., Madoff D.C., Manley B., Michaelson M.D., Mortazavi A., Nandagopal L., Plimack E.R., Ponsky L., Ramalingam S., Shuch B., Smith Z.L., Sosman J., Dwyer M.A., Gurski L.A., Motter A. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in Oncology, J. Natl. Compr. Cancer netw. J. Natl. Compr. Cancer Netw. 2022;20:71–90. doi: 10.6004/jnccn.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F., Zhang C., Guo F., Ji J., Lyu J., Cao Z., Yang B. Navigation of intelligent/interactive qualitative and quantitative analysis three-dimensional reconstruction technique in laparoscopic or robotic assisted partial nephrectomy for renal hilar tumors. J. Endourol. 2019;33:641–646. doi: 10.1089/end.2018.0570. [DOI] [PubMed] [Google Scholar]
- 9.Lasser M.S., Doscher M., Keehn A., Chernyak V., Garfein E., Ghavamian R. Virtual surgical planning: a novel aid to robot-assisted laparoscopic partial nephrectomy. J. Endourol. 2012;26:1372–1379. doi: 10.1089/end.2012.0093. [DOI] [PubMed] [Google Scholar]
- 10.Marszalek M., Meixl H., Polajnar M., Rauchenwald M., Jeschke K., Madersbacher S. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur. Urol. 2009;55:1171–1178. doi: 10.1016/j.eururo.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 11.Jang H.A., Kim J.W., Byun S.S., Hong S.H., Kim Y.J., Park Y.H., Yang K.S., Cho S., Cheon J., Kang S.H. Oncologic and functional outcomes after partial nephrectomy versus radical nephrectomy in T1b renal cell carcinoma: a multicenter, matched case-control study in Korean patients. Cancer Res. Treat. 2016;48:612–620. doi: 10.4143/crt.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W.C., Elkin E.B., Levey A.S., Jang T.L., Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J. Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. ; discussion 61-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesage K., Joniau S., Fransis K., Van Poppel H. Comparison between open partial and radical nephrectomy for renal tumours: perioperative outcome and health-related quality of life. Eur. Urol. 2007;51:614–620. doi: 10.1016/j.eururo.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Volpe A., Blute M.L., Ficarra V., Gill I.S., Kutikov A., Porpiglia F., Rogers C., Touijer K.A., Van Poppel H., Thompson R.H. Renal ischemia and function after partial nephrectomy: a collaborative review of the literature. Eur. Urol. 2015;68:61–74. doi: 10.1016/j.eururo.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Shrivastava N., Sharma G., Ahluwalia P., Gautam G., Erdem S., Amparore D., Marchioni M., Pavan N., Marandino L., Roussel E., Campi R., Bertolo R. European association of Urology Young academic urologists renal cancer study group, off-clamp versus on-clamp robot-assisted partial nephrectomy: a systematic review and quantitative synthesis by the European association of Urology Young academic urologists renal cancer study group. Eur. Urol. Open Sci. 2023;58:10–18. doi: 10.1016/j.euros.2023.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lofaro D., Amparore D., Perri A., Rago V., Piana A., Zaccone V., Morelli M., Bisegna C., Suraci P.P., Conforti D., Porpiglia F., Di Dio M. Comparing perioperative complications of off-clamp versus on-clamp partial nephrectomy for renal cancer using a novel energy balancing weights method. Life Basel Switz. 2024;14:442. doi: 10.3390/life14040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong K.Y., Gan V.H.L., Lim B.J.H., Chan Y.H., Castellani D., Chen K., Tay K.J., Ho H.S.S., Yuen J.S.P., Aslim E., Teoh J., Lim E.J. Off-clamp vs on-clamp robot-assisted partial nephrectomy: a systematic review and meta-analysis. BJU Int. 2024;133:375–386. doi: 10.1111/bju.16250. [DOI] [PubMed] [Google Scholar]
- 18.Schiavina R., Bianchi L., Borghesi M., Chessa F., Cercenelli L., Marcelli E., Brunocilla E. Three-dimensional digital reconstruction of renal model to guide preoperative planning of robot-assisted partial nephrectomy. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2019;26:931–932. doi: 10.1111/iju.14038. [DOI] [PubMed] [Google Scholar]
- 19.Komai Y., Sakai Y., Gotohda N., Kobayashi T., Kawakami S., Saito N. A novel 3-dimensional image analysis system for case-specific kidney anatomy and surgical simulation to facilitate clampless partial nephrectomy. Urology. 2014;83:500–506. doi: 10.1016/j.urology.2013.09.053. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Li H., Wu D., Bi K., Liu C. Surgical planning and manual image fusion based on 3D model facilitate laparoscopic partial nephrectomy for intrarenal tumors. World J. Urol. 2014;32:1493–1499. doi: 10.1007/s00345-013-1222-0. [DOI] [PubMed] [Google Scholar]
- 21.Shao P., Tang L., Li P., Xu Y., Qin C., Cao Q., Ju X., Meng X., Lv Q., Li J., Zhang W., Yin C. Application of a vasculature model and standardization of the renal hilar approach in laparoscopic partial nephrectomy for precise segmental artery clamping. Eur. Urol. 2013;63:1072–1081. doi: 10.1016/j.eururo.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Huang M.-W., Liu S.-M., Zheng L., Shi Y., Zhang J., Li Y.-S., Yu G.-Y., Zhang J.-G. A digital model individual template and CT-guided 125I seed implants for malignant tumors of the head and neck. J. Radiat. Res. 2012;53:973–977. doi: 10.1093/jrr/rrs046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu J., Bo X.F., Xiong C.Y., Wu A.W., Zhang X.P., Li M., An Q., Fang J., Li J., Zhang X., Wang H.Y., Gao F., You W.C. Defining pelvic factors in sphincter-preservation of low rectal cancer with a three-dimensional digital model of pelvis. Dis. Colon Rectum. 2006;49:1517–1526. doi: 10.1007/s10350-006-0665-4. [DOI] [PubMed] [Google Scholar]
- 24.Arora S., Rogers C. Partial nephrectomy in central renal tumors. J. Endourol. 2018;32:S63–S67. doi: 10.1089/end.2018.0046. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi L., Schiavina R., Bortolani B., Cercenelli L., Gaudiano C., Mottaran A., Droghetti M., Chessa F., Boschi S., Molinaroli E., Balestrazzi E., Costa F., Rustici A., Carpani G., Piazza P., Cappelli A., Bertaccini A., Golfieri R., Marcelli E., Brunocilla E. Novel volumetric and morphological parameters derived from three-dimensional virtual modeling to improve comprehension of tumor's anatomy in patients with renal cancer. Eur. Urol. Focus. 2022;8:1300–1308. doi: 10.1016/j.euf.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Porpiglia F., Checcucci E., Amparore D., Piramide F., Volpi G., Granato S., Verri P., Manfredi M., Bellin A., Piazzolla P., Autorino R., Morra I., Fiori C., Mottrie A. Three-dimensional augmented reality robot-assisted partial nephrectomy in case of complex tumours (PADUA ≥10): a new intraoperative tool overcoming the ultrasound guidance. Eur. Urol. 2020;78:229–238. doi: 10.1016/j.eururo.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Li K.-P., Wan S., Wang C.-Y., Chen S.-Y., Yang L. Perioperative, functional, and oncologic outcomes of robot-assisted versus open partial nephrectomy for complex renal tumors (RENAL score ≥ 7): an evidence-based analysis. J. Robot. Surg. 2023;17:1247–1258. doi: 10.1007/s11701-023-01565-3. [DOI] [PubMed] [Google Scholar]
- 28.Shen Z., Xie L., Xie W., Hu H., Chen T., Xing C., Liu X., Xu H., Zhang Y., Wu Z., Tian D., Wu C. The comparison of perioperative outcomes of robot-assisted and open partial nephrectomy: a systematic review and meta-analysis. World J. Surg. Oncol. 2016;14:220. doi: 10.1186/s12957-016-0971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hori S., Sakamoto K., Onishi K., Tomizawa M., Morizawa Y., Gotoh D., Nakai Y., Miyake M., Torimoto K., Yoneda T., Tanaka N., Fujimoto K. Perioperative outcomes of open and robot-assisted partial nephrectomy in patients with renal tumors of moderate to high complexity. Asian J. Surg. 2023;46:2310–2318. doi: 10.1016/j.asjsur.2022.09.155. [DOI] [PubMed] [Google Scholar]
- 30.McGrath S., Katzenschlager S., Zimmer A.J., Seitel A., Steele R., Benedetti A. Standard error estimation in meta-analysis of studies reporting medians. Stat. Methods Med. Res. 2023;32:373–388. doi: 10.1177/09622802221139233. [DOI] [PubMed] [Google Scholar]
- 31.Wu C., Guo S., Zhuo S., Wang Y., Ye Y., Li Z., Mou Y., Yang X., Zhang Z., Dong P., Zhou F., Han H. Better specificity and less ischemia: three-dimensional reconstruction is superior to routine computed tomography angiography in navigation of super-selective clamping robot-assisted laparoscopic partial nephrectomy. Transl. Androl. Urol. 2023;12:97–111. doi: 10.21037/tau-22-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger L., Gulamhusein A., Hyde E., Gibb M., Kuusk T., Neves J., Silva P., Marchetti M., Barod R., Tran M., Patki P., Bex A., Ourselin S., Dasgupta P., Mumtaz F. Clinical experience of using virtual 3D modelling for pre and intraoperative guidance during robotic-assisted partial nephrectomy. J. Clin. Urol. 2022;15:323–330. doi: 10.1177/20514158211000204. [DOI] [Google Scholar]
- 33.Li L., Zeng X., Yang C., Un W., Hu Z. Three-dimensional (3D) reconstruction and navigation in robotic-assisted partial nephrectomy (RAPN) for renal masses in the solitary kidney: a comparative study. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS. 2022;18 doi: 10.1002/rcs.2337. [DOI] [PubMed] [Google Scholar]
- 34.Michiels C., Khene Z.-E., Prudhomme T., Boulenger de Hauteclocque A., Cornelis F.H., Percot M., Simeon H., Dupitout L., Bensadoun H., Capon G., Alezra E., Estrade V., Bladou F., Robert G., Ferriere J.-M., Grenier N., Doumerc N., Bensalah K., Bernhard J.-C. 3D-Image guided robotic-assisted partial nephrectomy: a multi-institutional propensity score-matched analysis (UroCCR study 51) World J. Urol. 2023;41:303–313. doi: 10.1007/s00345-021-03645-1. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi S., Cho B., Mutaguchi J., Inokuchi J., Tatsugami K., Hashizume M., Eto M. Surgical navigation improves renal parenchyma volume preservation in robot-assisted partial nephrectomy: a propensity score matched comparative analysis. J. Urol. 2020;204:149–156. doi: 10.1097/JU.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 36.Shirk J.D., Thiel D.D., Wallen E.M., Linehan J.M., White W.M., Badani K.K., Porter J.R. Effect of 3-dimensional virtual reality models for surgical planning of robotic-assisted partial nephrectomy on surgical outcomes: a randomized clinical trial, jama netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirk J.D., Kwan L., Saigal C. The use of 3-dimensional, virtual reality models for surgical planning of robotic partial nephrectomy. Urology. 2019;125:92–97. doi: 10.1016/j.urology.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Maddox M.M., Feibus A., Liu J., Wang J., Thomas R., Silberstein J.L. 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: a feasibility study. J. Robot. Surg. 2018;12:27–33. doi: 10.1007/s11701-017-0680-6. [DOI] [PubMed] [Google Scholar]
- 39.Porpiglia F., Fiori C., Checcucci E., Amparore D., Bertolo R. Hyperaccuracy three-dimensional reconstruction is able to maximize the efficacy of selective clamping during robot-assisted partial nephrectomy for complex renal masses. Eur. Urol. 2018;74:651–660. doi: 10.1016/j.eururo.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 40.Lane B.R., Gill I.S. 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J. Urol. 2010;183:473–479. doi: 10.1016/j.juro.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Van Poppel H., Da Pozzo L., Albrecht W., Matveev V., Bono A., Borkowski A., Colombel M., Klotz L., Skinner E., Keane T., Marreaud S., Collette S., Sylvester R. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur. Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Huang W.C., Levey A.S., Serio A.M., Snyder M., Vickers A.J., Raj G.V., Scardino P.T., Russo P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 44.Rais-Bahrami S., Lima G.C., Varkarakis I.M., Romero F.R., Trock B., Jarrett T.W., Kavoussi L.R. Intraoperative conversion of laparoscopic partial nephrectomy. J. Endourol. 2006;20:205–208. doi: 10.1089/end.2006.20.205. [DOI] [PubMed] [Google Scholar]
- 45.Kaouk J.H., Khalifeh A., Hillyer S., Haber G.-P., Stein R.J., Autorino R. Robot-assisted laparoscopic partial nephrectomy: step-by-step contemporary technique and surgical outcomes at a single high-volume institution. Eur. Urol. 2012;62:553–561. doi: 10.1016/j.eururo.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Aboumarzouk O.M., Stein R.J., Eyraud R., Haber G.-P., Chlosta P.L., Somani B.K., Kaouk J.H. Robotic versus laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur. Urol. 2012;62:1023–1033. doi: 10.1016/j.eururo.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 47.Deng H., Fan Y., Yuan F., Wang L., Hong Z., Zhan J., Zhang W. Partial nephrectomy provides equivalent oncologic outcomes and better renal function preservation than radical nephrectomy for pathological T3a renal cell carcinoma: a meta-analysis. Int. Braz J Urol Off. J. Braz. Soc. Urol. 2021;47:46–60. doi: 10.1590/S1677-5538.IBJU.2020.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mir M.C., Derweesh I., Porpiglia F., Zargar H., Mottrie A., Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur. Urol. 2017;71:606–617. doi: 10.1016/j.eururo.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 49.Kutikov A., Uzzo R.G., The R.E.N.A.L. Nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 50.Ficarra V., Novara G., Secco S., Macchi V., Porzionato A., De Caro R., Artibani W. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur. Urol. 2009;56:786–793. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 51.Funahashi Y., Hattori R., Yamamoto T., Kamihira O., Kato K., Gotoh M. Ischemic renal damage after nephron-sparing surgery in patients with normal contralateral kidney. Eur. Urol. 2009;55:209–215. doi: 10.1016/j.eururo.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 52.Mir M.C., Ercole C., Takagi T., Zhang Z., Velet L., Remer E.M., Demirjian S., Campbell S.C. Decline in renal function after partial nephrectomy: etiology and prevention. J. Urol. 2015;193:1889–1898. doi: 10.1016/j.juro.2015.01.093. [DOI] [PubMed] [Google Scholar]
- 53.Liu W., Li Y., Chen M., Gu L., Tong S., Lei Y., Qi L. Off-clamp versus complete hilar control partial nephrectomy for renal cell carcinoma: a systematic review and meta-analysis. J. Endourol. 2014;28:567–576. doi: 10.1089/end.2013.0562. [DOI] [PubMed] [Google Scholar]
- 54.Franco A., Riolo S., Tema G., Guidotti A., Brassetti A., Anceschi U., Bove A.M., D'Annunzio S., Ferriero M., Mastroianni R., Misuraca L., Guaglianone S., Tuderti G., Leonardo C., Cicione A., Licari L.C., Bologna E., Flammia R.S., Nacchia A., Trucchi A., Lombardo R., Franco G., Tubaro A., Simone G., De Nunzio C. Renal function preservation in purely off-clamp sutureless robotic partial nephrectomy: initial experience and technique. Diagn. Basel Switz. 2024;14:1579. doi: 10.3390/diagnostics14151579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mari A., Morselli S., Sessa F., Campi R., Di Maida F., Greco I., Siena G., Tuccio A., Vittori G., Serni S., Carini M., Minervini A. Impact of the off-clamp endoscopic robot-assisted simple enucleation (ERASE) of clinical T1 renal tumors on the postoperative renal function: results from a matched-pair comparison. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2018;44:853–858. doi: 10.1016/j.ejso.2018.01.093. [DOI] [PubMed] [Google Scholar]
- 56.Tuderti G., Mastroianni R., Anceschi U., Bove A.M., Brassetti A., Ferriero M., Misuraca L., Guaglianone S., Costantini M., Torregiani G., Saidian A., Mari A., Narasimhan R., Derweesh I., Minervini A., Gallucci M., Simone G. Assessing the trade-off between the safety and effectiveness of off-clamp robotic partial nephrectomy for renal masses with a high renal score: a propensity score-matched comparison of perioperative and functional outcomes in a multicenter analysis. Eur. Urol. Focus. 2023;9:1037–1043. doi: 10.1016/j.euf.2023.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Brassetti A., Cacciamani G.E., Mari A., Garisto J.D., Bertolo R., Sundaram C.P., Derweesh I., Bindayi A., Dasgupta P., Porter J., Mottrie A., Schips L., Rah K.H., Chen D.Y.T., Zhang C., Jacobsohn K., Anceschi U., Bove A.M., Costantini M., Ferriero M., Mastroianni R., Misuraca L., Tuderti G., Kutikov A., White W.M., Ryan S.T., Porpiglia F., Kaouk J., Minervini A., Gill I., Autorino R., Simone G. On-clamp vs. Off-clamp robot-assisted partial nephrectomy for cT2 renal tumors: retrospective propensity-score-matched multicenter outcome analysis. Cancers. 2022;14:4431. doi: 10.3390/cancers14184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silberstein J.L., Maddox M.M., Dorsey P., Feibus A., Thomas R., Lee B.R. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: a pilot study. Urology. 2014;84:268–272. doi: 10.1016/j.urology.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 59.Knoedler M., Feibus A.H., Lange A., Maddox M.M., Ledet E., Thomas R., Silberstein J.L. Individualized physical 3-dimensional kidney tumor models constructed from 3-dimensional printers result in improved trainee anatomic understanding. Urology. 2015;85:1257–1261. doi: 10.1016/j.urology.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 60.Komai Y., Sugimoto M., Gotohda N., Matsubara N., Kobayashi T., Sakai Y., Shiga Y., Saito N. Patient-specific 3-dimensional printed kidney designed for “4D” surgical navigation: a novel aid to facilitate minimally invasive off-clamp partial nephrectomy in complex tumor cases. Urology. 2016;91:226–233. doi: 10.1016/j.urology.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y., Ge H., Li N., Yu C., Guo H., Jin S., Liu J., Na Y. Evaluation of three-dimensional printing for laparoscopic partial nephrectomy of renal tumors: a preliminary report. World J. Urol. 2016;34:533–537. doi: 10.1007/s00345-015-1530-7. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z., Qi L., Yuan P., Zu X., Chen W., Cao Z., Li Y., Wang L. Application of three-dimensional visualization technology in laparoscopic partial nephrectomy of renal tumor: a comparative study. J. Laparoendosc. Adv. Surg. Tech. 2017;27:516–523. doi: 10.1089/lap.2016.0645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.