Abstract
Background
The occurrence of co-infections during schistosomiasis, a neglected tropical disease, with other parasites have been reported suggesting an impaired host immune defense. Macrophage purinergic P2X7 receptor (P2X7R) plays an important role against intracellular pathogens. Therefore, we investigated the P2X7R-mediated phagocytosis and killing capacity of Leishmania amazonensis by macrophages during schistosomiasis in vitro and in vivo.
Methods
Swiss and C57BL/6 (Wild type) and P2X7R−/− were randomized in two groups: control (uninfected) and Schistosoma mansoni-infected. Alternatively, control Swiss and S. mansoni-infected mice were also infected with L. amazonensis.
Results
The pre-treatment of control macrophages with the P2X7R antagonist (A74003) or TGF-β reduced the phagocytosis index, mimicking the phenotype of cells from S. mansoni-infected mice and P2X7R−/− mice. Apyrase also reduced the phagocytosis index in the control group corroborating the role of ATP to macrophage activation. Moreover, l-arginine-nitric oxide pathway was compromised during schistosomiasis, which could explain the reduced killing capacity in response to ATP in vitro and in vivo. We found an increased extracellular nucleotide (ATP, ADP and AMP) hydrolysis along with an increased frequency of F4/80+ CD39+ macrophages from the S. mansoni-infected group. Moreover, the content of adenosine in the cell supernatant was higher in the S. mansoni-infected group in relation to controls. Schistosomiasis also increased the expression of macrophage adenosine A2BR. In good accordance, both ADA and the selective A2BR antagonist restored the phagocytosis index of macrophages from S. mansoni-infected group.
Conclusions
Altogether, the altered P2X7R and A2BR signaling limits the role of macrophages to host defense against L. amazonensis during schistosomiasis, potentially contributing to the pathophysiology and clinically relevant co-infections.
Keywords: Macrophages, Schistosomiasis, Host defense, Adenosine receptor, P2X7 receptor, Leishmaniasis
Graphical abstract
Highlights
-
•
Schistosomiasis downregulates P2X7 receptor function limiting host defense
-
•
Macrophage P2X7-A2B receptors signaling is altered in schistosomiasis
-
•
Blockage of A2B receptors restores macrophage phagocytosis during schistosomiasis
1. Introduction
Schistosomiasis is an intravascular and potentially lethal infectious disease. According to the World Health Organization (WHO) schistosomiasis affects approximately 250 million people worldwide, mainly in middle- and low-income countries leading to a significative socioeconomic burden, being defined by as a neglected tropical disease [1].
After infection with Schistosoma mansoni, the immature schistosomulum reaches the vascular system beginning a journey to the mesenteric system, where worms mature, mate, and the females start eggs deposition [2]. In turn, eggs secrete proteolytic enzymes that, along with host immune responses, cause gastrointestinal inflammation favoring endothelial extravasation to the intestinal lumen [2,3]. Eventually, eggs reach the peritoneum or are embolized to hepatic sinusoids causing inflammation [2,4].
The recognition by host innate immune cells of pathogen-associated molecular patterns (PAMPs) released by worms and eggs, such as soluble antigens, miRNA and exosomes, triggers an adaptative immune response [4].
Briefly, soon after infection, during the acute phase of the disease, a CD4+ type 1 biased (Th1) cell response emerges characterized by interferon (INF)-γ, tumor necrosis factor (TNF)-α, and nitric oxide (NO) release, which may limit early liver damage [2,5]. With the onset of egg release, a Th2-driven immune response starts. IL-4 and IL-13 induce a chronic infection and a granulomatous response in liver and gut [2,4]. Initially, the Th2 response reduces immune-mediated tissue damage and acute deaths; however, chronic Th2 response may be highly pathogenic and fatal in long term [6].
Macrophages have an important and plastic role in host defense during schistosomiasis and, depending on the cytokine environment, present classically (M1) or alternatively activated (M2) phenotypes [6]. The M1 phenotype is of fundamental importance to host defense, while M2 phenotype is involved in tissue remodeling [7]. Therefore, the interplay between Th1 and Th2 cytokines determines disease outcomes.
Schistosome-host interactions damage cells triggering the modulation of host immune system through the release of damage-associated molecular patterns (DAMPs). The release of extracellular ATP (a DAMP) initiates purinergic signaling through the action of purinergic receptors and nucleotide metabolizing enzymes (or ectonucleotidases; CD39 and CD73). Extracellular ATP and its metabolite adenosine signal through P2 and adenosine (P1) receptors, respectively, with distinct physiological actions [8,9]. To date, seven P2X1-7 and four adenosine A1, A2A, A2B and A3 receptor subtypes have been described [10].
P2X7 is a ligand-gated ionotropic receptor activated by ATP. Upon stimulation, P2X7 receptors trigger Ca2+ and Na + influx, along with K+ efflux modulating cell responses. For instance, P2X7 receptor-mediated Ca2+ influx potentiates macrophage phagocytosis [11,12]. Besides, macrophages primed by PAMPs and treated with ATP or the selective P2X7 receptor agonist benzoyl-ATP release interleukin (IL)-1β secondary to inflammasome activation, which is also important for host defense [8,13]. In turn, ATP hydrolysis by ectonucleotidases may limit P2X7 inflammatory signaling by generating adenosine, which promotes alternatively macrophage activation via A2A and A2B receptors [7]. Therefore, the fine tune of purinergic signaling drives distinct modulations of host defense.
The occurrence of coinfections during schistosomiasis with intracellular pathogens, especially in endemic areas, has been reported, which may impact disease severity [[14], [15], [16]]. However, the putative immunomodulatory role of purinergic signaling in such coinfections has not been addressed yet. We showed previously that the macrophage P2X7 receptor function is damped during chronic schistosomiasis [17]. Therefore, we hypothesized that the reduced macrophage P2X7 receptor function could impair host defense against a second infection. Present data revealed that macrophages harvested from S. mansoni-infected mice and exposed to Leishmania amazonensis failed to kill the protozoa in response to P2X7 receptor activation, leading to an increased protozoan load in vivo. Moreover, these macrophages showed an increased ATP hydrolysis driving to an increased A2B receptor signaling. Altogether, the alterations of macrophage P2X7 and A2B receptors signaling during schistosomiasis may explain the occurrence of coinfections during chronic schistosomiasis.
2. Materials and Methods
2.1. Chemicals
Dulbecco's Modified Eagle's Medium (DMEM), Medium 199, fetal bovine serum (FBS), penicillin, and streptomycin were obtained from Thermo Fisher Scientific (Brazil). ATP, ADP, AMP, adenosine, TGF-β, apyrase, and ADA were obtained from Sigma-Aldrich® (USA). A740003, CGS-21680 and MRS1706 were obtained from Tocris (UK). All other reagents used were of analytical grade.
2.2. Animals and S. mansoni infection
Swiss and C57BL/6 (Wild type and P2X7R knockout (P2X7R−/−; Jackson Laboratory (Bar Harbor, ME, USA)) male mice were used. The animals were housed in a temperature-controlled room (22 °C) with a light/dark cycle (12 h) and received food and water ad libitum. All procedures using animals followed the National Council for the Control of Animal Experimentation (CONCEA, Brazil) guidelines (licenses 048/16, 124/22 and IBCCF 154, UFRJ) and ARRIVE guidelines. Briefly, newborn animals (7–10 days old/Swiss, WT, P2X7R−/−) were infected percutaneously with 80 cercariae of S. mansoni for 8 min. S. mansoni-infected and uninfected (control) animals were maintained under a 12/12 h light/dark cycle and had access to water and food ad libitum. Age-matched male animals were used from 8 to 10 weeks post-infection [17]. All efforts were made to minimize suffering and the number of animals used based on G∗Power software (version 3.1.9.4) for statistical evaluation (Franz Faul, Kiel University, Germany).
2.3. Peritoneal macrophages
Euthanasia was performed in a CO2 chamber. Resident peritoneal macrophages from control and S. mansoni-infected groups were obtained from peritoneal cavity wash with 5 mL of cold sterile phosphate-buffered saline (PBS). In all groups, cell viability following peritoneal wash was over 95%. The peritoneal exudate was centrifuged (350 × g, for 5 min, at 4 °C). Cell suspensions (2 × 105 cells/well) were distributed in flat bottom 24- or 96-well plates and incubated for 1 h in serum-free DMEM (1% penicillin/streptomycin) at 37 °C under 5% CO2 atmosphere. After this period, cells were washed with sterile PBS at 37 °C to remove non-adherent cells, and the adherent cells were cultured in complete DMEM (10% heat-inactivated FBS, 1 mM l-glutamine, 1% penicillin/streptomycin) for 24 h [18] and then used in qRT-PCR, flow cytometry or phagocytosis assays.
2.4. Macrophage exposure to Leishmania amazonensis and in vitro phagocytosis assay
L. amazonensis (MHOM/BR/Josefa strain) was cultured as previously described [18] and used for in vitro and in vivo assays.
Resident peritoneal macrophages from control and S. mansoni-infected groups were obtained and cultured as described in 2.3 section. Macrophage infection with L. amazonensis was conducted at a multiplicity of infection (MOI) of 10:1 (10 promastigotes for each macrophage). L. amazonensis promastigotes in the growth stationary phase were added to the macrophage culture for 4 h in the presence of complete DMEM. Then, macrophages were washed three times with sterile PBS at 37 °C to remove non-internalized promastigotes, fixed with the Panotic kit (Laborclin®), and imaged under light microscopy (40X). The infection index was calculated as previously described [18]. In the case of pharmacological treatment, macrophages were incubated for 30 min with drugs or vehicle before the addition of L. amazonensis promastigotes (MOI 10:1) for 4 h. Alternatively, plated macrophages were incubated with L. amazonensis for 4 h in complete DMEM, washed to remove non-internalized promastigotes and incubated for 24 h. Then, these macrophages were treated with vehicle or ATP 500 μM for 30 min and the killing response in vitro was analyzed 24 h later [18].
2.5. In vivo infection with L. Amazonesis
Adult Swiss mice (control and S. mansoni-infected groups) were submitted to a subcutaneous injection with 106 L. amazonesis promastigotes in the right footpad. The number of L. amazonensis parasites in the popliteal lymph nodes at 35 d.p.i. was determined by the limiting dilution assay, as described previously [18]. Briefly, after euthanasia, the popliteal lymph nodes were collected, weighed, and dissociated in Medium 199 supplemented with 10% FBS, 1% l-glutamine, 0.25% hemin using a cell strainer BD® (40 μm). Tissue debris was removed by centrifugation at 150 × g and then, cells were pelleted by centrifugation at 2000 × g for 10 min and resuspended in complete Medium 199. Fifty microliters (50 μL) aliquots of cell suspension (with an initial density of 0.25–1 × 106 cells/mL) were plated in flat bottom 96-well plates containing Medium 199, at 4:1 serial dilution in Medium 199, in triplicate. Samples were cultured at 26–28 °C for at least 7 days, when the wells were examined by phase contrast microscopy under an inverted microscope (NIKON TMS, JP) and scored as “positive” or “negative” for the presence of parasites. Wells were classified as "positive" when at least one parasite per well was observed, and the parasite load (L. amazonensis count) was estimated according to the highest dilution at which the parasites could still be detected [18].
2.6. Phenotypic analysis by flow cytometry
Cells (106 cells/well) were transferred to a 96-well U-bottom plate and centrifuged for 5 min at 300 × g. Then, cells were suspended in 100 μL of the FACS Buffer and subjected to Fc portion blocking (CD16/CD32 clone 93 in labeling buffer (eBioscience, CA, USA) for 20 min at 4 °C. After incubation, the plate was centrifuged again (5 min, 300 x g) before the addition of one of the specific anti-mouse antibodies (1.0 μg/mL, 30 min at 4 °C in the dark) conjugated to the respective fluorochrome in labeling buffer: Alexa Fluor® 488 anti-F4/80 (clone BM8; FL-1), Alexa Fluor® 647 anti-CD39 (clone A1; FL-4), and isotypes (eBioscience, CA, USA). Samples were analyzed by flow cytometry in FACScalibur (BD Biosciences, CA, USA) acquiring 10,000 events. Data were analyzed using Summit 2.3 software and expressed as the number of positive cells [18].
2.7. RNA isolation and real-time quantitative PCR (RT-qPCR)
Total RNA was isolated from macrophages using the TRIzol® reagent (Thermo Fisher Scientific, Rockford, IL, USA) according to the manufacturer's instructions. RNA samples were quantified, and the purity was assessed using a Nanodrop Lite spectrophotometer (Thermo Scientific, NJ, USA). The synthesis of cDNA was performed using 1 μg of total RNA using the High-Capacity Reverse Transcription Kit with RNase Inhibitor (Thermo Fisher Scientific, NJ, USA) according to the manufacturer's instructions in a Master Cycler Gradient thermocycler (Eppendorf, Hamburg, Germany).
The HOT FIREPol® Evagreen® qPCR Supermix (Solis Biodyne, Denmark) was used for RT-qPCR to detect double-stranded DNA synthesis in a QuantStudio 3 Real-Time PCR System (Thermo Scientific, NJ, USA). The reactions were performed in a final volume of 10 μL, using 2 μL of diluted cDNA (1:10) and 300 nM of each primer forward and reverse of the genes. The specificity of amplification and absence of primer-dimer was confirmed using melting curve analysis at the end of each run.
The following forward and reverse primer sequences were used: Adora1 5′-ACAAAAACCAGTGGTGGAGTGA-3′ and 5′-TCTGTCCCCTCCCCTTGTC-3’; Adora2a 5′-TGAAGGCGAAGGGCATCA-3′ and 5′-GGGTCAGGCCGATGGC-3’; Adora2b 5′-ACGTGGCCGTGGGACTC-3′ and 5′-GCAGAAGCCCAAGCTGATG-3’; Adora3 5′-GAGACCTGCATCCTCCAGGTT-3′ and 5′-GGCCTGTTACAGGACCATCAA-3’; Il10 5′-GCTGGACAACATACTGCTAACC-3′ and 5′-ATTTCCGATAAGGCTTGGCAA-3’; Arg1 5′-GAAAGTTCCCAGATGTACCAG-3′ and 5′-CCAGGGTCTACGTCTCGC-3’; Nos2 5′-ACATCGACCCGTCCACAGTAT-3′ and 5′-CAGAGGGGTAGGCTTGTCTC-3’; and Actb 5′-TATGCCAACACAGTGCTGTCTGG-3′ and 5′-TACTCCTGCTTGCTGATCCACAT-3’.
The relative cDNA expression was calculated using the comparative cycle threshold (CT) method. The Actb gene was used as an endogenous control. The results were expressed as relative expression of the gene of interest/endogenous control.
2.8. Determination of cytokine levels
The concentrations of IL-6 in the supernatant of macrophage cells were determined by the ELISA method according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). The plate was sensitized overnight with the anti-cytokine capture antibody. After this period, the plate was washed four times (0.05% Tween 20 in PBS) and then incubated for 2 h with blocking buffer (1% BSA in PBS). Then, the plate was washed again four times before adding the samples and the standards (triplicate) and incubated for 2 h. After washings (four times), the detection antibody (0.5 μg/mL) was added and incubated for 2 h. Finally, the plate was washed, incubated with streptavidin for 30 min at room temperature, washed, and the reading was performed at 450 nm.
2.9. Inorganic phosphate release assay (ectonucleotidase activity)
The activities of the ectonucleotidases in peritoneal macrophages from control and S. mansoni-infected animals were determined as previously described [19]. Plated cells (2 × 105/well; 96-well plates) were incubated in a reaction medium (20 mM HEPES (pH 7.5), 120 mM NaCl, 5 mM KCl, 60 mM glucose, 1 mM sodium azide, 0.1% albumin, 1 mM CaCl2 (for ATP) or MgCl2 (for AMP)) in a final volume of 160 μL per well for 10 min at 37 °C. The reactions were initiated by adding nucleotides (ATP, ADP, AMP) at a final concentration of 2 mM for 30 min. After 30 min, the reaction was stopped by combining an ice bath with the addition of 10% TCA. The inorganic phosphate (Pi) release was measured by the colorimetric assay described by Chan et al. [20] using KH2PO4 as a Pi standard. The non-enzymatic hydrolysis was evaluated in the absence of cells. Assays were performed in triplicate, and enzymes activities were reported as nmol of Pi released/min/2 × 105 cells.
2.10. Adenosine quantification by LC/MS/MS
Macrophages from control and S. mansoni-infected mice (2 × 105 cells/well) were plated. After 24 h, cell supernatants from both groups were collected and stored at −80 ° C until use. Adenosine quantification was performed by LC/MS/MS system. Adenosine (Sigma Aldrich, USA) was used for the standard calibration curve. The analyte peak area was divided by the standard area. The integrated areas ratio between adenosine and internal standards were used in the results interpretation. The chromatographic separation was performed in a hydrophilic interaction liquid chromatography (HILIC) column (ACE-HILIC-A 3 μm, 100 mm × 2.1 mm) at 40 °C. The mobile phases were composed of (A) H2O with 5 mM ammonium formate and 0.1% formic acid, and (B) acetonitrile with 0.1% formic acid. The flow rate was set at 400 μL/min. The elution profile was 0–8.5 min, 97-50% A; 8.5–9 min, 50% A; 9–9.5 min, 50–97% A; 9.5–11 min, 97% A to equilibrate to the initial conditions. The overall run time was 11 min, and the injection volume was 10 μL. The LC effluent was pumped to a Q-Exactive mass spectrometer (Q Exactive Plus - Ultimate 3000 HPLC system, Thermo Scientific, Germany) operating in positive and negative ionization mode. The spray voltage was set at 2.9 kV. The capillary temperature was 380 °C, and the S-lens radio frequency (RF) level was set at 50 (arbitrary units). The nitrogen sheath and auxiliary gas flow rates were set at 60 and 20 (arbitrary units), respectively. To ensure mass accuracies below 6 ppm, the instrument was calibrated in positive and negative mode using the manufacture's calibration solutions (Thermo Fisher Scientific, Germany). The mass spectrometer acquired FullMS at resolution of 35,000 full width at half maximum (FWHM) and with an automatic gain control (AGC) of 106, maximum IT 75 ms. The target mass for positive mode was m/z 268,1040 to adenosine and to internal standard 7-propilteofilina m/z 223.1189. The lower limit of detection was 10 ng/mL.
2.11. Data analysis
Results were expressed as mean and SEM. Differences between two or more groups were analyzed by unpaired Student's t-test or one way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test, respectively, considering p < 0.05 (GraphPad Prism 7.0, USA).
3. Results
3.1. Schistosomiasis-induced P2X7 receptor impairment reduces macrophage phagocytic activity
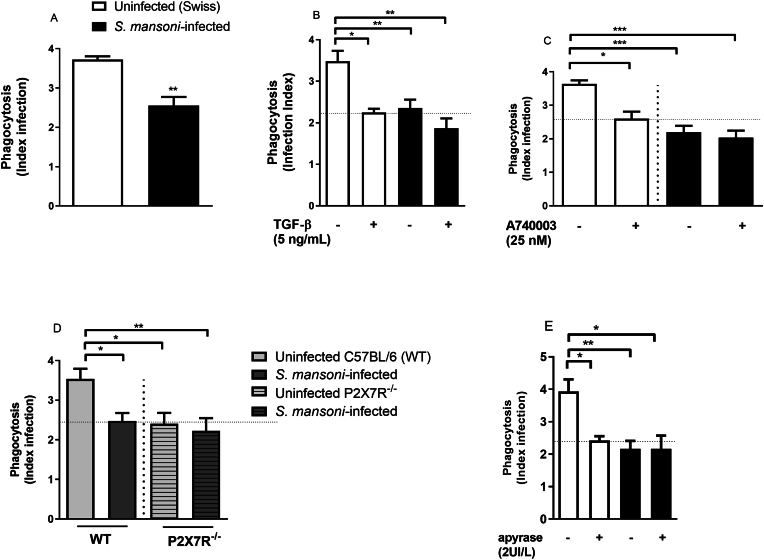
Peritoneal macrophages from S. mansoni-infected mice show a reduced P2X7 receptor function [17]. Therefore, we initially isolated peritoneal macrophages from S. mansoni-infected or uninfected (control) mice. Subsequently, we exposed these cells to L. amazonensis to assess their phagocytic activity. Interestingly, we found a significant decreased L. amazonensis phagocytosis index in macrophages from S. mansoni-infected mice compared to cells from uninfected (i.e., control) mice [Fig. 1A]. As this P2X7 receptor impairment could be related to the elevated peritoneal levels of the Th2 cytokine TGF-β [17], we evaluated the impact of exogenous TGF-β on macrophage phagocytic activity. We observed that the treatment of peritoneal macrophages from control mice with TGF-β (5 ng/mL overnight) mimicked the phenotype of cells obtained from S. mansoni-infected group [Fig. 1B] characterized by a reduced phagocytosis index. However, TGF-β had no effect on phagocytic activity in macrophages from S. mansoni-infected group [Fig. 1B]. Moreover, the treatment of macrophages with the selective P2X7 receptor antagonist (A740003, 25 nM added 30 min before exposition to L. amazonensis) reduced the phagocytosis in the control group (cells from Swiss and WT C57BL/6 mice not infected with S. mansoni; [Fig. 1C]) mimicking the profile of P2X7R−/− mice [Fig. 1D]. However, A740003 did not show additional effects in the decreased phagocytic activity of cells from S. mansoni-infected mice [Fig. 1C]. We also evaluated the effect of apyrase, an enzyme which degrades the P2X7 ligand (ATP) and other extracellular nucleotides. In macrophages from the uninfected group, apyrase treatment (2U/L) reduced phagocytic activity [Fig. 1E] to the same level observed after the selective P2X7 receptor antagonist A740003 pre-treatment [Fig. 1C] or to the levels observed in macrophages from P2X7R−/− mice. However, apyrase did not alter the phagocytosis index in macrophages from S. mansoni-infected group [Fig. 1E]. Interestingly, uninfected cells treated with apyrase and cells from S. mansoni-infected mice showed similar levels of phagocytosis.
Fig. 1.
Macrophages with reduced P2X7 receptor function due to schistosomiasis showed a reduced phagocytosis index of L. amazonensis in vitro. Macrophages from Swiss control and S. mansoni-infected mice were incubated with TGF-β (5 ng/mL, 12h), with A740003 (25 nM, 30 min) or apyrase (2UI/L, 30 min) before exposition to L. amazonensis. White and light gray bars = uninfected (control) Swiss (A, B, C, E) or WT C57BL/6 (D) mice, respectively. Black bars = S. mansoni-infected mice. Light and dark gray hatched bars = P2X7R −/− uninfected or S. mansoni-infected mice, respectively (D). Data expressed as mean and SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 4–5 individual animals for each group, Student's t-test (A) or one way ANOVA followed by Tukey's multiple comparisons test (B–D). E) ∗p < 0.05, ∗∗p < 0.01, n = 5–7 individual animals for each group, one way ANOVA followed by Tukey's multiple comparisons test.
3.2. Schistosomiasis impairs P2X7R-mediated L. amazonensis killing in vitro and in vivo
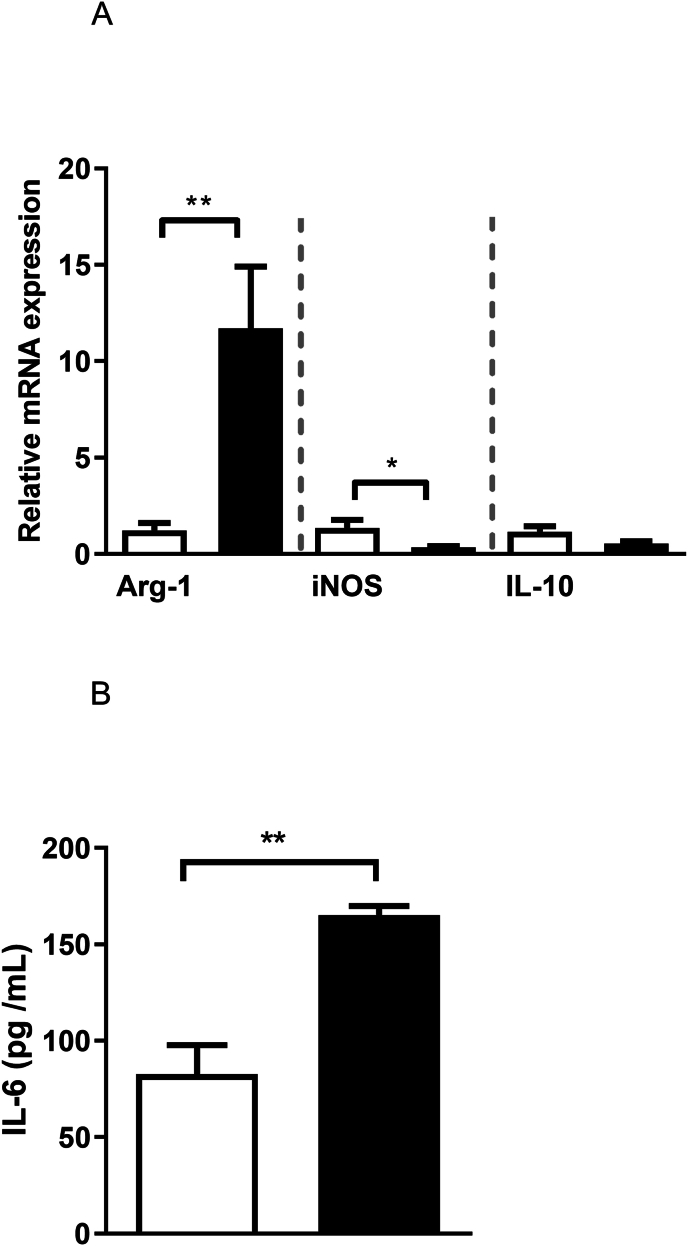
Schistosomiasis reduced the macrophage expression of the inducible nitric oxide synthase (iNOS; p = 0.02), while upregulated the expression of arginase (Arg)-1 in these cells ([Fig. 2A]; p = 0.01). These cells also released high levels of IL-6 [Fig. 2B], while no alterations for IL-10 transcripts were observed ([Fig. 2A]; p = 0.065). Since these data suggest that macrophages from S. mansoni-infected mice have a decreased capacity to produce important microbicidal mediators, we investigated their killing capacity in response to P2X7 receptor activation.
Fig. 2.
Schistosomiasis alters macrophage expression of enzymes regulating the l-arginine-NO pathway and immunomodulatory cytokines. A) Relative mRNA expression of Arg-1, iNOS and IL-10. B) IL-6 concentration (ELISA). White and black bars = uninfected or S. mansoni-infected mice, respectively. Data expressed as mean and SEM. ∗p < 0.05, ∗∗p < 0.01, n = 5 individual animals for each group, Student's t-test.
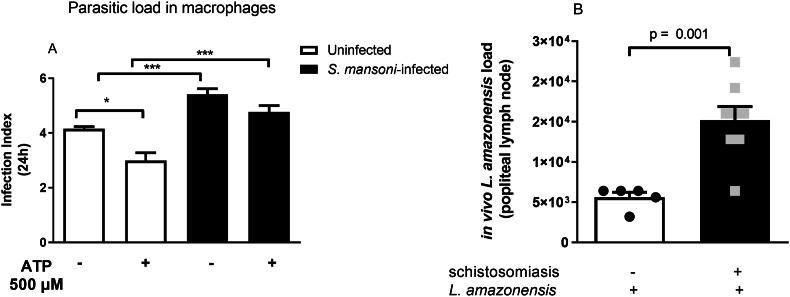
After 24 h of the exposition to L. amazonensis, macrophages were challenged with 500 μM ATP for 30 min, a condition which activates P2X7 receptor and Ca2+ influx [17], and analyzed 24 h later. As depicted in [Fig. 3A], macrophages from the S. mansoni-infected mice failed to kill L. amazonensis in response to ATP. In addition, the parasitic load in this group was higher than in the control group, both in the absence or presence of ATP (p < 0.001).
Fig. 3.
The reduced macrophage P2X7 receptor function during schistosomiasis impairs L. amazonensis killing in response to ATP in vitro and in vivo. A) Macrophages from control and S. mansoni-infected mice were exposed to L. amazonensis for 4 h (MOI 10:1) and washed to remove non-internalized protozoan. After 24 h, cells were treated with 500 μM ATP for 30 min, and 24 h later, cells were washed, fixed, and imaged to calculate the infection index (see Methods section). Data expressed as mean and SEM. ∗p < 0.05, ∗∗∗p < 0.001, n = 4–5 individual animals for each group, one way ANOVA followed by Tukey's multiple comparisons test. B) Reduced control of L. amazonensis proliferation during schistosomiasis in vivo. Control and S. mansoni-infected mice were infected through an intradermal injection of 106 L. amazonensis promatigotes. The popliteal lymph nodes were analyzed 35 days later. n = 5–8 individual animals for each group, p = 0.001, Student's t-test.
The killing failure observed in the S. mansoni-infected group had in vivo consequences for host defense since the parasitic load in the popliteal lymph node was higher in the S. mansoni-infected group than in the controls [Fig. 3B]. Taken together, these data suggest that the reduced P2X7 receptor signaling due to schistosomiasis may contribute to impaired host defense against L. amazonensis.
3.3. Schistosomiasis increases adenosine generation and A2B receptor signaling damping host defense against L. amazonensis
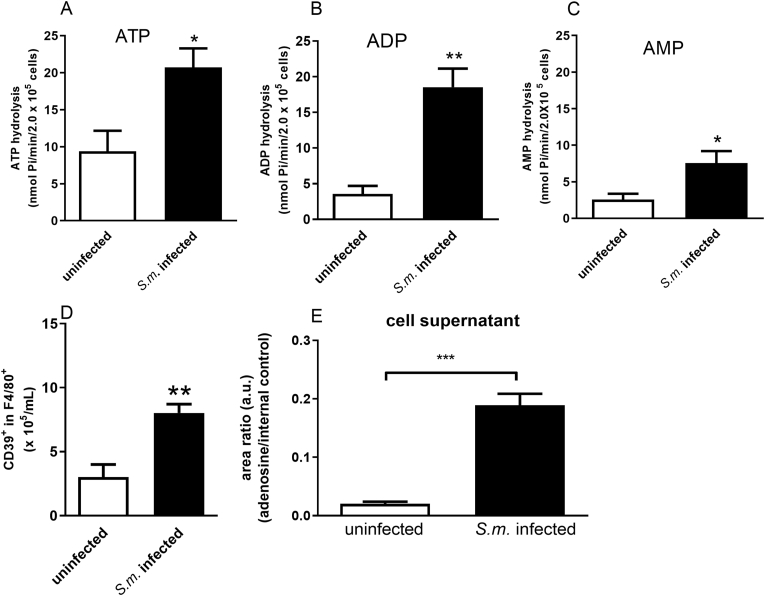
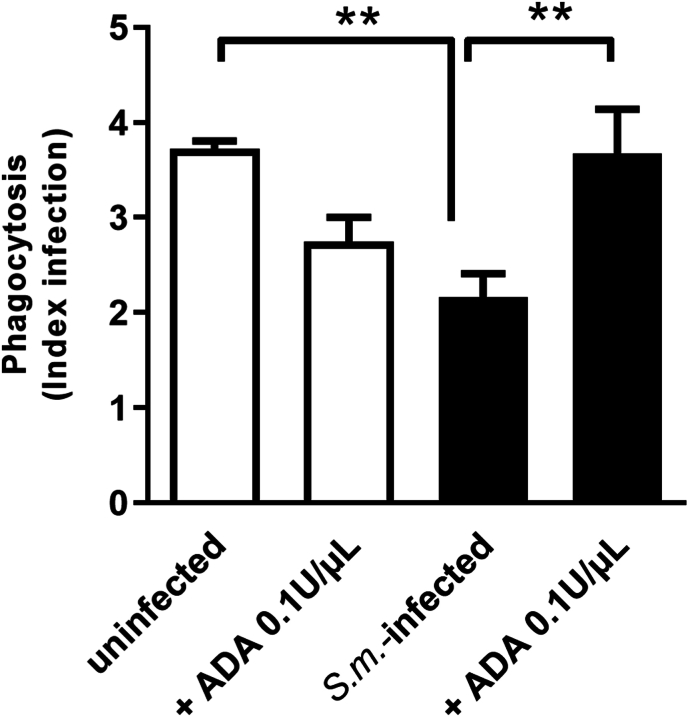
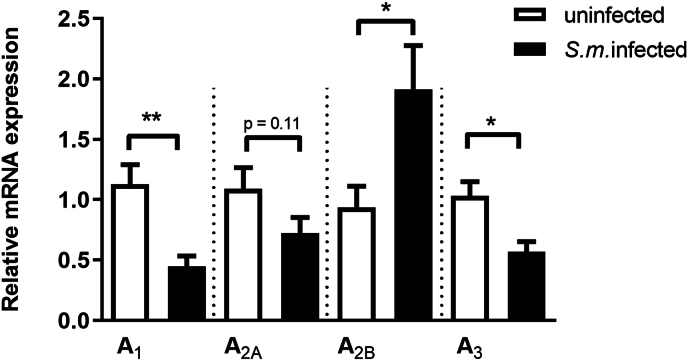
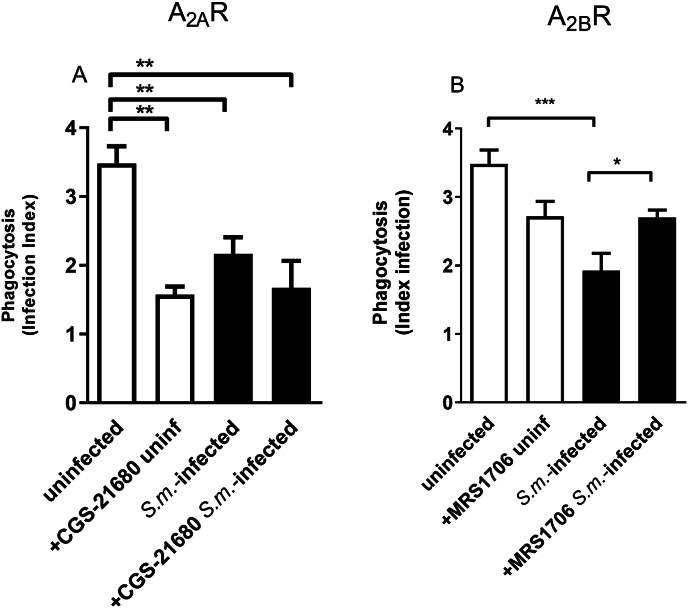
Ectonucleotidases are critical enzymes that regulate the levels of extracellular nucleotides and nucleosides. Therefore, we performed ectonucleotidase assays in macrophages from S. mansoni-infected mice. We found an increased ATP, ADP, and AMP hydrolysis compared to the control group [Fig. 4A–C]. Also, these F4/80+ cells from S. mansoni-infected mice express higher levels of CD39 than the control group [Fig. 4D]. Moreover, the content of adenosine in cell supernatants of the macrophages from S. mansoni-infected group was higher than in the control group [Fig. 4E]. To evaluate if macrophage-derived adenosine could be involved in the reduced phagocytic index of the S. mansoni-infected group, we treated cells from both groups with adenosine deaminase (ADA, 0.1 U/μL). In the macrophages from S. mansoni-infected group, ADA restored the phagocytic index [Fig. 5]. Following, we investigated the subtypes of adenosine receptors expressed in the peritoneal macrophages. We found that peritoneal macrophages express the transcript of all adenosine receptor subtypes, and schistosomiasis induced different patterns of expression [Fig. 6]. A1 and A3 receptor expressions were reduced by the disease. At the same time, we found an increased level of transcripts for A2B receptors and similar levels for A2A receptors in macrophages from the S. mansoni-infected mice [Fig. 6]. The activation of A2A receptors with CGS-21680 reduced the phagocytic index in the control group reaching similar values of the S. mansoni-infected group [Fig. 7A]. CGS-21680 did not alter phagocytosis in the infected group. On the other hand, the blockage of A2B receptors with MRS1706 restored the phagocytic index in cells from S. mansoni-infected mice [Fig. 7B].
Fig. 4.
Schistosomiasis alters nucleotide hydrolysis and increases extracellular adenosine. Macrophages from control and S. mansoni-infected mice (2 × 105 cells/well) were plated for enzymatic activity (A–C), CD39 expression (D) or adenosine quantification (E). Cells were incubated with 1 mM ATP, ADP or AMP for 30 min. Data expressed as nmol of Pi released/min/2 × 105 cells (mean and SEM). A-C) n = 4–6 individual animals for each group, ∗p < 0.05, ∗∗p < 0.01, Student's t-test. D) CD39+ cells in the gate of F4/80+ cells, n = 3–4 individual animals for each group, ∗∗p < 0.01, Student's t-test. E) n = 12 samples, ∗∗∗p < 0.001, Student's t-test. S.m. = S. mansoni.
Fig. 5.
Adenosine deaminase (ADA) restores the phagocytic index of macrophages from S. mansoni-infected mice. Macrophages from control and S. mansoni-infected groups were incubated with vehicle or ADA (0.1U/μL) for 30 min before exposition to L. amazonensis. Data expressed as mean and SEM. n = 4–7 individual animals for each group, ∗∗p < 0.01, one way ANOVA followed by Tukey's multiple comparisons test. S.m. = S. mansoni.
Fig. 6.
Schistosomiasis alters the macrophage expression of purinergic A1, A2B and A3 receptors. Data expressed as mean and SEM. n = 5–6 individual animals for each group, ∗p < 0.05, ∗∗p < 0.01, Student's t-test. S.m. = S. mansoni
Fig. 7.
The pharmacological blockage of purinergic A2B receptor restores the phagocytic index of macrophages from S. mansoni-infected mice. Macrophages from both groups were incubated with the selective A2A receptor agonist CGS21680 (1 μM, A) or with the selective A2B receptor antagonist MRS1706 (1 μM, B) for 30 min before exposition to L. amazonensis. Data expressed as mean and SEM. n = 5–8 individual animals for each group, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, one way ANOVA followed by Tukey's multiple comparisons test (A, B) or Student's t-test (B, S.m.-infected + MRS1706). S.m. = S. mansoni
4. Discussion
Schistosomiasis is an intravascular disease related to cell damage, a condition that is classically linked to the release of DAMPs. Macrophage phenotypes vary during schistosomiasis driving host inflammation and fibrosis. Chronic disease induces a Th2-biased immunity and co-infections have been reported [6,16,21]. ATP is an important DAMP with autocrine/paracrine actions shaping macrophage function through purinergic P2 receptors. In the present work we unveiled that macrophage phagocytic and killing capacity is reduced during schistosomiasis due to alterations of purinergic signaling. The main findings observed during schistosomiasis were a reduced P2X7 receptor function in response to ATP and an increased expression of adenosine A2B receptors resulting in an increased L. amazonensis load in vitro and in vivo.
S. mansoni worms exploit highly vascularized tissues. Living with a chronic parasitic disease request that host immune system tightly control immune responses. Briefly, after an initial Th1 immunity, as the parasites reach the mesenteric vessels and start egg deposition, a new polarization occurs with the production of key Th2 cytokines such as IL-4 (and IL-13), which is linked to STAT6-dependent alternatively activated macrophages [22]. In turn, intravascular eggs may be embolized to the liver and lungs, extravasate to peritoneum or to the intestinal lumen (mainly colon and rectum), causing a granulomatous inflammation in the target organs [4]. However, a maladaptive response during chronic helminth-induced immunomodulation may favor co-infections which would be detrimental to host [16,22]. Therefore, the balance between Th1 and Th2 immunity determines disease outcomes [6].
ATP release during inflammation depends on controlled mechanisms upon cell activation, or on membrane disruption of dying cells [23]. In the context of schistosomiasis, it is hypothesized that cell stress and tissue damage induced by worms and eggs, and the recognition of their antigens and worm defense molecules by host innate immune cells could release ATP, the main agonist of purinergic signaling with immunomodulatory actions [24]. However, the knowledge about the impact of schistosomiasis on macrophage purinergic signaling is sparse.
Previously, we showed that schistosomiasis reduces macrophage P2X7 receptor function [17]. Moreover, macrophage P2X7 receptors are important for host resistance to intracellular pathogens such as L. amazonensis, the etiological agent of leishmaniasis [25]. In the present work, peritoneal macrophages harvested from S. mansoni-infected mice and exposed to L. amazonensis showed a reduced phagocytic capacity, which was similar to macrophages from P2X7−/− receptor mice or macrophages from control group treated with the selective P2X7 receptor antagonist A740003. Apyrase also reduced the phagocytosis of macrophages from control group mimicking the profile of cells from S. mansoni-infected mice.
According to previous data, schistosomiasis increases TGF-β levels, and the treatment of macrophages from control mice with TGF-β reduced membrane expression of P2X7 receptors and Ca2+ influx [17]. Since P2X7 receptor-mediated increase of intracellular Ca2+ in macrophages potentiates phagocytosis [11], we investigated this cell function. In the present work, the treatment of control macrophages with 5 ng/mL TGF-β, a concentration similar to the serum levels found in patients with chronic schistosomiasis [17], recapitulated the cell phenotype of macrophages from S. mansoni-infected mice characterized by a reduced phagocytosis. In good accordance with our data, Oswald et al. [26] showed that in vitro TGF-β synergizes with IL-10 reducing the killing capacity of INF-γ-stimulated murine macrophages. Moreover, macrophages harvested from S. mansoni-infected mice and challenged with 500 μM ATP, a condition that activates P2X7 receptor, showed reduced L. amazonensis killing, and S. mansoni-primed cells failed to release IL-1β (data not shown). Macrophages from schistosomiasis group also expressed reduced transcriptional levels of iNOS. On the contrary, those cells expressed higher levels of Arg-1 compared to controls. In good accordance with our in vitro data, we observed in vivo an increased L. amazonensis load in the schistosomiasis group. Since Arg-1 and iNOS compete for the common substrate, l-arginine, these data could partially explain the reduced protozoan killing in the schistosomiasis group due to an impaired release of NO. Moreover, Arg-1 favors polyamine pathway supporting Leishmania survival [27]. Previous studies have shown that TGF-β favors Leishmania infection [28], and pre-existing schistosomiasis reduces anti-L. donovani immunity [29]. Even though we did not observe increased levels of IL-10 mRNA in the schistosomiasis group, as mentioned earlier, TGF-β and IL-10 have synergistic immunosuppressive effects [26]. Based on our data, those findings could be partially related to the downregulation of P2X7 receptors mediated by TGF-β [17]. Altogether, schistosomiasis impairs host immune response to L. amazonensis. Furthermore, besides protozoan infections, schistosomula killing also depends on NO-derived species [30].
We observed that schistosomiasis increased the release of IL-6 by peritoneal macrophages, a cytokine that has been shown to alter purinergic signaling [31,32] and implicated as a risk factor for leishmaniasis severity in patients [33]. Previous work by Sanmarco et al. [31] showed that IL-6 treatment reduced NO release and increased the percentage of CD39+ macrophages. Since ectonucleotidase (CD39) hydrolyzes extracellular ATP and governs P2X7 receptor-dependent functions in murine macrophages [34], we evaluated enzyme expression and nucleotide hydrolysis by macrophages from control and S. mansoni-infected mice. Cells F4/80+ from the infected group expressed higher transcriptional levels of CD39 and showed higher ATP, ADP and AMP hydrolysis than controls. Previously, we showed that mesenteric endothelial cells from infected mice also expressed increased levels of CD39 [35] and reduced P2X7 receptor function [36], which may suggest an adaptative response to downmodulate P2X7 receptor signaling during schistosomiasis. Moreover, in macrophages harvested from infected mice, we found that extracellular adenosine formation is increased during this stage of the disease, and cell treatment with ADA restored the phagocytic index to control levels. Adult schistosomes may also increase host adenosine production [37], suggesting that adenosine is crucial to infection.
In our model, the peritoneal macrophages express the four adenosine receptor subtypes, but the disease affects differently their expressions. In the schistosomiasis group, we found reduced transcriptional levels of A1 and A3 receptors, and increased levels of A2B receptor as compared to controls. However, there was no alteration of A2A receptor expression between groups. As A2A and A2B receptors are major regulators of the immune system, we used pharmacological tools to investigate their roles. In the control group, the selective A2A receptor agonist CGS21680 reduced the phagocytic capacity to values like the ones observed in the S. mansoni-infected group, which is consistent to the inhibition of macrophage activation [38]. However, CGS21680 did not affect cell phagocytosis in the S. mansoni-infected group. On the other hand, the blockage of A2B receptor with the selective antagonist MRS1706 restored the phagocytic activity of macrophages from the S. mansoni-infected group. These data are in good accordance with A2B receptor role as an inhibitor of iNOS expression [39] and macrophage activation [38], resulting in immunosuppression. Altogether, the increased A2B receptor signaling during schistosomiasis may favor Arg-1 pathway impairing host defense [40]. These data may also be relevant for patients since an increased monocyte A2B receptor expression has been correlated with an increased protozoan load and disease severity in visceral leishmaniasis [41].
In the present work we also observed a reduced expression of adenosine A1 and A3 receptors on macrophages from S. mansoni-infected mice compared to controls. A previous investigation into intestinal purinergic signaling during schistosomiasis revealed a diminished function of A1 receptors in mice after 10 weeks of infection [42], coinciding with the duration of infection in our model. This may suggest a potential immunomodulatory role for these receptors. Therefore, additional experiments are warranted to investigate the putative roles of A1 and A3 receptors on macrophages function during schistosomiasis.
Recent studies have suggested that TGF-β can modulate the expression of adenosine receptors in different cell types. For instance, in human lung epithelial cells TGF-β has been shown to upregulate or downregulate the expressions of adenosine A1 and A2A receptors, respectively, without affecting the other adenosine receptor subtypes [43]. However, here we observed an opposite profile of expression of these receptors, which may reflect the complex and dynamic cytokine profile of schistosomiasis, with different cytokines (and immune cells) and microbiome playing different roles at different stages of infection [44].
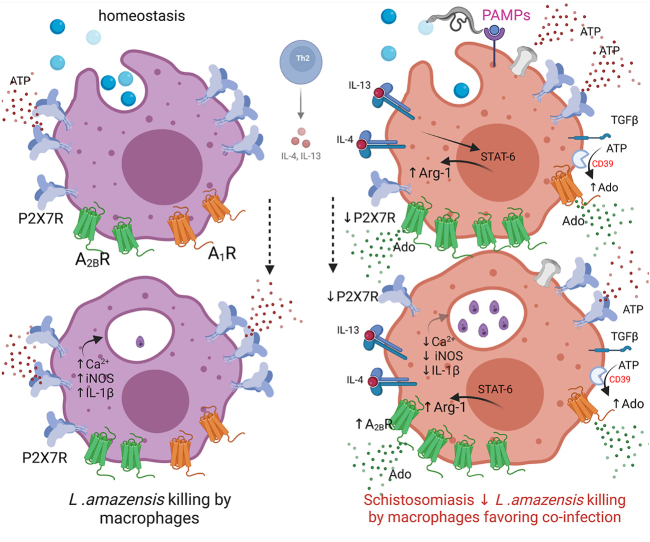
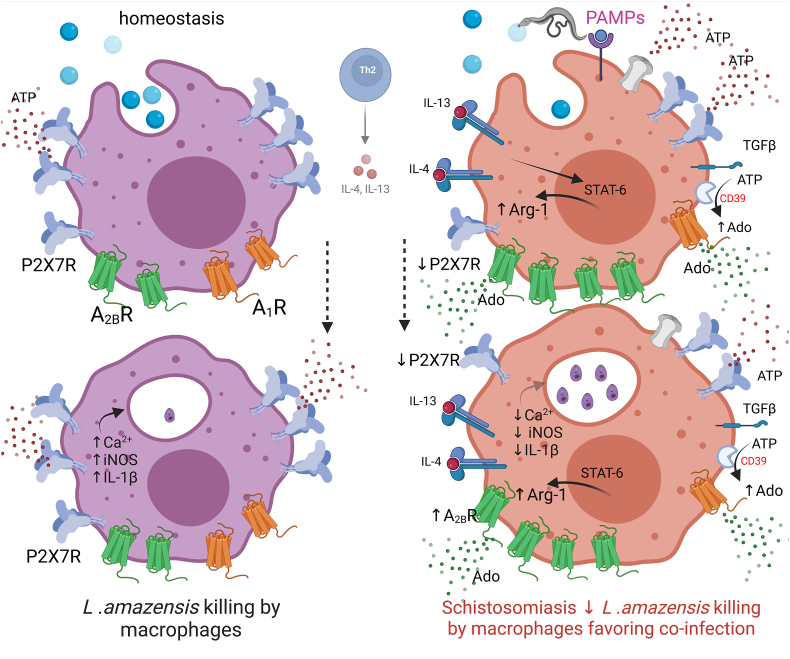
Macrophages have a plastic role in host defense. They determine the fate of leishmaniasis by limiting or allowing the growth of parasites [45]. In summary, our findings shed light on the intricate dynamics of macrophage responses in the context of schistosomiasis [Fig. 8]. Present work unveiled that schistosomiasis dampens macrophage P2X7 receptor and upregulates A2B receptor signaling, which reduced the killing of L. amazonensis in vitro and in vivo. The blockage of A2B receptors restored macrophage phagocytosis of L. amazonensis in vitro during schistosomiasis. The implications of these purinergic signaling alterations may potentially explain the molecular basis of a clinically relevant co-infection observed in patients with chronic schistosomiasis.
Fig. 8.
Schistosomiasis negatively impacts macrophage purinergic P2X7 receptors and elevates A2B receptor signaling, leading to a decreased ability to eliminate L. amazonensis. Extracellular ATP is an important DAMP (damage-associated molecular pattern) and the main agonist of purinergic signaling. Under normal conditions (homeostasis), macrophage P2X7 receptors trigger Ca2+ influx and inflammasome activation, resulting in IL-1β release, NO production, and efficient phagocytosis of promastigotes (blue circles) and killing of L. amazonensis. However, in the context of chronic schistosomiasis, the recognition by host innate immune cells of pathogen-associated molecular patterns (PAMPs; pink) released by S. mansoni and eggs triggers an adaptative immune response that reshapes the host's purinergic signaling. Elevated TGF-β levels diminish macrophage P2X7 receptor signaling. We also observed an increase in extracellular ATP hydrolysis by CD39 favoring adenosine (Ado) formation. Additionally, increased IL-6 levels, A2B receptor (green) and Arg-1 expressions, and decreased iNOS expression compromised microbicidal mechanisms, fostering a favorable environment for L. amazonensis co-infection in vitro and in vivo. The blockage of A2B receptors restored macrophage phagocytosis during schistosomiasis. These alterations in purinergic signaling may offer insights into the molecular basis of co-infections observed in individuals enduring chronic schistosomiasis. Understanding these mechanisms not only enhances our comprehension of host-pathogen interactions but also opens avenues for developing targeted therapeutic strategies to address co-infections in patients with chronic schistosomiasis. Created with Biorender.
Declaration of competing interest
none.
Acknowledgements
The authors thank Dr. Silvana C. Thiengo (Malacology Dept., Fiocruz Foundation, Rio de Janeiro, Brazil) for cercariae donation; Orlando R. Moreira (ICB/UFRJ) for technical assistance. This work was supported by the National Council for Scientific and Technological Development (CNPq; Brazil; 311402/2019-3; 312051/2023-9); Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ, Brazil; E-26/200.947/2021; /299.732/2024; /002.985/2014); Federal Agency for Support and Evaluation of Graduate Education (CAPES/PNPD, UFRJ, Brazil, 621/2018). NFO is a fellow from CAPES Brazil. CLMS, LEBS and RCS are senior fellows from CNPq. Funding institutions had no role in the study design, data analysis, conclusions, and decision to publish the manuscript. All authors made substantial contributions to the study.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.King CH. Health Metrics for helminth infections. Acta Trop. 2015;141:150–160. doi: 10.1016/j.actatropica.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunne DW, Cooke A. A worm’s eye view of the immune system: consequences for evolution of human autoimmune disease. Nature Rev Immunol. 2005;5(5):420–426. doi: 10.1038/nri1601. [DOI] [PubMed] [Google Scholar]
- 3.Silva CLM., Morel N, Lenzi HL, Noël F. Increased reactivity to 5-hydroxytryptamine of portal veins from mice infected with Schistosoma mansoni. Comp Biochem Physiol Mol Integr Physiol. 1998;120(3):417–423. doi: 10.1016/s1095-6433(98)10041-7. [DOI] [PubMed] [Google Scholar]
- 4.Costain AH, MacDonald AS, Smits H. Schistosoma egg migration: mechanisms, pathogenesis and host immune responses. Frontiers Immunol. 2018;9:3042. doi: 10.3389/fimmu.2018.03042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet RL, Beall M, Dunne DW, Pearce EJ. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J Immunol. 1999;163(9):4976–4984. [PubMed] [Google Scholar]
- 6.Barron L, Wynn TA. Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur J Immunol. 2011;41(9):2509–2514. doi: 10.1002/eji.201141869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26(1):376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savio LE, Mello PA, da Silva CG, Coutinho-Silva R. The P2X7 receptor in inflammatory diseases: Angel or Demon? Frontiers Pharmacol. 2018;9:52. doi: 10.3389/fphar.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann H. Extracellular ATP and other nucleotides-ubiquitous triggers of intercellular messenger release. Purinergic Signal. 2016;12(1):25–57. doi: 10.1007/s11302-015-9483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander SP, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, et al. The concise guide to pharmacology 2021/22: G protein-coupled receptors. Br J Pharmacol. 2021;176(Suppl 1):S27–156. [Google Scholar]
- 11.Zumerle S, Calı B, Munari F, Angioni R, Di Virgilio F, Molon B, et al. Intercellular Calcium signaling induced by ATP potentiates macrophage phagocytosis. Cell Rep. 2019;27(1):1–10.e4. doi: 10.1016/j.celrep.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques-da-Silva C, Burnstock G, Ojcius DM, Coutinho-Silva R. Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology. 2011;216(1–2):1–11. doi: 10.1016/j.imbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1b release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159(3):1451–1458. [PubMed] [Google Scholar]
- 14.Bajinka O, Qi M, Barrow A, Touray AO, Yang L, Tan Y. Pathogenicity of salmonella during Schistosoma-Salmonella co-infections and the importance of the gut microbiota. Curr Microbiol. 2022;79(1):26. doi: 10.1007/s00284-021-02718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chacha F, Mshana SE, Mirambo MM, Mushi MF, Kabymera R, Gerwing L, et al. Salmonella Typhi meningitis in a 9-year old boy with urinary schistosomiasis: a case report. BMC Res Notes. 2015;8:64. doi: 10.1186/s13104-015-1030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neal SE, Guimarães LH, Machado PR, Alcântara L, Morgan DP, Passos S, et al. Influence of helminth infections on the clinical course of immune response to Leishmania braziliensis cutaneous Leishmaniasis. J Infect Dis. 2007;195(1):142–148. doi: 10.1086/509808. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira SD, Nanini HF, Savio LE, Waghabi MC, Silva CL, Coutinho-Silva R. Macrophage P2X7 receptor function is reduced during schistosomiasis: putative role of TGF- β1. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/134974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorstenberg ML, Martins MDA, Figliuolo V, Silva CLM, Savio LEB, Coutinho-Silva R. P2Y2 receptor induces L. Amazonensis infection control in a mechanism dependent on Caspase-1 activation and IL-1β secretion. Mediators Inflamm. 2020 doi: 10.1155/2020/2545682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savio LE, Mello PA, Figliuolo VR, Almeida TFA, Santana PT, Oliveira SDS, et al. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J Hepatol. 2017;67(4):716–726. doi: 10.1016/j.jhep.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan KM, Delfert D, Junger KD. A direct colorimetric assay for Ca2+ -stimulated ATPase activity. Anal Biochem. 1986;157(2):375–3780. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 21.Martínez DY, Verdonck K, Kaye PM, Adaui V, Polman K, Llanos-Cuentas A, et al. Tegumentary leishmaniasis and coinfections other than HIV. PLoS Negl Trop Dis. 2018;12(3) doi: 10.1371/journal.pntd.0006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damania B, Dittmer DP. What lies within: coinfections and immunity. Cell Host Microbe. 2014;16(2):145–147. doi: 10.1016/j.chom.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosch M, Gerber J, Jebbawi F, Beldi G. Mechanisms of ATP release by inflammatory cells. Int J Mol Sci. 2018;19(4):1222. doi: 10.3390/ijms19041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acharya S, Da’dara AA, Skelly PJ. Schistosome immunomodulators. PLoS Pathog. 2021;17(12) doi: 10.1371/journal.ppat.1010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figliuolo VF, Chaves SP, Savio LEB, Thorstenberg MLP., Salles EM, Takiya CM, et al. The role of the P2X7 receptor in murine cutaneous leishmaniasis: aspects of inflammation and parasite control. Purinergic Signal. 2017;13(2):143–152. doi: 10.1007/s11302-016-9544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oswald IP, Gazzinelli RT, Sher A, James SL. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992;148(11):3578–3582. [PubMed] [Google Scholar]
- 27.Acuña SM, Aoki JI, Laranjeira da Silva MF, Zampieri RA, Fernandes JC, Muxel SM, et al. Arginase expression modulates nitric oxide production in Leishmania amazonensis. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Hunter CA, Farrel JP. Anti-TGF-β treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J Immunol. 1999;162(2):974–979. [PubMed] [Google Scholar]
- 29.Hassan MF, Zhang Y, Engwerda CR, Kaye PM, Sharp H, Bickle QD. The Schistosoma mansoni hepatic egg granuloma provides a favorable microenvironment for sustained growth of Leishmania donovani. Am J Pathol. 2006;169(3):943–953. doi: 10.2353/ajpath.2006.051319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James SL., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143(12):4208–4212. [PubMed] [Google Scholar]
- 31.Sanmarco LM, Ponce NE, Visconti LM, Eberhardt N, Theumer MG, Minguez AR, et al. IL-6 promotes M2 macrophage polarization by modulating purinergic signaling and regulates the lethal release of nitric oxide during Trypanosoma cruzi infection. Biochim Biophys Acta. 2017;1863(4):857–869. doi: 10.1016/j.bbadis.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4(162):ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 33.Dos Santos PL, Oliveira F, Santos MLB, Cunha LCS, Lino MTB, Oliveira MFS, et al. The severity of visceral leishmaniasis Correlates with elevated levels of serum IL-6, IL-27 and sCD14. PLoS Negl Trop Dis. 2016;10(1) doi: 10.1371/journal.pntd.0004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lévesque SA, Kukulski F, Enjyoji K, Robson SC, Sévigny J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol. 2010;40(5):1473–1485. doi: 10.1002/eji.200939741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira SD, Oliveira NF, Meyer-Fernandes JR, Savio LE, Ornelas FG, Ferreira ZS, et al. Increased expression of NTPDases 2 and 3 in mesenteric endothelial cells during schistosomiasis favors leukocyte adhesion through P2Y1 receptors. Vascul Pharmacol. 2016;82:66–72. doi: 10.1016/j.vph.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira SD, Coutinho-Silva R, Silva CL. Endothelial P2X7 receptors’ expression is reduced by schistosomiasis. Purinergic Signal. 2013;9(1):81–89. doi: 10.1007/s11302-012-9332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhardwaj R, Skelly PJ. Characterization of schistosome tegumental alkaline phosphatase (SmAP) PLoS Negl Trop Dis. 2011;5(4) doi: 10.1371/journal.pntd.0001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haskó G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xaus J, Mirabet M, Lloberas J, Soler C, Lluis C, Franco R, et al. IFN-g up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol. 1999;162(6):3607–3614. [PubMed] [Google Scholar]
- 40.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16(3):177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 41.Vijayamahantesh, Amit A, Kumar S, Dikhit MR, Jha PK, Singh AK, et al. Up regulation of A2B adenosine receptor on monocytes are crucially required for immune pathogenicity in Indian patients exposed to Leishmania donovani. Cytokine. 2016;79:38–44. doi: 10.1016/j.cyto.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 42.De Man JG, Seerden TC, De Winter BY, Van Marck EA, Herman AG, Pelckmans PA. Alteration of the purinergic modulation of enteric neurotransmission in the mouse ileum during chronic intestinal inflammation. Br J Pharmacol. 2003;139(1):172–184. doi: 10.1038/sj.bjp.0705218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giacomelli C, Daniele S, Romei C, Tavanti L, Neri T, Piano I, et al. The A2B adenosine receptor modulates the epithelial-mesenchymal transition through the balance of cAMP/PKA and MAPK/ERK pathway activation in human epithelial lung cells. Front Pharmacol. 2018;(9):54. doi: 10.3389/fphar.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marinho Y, Villarreal ES, Aboagye SY, Williams DL, Sun J, Silva CLM, et al. Schistosomiasis-associated pulmonary hypertension unveils disrupted murine gut-lung microbiome and reduced endoprotective Caveolin-1/BMPR2 expression. Front Immunol. 2023;14:1254762. doi: 10.3389/fimmu.2023.1254762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goto Y, Mizobuchi H. Pathological roles of macrophages in Leishmania infections. Parasitol Int. 2023;94 doi: 10.1016/j.parint.2023.102738. [DOI] [PubMed] [Google Scholar]