Abstract
Purpose
Several international organizations advocate for monitoring of adherence to direct oral anticoagulants (DOACs), given the prevalent issue of suboptimal adherence to DOACs. The aim was to investigate intake patterns in patients on DOAC therapy by electronic monitoring of medication adherence in community pharmacies (using a Medication Event Monitoring System® (MEMS®)-device), and to assess patients’ experiences with this device.
Patients and Methods
Patients using apixaban, rivaroxaban or edoxaban and visiting a community pharmacy, were included. Adherence was electronically monitored over a twelve-week period. Pharmacists conducted data readings from the electronic device at six and twelve weeks, and discussed these data with the patients. At the beginning and end of the study, patients completed a questionnaire about their expectations and experiences respectively.
Results
Eighty-nine patients were included and high taking adherence rates were observed (median adherence of 100% for once-daily dosed patients and 96.7% for twice-daily dosed patients), but more than half of the patients took at least one dose too late or skipped at least one dose, possibly resulting in temporarily reduced protection against thromboembolic events. Most patients who felt that their adherence had improved, believed this was due to the combination of the electronic device and the personal follow-up by the pharmacist. Although most patients stated that medication adherence is their own responsibility, they were grateful for the support they received from their community pharmacist.
Conclusion
High adherence rates were observed, but there was still room for improvement regarding intake moments. Positive experiences with an electronic device for medication adherence monitoring were reported.
Keywords: adherence, electronic adherence monitoring, community pharmacist, anticoagulation
Introduction
Direct oral anticoagulants (DOACs) are important assets in the prevention and treatment of thromboembolic diseases due to the unrivalled combination of ease of use, safety and effectiveness compared to other antithrombotic treatments. Because of the relative short half-life of DOACs and rapid offset of action compared to vitamin K antagonists and the important consequences of therapy failure, several international organizations recommend the monitoring of adherence to these molecules.1–5 Moreover, several studies have shown a suboptimal adherence to DOACs that often goes unnoticed in daily practice.6–13 Adherence data vary depending on the type of DOAC, definition of adherence used, clinical indication, measurement method and the duration of DOAC intake.3,6–9,11,12,14–16
Adherence is defined by the World Health Organization (WHO) as
the extent to which a person’s behavior – taking medication, following a diet, and/or executing lifestyle changes – corresponds with agreed recommendations from a healthcare provider.17
Three phases of adherence can be distinguished, namely initiation, implementation and discontinuation.10 Adherence can be measured by subjective methods (eg pill counts or self-reporting) and objective methods (eg blood concentration monitoring or electronic monitoring).3,7,18–23 One example of electronic monitoring is the Medication Event Monitoring System® (MEMS®, AARDEX Group, Belgium), used in this study, an electronic cap secured on top of a plastic medication bottle that records the date and time the pill container is opened. An overview of all dates and times the device is opened, as well as a visual overview of these time points, can be obtained.24–27 Recently, the International Society for Medication Adherence (ESPACOMP) stated that electronic medication monitoring should be considered as a gold standard to assess medication adherence.28 Although there are some studies evaluating the impact of electronic monitoring on DOAC therapy, no studies are available yet as far as we know on the use of electronic monitoring to measure DOAC adherence in primary care services.15,29
Therefore, the primary aim of this study was to investigate intake patterns in patients on DOAC therapy by electronic monitoring in community pharmacies. A second objective was to assess patients’ experiences with DOAC adherence follow-up by an electronic device.
Materials and Methods
Study Design
A mixed-method observational study was conducted in Belgian community pharmacies. Data collection was done in two ways namely through the use of the electronic devices and questionnaires. The combination of both methods resulted in the collection of quantitative and qualitative data.
Study Population
Patients aged 18 years or older, visiting one of the participating community pharmacies with a prescription for apixaban, edoxaban or rivaroxaban were consecutively included between October 1st, 2020 and February 28th, 2021. Because of its hygroscopic character, dabigatran could not be repackaged in an electronic device, which is the reason why patients on dabigatran could not be included in this study.30 There were no restrictions regarding the indication for or duration of DOAC use. A maximum number of 20 patients per pharmacy could be included. Participating community pharmacists were recruited from the network of the “Koninklijke Apothekersvereniging van Antwerpen” (KAVA), a local Belgian professional association of community pharmacists. Attendees in a training event on DOACs were approached for voluntary participation.
By signing an informed consent, patients gave permission to access their shared pharmaceutical file ie, a file containing all products dispensed in Belgian community pharmacies. Patients also gave permission to contact their general practitioner (GP).
Role of Pharmacists
At least one pharmacist from each participating community pharmacy received training on the interpretation of electronic data and motivational communication skills.
At every inclusion, DOAC therapy was repackaged into an electronic device containing sufficient supply for 6 weeks. The pharmacist explained the use of the device thoroughly to the patient. When the patient came back to the community pharmacy after 6 weeks, the electronic device was read out and refilled by the pharmacist for another 6 weeks. The adherence results were shown to and discussed with the patient. A discussion guide was available for the pharmacists.
After 12 weeks, the study was concluded with a third visit by the patient to the community pharmacy. The electronic device was read out and results were again discussed between the patient and pharmacist. At this visit, the electronic devices were handed in and the patient received DOAC therapy as before in the original medication packages.
Data Collection and Analysis
Data from the Electronic Device
Based on the data obtained from the electronic device, the DOAC intake pattern of each participating subject was characterized.
Adherence was measured by taking adherence and timing adherence. Taking adherence was defined as the percentage of prescribed doses a subject took, while timing adherence was defined as the percentage of doses correctly taken within a pre-defined window.31 For this study, this window was set between 12h and 36h (24h ± 12h) around the median intake time for once-daily dosed (QD) DOAC patients and between 6h and 18h (12h ± 6h) around the median intake time for twice-daily dosed (BID) patients. Changes in intake pattern before and after discussion with the pharmacist after the first six weeks of using the electronic device were analyzed using a paired T-test (if normally distributed) or a Wilcoxon signed rank test (if not normally distributed). Differences in timing adherence between QD and BID patients were analyzed using a Mann–Whitney U-test. Differences in the proportion of QD and BID patients missing at least one dose were investigated using a Chi-square test. Normality was tested using the Shapiro–Wilk test (if n < 50) or the Kolmogorov–Smirnov test (if n ≥ 50). A two-sided p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 29.0.0.0.
Experiences and Expectations
At the start of the study, the patient completed a first questionnaire about his/her medication use and his/her expectations towards electronic monitoring of medication adherence. At the end of the study (week 12), the patient completed a second questionnaire about his/her experiences.
The first questionnaire was based on the validated MARS-5 questionnaire,32 combined with demographic and medical data. The second questionnaire included a mix of open-ended and multiple-choice questions. Both questionnaires were initially developed by the first author (SD). Subsequently, the entire research team revised the questionnaires followed by a third revision carried out by the participating community pharmacists, academics and by representatives of professional associations. Depending on the patient’s preference, a paper or an electronic version of the questionnaires was provided. Both questionnaires are available in the Supplementary Materials.
Ethics Approval
The study was approved by the Ethics Committee of UZ Brussel (2020/346 – 30/09/2020).
Results
In total, 13 community pharmacies participated in this project. The participating pharmacies had a median employee number of 2.5 full time equivalents (both pharmacists and pharmacy technicians) with an interquartile range (IQR) of 2.5–3.0, a median number of 105 visiting patients per day (IQR: 100–120) and included a median number of 6 patients (IQR: 5.0–8.5) each in this study.
In total, 158 patients were eligible for inclusion and were asked to participate, of whom 89 (56.3%) agreed. Patients most frequently agreed to participate out of sympathy for the pharmacist (n = 58; 65.2%), while for only 7 patients (7.9%), the primary objective for participation was to improve their medication adherence. The most common reason for refusal was that a pill organizer or similar system was already in use (n = 30; 43.5%), preventing the re-packaging of the drug in the electronic device. Table 1 summarizes the reasons for refusal.
Table 1.
Reasons for Patients’ Refusal (n = 69)
| Use of pill organizer or similar system | 30 (43.5%) |
| Not interested due to lack of added value | 18 (26.1%) |
| Research context does not feel comfortable | 8 (11.6%) |
| Too difficult for the patient | 8 (11.6%) |
| Patient is too busy | 3 (4.3%) |
| Other reasons | 2 (2.9%) |
Note: All data are represented as numbers and corresponding percentages.
The median age of participating patients was 71.0 years (IQR: 66.0–78.0) and 66.3% (n = 59) were male. The majority of patients lived together with their partner (n = 63; 70.8%) and 75.3% of patients (n = 67) finished at least secondary school. Most patients were treated with a DOAC for thromboembolic (stroke) prevention because of atrial fibrillation (n = 63; 70.8%). Sixty-nine patients (77.5%) had a QD dose regimen, while 20 patients (22.5%) took a DOAC BID. Data from the electronic device were obtained in 87 patients, among whom 67 (77.0%) had a QD and 20 (23.0%) a BID dose regimen. Additional patient characteristics are summarized in Table 2.
Table 2.
Patient Characteristics (n = 89)
| Age (years) | 71.0 (66.0–78.0) | |
| Sex | Male | 59 (66.3%) |
| Female | 30 (33.7%) | |
| Living situation | Living together with partner | 63 (70.8%) |
| Living together with partner and children | 9 (10.1%) | |
| Single without children | 13 (14.6%) | |
| Single with children | 3 (3.4%) | |
| Living with parents | 1 (1.1%) | |
| Highest education | Primary school | 22 (24.7%) |
| Secondary school | 44 (49.5%) | |
| Higher education | 23 (25.8%) | |
| CHA2DS2-VASc scorea | 3 (2–4) | |
| HAS-BLED scorea | 2 (1–2.5) | |
| Indication for DOAC useb | Non-valvular atrial fibrillation | 63 (70.8%) |
| Secondary prevention of venous thromboembolism (VTE) | 19 (21.3%) | |
| Other | 6 (6.7%) | |
| Unknown | 3 (3.4%) | |
| Apixaban | 2.5 mg | 2 (2.2%) |
| 5 mg | 16 (18.0%) | |
| Edoxaban | 30 mg | 7 (7.9%) |
| 60 mg | 34 (38.2%) | |
| Rivaroxaban | 2.5 mg | 2 (2.2%) |
| 10 mg | 1 (1.1%) | |
| 15 mg | 3 (3.4%) | |
| 20 mg | 24 (27.0%) |
Notes: All data are represented as numbers and corresponding percentages except for age, CHA2DS2-VASc score and HAS-BLED score that are represented as a median with corresponding interquartile range. aOnly calculated for AF-patients, based on self-reported data; bDifferent reasons possible, self-reported.
Taking Adherence
The median taking adherence was significantly higher among patients using a DOAC QD (100%; IQR: 97.1–100%) compared to patients using a DOAC BID (96.7%; IQR: 93.8–99.3%) (Mann–Whitney U-test; p = 0.001).
Of the 67 QD patients, 22 patients (32.8%) missed at least one dose during the 12-week study period. After six weeks of using the electronic device, 57 of these patients (85.1%) had a discussion moment with the pharmacist. No statistically significant difference in taking adherence before and after the discussion moment with the pharmacist was observed (Wilcoxon signed-rank test; p = 0.893).
Of the 20 BID patients, 14 patients (70.0%) missed at least one dose during the 12-week study period, which is significantly higher than for the QD patients (Chi-square test; p = 0.003). After six weeks, all these patients had a discussion moment with the pharmacist. Taking adherence did not significantly change after the discussion moment with the community pharmacist after six weeks (Wilcoxon signed-rank test; p = 0.055).
Among most patients (n = 19; 95.0%) using a DOAC BID, taking adherence in the morning was similar to that in the evening. Likewise, time variances when taking the morning dose were similar to those of the evening dose.
Timing Adherence
Patients on a QD regimen had a significantly higher median timing adherence (98.6%; IQR: 96.3–100%) compared to patients on a BID regimen (97.1%; IQR: 94.2–98.9%) (Mann–Whitney U-test; p = 0.043).
Once-Daily Dose Regimen
Most patients using a DOAC QD took the DOAC in the morning between 8 am and 11 am (n = 54, 80.6%), while 11 patients (16.4%) took the DOAC in the evening (between 6 pm and 10 pm) and only two patients (3.0%) took the DOAC around 1 pm.
In the QD regimen, the median interval between two consecutive intakes was 24h for most patients (n = 39; 58.2%). However, 14 patients (20.9%) had a median interval shorter than 24h (with a minimum of 23.4h), whereas another 14 patients (20.9%) had a median interval longer than 24h (with a maximum of 24.2h). There were no significant changes in the median interval between two intakes before and after the discussion moment with the pharmacist (Wilcoxon signed-rank test; p = 0.062).
During the 12-week study period, 34 QD patients (50.7%) had at least one dosing interval of more than 36h, with one patient having 11.3% of his intakes too late by at least 12h. There were no significant changes in the number of doses taken too late before and after the discussion moment with the pharmacist (Wilcoxon signed-rank test; p = 0.165).
Similarly, 30 patients (44.8%) had at least one dosing interval less than 12h and thus took the medication too early, with one patient taking his DOAC too early by at least 12h in 21.7% of cases. After the six weeks-discussion moment with the pharmacist, the number of doses that were taken too early, decreased significantly (Wilcoxon signed-rank test; p = 0.006).
Twice-Daily Dose Regimen
In most patients using a DOAC BID (n = 14; 70.0%), the median interval between two intakes ranged between 11.5h and 12.5h. Nine patients (45.0%) had a median interval between two consecutive intakes of 12.0h. however, five patients (25.0%) had a median interval shorter than 12.0h (with a minimum of 10.1h), whereas another five patients (25.0%) had a median interval longer than 12.0h (with a maximum of 13.5h) and one patient (5.0%) had a median interval of 21.1h.
During the 12-week study period, 18 patients (90.0%) had at least one intake more than 18h after the previous one (and thus 6h after the planned intake), with one patient taking his DOAC too late by at least 6h in 57.9% of intake moments. There were no significant differences before and after the discussion moment with the pharmacist (Wilcoxon signed-rank test; p = 0.078).
Similarly, 11 patients (55.0%) using a DOAC BID had at least one intake less than 6h after the previous intake and thus took their medication too early by at least 6h. After the six weeks-discussion moment with the pharmacist, the number of doses that were taken too early, decreased significantly (Wilcoxon signed-rank test; p = 0.004).
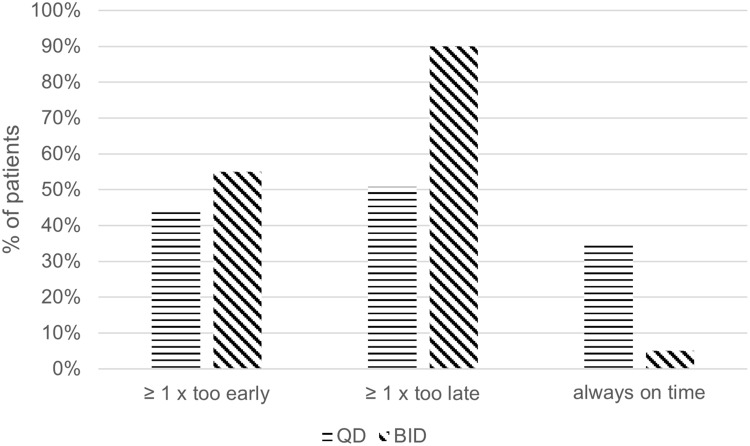
Figure 1 shows the timing adherence in QD and BID patients. The percentage of patients with at least one intake too early, one intake too late or without any mistakes in timing adherence are shown.
Figure 1.
Timing adherence in QD and BID patients. Percentage of patients with at least one intake too early or too late compared to the planned intake.
Patient Experiences and Expectations
At the end of the study, 77 patients (86.5%) completed the questionnaire about their experiences and expectations towards healthcare providers concerning medication adherence. Although 51 of these patients (66.2%) believed that their adherence remained unchanged during this study, 26 patients (33.8%) stated that their adherence increased. Sixteen of these 26 patients (61.5%) believed that their adherence increased due to the combination of follow-up with the electronic device and the discussion with the pharmacist. Six of these 26 patients (23.1%) reported that the increase was solely due to the discussion with the pharmacist, while four patients (15.4%) mentioned the follow-up with the electronic device as the reason for the increased adherence. None of the patients stated that their adherence decreased during the study period.
Two-thirds of patients (n = 51; 66.2%) reported that medication intake and adherence is their own responsibility. Nevertheless, more than half of them (n = 29; 56.9%) were grateful for the support they received from the healthcare providers (pharmacist and/or GP).
The remaining one-third of patients (n = 26; 33.8%) stated that correct medication intake is a shared responsibility between the patient, GP and pharmacist.
Sixty-two patients (80.5%) expressed that they gained more insight into their medication use due to the discussion with the pharmacist. Sixty-three patients (81.8%) reported that the pharmacist can give more personal advice based on the data obtained with the electronic devices and 66 patients (85.7%) stated that adherence monitoring should be carried out by both GPs and pharmacists.
More than half of the patients (n = 44; 57.1%) were only willing to come to the pharmacy for a refill of the electronic device when they also needed a new supply of the medication. Forty-six patients (59.7%) stated that adherence follow-up, including the use of the electronic device, should be fully reimbursed. Regarding a personal contribution, 18 patients (23.4%) were willing to pay up to €5; 10 patients (13.0%) up to €20 and only three patients (3.9%) were willing to pay up to €50 for this service. None of the patients was willing to pay more than €50 for this follow-up.
Discussion
In this study, two main aspects of real-life electronic monitoring of DOAC adherence in community pharmacies using an electronic device were investigated, ie (1) intake patterns of patients on DOAC therapy, and (2) patients’ experiences with DOAC adherence follow-up by their pharmacists based on the results of the electronic device.
Electronic Monitoring of DOAC Adherence: Intake Patterns
Higher adherence rates were seen in patients with a QD compared to BID dose regimen, which is in line with previous findings.7,13,33 The half-lives of DOACs vary between 9h to 14h in patients with a normal renal function. In a BID regimen, doses were skipped more often and the correct timing of intake was less respected, but skipping one dose in a BID dose regimen is expected to have less impact on DOAC blood levels compared to a QD dose regimen.7 Although our study showed overall high taking adherence rates, an important part of the patients missed at least one dose during the 12-week study period (32.8% of the QD patients and 70.0% of the BID patients). Given the relatively short half-life of DOACs and rapid offset of action, a missed dose may increase the risk of thromboembolic events, especially in a QD dose regimen. The European Heart Rhythm Association (EHRA)-guidelines clearly state that a missed DOAC dose may be taken until half of the dosing interval has passed. After this time point, the missed dose should be skipped to avoid an increased bleeding risk.2
In our study, we frequently observed incorrect intake of missed DOAC doses, with 50% of the QD dosed patients and 90% of the BID dosed patients having at least one intake of more than half the dosing interval after the planned intake and hence a higher thromboembolic risk susceptibility.
Conversely, an important part of the patients took at least one dose of their medication too early (44.8% of QD patients and 55.0% of BID patients), resulting in a higher bleeding risk. The percentage of doses taken too early was reduced after pharmacist intervention.
Electronic Monitoring of DOAC Adherence: Patient Satisfaction
According to the literature, medication education and a good relationship with the healthcare provider are important factors contributing to medication adherence.20,34–36 Our study showed that all patients were highly satisfied with the follow-up by the community pharmacist and the electronic device was considered user-friendly. It has previously been shown that feedback from electronic monitoring data is an important factor influencing medication adherence.7 Such feedback moments do not only involve a discussion about specific medication intake patterns, but also include elements of education and motivation.7 Therefore, monitoring and managing medication adherence should become a standard practice for patients with different types of medication, especially for those struggling with a correct intake of chronic medications. Subject to a financial compensation, electronic monitoring of medication adherence could be part of community pharmacists’ remit. However, the most important challenge may remain to target the patients that will benefit most from this kind of follow-up.
Strengths and Weaknesses
In our study, electronic monitoring of DOAC adherence was used in a primary care setting. Adherence to DOAC therapy is a key area where there is still much to be gained. DOACs can be considered high risk medication due to their low drug forgiveness, therefore, follow up of their correct intake is of major importance.
For several reasons, no firm conclusions could be drawn about the direct impact of the electronic monitoring on DOAC adherence. First, from the start of the study (with baseline electronic measurement after six weeks), adherence among the included patients was already high compared to other studies,6–9 but comparable to another Belgian study using electronic monitoring.33 The high baseline adherence rates may be partly explained by the Hawthorne effect, which makes patients observed in a study setting behave better.37 Additionally, approximately one out of two invited patients (56.3%) agreed to participate in this study and we have no information on the adherence rates of patients who refused to participate. Two-thirds of patients mentioned that the most important reason to participate was out of sympathy for their community pharmacist. The existing good relationship with the pharmacist may have been a source of bias that contributed to the observed high adherence rate, as this may have mainly led to the inclusion of patients with a high baseline adherence. Moreover, opening a package does not always mean that the medication is actually taken.28 Despite the high adherence rate in this study population, there is still room for improvement, especially with respect to timing adherence.
Second, there was probably also a bias in pharmacist participation, as community pharmacists voluntarily participated in this study, presumably attracting mainly the highly motivated. These pharmacists may already provide excellent pharmaceutical care in daily practice with due consideration for patient adherence.
Third, the intervention consisted of two parts, namely the electronic monitoring and the intervention by the pharmacist. Because of this dual intervention, no conclusions could be drawn about the effect of either alone.
Moreover, as the questionnaires were not reviewed by a patient population, there may have been difficulties in interpreting the questions. However, we have no evidence in the form of unanswered or additional questions that patients experienced interpretation difficulties.
Implementation Barriers
Two main barriers to practical implementation of measuring DOAC adherence using the electronic device were mentioned. First, many patients were polymedicated. Adherence to DOAC was measured using a smart bottle cap, which implied storing the DOAC in a specific, separate medication bottle. Adding an extra device for a single medication is not desirable. Some patients refused participation for this reason. Although we had no information on the adherence of polymedicated patients specifically, it is possible that this subgroup of patients could benefit most from adherence monitoring facilitated by a healthcare provider. To overcome this limitation, another type of electronic monitor could be used for polymedicated patients, for instance a smart button that can be stuck on a pill organizer. However, interpretation of data on polymedicated patients will be difficult, because there may be different dosing regimens and different times of intake. Therefore, it is of major importance to select the most eligible patient population that would benefit most from electronic monitoring. Possible patient profiles could be patients with suspected non-intentional non-adherence, patients starting a therapy (to create habits) or patients on medication with low drug forgiveness such as DOACs. Additionally, it can be of interest to monitor adherence before changing therapy in case of treatment failure.
A second barrier was the cost of the device. Patients agreed that the government should reimburse this device or its use, although some patients were also willing to partially pay for such a service themselves. Additionally, time management can be a challenge, providing another reason to select a high risk population and to implement electronic monitoring in these patients to prevent pharmacotherapeutic escalation and hospitalizations.
Further Research
An interesting possibility for further research could be to focus on the use of a smart button stuck on a medication box in polymedicated patients. With this button (instead of the bottle cap used in this study), adherence to all medications a patient takes could be tracked. DOAC patients are often polymedicated and considering the importance of other medication classes in these patients, an overall view of their medication intake behavior can provide important information about their (cardiovascular) risk. In addition, factors contributing to poor medication adherence can be investigated and the impact of the electronic device or button in these specific patients can be studied.
Conclusion
In this study, high DOAC adherence rates were observed, but more than half of the patients took at least one DOAC dose too late or skipped at least one dose during the 12-week study period. This can potentially result in suboptimal protection against thromboembolic events. Consequently, there is still room for improvement regarding the correct intake and timing adherence of DOACs. Positive experiences with an electronic device for medication adherence follow-up were reported. Moreover, this study found that patients appreciated the support of healthcare providers in monitoring their adherence.
Acknowledgments
We would like to thank all participating patients and pharmacists for their participation in this study, Prof. Bernard Vrijens for training the pharmacists on interpretation of MEMS® data and Prof. Lies Leemans for training the pharmacists on motivational communication skills.
Funding Statement
This work was supported by Provincie Antwerpen (Belgium) – Innovation in Care.
Abbreviations
BID, twice-daily dosed; DOAC, direct oral anticoagulant; EHRA, European Heart Rhythm Association; ESPACOMP, International Society for Medication Adherence; GP, general practitioner; IQR, interquartile range; KAVA, Koninklijke Apothekersvereniging van Antwerpen; MEMS®, Medication Event Monitoring System; QD, once-daily dosed; WHO, World Health Organization.
Data Sharing Statement
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of UZ Brussel (2020/346).
Informed consent was obtained from all individual participants included in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Antoine Pironet reports employment relationship with AARDEX Group. The other authors have no relevant financial or non-financial interests to disclose for this work.
References
- 1.Damen NL, Van den Bemt BJF, Hersberger KE, et al. Creating an interprofessional guideline to support patients receiving oral anticoagulation therapy: a Delphi exercise. Int J Clin Pharm. 2019;41(4):1012–1020. doi: 10.1007/s11096-019-00844-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–1676. doi: 10.1093/europace/euab065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toorop MMA, van Rein N, Nierman MC, et al. Self-reported therapy adherence and predictors for nonadherence in patients who switched from vitamin K antagonists to direct oral anticoagulants. Res Pract Thromb Haemost. 2020;4(4):586–593. doi: 10.1002/rth2.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruff C, Koukalova L, Haefeli WE, Meid AD. The role of adherence thresholds for development and performance aspects of a prediction model for direct oral anticoagulation adherence. Front Pharmacol. 2019;10:113. doi: 10.3389/fphar.2019.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wirbka L, Haefeli WE, Meid AD. Estimated thresholds of minimum necessary adherence for effective treatment with direct oral anticoagulants—a retrospective cohort study in health insurance claims data. Patient Prefer Adherence. 2021;15:2209–2220. doi: 10.2147/PPA.S324315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozaki AF, Choi AS, Le QT, et al. Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2020;13(3):e005969. doi: 10.1161/CIRCOUTCOMES.119.005969 [DOI] [PubMed] [Google Scholar]
- 7.Vrijens B, Heidbuchel H. Non-vitamin K antagonist oral anticoagulants: considerations on once- vs. twice-daily regimens and their potential impact on medication adherence. Europace. 2015;17(4):514–523. doi: 10.1093/europace/euu311 [DOI] [PubMed] [Google Scholar]
- 8.Beyer-Westendorf J, Ehlken B, Evers T. Real-world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace. 2016;18(8):1150–1157. doi: 10.1093/europace/euv421 [DOI] [PubMed] [Google Scholar]
- 9.Obamiro KO, Chalmers L, Bereznicki LR. A summary of the literature evaluating adherence and persistence with oral anticoagulants in atrial fibrillation. Am J Cardiovasc Drugs. 2016;16(5):349–363. doi: 10.1007/s40256-016-0171-6 [DOI] [PubMed] [Google Scholar]
- 10.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raparelli V, Proietti M, Cangemi R, Lip GY, Lane DA, Basili S. Adherence to oral anticoagulant therapy in patients with atrial fibrillation. Focus on non-vitamin K antagonist oral anticoagulants. Thromb Haemost. 2017;117(2):209–218. doi: 10.1160/TH16-10-0757 [DOI] [PubMed] [Google Scholar]
- 12.Moudallel S, van den Bemt BJF, Zwikker H, et al. Association of conflicting information from healthcare providers and poor shared decision making with suboptimal adherence in direct oral anticoagulant treatment: a cross-sectional study in patients with atrial fibrillation. Patient Educ Couns. 2021;104(1):155–162. doi: 10.1016/j.pec.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 13.Grymonprez M, Capiau A, Steurbaut S, et al. Adherence and persistence to oral anticoagulants in patients with atrial fibrillation: a Belgian nationwide cohort study. Front Cardiovasc Med. 2022;9:994085. doi: 10.3389/fcvm.2022.994085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capiau A, Mehuys E, Van tongelen I, et al. Community pharmacy-based study of adherence to non-vitamin K antagonist oral anticoagulants. Heart. 2020;106(22):1740–1746. doi: 10.1136/heartjnl-2020-316781 [DOI] [PubMed] [Google Scholar]
- 15.Shiga T, Kimura T, Fukushima N, et al. Electronic monitoring of adherence to once-daily and twice-daily direct oral anticoagulants in patients with atrial fibrillation: baseline data from the SMAAP-AF trial. J Arrhythm. 2021;37(3):616–625. doi: 10.1002/joa3.12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moudallel S, van Laere S, Cornu P, Dupont A, Steurbaut S. Assessment of adherence, treatment satisfaction and knowledge of direct oral anticoagulants in atrial fibrillation patients. Br J Clin Pharmacol. 2022;88(5):2419–2429. doi: 10.1111/bcp.15180 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. [Google Scholar]
- 18.Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117–122. doi: 10.15386/mpr-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Escamilla B, Franco-Trigo L, Moullin JC, Martínez-Martínez F, García-Corpas JP. Identification of validated questionnaires to measure adherence to pharmacological antihypertensive treatments. Patient Prefer Adherence. 2015;9:569–578. doi: 10.2147/PPA.S76139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 21.Chiu PE, Tsao HM, Tsai CH. Discrepancy among self-reported adherence, prescription refills, and actual anticoagulant control. J Nurs Res. 2020;28(1):e63. doi: 10.1097/jnr.0000000000000374 [DOI] [PubMed] [Google Scholar]
- 22.Chan AHY, Horne R, Hankins M, Chisari C. The medication adherence report scale: a measurement tool for eliciting patients’ reports of nonadherence. Br J Clin Pharmacol. 2020;86(7):1281–1288. doi: 10.1111/bcp.14193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011;11:149. doi: 10.1186/1471-2288-11-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vrijens B, Goetghebeur E. Comparing compliance patterns between randomized treatments. Control Clin Trials. 1997;18(3):187–203. doi: 10.1016/S0197-2456(96)00235-8 [DOI] [PubMed] [Google Scholar]
- 25.El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82(1):268–279. doi: 10.1111/bcp.12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeller A, Schroeder K, Peters TJ. Electronic pillboxes (MEMS) to assess the relationship between medication adherence and blood pressure control in primary care. Scand J Prim Health Care. 2007;25(4):202–207. doi: 10.1080/02813430701651954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartman L, Lems WF, Boers M. Outcome measures for adherence data from a medication event monitoring system: a literature review. J Clin Pharm Ther. 2019;44(1):1–5. doi: 10.1111/jcpt.12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dima AL, Allemann SS, Dunbar-Jacob J, Hughes DA, Vrijens B, Wilson IB. Methodological considerations on estimating medication adherence from self-report, electronic monitoring and electronic healthcare databases using the TEOS framework. Br J Clin Pharmacol. 2023;89:1918–1927. doi: 10.1111/bcp.15375 [DOI] [PubMed] [Google Scholar]
- 29.Shiga T, Kimura T, Fukushima N, et al. Effects of a pharmacist-led educational interventional program on electronic monitoring–assessed adherence to direct oral anticoagulants: a randomized, controlled trial in patients with nonvalvular atrial fibrillation. Clin Ther. 2022;44(11):1494–1505. doi: 10.1016/j.clinthera.2022.09.011 [DOI] [PubMed] [Google Scholar]
- 30.Robertson SG, Glass BD. Stability of repackaged dabigatran etexilate capsules in dose administration aids. Eur J Hosp Pharm. 2018;25(e2):e93–e97. doi: 10.1136/ejhpharm-2017-001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demonceau J, Ruppar T, Kristanto P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73(6):545–562. doi: 10.1007/s40265-013-0041-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new medication adherence rating scale (Mars) for the psychoses. Schizophr Res. 2000;42(3):241–247. doi: 10.1016/S0920-9964(99)00130-9 [DOI] [PubMed] [Google Scholar]
- 33.Knaepen L, Delesie M, Vijgen J, et al. Adherence to oral anticoagulation measured by electronic monitoring in a Belgian atrial fibrillation population. Clin Res Cardiol. 2023;112(12):1812–1823. doi: 10.1007/s00392-023-02261-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patton K, Meyers J, Lewis BE. Enhancement of compliance among patients with hypertension. Am J Manag Care. 1997;3(11):1693–1698. [PubMed] [Google Scholar]
- 35.Ran MS, Xiang MZ, Chan CL, et al. Effectiveness of psychoeducational intervention for rural Chinese families experiencing schizophrenia--a randomised controlled trial. Soc Psychiatry Psychiatr Epidemiol. 2003;38(2):69–75. doi: 10.1007/s00127-003-0601-z [DOI] [PubMed] [Google Scholar]
- 36.Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002;2:CD000011. [DOI] [PubMed] [Google Scholar]
- 37.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.