Abstract
Background
Icodec, once-weekly basal insulin, aims to simplify therapy management by reducing injection frequency for diabetic patients. The efficacy and safety of icodec were evaluated in the ONWARDS clinical development program. This study evaluates icodec economic and quality of life impact from the Italian National Healthcare System (NHS) perspective.
Materials and Methods
A pharmacoeconomic study was developed to assess the once-weekly insulin icodec value, highlighting its potential to decrease needle use while improving adherence and quality of life. In the base case, a differential cost and cost-utility analysis over one year compared to once-daily insulin degludec were developed. Based on the comparison with degludec, a scenario analysis was planned between icodec and the mix of basal insulins available on the market. Economic evaluations included drug and administration costs, needles, and impact on adherence. The cost-utility analysis measured the utility associated with the weekly injection compared to the daily ones, resulting in an incremental cost-effectiveness ratio (ICER), measured as Δ€/ΔQALY (Quality Adjusted Life Years). To assess the robustness of the results, a deterministic one-way sensitivity analysis and a probabilistic sensitivity analysis were carried out.
Results
At an annual cost 25% higher than degludec, considering the economic benefits generated by the needle use reduction (-€51.10) and adherence improvement (-€54.85), once-weekly icodec grants no incremental cost and even potential savings per patient. Furthermore, icodec reported a utility advantage (0.023). It achieved a dominant incremental cost-effectiveness ratio (ICER) compared to degludec. The comparison with the mix of basal insulins also reported a cost-effectiveness profile. Sensitivity tests conducted confirmed the robustness of the findings, highlighting the key drivers of the analysis.
Conclusion
Icodec represents a new therapeutic option to simplify basal insulin treatment. It also improves the patient’s management and his quality of life, without increasing the economic burden for the Italian NHS, while guaranteeing an excellent cost-effectiveness profile.
Keywords: icodec, once-weekly basal insulin, diabetes, differential cost analysis, cost-utility analysis, adherence improvement
Introduction
Icodec is a novel once-weekly basal insulin analogue. It has been re-engineered while increasing the albumin binding and reducing the insulin receptor affinity to obtain a plasmatic half-life of 196 hours, suitable for one-weekly subcutaneous injection.1 The efficacy and safety of icodec were evaluated in the ONWARDS clinical development program.2–7 Once-weekly icodec treatment was compared with once-daily insulins (glargine, and degludec), in basal insulin-treated type 2 diabetes, insulin-naive type 2 diabetes and in adults with type 1 diabetes. However, degludec is the only ultra-long-acting insulin available on the market.2–7
Several studies demonstrated good tolerance profile and effective glycemic control in comparison with once-daily basal insulin analogues; furthermore, incidence and rates of adverse events, as well as hypoglycemic episodes, were almost overlapping.3–5,8 This once-weekly basal insulin can be used both in switching from once-daily analogue in patients on a basal-bolus or basal-plus regimen and in patients treated with daily basal analogue plus other antidiabetic drugs.7,9 Its use as a starting basal insulin compared to an insulin daily administration in patients without previous insulin treatment has also been considered.10 Treatment with icodec demonstrated non-inferiority in all studies, and in the most superiority in reducing glycosylated hemoglobin (HbA1c) values.
Once-daily insulin degludec is the only ultra-long basal insulin on the market and represents the closest analogue to icodec in terms of pharmacological characteristics. In the ONWARDS 2, 3 and 6 RCTs the primary end point was the change into HbA1c from baseline to week 26 (noninferiority margin, 0.3% percentage points) of once-weekly icodec compared to once-daily insulin degludec, respectively, in basal insulin-treated type 2 diabetes, insulin-naive type 2 diabetes and in type 1 diabetes patients.
In basal insulin-treated type 2 diabetes (ONWARDS 2) HbA1c was reduced to a greater extent with icodec than degludec (7.20% compared to 7.42% [55.2 vs 57.6 mmol/mol], respectively) at week 26, with an estimated treatment difference (ETD) of −0.22 percentage points (95% CI −0.37 to −0.08) or −2.4 mmol/mol (95% CI −4.1 to −0.8), demonstrating non-inferiority (p<0·0001) and superiority (p = 0·0028).3
In insulin-naive type 2 diabetes (ONWARDS 3), mean HbA1c level decreased from 8.6% to 7.0% at 26 weeks in the icodec group and from 8.5% to 7.2% in the degludec group, with an estimated treatment difference [ETD] of −0.2 [95% CI, −0.3 to −0.1] percentage points, confirming non-inferiority (P < 0.001) and superiority (P = 0.002).4 Among type 1 diabetes adult patients (ONWARDS 6), once-weekly icodec showed non-inferiority to once-daily degludec in HbA1c reduction at week 26. For icodec, time below 3.0 mmol/L (<54 mg/dL) was at the threshold of the internationally recommended target (<1%) during weeks 22–26 and below target during weeks 48–52.7
Incidence of hypoglycemic events was comparable to that of the currently available standard basal insulins in all studies regarding T2 and T1. Treat-to-target studies involving type 1 patients highlight higher rates of combined clinically significant hypoglycemia or severe one as direct consequence of the study design.7 We must consider that the high dosages adopted in the above reported studies are uncommon in clinical practice; consequently, such effects are not expected in real-life context.
In all patients with diabetes, using icodec, the number of insulin administrations per year drops from 365 to 52, resulting in a savings of 313 injections. Reducing the frequency of insulin injections decreases the burden related to care operations both for type 1 and type 2 insulin-treated diabetic patients. This may lead to an increase in treatment persistence and adherence and hence to better clinical outcomes.11 Additionally, once-weekly glucose-lowering medications are perceived positively by type 2 diabetic patients, especially if they are current injection users.12
Flexible basal insulin injections were associated with 0.016 and 0.013 higher utility versus a fixed time administration for basal-only and basal-bolus regimens, respectively, inducing a positive impact on quality of life and potentially on adherence as well, whereas weekly injections were associated with an average added utility of 0.023 compared to daily injections (p = 0.01).13,14 Furthermore, a systematic literature review involving several database and regarding 30 studies about missed and mistimed doses in performing insulin therapy highlighted an unmet need in simplifying insulin administration, to empower diabetic patients in managing their therapy.15 Another study on 3042 patients treated with basal insulin analogue reported that 38% of patients experienced dosing irregularity during the last 30 days and specifically a 22% of missing basal insulin doses.16 Two studies regarding once-weekly compared to daily GLP-1 receptor agonist injection demonstrated, respectively, a significant, higher persistence (333 vs 269 days; HR 0.80, 95% CI: 0.71–0.9, p < 0.01) and an 11% lower risk of non-adherence (risk ratio 0.89; 95% CI: 0.83–0.95, I²=89%.17,18
Moreover, the once-weekly basal insulin formulation has a positive impact not only on improving quality of life and adherence but also on environmental sustainability as has recently been documented.19
The aim of our study is to evaluate the potential economic impact of icodec in Italy, expressed in terms of direct healthcare costs and patient’s quality of life from the Italian National Healthcare System (NHS), testing price simulations for the new product.
Material and Methods
Model Structure
A pharmacoeconomic evaluation based on a decision tree model was conducted to capture the expected benefit from new once-weekly insulin adoption in adult patients with diabetes eligible for insulin treatment while providing a justifiable price for icodec, considering its clinical and economic value compared to the currently available basal insulins. The model considered the potential of once-weekly administration on reducing needle consumption and improving the adherence profile, with consequent effects on disease management and costs.
The impact of weekly administration on quality of life was also evaluated. Based on these premises, a differential cost analysis and a cost-utility one were developed to establish the costs related to icodec use while identifying the cost-effectiveness profile.
The analysis was conducted according to the CHEERS 2022 ISPOR Good Research Practices Task Force for health economic evaluations.20 The economic evaluation was developed from the NHS perspective and developed in Microsoft Excel®, adopting a 1-year time horizon. A discount rate for outcomes and costs was not included, given the short-time horizon. The two analyses aimed at defining a hypothetical estimate of the financial impact and the annual cost per patient. Differences in expected costs between technologies can be justified in terms of changes in estimated health effects. Health effects should be expressed in terms of quality-adjusted life years (QALYs). In the economic evaluations, basal insulins available on Italian market were considered as comparator. In the base case scenario, once-daily insulin degludec was adopted as comparator, representing the only ultra-long basal insulin on the market and the closest analogue to icodec in terms of pharmacological characteristics. Moreover, by establishing an estimate of the annual icodec cost, based on the comparison with degludec, a scenario analysis was developed. For this analysis, a comparison between icodec and the mix of basal insulins on the market was planned. The ONWARDS clinical trial program was designed to demonstrate icodec non-inferiority to the basal insulins degludec and glargine U100 as primary endpoint (change from baseline in HbA1c, margin of 0.3 percentage points).2–7 In addition to the confirmation of non-inferiority, superiority was demonstrated in most trials.2–4,6 However, conservatively, the outcomes of the reduction of HbA1c and other secondary outcomes were not considered in this pharmacoeconomic evaluation, and comparable efficacy and safety profiles were assumed among the comparators.
In the cost-utility analysis, the utility associated with the weekly injection compared to the daily one was considered as a measure of effectiveness. Consistently, in the economic evaluations, the costs of drug and administration, needles for the administration, and costs related to the impact of icodec on adherence were considered, since the costs have a significant impact on the comparison between weekly insulin and daily one. Thus, equal efficacy between the treatments, equal effect on hypoglycaemic events and comparable use of other hypoglycaemic drugs in the two arms were conservatively assumed, since this is a differential cost analysis, these costs, common to the two comparisons, do not have been considered. For the economic evaluation, 2024 was used as the index year and indirect costs were not considered, in accordance with the NHS perspective.
Based on the relationship between incremental costs and benefits between icodec and comparators, the model allowed to calculate the incremental cost-effectiveness ratio (ICER), measured as Δ€/ΔQALY (Quality Adjusted Life Years).
Finally, the cost per QALY acceptability was assessed based on the willingness to pay (WTP) threshold considered by the Italian Medicines Agency (AIFA). For the analysis, a WTP value of €30,000 was adopted, considering the results of a recent study, based on the data reported in 48 negotiation procedures, reporting an average acceptability value (ICER) of 33,004 euro/QALY.21
Target Population
The target population, potentially icodec eligible, is represented by adult diabetic patients in Italy treated with basal insulins. This population can be obtained based on Italian epidemiological data, and data on type 1 and 2 diabetes reported by institutional sources,22–24 as well as data on insulin consumption reported in AMD (diabetologists medical associations) reports.25,26
Comparator
In the base case scenario, once-daily insulin degludec was used as the appropriate comparator. Degludec is the only ultra-long-acting basal insulin on the market and represents the closest analogue to icodec in terms of pharmacological characteristics. For this reason, it was considered the most suitable comparator for annual treatment cost. A scenario analysis was also developed, in which the comparator was identified as the mix of basal insulins available on the market in Italy, degludec included, and, conservatively, with the exception of pre-mixed fixed-ratio combinations of basal insulins and glucagon-like peptide-1 receptor agonists (degludec/liraglutide and glargine/lixisenatide). Market data were adopted to define the mix of basal insulin in use and specific market shares.27 These data reported insulin glargine 100 U (originator and biosimilar), insulin glargine 300 U, insulin degludec 100 U, and detemir 100 U.
Cost Data
Drug and Administration
The analysis included the following input costs for the comparative evaluation: purchasing drugs costs, administration costs, for which the needle cost was considered and costs associated with the variation in the degree of adherence, based on the type of administration regimen (daily vs weekly). Only the direct costs relevant for the comparison of alternatives were included. Therefore, the costs assumed as common between the two arms were not included in the differential cost analysis (eg adverse events, hypoglycaemic events, other hypoglycaemic treatments, costs of visits and monitoring).
The cost input data were referred to the specific Italian context, when available. 2024 was adopted as the index year. To quantify the purchasing cost of basal insulins on the market in Italy, the ex-factory prices published in Gazzetta Ufficiale (with −5%–5% mandatory rebates), derived from software Gallery® Farmadati,28 were considered.
In Italy, ex-factory prices (manufacturer prices) of medicines included into reimbursement are negotiated between the AIFA and the marketing authorisation holder. Following the negotiation and its outcome, it is established whether the medicine will be reimbursed or not, and the ex-factory prices are made public. Pharmaceutical companies are expected to grant a mandatory cumulative discount to the NHS on the ex-factory price (−5%–5%), unless a request for suspension of the 5% price reduction is made upon payment (payback) of the relevant equivalent value.29,30 In addition to these mandatory and known discounts, unpublished negotiation discounts may be agreed upon during the negotiation process.
Since icodec is not yet available in Italy, and an ex-factory price has not yet been published for this new product, cost simulations were conducted based on the expected value that the first once-weekly insulin will generate for NHS. In particular, simulations were conducted to define the cut-off value, at which, despite savings in needle consumption and benefits in adherence, icodec does not lead to an increase in NHS healthcare spending. This can help decision makers better understand the value of icodec. The icodec annual cost simulation was set to compare with degludec, to understand which cost increase can be sustainable for icodec compared to the comparator. Different annual cost assumptions were tested in sensitivity analyses. To determine the annual basal insulin therapies cost, in addition to the ex-factory price per pack, the number of dosage units contained, the total number of insulin units (IU) per pack and a 20 IU average daily dosage of basal insulin were considered. A titration of 20 IU was considered, corresponding to an average daily dosage consistent with that recorded in real-life.31–33 This daily dosage was derived from real practice consumption data, which reports values well below those reported in clinical studies where demanding titration schemes are adopted.31–33 In addition to drug cost, administration cost related to insulin needle use was included. This cost was derived based on the frequency of administration according to SmPCs and needle unit cost. Since there is no single-unit cost available in Italy for the insulin needle, and based on the wide regional price variability, an average unit cost (€0.14) was obtained from software Gallery® Farmadati.28 To calculate the total number of needles per day, we considered it equals the number of daily administrations. This number is equivalent to weekly use in the case of icodec and to daily use for other basal insulins (1 compared to 7 injections per week). Furthermore, to evaluate the icodec administration cost, the needle cost has not been considered, as it will be supplied inside the package. Table 1 describes the basal insulin included in the base case analysis based on comparative appropriateness, detailing packaging, number of dosage units, total IU and cost per package.
Table 1.
Cost of the Comparator: Characteristics and Price per Pack
| Treatment | Product | Content | Dose per | Total IU per pack | Ex-factory |
|---|---|---|---|---|---|
| specifications | per pack | unit (mg/IU) | price (−5%-5%) per pack | ||
| Degludec | Tresiba® | 5 pens 3mL | 300 U | 1500 U | €83.06 |
Adherence
The analysis has also considered the potential economic benefits of adherence profile improvement associated with icodec once-weekly subcutaneous administration, compared to daily administration of the other basal insulin. This evaluation was conducted starting from literature data, meta-analysis evidence has shown that once-weekly injection of hypoglycaemic agents was associated with an 11% lower risk of non-adherence compared to the daily regimen (hazard ratio = 0.89; 95% confidence interval = 0.83–0.95; I2 = 89%).17,18 As no specific study is available for the Italian context, the data from a large meta-analysis (n = 75159 patients) were considered, reporting a proportion of patients with non-adherence, measured by the proportion of days covered (PDC < 80), ranged from 51% to 72% across 7 included studies.18 The average value was obtained from this range, weighted by the proportion of patients treated with daily (65.6%) and weekly (34.4%) administration. To correlate the administration-type impact to the adherence profile and costs, the information collected in the meta-analysis was combined with further literature data reporting the association between adherence to glucose-lowering agents and patient outcomes, including costs, acute-care resource utilization, and complications.34 From this source, the correlation between a 1% increase in adherence in 1000 patients and all-cause savings of $65,464 over a 3-year period ($21.82/patient/year) was derived.34 It was chosen to transfer this evidence to the Italian context. To estimate the potential impact in Italy, an Italian diabetes cost of illness study was adopted and compared with an American one.35,36 Based on this comparison, the average annual costs were proportioned.
Utility Data
The utility data adopted for the evaluation refer to the quality of life perceived by the patient as a function of the insulin frequency of administration. For the analysis, an incremental utility benefit value of 0.023 taken from the literature was considered.14 Weekly injections were associated with an average added utility of 0.023 compared to daily injections (p = 0.01), assuming the same effect reported in the study for the weekly insulin treatment.
Sensitivity Analysis
In order to explore the uncertainty of the input parameters, identify the drivers of the model and test its robustness, univariate deterministic sensitivity analysis (OWSA, one-way sensitivity analysis) and probabilistic sensitivity analysis (PSA) were developed. The analyses assessed the impact on the results of the variation in the main input data and the assumptions considered for the definition of the base case. The OWSA sensitivity analysis was developed considering per patient direct annual cost difference between icodec and degludec as reference indicator against which the outcomes in the proposed univariate scenarios were compared. The sensitivity analysis tested different simulation scenarios considering the impact of average annual cost of icodec higher than degludec, respectively, of 20% and 30%, a reduction of 20% and 30% in the price per pack of degludec, a ±20% variation in the unit cost of the needle, a ±20% change in the adherence variation related to once-weekly administration, and an increase in the average daily dose of +10% and +20%.
Probabilistic sensitivity analysis was conducted using the gamma distribution and the reiteration of 1000 simulations. The parameters of the model assed were varied within a plausible range, based on input value, standard deviation (SD) and an appropriate uncertainty distribution. PSA involved extracting values for each parameter from its individual uncertainty distribution. The PSA sensitivity analysis also adopted the difference in direct annual cost per patient between icodec and degludec as reference indicator. However, unlike OWSA, probabilistic analyses were performed simultaneously for all selected parameters, recording the resulting outcomes.
PSA extracts a value for each parameter from the assumed probability distributions 1000 times, evaluating the per patient direct annual cost difference obtained with each iteration, providing a distribution of outcomes and, consequently, an estimate of the overall uncertainty surrounding the model results.
Table 1S, available in the supplementary material, shows the main input data adopted for the analysis, the lower and upper limits of the variables used for the deterministic sensitivity analyses, the distributions used for the probabilistic sensitivity, as well as the sources.
Results
It was estimated the average costs per patient per year relating to basal insulin therapy, the consumption of needles for administration and the impact of the therapeutic regimen on adherence, Table 2. For degludec, an annual direct drug cost per patient of €404.50 was calculated. In addition, an annual expense for insulin needles equal to €51.10 was obtained. Overall, an annual cost per patient of €455.60 was reported for degludec, considering the estimated administration of 20 IU/day. For icodec, a reduction in the administration cost was calculated (-€51.10; €0 for icodec vs 51.10 for degludec), it has been hypothesized that there are no costs associated with administration, as the needle is expected to be provided in the package. Furthermore, an annual saving of €54.85 was calculated, connected to the benefits deriving from the greater degree of adherence to once-weekly therapy.
Table 2.
Differential Annual Cost per Patient: Icodec vs Degludec
| Treatments | Drug cost | Needles cost | Adherence cost | Total |
|---|---|---|---|---|
| Icodec | €505.63 | €0.0 | – €54.85 | €450.77 |
| Degludec | €404.50 | €51.10 | – | €455.60 |
| Differential cost | €101.13 | – €51.10 | – €54.85 | – €4.83 |
Overall, the savings generated by the reduction in needle consumption and the improvement in the adherence resulted in an annual economic benefit per patient of €105.95, related to the once-weekly administration benefits, not considering the costs of the drugs.
Consistent with savings on needles and improved adherence, the maximum annual cost of icodec was calculated in a way to keep healthcare spending almost unchanged after product introduction, with an overall impact comparable to that of degludec, the only ultra-long basal insulins currently available.
At an annual cost 25% higher than the cost of degludec, considering the economic benefits generated by weekly administration on needle use and adherence improvement, icodec does not entail incremental NHS cost (indeed generating savings of -€4.83 per patient).
This differential cost was related to the differential utility achieving a dominant incremental cost-effectiveness ratio (ICER), Table 3. This result has shown economic benefit associated with the use of icodec treatment at an annual cost 25% higher than degludec with a 20 IU daily dose.
Table 3.
Incremental Cost-Effectiveness Ratio: icodec compared to degludec
| Basal insulins | Δ Cost | Δ Utilities | ICER |
|---|---|---|---|
| Icodec | – €4.83 | +0.023 | Icodec dominant |
| Degludec |
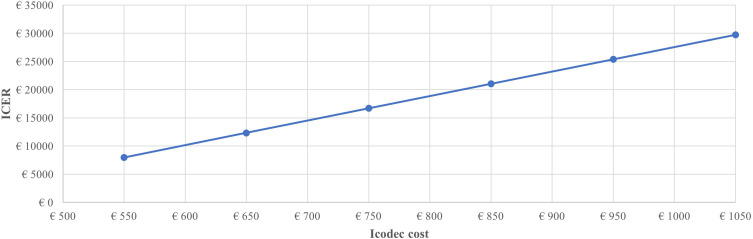
For the analysis, various annual cost increases were tested compared to the baseline value, reporting ICER values below the average threshold of about €30,000,34 recently highlighted for Italy, up to €1150, Figure 1.
Figure 1.
Incremental cost-effectiveness ratio: icodec annual cost impact.
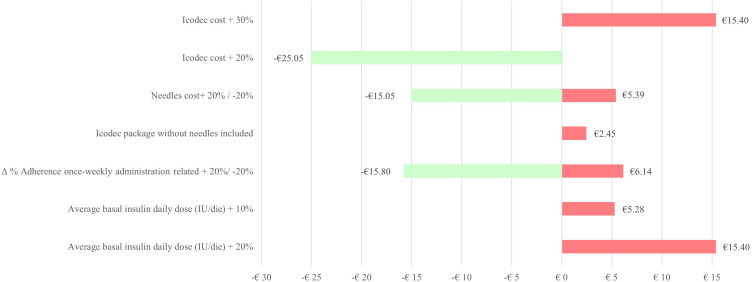
Deterministic sensitivity analysis, developed considering per patient direct annual cost difference between icodec and degludec as reference indicator (-€4.83), has shown the sensitivity of the result in the variability of the main input parameters in the model, Figure 2. The condition of substantially equal cost between the treatments is determined in the base case scenario by an increase in the annual cost of icodec, compared to degludec, of 25% and by an average daily insulin consumption of 20 IU. The impact of a scenario including the icodec administration costs was also considered, assuming the cost of a needle per week, if it is not included in the package.
Figure 2.
Deterministic sensitivity analysis results.
Key driver analysis resulted annual icodec cost variation, needle unit cost, percentual change in adherence once-weekly administration related and average basal insulin daily dose, Figure 2.
Figure 2 has shown possible oscillations as these values vary, and all the simulations implemented, in any case, resulted in an ICER dominant (green bars in the tornado diagram) or cost-effective (red bars) compared to degludec.
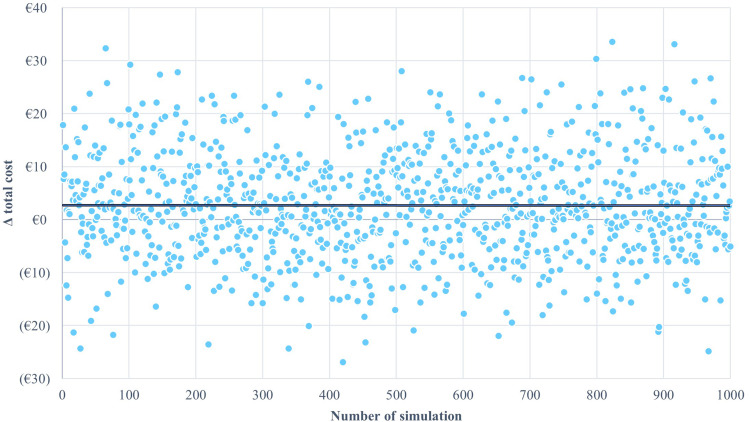
The robustness of the analysis was also confirmed by the probabilistic sensitivity analysis, with approximately 40% of the values below 0, Figure 3.
Figure 3.
Probabilistic sensitivity analysis results.
Scenario Analysis
The annual cost estimate, obtained by the comparison with degludec, was then used for the comparison with basal insulin mix on market in the further simulation scenario.
Since all basal insulins on the market have a daily-administration frequency, the same effects obtained with degludec were reported for the mix in terms of annual expense for insulin needles. Overall, the savings generated by the reduction in needle consumption and the improvement in the adherence resulted, also with this comparator, in an annual economic benefit per patient of €105.95 related to the once-weekly administration benefits, not considering the costs of the drugs. Despite the lower annual cost of the basal insulin mix, thanks to the advantage of weekly administration, icodec reported a cost-effective profile, reporting an ICER of €6047.66, well below the willingness to pay threshold. This scenario includes basal insulins on the market, conservatively, with the exception of pre-mixed fixed-ratio combinations of basal insulins and glucagon-like peptide-1 receptor agonists (degludec/liraglutide and glargine/lixisenatide). If these were also included, the ICER would be dominated in favor of icodec.
The deterministic and probabilistic sensitivity analyses developed for the alternative scenario, as well as for the base case, confirmed the robustness of the results.
Discussion
The economic evaluation allowed the analysis of the potential impact of icodec on NHS and the potential savings due to the reduction in needle consumption and an improved adherence related to icodec, compared to basal insulins currently available in Italy. Furthermore, the impact of the icodec treatment on improving the quality of life was evaluated. The highlighted benefits are connected to the advantages of icodec, which represents the first once-weekly basal insulin analogue for diabetes management.
Diabetic patients will be able to reduce the number of insulin injections from 365 to 52 in a year, a truly positive revolution in insulin therapy. This new therapeutic option has the potential to simplify insulin treatment for diabetes by eliminating the discomfort of daily injections for patients and thus increasing adherence, resulting in a relevant change, a marked improvement in the quality of life of diabetic patients, and potential saving for NHS.
Since the price of icodec has not yet been established, evidence from this analysis might provide an indication of justifiable price, considering clinical and economic value impact of the first once-weekly basal insulin analogue on patient society and NHS, compared to the other insulins.
The base case scenario, developed by comparing icodec with degludec, reported a justifiable price, guaranteeing healthcare spending to be almost unchanged after product introduction, in consideration of the advantages on saving needles and adherence improvement. The higher annual drug cost reported was offset by the reduction in healthcare costs related to once-weekly administration.
The minimal total annual differential cost, in favor of icodec, obtained considering drug cost, administration cost and adherence impact on disease management cost, allows the definition of a dominant cost-effectiveness ratio compared to degludec, due to a once-weekly injection utility advantage of 0.023 compared to daily injections.14 Moreover, the scenario analysis confirmed the potential benefit of icodec. In fact, despite a small annual increase in cost compared to basal insulin mix, an excellent cost-effectiveness profile can be granted.
The present economic evaluation allows a first test of the robustness of the economic benefits associated with icodec treatment, considering only the potential price and benefits on needle consumption and adherence, representing a useful tool for the evaluation of decision makers.
To define the average annual cost of available basal insulins, ex-factory prices net of mandatory discounts (−5%, -5%) were adopted. However, these products have confidential discounted prices, which would have allowed a more advantageous scenario in the NHS budget, compared to the those reported here. Therefore, the analysis is conservative and does not include among the comparators fixed-dose formulations of basal insulin and GLP-1 (glucagon-like peptide 1), since they have higher costs than extemporaneous insulins.
The analysis focused on the benefits of icodec related to weekly administration, while it did not consider the potential benefits associated with the positive results emerging from the clinical development program regarding the glycated haemoglobin reduction from baseline in the icodec group, and increase in TIR compared with once-daily insulin was not considered, result that could indicate a potential reduction in diabetes complications. This constitutes a limitation of the analysis, but also makes the study conservative and allows for the evaluation of further investigations. The choice of a time horizon of one year can be seen as a limit, and certainly, it could be of interest to evaluate the effects of the treatment beyond this horizon, which, however, was useful for producing estimates of the icodec price.
Furthermore, indirect costs were not included, and the benefits of optimization in the management of long-term hospitalized patients or in retirement home were not quantified. Considering an average time of 3.5 minutes to perform an insulin administration, the patient, or his caregiver, could save over 18 hours per year due to icodec once-weekly administration, corresponding to a waking day. In the future, these elements could be considered as further benefits to be evaluated, related to the use of icodec in diabetic patients, with more positive results.
The analysis involved some assumptions or international data use, in case of unavailability of national reference data. The assumptions made were validated by the opinion of clinical experts, and the quality of the international evidence and transferability to the national context was assessed.
In the absence of specific data on adherence, the analysis assumed the same adherence outcome obtained with the switch from daily to weekly administration, as observed with once-weekly glucagon-like peptide 1 (GLP-1) injections.17,18,37,38 Indeed, studies comparing once-weekly and once-daily injectable GLP-1 showed an increase in treatment adherence and patient satisfaction.37,39–42 Furthermore, patient satisfaction data were also reported among the results of the ONWARDS 2 and 5 clinical trials, confirming greater treatment satisfaction with once-weekly basal insulin compared to insulin once daily, measured by Diabetes Treatment Satisfaction Questionnaire (DTSQ).3,6
Benefits of adherence were observed both in patients who had never previously undergone injection treatment and in patients previously treated with daily injections who switched to weekly therapy.37,42 The adherence impact on clinical and economic outcomes of diabetic patients is widely documented in the literature.34,37,43–57 Associations between adherence among type 2 diabetic patients and both clinical (i.e glycated hemoglobin – HbA1c) and non-clinical outcomes, as healthcare resource consumption and work impairment, have been highlighted.58 A study on type 2 diabetic patients using basal insulin reported a 0.21 increase in HbA1c for each point increase in the level of non-adherence as well as a corresponding increase of 4.6%, 20.4% and 20.9% in physician visits, emergency room visits and hospitalizations, respectively.59 In another study on type 2 diabetes patients (N = 123,235) higher adherence to glucose-lowering agents was associated with substantially less need for acute care, reporting a reduced likelihood of hospitalization or emergency room use, a reduced risk of acute complications, and a decrease in the number of hospitalizations, emergency room visits and days of hospitalization.34 Results suggest that higher glucose-lowering agent adherence is associated with significant benefits for payers and patients due to lower all-cause and diabetes-related total costs, despite higher drug costs. The correlation between a 1 percentage point improvement in adherence and the relative economic savings per patient ($62) was also taken from the same article.34 Starting from this evidence, and after comparing the economic impact of the diabetes in the USA and Italian context, the transferability of this correlation was assumed, subject to adjustment of the Italian costs, with possible adaptation also to other settings.
The data relating to suboptimal adherence to the administration of injectable antidiabetics was taken from a meta-analysis18 and assumed transferable to Italy, after verifying data on drug consumption at a national level.60
The assumptions adopted for the analysis made it possible to evaluate the impact of the use of weekly administration, compared to daily, on the improvement of the adherence profile, with consequent economic benefit. The validation of the assumptions and the evaluation of the robustness of the results through sensitivity analysis allow us to consider the methodology transferable to other settings, following adaptation of the data.
It is also noteworthy to consider that icodec facilitates the start of treatment in patients with type 2 diabetes who have not previously taken insulin given the lower number of injections, decreasing clinical inertia and promoting better acceptance of insulin therapy.37,61 Early glucose control has been associated with a reduction in microvascular complications and major adverse cardiovascular events (MACE) due to glycemic legacy or metabolic memory.62–65 Highly prevalent clinical inertia for insulin initiation and/or intensification is internationally recognized. Reducing the delay in treatment could lead to an improvement in complications and better management of the disease.66–69 Therefore, the availability of icodec, as a new therapeutic option capable of decreasing clinical inertia, could lead to promising outcomes in the management of TDM2, in naïve patients, as well as in subjects already treated and not sufficiently controlled.37
Moreover, icodec contributes to environmental sustainability in the healthcare sector by decreasing medical waste production and resource consumption due to the reduced use of pens and needles for administration. The healthcare industry should not ignore the needs for a change towards greater environmental sustainability, accounting for a substantial proportion of global carbon emissions.70,71
The World Health Organization (WHO) emphasizes the importance of environmental factors as significant influences on human health. In this context, Health Technology Assessment (HTA) is evolving to include the evaluation of environmental impact in healthcare decision-making processes, viewing these elements as closely related rather than distinct aspects. The goal is to inform decision-makers to promote an equitable, efficient, and high-quality health system.72,73 Once-weekly icodec basal insulin option, reducing the frequency of administration and, therefore, the consumption of healthcare waste moves in this direction, contributing to environmental sustainability. Regarding this, a recent HTA report, about icodec use for the management of patients with diabetes in Italy, has analysed the reduction in CO2 emissions linked to the decreased number of administrations, dropping from 365 per year with traditional insulins to just 52 with Icodec.19 Specifically, the icodec adoption results in a 62% reduction in CO2 emissions, from 12 kg of CO2 per patient per year with daily administrations to 4.6 kg of CO2 with weekly administration.
At an aggregate level, it is estimated that the use of icodec for 100,000 patients in one year could reduce CO2 emissions by approximately 740 tons, equivalent to the annual electricity consumption of 100 families or 671 round-trip flights from Rome to New York.19
The document also highlights how improved treatment adherence, facilitated by the reduced administration frequency with icodec, leads to a fewer visits to healthcare centres and, consequently, fuel savings.
This information is a corollary to the results of the present analysis, as a significant added value to support the evaluation of icodec use compared to the basal insulins currently on the market.
The analysis highlighted the possibility of benefiting from the multiple advantages of once-weekly basal insulin administration. The increase in the annual drug cost reported can be considered justifiable and sustainable in consideration of the savings generated by the new therapeutic option, as a result of the reduction in administration costs and the improvement in adherence and quality of life.
Deterministic and probabilistic sensitivity analyses allowed to test the robustness of the results. The driver parameters for the analysis were highlighted and all the simulations implemented resulted in an ICER dominant or cost-effective for icodec, respectively, compared to degludec and basal insulin mix.
Conclusion
Icodec is expected to be a significantly efficient therapeutic option for patients needing basal insulin therapy. With its innovative once-weekly administration, it will have a positive impact on diabetes management in patients with only basal insulin treatment, improving patients’ adherence, QoL, overall healthcare efficacy, simplifying the therapy management, and also contributing to environmental sustainability. Moreover, it could reduce the time spent on insulin administration and related tasks. This condition could improve the mental burden and psychological impact associated with daily diabetes management. Greater flexibility in planning daily activities could help improve overall well-being and adaptation to diabetes treatment. Furthermore, the product characteristics may allow an improvement in adherence, with the clinical and economic benefits that this entails. Finally, all these outcomes can be achieved without increasing the economic burden for the NHS in terms of direct costs, in respect of an improvement in the patient’s quality of life, better patient management and healthcare resources savings, guaranteeing an excellent cost-effective profile.
Funding Statement
This study was funded by unconditional grant from NovoNordisk. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Disclosure
Professor Giorgio Lorenzo Colombo reports grants from NovoNordisk, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Kjeldsen TB, Hubálek F, Hjørringgaard CU, et al. Molecular engineering of insulin icodec, the first acylated insulin for once-weekly administration in humans. J Med Chem. 2021;64:8942–8950. doi: 10.1021/acs.jmedchem.1c00257 [DOI] [PubMed] [Google Scholar]
- 2.Rosenstock J, Bain SC, Gowda A, et al. ONWARDS 1 trial investigators. weekly icodec versus daily glargine U100 in type 2 diabetes without previous insulin. N Engl J Med. 2023;389(4):297–308. doi: 10.1056/NEJMoa2303208 [DOI] [PubMed] [Google Scholar]
- 3.Philis-Tsimikas A, Asong M, Franek E, et al. Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol. 2023;11(6):414–425. doi: 10.1016/S2213-8587(23)00093-1 [DOI] [PubMed] [Google Scholar]
- 4.Lingvay I, Asong M, Desousa C, et al. Once-weekly insulin icodec vs once-daily insulin degludec in adults with insulin-naive type 2 diabetes the ONWARDS 3 randomized clinical trial. JAMA. 2023;330(3):228–237. doi: 10.1001/jama.2023.11313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu C, Ásbjörnsdóttir B, Bajaj HS, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open label, multicentre treat-to-target, non inferiority trial. Lancet. 2023;401:1929–1940. doi: 10.1016/S0140-6736(23)00520-2 [DOI] [PubMed] [Google Scholar]
- 6.Bajaj HS, Aberle J, Davies M, et al. Once-weekly insulin icodec with dosing GIUde app versus once-daily basal insulin analogues in insulin-naive type 2 diabetes (ONWARDS 5). Ann Intern Med. 2023;176(11):1476–1485. doi: 10.7326/M23-1288 [DOI] [PubMed] [Google Scholar]
- 7.Russel-Jones D, Babazono T, Cailletau R, et al. Once-weekly insulin icodec versus once-daily insulin degludec as part of a basal-bolus regimen in individuals with type 1 diabetes (ONWARDS 6): a phase 3a, randomised, open-label, treat-to-target trial. Lancet. 2023;402(10413):1636–1647. doi: 10.1016/S0140-6736(23)02179-7 [DOI] [PubMed] [Google Scholar]
- 8.Lingvay I, Buse JB, Franek E, et al. A randomized, open-label comparison of once-weekly insulin icodec titration strategies versus once-daily insulin glargine U100. Diabetes Care. 2021;44:1–9. doi: 10.2337/dc20-2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajaj HS, Bergenstal RM, Christoffersen A, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in type 2 diabetes inadequately controlled on daily basal insulin: a Phase 2 randomized controlled trial. Diabetes Care. 2021;44:1–9. doi: 10.2337/DC20-2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenstock J, Bajaj H, Janež A, et al. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Eng J Med. 2023;389(4):297–308. [DOI] [PubMed] [Google Scholar]
- 11.Alsaidan AA, Alsaidan QA, Mallhi TH, et al. Assessment of adherence to insulin injections among diabetic patients on basal-bolus regimen in primary and secondary healthcare centers in Al-Jouf Region of Saudi Arabia. A descriptive analysis. J Clin Med. 2023;12:3474. doi: 10.3390/jcm12103474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polonsky WH, Fisher L, Hessler D, et al. Patient perspectives on once-weekly medications for diabetes. Diabetes Obesity Metab. 2011;13(2):144–149. doi: 10.1111/j.1463-1326.2010.01327.x [DOI] [PubMed] [Google Scholar]
- 13.Evans M, Jensen HH, Bøgelund M, et al. Flexible insulin dosing improves health-related quality-of-life (HRQoL): a time trade-off survey. J Med Eco. 2013;16(11):1357–1365. doi: 10.3111/13696998.2013.846262 [DOI] [PubMed] [Google Scholar]
- 14.Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12:219–230. doi: 10.1007/s10198-010-0224-8 [DOI] [PubMed] [Google Scholar]
- 15.Robinson S, Newson RS, Liao B, et al. Missed and mistimed insulin doses in people with diabetes: a systematic literature review. Diabetes Technol. Ther. 2021;12(23):844–856. doi: 10.1089/dia.2021.0164 [DOI] [PubMed] [Google Scholar]
- 16.Brod M, Rana A, Barnett AH, et al. Adherence patterns in patients with type 2 diabetes on basal insulin analogues: missed, mistimed and reduced doses. Curr Med Res Opin 2012;28(12):1–14. doi: 10.1185/03007995.2012.743458 [DOI] [PubMed] [Google Scholar]
- 17.Polonsky WH, Arora R, Faurby M, et al. Higher rates of persistence and adherence in patients with type 2 diabetes initiating once-weekly vs daily injectable glucagon-like peptide-1 receptor agonists in US clinical practice (STAY study). Diabetes Ther. 2022;13(1):175–187. doi: 10.1007/s13300-021-01189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weeda ER, Muraoka AK, Brock MD, et al. Medication adherence to injectable glucagon-like peptide-1 (GLP-1) receptor agonists dosed once weekly vs once daily in patients with type 2 diabetes: a meta-analysis. Int J Clin Pract. 2021:1–6. doi: 10.1111/ijcp.14060 [DOI] [PubMed] [Google Scholar]
- 19.Basile M, Fortunato A, Antonini D, et al. Health Technology Assessment di icodec per la gestione dei pazienti affetti da diabete in Italia. Quaderni dell’Italian J Public Health. 2024;12(1):1. [Google Scholar]
- 20.Husereau D, Drummond M, Augustovski F, et al. CHEERS 2022 ISPOR good research practices task force. consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG. 2022;129(3):336–344. doi: 10.1111/1471-0528.17012 PMID: 35014160. [DOI] [PubMed] [Google Scholar]
- 21.Russo P, Zanuzzi M, Carletto A, Sammarco A, Romano F, Manca A. Role of economic evaluations on pricing of medicines reimbursed by the Italian national health service. Pharmacoeconomics. 2023;41(1):107–117. Italian. doi: 10.1007/s40273-022-01215-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ISTAT. Popolazione residente per sesso, età e stato civile al 1° gennaio 2023; “Resident population by sex, age and marital status as of 1 January 2023”. Available from: https://demo.istat.it/app/?i=POS&l=it. Accessed October 16, 2024.
- 23.ISTAT. Aspetti della vita quotidiana: stato di salute - età dettaglio; “Aspects of daily life: health state - age detail”. Available from: http://dati.istat.it/Index.aspx?QueryId=15445. Accessed October 16, 2024. Italian.
- 24.Ministero della Salute. Legge 16 marzo 1987, 115, recante “Disposizioni per la prevenzione e la cura del diabete mellito” Relazione 2021 Stato delle conoscenze e delle nuove acquizioni in tema di diabete mellito. Legge 16 marzo: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3229_allegato.pdf. Accessed October 16, 2024. Italian. [Google Scholar]
- 25.AMD. Annali AMD 2022_Diabete T1. Annali_2022_diabete_T1_prot_v2.pdf (aemmedi.it). Available from: https://aemmedi.it/wpcontent/uploads/2023/10/Annali_2022_diabete_T1_prot_v2.pdf. Accessed October 16, 2024. Italian.
- 26.AMD. Annali AMD 2022_Diabete T2. Annali_2022_diabete_T2_prot_v2.pdf (aemmedi.it). Available from: https://aemmedi.it/wp-content/uploads/2023/05/Annali_2022_diabete2-prot.pdf. Accessed October 16, 2024. Italian.
- 27.IQVIA market data: basal insulin market. (Market shares – data updated December 2023).
- 28.Farmadati Italia Srl. Software Gallery® BD 2.0. Available from: https://gallery.farmadati.it/Home.aspx. Accessed October 16, 2024.
- 29.Agenzia Italiana del Farmaco. AIFA. Determinazione 3 luglio 2006. Elenco dei medicinali di classe a) rimborsabili dal Servizio sanitario nazionale (SSN) ai sensi dell’articolo 48, comma 5, lettera c), del decreto-legge 30 settembre 2003, 269, convertito, con modificazioni, nella legge 24 novembre 2006, 326. Prontuario farmaceutico nazionale 2006). (GU Serie Generale n.156 del 07-07-2006 - Suppl. Ordinario n. 161. Prontuario farmaceutico nazionale 2006). (GU Serie Generale n.156 del 07-07-2006 - Suppl. Ordinario n. 161: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2006-07-07&atto.codiceRedazionale=06A06166&elenco30giorni=false. Accessed October 16, 2024. Italian.
- 30.Agenzia Italiana del Farmaco. AIFA. Determinazione 27 settembre 2006. Manovra per il governo della spesa farmaceutica convenzionata e non convenzionata. (GU Serie Generale n.227 del 29-09-2006). GU Serie Generale n.227 del 29-09-2006: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2006-09-29&atto.codiceRedazionale=06A08826&elenco30giorni=false. Accessed October 16, 2024. Italian.
- 31.Ponzani P, Berra C, Di Lelio A, et al. Impact of insulin degludec in type 2 diabetes: real-world data on effectiveness and safety. Diabetes Ther. 2018;9(6):2209–2218. doi: 10.1007/s13300-018-0511-4 Epub 2018 Sep 21. PMID: 30242611; PMCID: PMC6250625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kesavadev J, Murthy LS, Chaudhury T, et al. One-year safety and effectiveness of insulin degludec in patients with diabetes mellitus in routine clinical practice in India-TRUST (Tresiba real-world use study). Metabol Open. 2022;14:100184. doi: 10.1016/j.metop.2022.100184 PMID: 35496980; PMCID: PMC9046940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fadini GP, Buzzetti R, Fittipaldi MR, et al. REX study group. IDegLira for the real-world treatment of type 2 diabetes in Italy: protocol and Interim results from the REX observational study. Diabetes Ther. 2022;13(8):1483–1497. doi: 10.1007/s13300-022-01287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boye KS, Curtis SE, Lage MJ, Garcia-Perez LE. Associations between adherence and outcomes among older, type 2 diabetes patients: evidence from a medicare supplemental database. Patient Prefer Adherence. 2016;10:1573–1581. doi: 10.2147/PPA.S107543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrestha SS, Honeycutt AA, Yang W, et al. Economic costs attributable to diabetes in each U.S. state. Diabetes Care. 2018;41(12):2526–2534. doi: 10.2337/dc18-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagano E, De Rosa M, Rossi E, et al. The relative burden of diabetes complications on healthcare costs: the population-based CINECA-SID ARNO diabetes observatory. Nutr Metab Cardiovasc Dis. 2016;26(10):944–950. doi: 10.1016/j.numecd.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 37.Silva R RE, de Miranda Gauza M, GIUsso MES, da Silva JON, Kohara SK. Once-weekly insulin icodec vs. once-daily insulin glargine U100 for type 2 diabetes: a systematic review and meta-analysis of phase 2 randomized controlled trials. Arch Endocrinol Metab. 2023;67(5):e000614. doi: 10.20945/2359-3997000000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao Q, Ouwens MJ, Grandy S, Johnsson K, Kostev K. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes. 2016;9:201–205. doi: 10.2147/DMSO.S99732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wettergreen SA, Stewart MP, Kennedy K, Trujillo JM. Comparison of the usability, accuracy, preference, and satisfaction of three once-weekly glucagon-like peptide 1 receptor agonist pen devices in people with type 2 diabetes. Diabetes Spectr. 2023;36(1):5–13. doi: 10.2337/ds21-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Billings LK, Handelsman Y, Heile M, Schneider D, Wyne K. Health-related quality of life assessments with once-weekly glucagon-like peptide-1 receptor agonists in type 2 diabetes mellitus. J Manag Care Spec Pharm. 2018;24(9–a Suppl):S30–S41. doi: 10.18553/jmcp.2018.24.9-a.s30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12‐month follow‐up in a real‐world setting in the United States. Diabetes Obes Metab. 2019;21(4):920–9.12. doi: 10.1111/dom.13603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takase T, Nakamura A, Yamamoto C, et al. Improvement in treatment satisfaction after switching from liraglutide to dulaglutide in patients with type 2 diabetes: a randomized controlled trial. J Diabetes Investig. 2019;10(3):699–705. doi: 10.1111/jdi.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Nieves M, Boye KS, Kiljanski J, Cao D, Lage MJ. Adherence to basal insulin therapy among people with type 2 diabetes: a retrospective cohort study of costs and patient outcomes. Diabetes Ther. 2018;9(3):1099–1111. doi: 10.1007/s13300-018-0421-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33(1):74–109. doi: 10.1016/j.clinthera.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 45.Han E, Suh D-C, Lee S-M, Jang S. The impact of medication adherence on health outcomes for chronic metabolic diseases: a retrospective cohort study. Res Soc Adm Pharm. 2014;10(6):e87–98. doi: 10.1016/j.sapharm.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 46.Kennedy-Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. 2017;11:1103–1117. doi: 10.2147/PPA.S136639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadjiyianni I, Desai U, Suzuki S, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation: a retrospective database study among people with Type 2 diabetes mellitus in Japan. Diabetes Ther. 2017;8(1):149–166. doi: 10.1007/s13300-016-0215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Nieves M, Kabul S, Desai U, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in the US. Curr Med Res Opin. 2016;32(4):669–80.35. doi: 10.1185/03007995.2015.1135789 [DOI] [PubMed] [Google Scholar]
- 49.Wong ES, Bryson CL, Hebert PL, Liu C-F. Estimating the impact of oral diabetes medication adherence on medical costs in VA. Ann Pharmacother. 2014;48(8):978–985. doi: 10.1177/1060028014536981 [DOI] [PubMed] [Google Scholar]
- 50.Chandran A, Bonafede MK, Nigam S, Saltiel-Berzin R, Hirsch LJ, Lahue BJ. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8(3):148–158. [PMC free article] [PubMed] [Google Scholar]
- 51.Egede LE, Gebreqziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012;35(12):2533–2539. doi: 10.2337/dc12-0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibson TB, Song X, Alemayehu B, et al. Cost sharing, adherence, and health outcomes in patients with diabetes. Am J Manag Care. 2010;16(8):589–600. [PubMed] [Google Scholar]
- 53.Jha AK, Aubert RE, Yao J, Teagarden JR, Epstein RS. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff. 2012;31(8):1836–1846. doi: 10.1377/hlthaff.2011.1198 [DOI] [PubMed] [Google Scholar]
- 54.Curtis SE, Boye KS, Lage MJ, Garcia-Perez L-E. Medication adherence and improved outcomes among patients with type 2 diabetes. Am J Manag Care. 2017;23(7):e208–14. [PubMed] [Google Scholar]
- 55.Juarez DT, Tan C, Davis J, Mau M. Factors affecting sustained medication adherence and its impact on health care utilization in patients with diabetes. J Pharm Health Serv Res. 2013;4(2):89–94. doi: 10.1111/jphs.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eby EL, Bajpai S, Faries DE, Haynes VS, Lage MJ. The association between adherence to insulin therapy and health care costs for adults with type 2 diabetes: evidence from a U.S. retrospective claims database. J Manag Care Spec Pharm. 2020;26(9):1081–1089. doi: 10.18553/jmcp.2020.26.9.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edelman SV, Ermakova A, Xiong Y, Sieradzan R, Taylor SD. Persistence with basal-bolus insulin therapy in patients with type 2 diabetes mellitus and effect on clinical and economic outcomes: a retrospective claims database study. J Manag Care Spec Pharm. 2019;25(12):1420–1431. doi: 10.18553/jmcp.2019.19097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukuda H, MIzobe M. Impact of nonadherence on complication risks and healthcare costs in patients newly-diagnosed with diabetes. Diab Res Clin Pract. 2017;123:55–62. doi: 10.1016/j.diabres.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 59.DiBonaventura M, Wintfeld N, Huang J, et al. The association between nonadherence and glycated hemoglobin among type 2 diabetes patients using basal insulin analogs. Pat Pref Adherence. 2014;8:873–882. doi: 10.2147/PPA.S55550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osservatorio Nazionale sull’impiego dei Medicinali. L’uso dei farmaci in Italia. Rapporto Nazionale Anno 2022[The Medicines Utilisation Monitoring Centre. National Report on Medicines Use in Italy]. 2023.
- 61.Rosenstock J, Bajaj HS, Janež A, et al. NN1436-4383 investigators. once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med. 2020;383(22):2107–2116. doi: 10.1056/NEJMoa2022474 [DOI] [PubMed] [Google Scholar]
- 62.Aroda VR, Eckel RH. Reconsidering the role of glycaemic control in cardiovascular disease risk in type 2 diabetes: a 21st century assessment. Diabetes Obes Metab. 2022;24(12):2297–2308. doi: 10.1111/dom.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valensi P, Prévost G, Schnell O, Standl E, Ceriello A. Targets for blood glucose: what have the trials told us. Eur J Prev Cardiol. 2019;26(2 Suppl):64–72. doi: 10.1177/2047487319885456 [DOI] [PubMed] [Google Scholar]
- 64.Prattichizzo F, de Candia P, De Nigris V, Nicolucci A, Ceriello A. Legacy effect of intensive glucose control on major adverse cardiovascular outcome: systematic review and meta-analyses of trials according to different scenarios. Metabolism. 2020;110:154308. doi: 10.1016/j.metabol.2020.154308 [DOI] [PubMed] [Google Scholar]
- 65.Whyte MB, Joy M, Hinton W, et al. Early and ongoing stable glycaemic control is associated with a reduction in major adverse cardiovascular events in people with type 2 diabetes: a primary care cohort study. Diabetes Obes Metab. 2022;24(7):1310–1318.ri. doi: 10.1111/dom.14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gavin JR, Abaniel RM, Virdi NS. Therapeutic inertia and delays in insulin intensification in type 2 diabetes: a literature review. Diabetes Spectr. 2023;36(4):379–384. doi: 10.2337/ds22-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lingvay I, Buse JB, Franek E, et al. A randomized, open-label comparison of once-weekly insulin icodec titration strategies versus once-daily insulin glargine U100. Diabetes Care. 2021;44(7):1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andreozzi F, Candido R, Corrao S, et al. Clinical inertia is the enemy of therapeutic success in the management of diabetes and its complications: a narrative literature review. Diabetol Metab Syndr. 2020;12:52. doi: 10.1186/s13098-020-00559-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cucinotta D, Nicolucci A, Giandalia A, et al. Temporal trends in intensification of glucose-lowering therapy for type 2 diabetes in Italy: data from the AMD annals initiative and their impact on clinical inertia. Diabet Res Clin Pract. 2021;181:109096. doi: 10.1016/j.diabres.2021.109096 [DOI] [PubMed] [Google Scholar]
- 70.Pinho-Gomes A-C, Yoo S-H, Allen A, Maiden H, Shah K, Toolan M. Incorporating environmental and sustainability considerations into health technology assessment and clinical and public health guidelines: a scoping review. Int J Tech Assess Health Care. 2022;38(1):e84,1–10. doi: 10.1017/S0266462322003282 [DOI] [PubMed] [Google Scholar]
- 71.Lenzen M, Malik A, Li M, et al. The environmental footprint of health care: a global assessment. Lancet Planet Health. 2020;4(7):e271–e279. doi: 10.1016/S2542-5196(20)30121-2 [DOI] [PubMed] [Google Scholar]
- 72.O’Rourke B, Oortwijn W, Schuller T, International Joint Task Group. The new definition of health technology assessment: a milestone in international collaboration. Int J Technol Assess Health Care. 2020;36(3):187–190. doi: 10.1017/S0266462320000215 [DOI] [PubMed] [Google Scholar]
- 73.Antonazzo Cortesi P, Ferrara P, Losa L, Mantovani LG, Iraldo F, Iraldo F. HTA179 environmental impact and health technology assessment: state of art and future perspectives. Value Health. 2023;26(12):S353. doi: 10.1016/j.jval.2023.09.1863 ISSN 1098-3015. [DOI] [Google Scholar]