Abstract
Background
The discovery of biomarkers in chronic subdural haematomas (CSDH) suggests that inflammation is part of CSDH pathophysiology. It is unknown whether inflammation reflects an independent CSDH driver or haematoma degeneration. This knowledge holds a potential target for anti-inflammatory treatment in patients at risk of CSDH. This study investigated the association of pro- and anti-inflammatory factors with CSDH development.
Methods
This cohort study included all individuals in Denmark over 50 years between 2007–2018. The outcome was first-time CSDH surgery. Hazard ratios (HR) according to potential risk factors were estimated using Cox regression, with age as underlying time scale.
Results
Among the 2,391,853 individuals, head trauma was registered in 427,612 individuals (17%), and among these, only 812 were operated for CSDH (0.18%). Among individuals without registered head trauma, the pro-inflammatory conditions of alcohol addiction, diabetes, anti-hypertensive treatment, and chronic hepatic disease were significantly associated with CSDH among individuals (50–74 years). The use of glucocorticoids displayed a decreased risk in cohort members aged 75 and older. Non-steroid anti-inflammatory drugs and statins appeared to be risk factors for CSDH in individuals between the ages of 50–64 and 65–74.
Conclusion
Although head trauma was a risk factor for CSDH, the absolute risk was low (0.18%), which does not support preventive measures after emergency room contacts for head trauma. Interestingly, pro-and anti-inflammatory factors were significantly associated with CSDH in patients without registered head trauma, and the pronounced age-dependency of the associations suggests that the pathophysiological mechanisms vary with age.
Keywords: head trauma, inflammation, trauma, anti-inflammatory treatment, risk factors
Background
The incidence of chronic subdural haematoma (CSDH) patients has increased significantly due to a continuing rise in life expectancy and an increasing use of anticoagulative medicine.1 The understanding of CSDH aetiology is undergoing a paradigm shift. Historically, CSDH was considered solely traumatic, formed by torn bridging veins causing subdural blood accumulation and degradation, and subsequently an osmotically driven enlargement.2 However, the often-seen long timespan from head trauma to CSDH development, as well as the lack of recollections of previous head trauma among many of the patients, call into question head trauma as the sole aetiological factor.3 A CSDH becomes symptomatic weeks to months after a head trauma, but venous bleeding would most likely accumulate quickly, causing symptoms within days.4 Easy access to computed tomography enabled early scans of head trauma patients, and several examples of haematoma absence in patients who later developed CSDH exist.5
Studies on the molecular composition of subdural fluid have demonstrated inflammatory biomarkers in both primary and recurrent haematomas, which suggest subdural inflammation somewhere along the CSDH formation.6,7 However, it remains to be established whether this inflammation reflects a physiological response to a degrading blood clot originating from trauma, or if inflammation is a generic driver of CSDH. These findings are not only interesting in the context of CSDH aetiology but also in the debated role of glucocorticoids in CSDH treatment. Hutchinson et al investigated the effect of glucocorticoids on the modified Rankin scale score after six months as the primary outcome, as well as the effect on CSDH recurrence as a secondary outcome, in the setting of a multicentre randomised clinical trial (RCT).8 Although some reduction in recurrences was found (5.4%, unadjusted), glucocorticoids negatively affected the functional outcome due to adverse effects, and they were therefore deemed unsuitable for treatment.9
The access to extensive register-based information on hospital diagnoses and prescribed medicine enabled us to investigate the associations between pro-inflammatory conditions, anti-inflammatory medications and CSDH development in a large, unselected, nationwide cohort of individuals with and without head trauma exposure.
Materials and Methods
Data Sources
Patient information was extracted from the Danish Civil Registration System, the National Patient Discharge Register and the Danish National Prescription Register. Each individual living in Denmark is assigned a personal identification number by the Danish Civil Registration System.10 This number allows cross-linkage between all Danish registers and is continuously updated with information on vital status and emigration.
The National Patient Discharge Register contains information on all hospital admittances with diagnoses and operations recorded according to the International Classification of Diseases (ICD) system.11 The Danish National Prescription Register records individual-level information on all prescribed medicines dispensed at community pharmacies in Denmark. The register contains drug information that includes product name, anatomical therapeutic chemical classification (ATC) code, date of dispensing, number of doses, package size, strength and several other factors.12 Denmark has a readily accessible, free healthcare system, and reporting to the national registers is mandatory and automated in Denmark, no private treatment options are available for CSDH patients.
Study Cohort
The cohort covered all individuals in Denmark aged 50 years and older between 2007 and 2017. The outcome was first-time surgery for CSDH during the period 2007–2018 to ensure one-year follow-up. Cohort members who underwent first-time surgery for CSDH were restricted by the distinctive procedure code for CSDH: KAAD10. Individuals were followed in the study until death, emigration, surgery for CSDH, or the end of data, whichever came first.
Exclusion criteria: Patients with intracranial tumours, arteriovenous malformations or spontaneous intracranial haemorrhage within 1 year of CSDH operation. The latter is due to possible misclassification since the occurrence of simultaneous spontaneous intracerebral haemorrhage and CSDH seems unlikely.
Because head trauma is an established aetiological factor for CSDH development, the cohort members were given a time-varying head trauma status dependent on being exposed a year after the registration of a head trauma. When considering the association between inflammatory factors and CSDH, we focused on the results of cohort members without registered head trauma. Similar results were also given for cohort members with registered head trauma (Supplementary Figures 1–3 and Supplementary Table 3). Head trauma diagnoses registered within 7 days of each other were considered the same diagnosis, and, in these circumstances, the date of the first head trauma was registered as the start of exposure. If a new head trauma occurred within the year after registration of the first head trauma, the individual’s exposure was extended by a year from the date of the new head trauma.
Potential Risk Factors Analysed in CSDH Development
The associations between factors with pro-and anti-inflammatory effects and CSDH were investigated in the primary analyses. Information on surgical treatment for CSDH and diagnoses of pro-inflammatory conditions were retrieved from the National Patient Discharge Register.11 The pro-inflammatory conditions diabetes, hypertension and alcohol addiction were identified by the ATC codes for prescribed anti-diabetic and antihypertensive medicine as well as oral disulfiram from the National Prescription Register.12 See Supplementary Table 1 for ICD-10 and ATC codes and Supplementary Table 2 for medical ATC codes, including information on package size and washout period.
Pro-Inflammatory Factors
Factors with a potentially pro-inflammatory effect included hypertension, diabetes mellitus, alcohol addiction, liver cirrhosis, renal insufficiency, and autoimmune disorders, including autoimmune hepatitis, thyroiditis, haemolytic anaemia, pancreatitis, adrenalitis and polyglandular insufficiency. Chronic hypertension entails inflammatory changes in the arterial vessels, ultimately leading to endothelial damage and vascular stiffness.13 Type 1 and type 2 diabetes mellitus,14 liver cirrhosis15 and renal insufficiency16 display increased tissue levels of inflammatory markers. Autoimmune disorders have dysregulated inflammation as a fundamental pathological mechanism.17 Alcohol addiction is a well-known risk factor for trauma in states of intoxication but has a continuous deregulatory immunological effect and was therefore considered a pro-inflammatory risk factor in this study.18
Anti-Inflammatory Factors
Similar to non-steroid anti-inflammatory drugs (NSAIDs)19 and glucocorticoids,20 lipid-modifying drugs (statins) have anti-inflammatory properties.21 According to a previous study, statins protect against recurrent CSDH following primary surgery.22 Altogether, these medications were investigated as potential protective factors against CSDH development.
Conditions Predisposing for Head Trauma, Intracranial Bleedings, and Anticoagulative Medicine
It is well known that even minor head traumas may initialize CSDH development but do not necessarily lead to a hospital contact and a registered head trauma diagnosis. Therefore, several other conditions considered predisposing for head trauma were investigated as potential risk factors. These included cerebral infarction, anticoagulative treatment, disposing neurological disorders (eg, Parkinson’s disease, degenerative central and peripheral neurological diseases, epilepsy, neuropathy), and other conditions predisposing for head trauma (for instance muscle spasms, walking impairments, sensory disturbances). Anticoagulative treatment is a well-known risk factor for CSDH because it causes a lower threshold for bleeding, particularly in relation to a head trauma.23 NSAIDs have an antithrombotic side effect, apart from being an anti-inflammatory agent, and may therefore enhance bleeding risk in relation to head trauma. Additionally, we included acute subdural haematoma (ASDH) and traumatic intracranial haemorrhage as potential risk factors for CSDH development, as ASDH in particular has demonstrated the capability to aggravate into chronic bleeding.24
Statistical Analysis
Prescribed medicines were considered to be either chronic or transient exposure. For patients with transient exposure, the washout period varied according to the type of disease and treatment. The Supplementary Methods section presents a detailed description of chronic and time-dependent medicine.
The head trauma variable was defined as time-varying with a 1-year exposure period that was extended when the individual received another head trauma diagnosis. The one-year cumulative incidence of CSDH among individuals with registered head trauma was estimated using the Aalen-Johansen estimator.
The risks of CSDH in exposed individuals (versus non-exposed), according to potential risk and protective factors, were estimated as hazard ratios (HRs) with 95% confidence intervals (95% CI). This was done using a Cox proportional hazard model with age as the underlying time scale, according to exposure to head trauma, and stratified by sex and calendar year (2007–2009, 2010–2013, 2014–2018).
Ethics
The study was approved by the Danish Data Protection Agency. According with §38, paragraph 8 of the Danish Protection Act, approval from the scientific ethics committee was not required, as the study was purely register-based.
Results
Study Population
Baseline characteristics for the full cohort are presented in Table 1. The full cohort consisted of 2,391,853 Danish individuals over the age of 50 between 2007 and 2018. Of these, 4086 individuals were operated on for a first-time CSDH. Within individuals of the full cohort, at least one head trauma was registered in 427,612 individuals (17%), and among these, only 812 were operated on for CSDH (0.18%). Due to these low numbers, the associations between pro-inflammatory conditions, anti-inflammatory medications and CSDH in patients with registered head trauma had limited statistical power but are presented in Supplementary Figures 1 and 2. As the baseline risk of CSDH is highly age-dependent, with increasing risk with older age, the risk was estimated in predefined age intervals. Full datasets for all risk factors divided into age groups are presented in Supplementary Table 3. The risk of developing CSDH in individuals without registered head trauma, according to the potential pro- and anti-inflammatory risk factors, is presented below.
Table 1.
Baseline Characteristics for the Full Cohort and for Patients Within the Full Cohort with at Least One Head Trauma
| Characteristics | Full Cohort N (%) | Patients with Head Trauma N (%) |
|---|---|---|
| Total | 2391853 | 427,612 |
| SDH evacuations | 4086 (0.17) | 812 (0.18) |
| Age at start of study (years) | ||
| 50–65 | 1,618,025 (67.6%) | 235,076 (55.0%) |
| 65–74 | 414,916 (17.3%) | 86,893 (20.3%) |
| 74–84 | 258,666 (10.8%) | 74,633 (17.5%) |
| 85+ | 100,246 (4.2%) | 31,010 (7.3%) |
| Sex | ||
| Female | 1252264 (52.4%) | 243,613 (57.0%) |
| Male | 1139589 (47.6%) | 183,999 (43.0%) |
| Comorbidities and co-medications | ||
| Alcohol addiction | 113110 (4.7%) | 38,311 (9.0%) |
| Chronic hepatic disease | 45526 (1.9%) | 12,862 (3.0%) |
| Renal insufficiency | 92521 (3.9%) | 23,116 (5.4%) |
| Anticoagulant treatment | 845407 (35.3%) | 197,122 (46.1%) |
| Diabetes mellitus | 279584 (11.7%) | 56,120 (13.1%) |
| Previous cerebral infarction | 104973 (4.4%) | 27,836 (6.5%) |
| Antihypertensive treatment | 1463967 (61.2%) | 303,087 (70.9%) |
| Conditions predisposing for head trauma | 11170 (0.5%) | 6539 (1.5%) |
| Disposing neurological disorders | 192231 (8.0%) | 65,553 (15.3%) |
| Statins | 452734 (18.9%) | 110,141 (25.8%) |
| Autoimmune disorders | 879263 (36.8%) | 177,485 (41.5%) |
| Glucocorticoids | 7507 (0.3%) | 1490 (0.3%) |
| NSAIDs* | 256,309 (10.7%) | 53,921 (12.6%) |
Abbreviations: CSDH, Chronic subdural haematoma; * NSAIDs, Non-steroid anti-inflammatory drugs.
Pro-Inflammatory Conditions
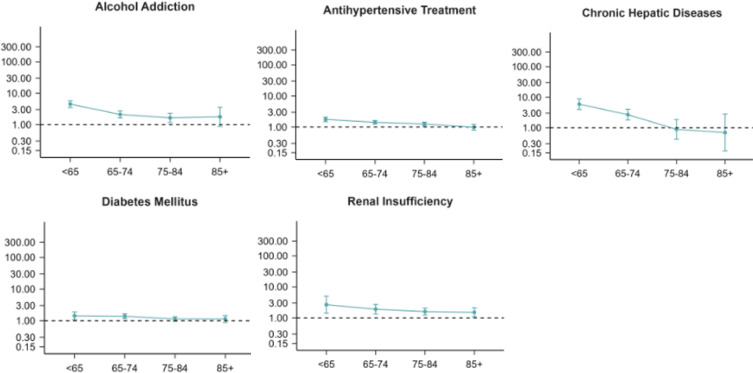
Among individuals without registered head trauma, the risk of developing a CSDH, according to the potential pro-inflammatory risk factors, according to age, is presented in Figure 1. A higher risk of CSDH was found in individuals with alcohol addiction compared to individuals without alcohol addiction for the age groups 50–64 years (HR 3.33, 95% CI 2.10–5.28), 65–74 years (HR 2.35, 95% CI 1.79–3.10) and 75–84 years (HR 1.59, 95% CI 1.24–2.03). We found a higher risk of CSDH among individuals with diabetes mellitus compared to individuals without diabetes mellitus in the age groups 50–64 years (HR 1.42, 95% CI 1.08–1.88) and 65–74 years (HR 1.38, 95% CI 1.17–1.64). The risk of CSDH among individuals with hypertension was higher compared to individuals without hypertension for age groups 50–64 years (HR 1.77, 95% CI 1.49–2.09), 65–74 years (HR 1.42, 95% CI 1.25–1.63) and 75–84 years (HR 1.26, 95% CI 1.09–1.45). Also, a higher risk of CSDH was found for individuals with chronic hepatic disease compared to individuals without chronic hepatic disease for age groups 50–65 years (HR 5.91, 95% CI 3.95–8.86) and 65–74 years (HR 2.68, 95% CI 1.78–4.02). The risk of CSDH in individuals with renal insufficiency was higher compared to individuals without renal insufficiency throughout all age groups: 50–64 years (HR 2.66, 95% CI 1.42–4.99), 65–74 years (HR 1.90, 95% CI 1.33–2.72), 75–84 years (HR 1.59, 95% CI 1.22–2.07) and 85 + years (HR 1.51, 95% CI 1.09–2.11).
Figure 1.
Hazard ratios (HR) with 95% confidence intervals displaying the risk of CSDH when comparing individuals exposed to pro-inflammatory conditions to unexposed individuals, according to age (years) for individuals without registered head trauma.
Anti-Inflammatory Medicine
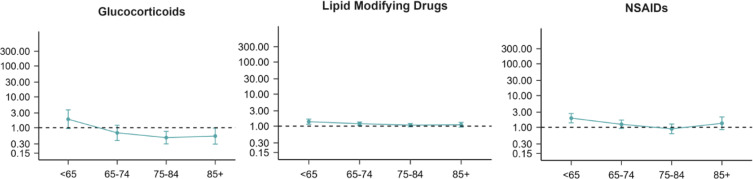
Among individuals without registered head trauma, the risk of developing a CSDH when comparing users to non-users of anti-inflammatory medicine, according to age, is presented in Figure 2. We observed a decreased risk of CSDH among users of glucocorticoids in the 75–84 years age group (HR 0.48, 95% 0.30–0.76) and the +85 years age group (HR 0.54, 95% CI 0.30–0.98). We found a higher risk of CSDH among individuals with statin intake compared to non-users for age groups 50–64 years (HR 1.38, 95% CI 1.15–1.66) and 65–74 years (HR 1.19, 95% CI 1.05–1.35). In line with this finding, higher risks of CSDH were seen for individuals with NSAID intake compared to non-users, but only for age group 50–64 years (HR 1.94, 95% CI 1.39–2.73).
Figure 2.
Hazard ratios (HR) with 95% confidence intervals displaying the risk of CSDH when comparing individuals exposed to pro-inflammatory conditions to unexposed individuals, according to age (years) for individuals without registered head trauma.
CSDH Risk Following Head Trauma
Although the absolute risk of CSDH after a registered head trauma per above was very low (0.18%), head trauma was still a highly significant risk factor for CSDH development when stratifying the analyses for age (ie, comparing the risk of CSDH in cohort members in a certain age group with unexposed individuals in the same age group). The risk was highly age-dependent, with higher risks in the younger part of the cohort: HR 50–64 years: 16.11, 95% CI 13.48–19.25; HR 65–74 years: 12.57, 95% CI 10.81–14.61; HR 75–84 years: 9.01, 95% CI 7.85–10.34; and HR +85 years: 5.56, 95% CI 4.65–6.64.
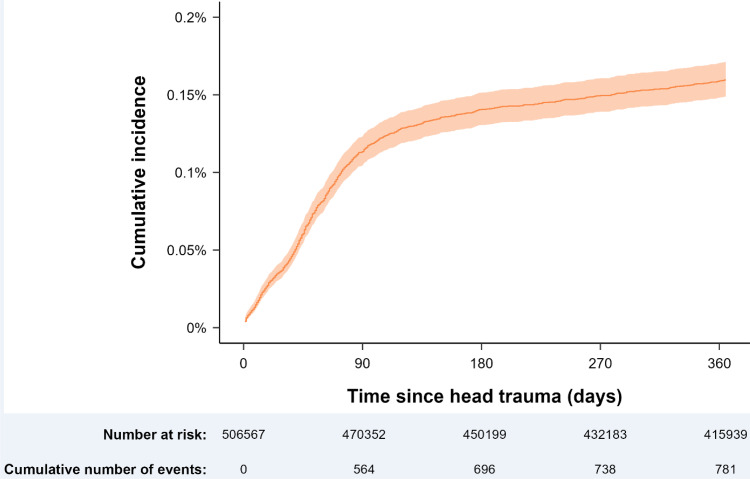
The cumulative 1-year incidence of CSDH following a registered head trauma is presented in Figure 3. The incidence of CSDH was temporally related to head trauma, with most cases occurring within 90 days after the registered trauma, after which the cumulative incidence displayed little increase (Figure 3, Supplementary Table 4).
Figure 3.
The cumulative incidence of CSDH operations in relation to time since registered head trauma. A rapid increase in incidences is observed the first 90 days following a registered head trauma and afterwards a flattened but continuous increase is seen throughout one-year follow-up. The x-axis is capped at a year for aesthetic purposes.
Sub-Analyses
Due to the previously mentioned assumption of unregistered head traumas, we included conditions predisposing for head traumas as potential risk factor as well as anticoagulative medications, ASDH and traumatic intracranial haemorrhage. The risks of CSDH, according to these proxy measures, are presented in Supplementary Figure 3 for cohort members with and Supplementary Figure 4 for cohort members without registered head trauma. All these factors were associated with CSDH, supporting the notion of undetected head trauma.
Discussion
This unselected, national cohort study encompassing all individuals in Denmark over the age of 50 was conducted to investigate the associations of pro- and anti-inflammatory factors and the development of CSDH in patients without registered head trauma. The pro-inflammatory conditions of alcohol addiction, hypertension, diabetes and chronic hepatic disease showed an increased risk of CSDH, especially among the younger individuals (50–74 years). NSAIDs and statins appeared to be risk factors for CSDH development, not protective factors as we hypothesized, but only among individuals between the ages of 50–64 years and 50–74 years. Interestingly, the use of glucocorticoids was associated with a significantly decreased risk among cohort members aged 75+.
Inflammation as a Risk Factor for CSDH
An ongoing focus point in CSDH pathophysiology is the role of inflammation.25 Inflammatory biomarkers have been identified in the subdural fluid, and anti-inflammatory treatment provided at the time of the primary evacuation as effective in reducing CSDH recurrence.6,8 These findings have shifted the pathophysiological understanding of CSDH from being solely traumatic to a perception of being inflammatory.25 However, coagulation and blood degeneration also augment inflammation by thrombin-induced secretion of pro-inflammatory cytokines and growth factors, including the activation of dendritic cells by platelets.26 Therefore, subdural inflammatory biomarkers would indeed be expected, but are not necessarily causal of CSDH.
In this study, we were able to investigate inflammatory risk factors early in the pathophysiological timeline, namely before development of the primary CSDH. All the investigated pro-inflammatory factors show a significant effect on CSDH risk, at least in younger age groups, which indeed supports that inflammation plays a part in CSDH development.25 Thus, all inflammatory risk factors had a greater impact on CSDH risk in patients younger than 75 years. It is well known that the inflammatory immune response becomes increasingly dysregulated with higher age, including both anti-and pro-inflammatory pathways serving as a central mechanism in many age-related diseases.27 As pro-inflammatory factors had less effect on CSDH development in the older age groups, this correlates well with a higher immunological and inflammatory response in the younger age groups.27
Anti-Inflammatory Medicine and the Risk of CSDH
As anti-inflammatory treatment applied at the time of the primary operation reduces the risk of a recurrent CSDH,8 it would be prudent to consider a prophylactic effect with an intake of anti-inflammatory medicine at the time of a registered head trauma. However, the statistical power to detect such associations in this subgroup was insufficient due to the low number of individuals who developed CSDH among this relatively low proportion (18%) of the cohort members with registered head trauma. Nevertheless, the finding of a protective effect of glucocorticoids in patients older than 75 years without registered head trauma may justify a clinical trial targeted at this age group investigating whether glucocorticoids prevent recurrence after a first surgery for CSDH. In the study by Hutchinson et al a starting dose of 8 mg dexamethasone was administered twice daily during 2 weeks to an unselected cohort of CSDH patient’s and the study reported a considerable risk of adverse events affecting the 6-month functional outcome on the modified ranking scale negatively in a large RCT, whereas the results suggest a somewhat smaller reduction (5.4%) in recurrences. The authors suggest that a future randomised study on CSDH patients older than 75 years use a smaller dosage and shorter duration of treatment.
Head Trauma Patients and the Risk of CSDH
A rather small proportion of patients with registered head trauma developed a CSDH (0.18%), yielding little evidence for prophylactic measures in patients with emergency room contacts for head trauma. Risk factors for CSDH in patients with head trauma have been investigated in several studies.28,29 These studies are based on radiological findings at the time of head trauma and clinical variables such as age, head trauma and co-morbidity, and can thus be compared to our sub-analysis of conditions predisposing for head traumas. Similar to our results, these studies found head trauma to be a significant risk factor for CSDH development, thereby confirming that regardless of our primary results demonstrating a pro-inflammatory effect on CSDH risk, the pathophysiological is not solely inflammatory, either.28 A possible explanation could be a pathophysiological timeline initiated with head trauma and, at some point, the launch of an inflammatory cascade. This would be in line with a previous study that found the formation of membranes within a CSDH a time-dependent process, indicating that a CSDH changes over time with fluctuating involvement of inflammatory factors.5
For all conditions predisposing for head traumas, similar to the pro-inflammatory factors, a profound impact of the risk factors was observed in younger individuals. This is interesting, especially considering that age-related cortical atrophy stretches the bridging veins, increasing the risk of rupture following head trauma.30 However, a likely explanation may be that CSDH in older patients is caused by less severe head trauma than in younger patients. In this study, only head trauma patients with head trauma registered in an emergency department were recorded. As the traumatic threshold for CSDH in elderly patients may be lower, a minor trauma may not lead to health care contact. This would result in fewer registered head traumas in the older age groups, deceptively reducing the effect of head traumas on CSDH development in the elderly. A study of CSDH risk among patients with minor head trauma without health care contact could be of interest yet challenging to perform.
Limitations and Strengths
An important limitation is the set of factors analysed in this study. The factors were selected from a clinical perspective to amplify the potential clinical relevance. Nevertheless, it is likely that other factors may have relevance in CSDH pathophysiology, including the composition of especially inflammatory biomarkers in the haematoma fluid of CSDH patients.6 This part of CSDH pathophysiology is not explored in this study. This limitation also highlights the possibility of residual confounders. Several of the investigated factors in this study may lead to cerebral atrophy, which is a known risk factor for CSDH.31 Assessment of cerebral atrophy is not possible with our study design, but are in our opinion one central residual confounder in this study.32
The reported findings regarding the systemic use of anti-inflammatory medicine inherently hold the bias of being treatment for pro-inflammatory autoimmune conditions such as rheumatoid arthritis, inflammatory bowel disease, eczema, and multiple sclerosis, diagnoses that are not necessarily registered in the hospital system. As presented in Table 1, only 0.3% of the full cohort had a registered diagnosis of an autoimmune disease (also 0.3% among cohort members with registered head trauma). Thus, the numbers were insufficient to conduct meaningful analyses of the association between autoimmune diseases and the risk of CSDH (Supplementary Table 3).
To avoid the influence of confounders such as hypertension, diabetes mellitus and renal insufficiency in cohort members treated with steroids and statins, it could be of clinical interest to further investigate interactions between these risk factors in the sub cohort using steroids or statins. However, a further subdivision of the cohort would result in insufficient numbers for the analyses. In Denmark, the free and accessible health care system provides a unique scientific platform for establishing a study cohort from an unselected population. This minimizes selection bias and provides a wide range of close-to-complete information on CSDH patients. Furthermore, this enables studies of risk factors involved in the pathophysiological timeline leading up to a CSDH operation, which hitherto was only sparsely explored. To our knowledge, this is the first unselected national cohort study of inflammatory and trauma-related risk factors for CSDH development.
Conclusion
Although head trauma was a highly significant risk factor for CSDH and was temporally associated with head trauma, the absolute risk was very low (0.18%). The low absolute risk does not support early diagnostic or preventive measures after emergency room contact for head trauma. Interestingly, pro-and anti-inflammatory factors were significantly associated with CSDH in patients without registered head trauma and the pronounced age-dependency of the associations suggests that the pathophysiological mechanisms vary with age.
Funding Statement
This study was funded by A. P. Moller Foundation (20-L-0165), Aase and Ejnar Danielsen Foundation (20-10-0202), Becket Foundation (20-2-6190) and Rigshospitalets Research Foundation.
Data-Sharing Statements
The datasets generated and/or analysed during the study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no competing interests in this work.
References
- 1.Gaist D. et al. Association of Antithrombotic Drug Use With Subdural Hematoma Risk. JAMA. 2017;317(8):836–846. [DOI] [PubMed] [Google Scholar]
- 2.Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54(5):637–645. doi: 10.3171/jns.1981.54.5.0637 [DOI] [PubMed] [Google Scholar]
- 3.Jensen TSR, et al. National randomized clinical trial on subdural drainage time after chronic subdural hematoma evacuation. J Neurosurg. 2021;137:1–8. [DOI] [PubMed] [Google Scholar]
- 4.Stroobandt G, Fransen P, Thauvoy C, et al. Pathogenetic factors in chronic subdural haematoma and causes of recurrence after drainage. Acta Neurochir. 1995;137(1–2):6–14. doi: 10.1007/BF02188772 [DOI] [PubMed] [Google Scholar]
- 5.Jensen TSR, Andersen-Ranberg N, Poulsen FR, et al. The Danish Chronic Subdural Hematoma Study-comparison of hematoma age to the radiological appearance at time of diagnosis. Acta Neurochir. 2020;162(9):2007–2013. doi: 10.1007/s00701-020-04472-w [DOI] [PubMed] [Google Scholar]
- 6.Stanisic M, Aasen AO, Pripp AH, et al. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: a prospective study. Inflamm Res. 2012;61(8):845–852. doi: 10.1007/s00011-012-0476-0 [DOI] [PubMed] [Google Scholar]
- 7.Jensen TSR, Binderup T, Olsen MH, et al. Subdural Levels of Interleukin 1-receptor Antagonist are Elevated in Patients with Recurrent Chronic Subdural Hematomas. Inflammation. 2023;46(4):1332–1342. doi: 10.1007/s10753-023-01811-8 [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson PJ, Edlmann E, Bulters D, et al. Trial of Dexamethasone for Chronic Subdural Hematoma. N Engl J Med. 2020;383(27):2616–2627. doi: 10.1056/NEJMoa2020473 [DOI] [PubMed] [Google Scholar]
- 9.Saul H, Gursul D, Cassidy S, et al. Dexamethasone should not be given to people with a chronic subdural haematoma. BMJ. 2022;377:o1302. doi: 10.1136/bmj.o1302 [DOI] [PubMed] [Google Scholar]
- 10.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 11.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 12.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 13.Franco C, Sciatti E, Favero G, et al. Essential Hypertension and Oxidative Stress: novel Future Perspectives. Int J Mol Sci. 2022;23(22):14489. doi: 10.3390/ijms232214489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera-Martínez AD, Herrero‐Aguayo V, Pérez‐Gómez JM, et al. Inflammasomes: cause or consequence of obesity-associated comorbidities in humans. Obesity. 2022;30(12):2351–2362. doi: 10.1002/oby.23581 [DOI] [PubMed] [Google Scholar]
- 15.Shearn CT, Anderson AL, Miller CG, et al. Thioredoxin reductase 1 regulates hepatic inflammation and macrophage activation during acute cholestatic liver injury. Hepatol Commun. 2023;7(1):e0020. doi: 10.1097/HC9.0000000000000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Li J, Song H, et al. Monocyte to Lymphocyte Ratio, A Novel Predictor of Acute Kidney Injury After Cardiac Valve Surgery. Heart Surg Forum. 2022;25(6):E833–e839. doi: 10.1532/hsf.5111 [DOI] [PubMed] [Google Scholar]
- 17.Benucci M, Bernardini P, Coccia C, et al. JAK inhibitors and autoimmune rheumatic diseases. Autoimmun Rev. 2023;22(4):103276. doi: 10.1016/j.autrev.2023.103276 [DOI] [PubMed] [Google Scholar]
- 18.Crotty K, et al. A critical review of recent knowledge of alcohol’s effects on the immunological response in different tissues. Alcohol Clin Exp Res. 2022;47:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen TSR, Mahmood B, Damm MB, et al. Combined activity of COX-1 and COX-2 is increased in non-neoplastic colonic mucosa from colorectal neoplasia patients. BMC Gastroenterol. 2018;18(1):31. doi: 10.1186/s12876-018-0759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berghauser Pont LM, Dirven CMF, Dippel DWJ, et al. The role of corticosteroids in the management of chronic subdural hematoma: a systematic review. Eur J Neurol. 2012;19(11):1397–1403. doi: 10.1111/j.1468-1331.2012.03768.x [DOI] [PubMed] [Google Scholar]
- 21.Araújo FA, Rocha MA, Mendes JB, et al. Atorvastatin inhibits inflammatory angiogenesis in mice through down regulation of VEGF, TNF-alpha and TGF-beta1. Biomed Pharmacother. 2010;64(1):29–34. doi: 10.1016/j.biopha.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 22.Jiang R, Zhao S, Wang R, et al. Safety and Efficacy of Atorvastatin for Chronic Subdural Hematoma in Chinese Patients: a Randomized ClinicalTrial. JAMA Neurol. 2018;75(11):1338–1346. doi: 10.1001/jamaneurol.2018.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younsi A, Riemann L, Habel C, et al. Relevance of comorbidities and antithrombotic medication as risk factors for reoperation in patients with chronic subdural hematoma. Neurosurg Rev. 2022;45(1):729–739. doi: 10.1007/s10143-021-01537-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JJ, Won Y, Yang T, et al. Risk Factors of Chronic Subdural Hematoma Progression after Conservative Management of Cases with Initially Acute Subdural Hematoma. Korean J Neurotrauma. 2015;11(2):52–57. doi: 10.13004/kjnt.2015.11.2.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edlmann E, Giorgi-Coll S, Whitfield PC, et al. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflamm. 2017;14(1):108. doi: 10.1186/s12974-017-0881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18(11):1478–1493. doi: 10.2174/138161212799504731 [DOI] [PubMed] [Google Scholar]
- 27.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi: 10.1038/nri3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng JH, Tseng M-Y, Liu A-J, et al. Risk factors for chronic subdural hematoma after a minor head injury in the elderly: a population-based study. Biomed Res Int. 2014;2014:218646. doi: 10.1155/2014/218646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komiyama K, Tosaka M, Shimauchi-Ohtaki H, et al. Computed tomography findings after head injury preceding chronic subdural hematoma. Neurosurg Focus. 2019;47(5):E12. doi: 10.3171/2019.8.FOCUS19535 [DOI] [PubMed] [Google Scholar]
- 30.Schievink WI, Maya MM, Pikul BK, et al. Spontaneous spinal cerebrospinal fluid leaks as the cause of subdural hematomas in elderly patients on anticoagulation. J Neurosurg. 2010;112(2):295–299. doi: 10.3171/2008.10.JNS08428 [DOI] [PubMed] [Google Scholar]
- 31.Yang AI, Balser DS, Mikheev A, et al. Cerebral atrophy is associated with development of chronic subdural haematoma. Brain Inj. 2012;26(13–14):1731–1736. doi: 10.3109/02699052.2012.698364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CL, Gilbert TJ, Daling JR. Maternal smoking and Down syndrome: the confounding effect of maternal age. Am J Epidemiol. 1999;149(5):442–446. doi: 10.1093/oxfordjournals.aje.a009831 [DOI] [PubMed] [Google Scholar]