Abstract
This report is of a systematic review and meta-analysis evaluating the efficacy and safety of transcatheter arterial embolization (TAE) for nonvariceal gastrointestinal bleeding (GIB) with n-butyl cyanoacrylate (NBCA) or coils as the primary embolic agent. The primary outcome was the clinical success rate. The secondary outcomes were technical success rates, 30-day rebleeding rates, major complication rates, and 30-day overall mortality rates. A systematic search was performed in PubMed, Embase, and Cochrane Library. Articles included had been published in English from January 2000 to August 2023 and assessed patients with nonvariceal upper and lower GIB (UGIB and LGIB) who received TAE with NBCA or coils. Single-arm meta-analyses were performed for these outcomes. Subgroup analyses comparing NBCA and coils were conducted if there were more than 10 articles selected for each outcome. Thirty-seven articles were selected for analysis. The pooled rates of TAE for UGIB and LGIB were clinical success 73.0% and 76.5%, technical success 94.9% and 91.4%, 30-day rebleeding 25.0% and 17.1%, major complications 3.5% and 10.0%, and 30-day overall mortality 20.7% and 11.4%, respectively. The subgroup analysis showed a significant difference only for the technical success rates of LGIB between NBCA and the coils (p < 0.001). The systematic review and meta-analysis indicate that TAE with NBCA or coils as the primary embolic agent is safe and effective for both UGIB and LGIB.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79133-4.
Keywords: Embolization, Therapeutic; Gastrointestinal Hemorrhage; Radiology, Interventional; Systematic Review; Meta-Analysis
Subject terms: Lower gastrointestinal bleeding, Upper gastrointestinal bleeding
Introduction
Gastrointestinal bleeding (GIB) is a common medical emergency associated with significant morbidity and mortality1. Transcatheter arterial embolization (TAE) is a minimally invasive therapy that is widely used to control both nonvariceal upper (UGIB) and lower GIB (LGIB), especially when prior therapeutic endoscopy cannot achieve hemostasis2,3. TAE for GIB is often performed with n-butyl cyanoacrylate (NBCA) or coils as the primary embolic agent4,5.
Coils are highly thrombogenic and result in the complete occlusion of the target vessel after appropriate packing4. However, there is a size limitation to their use as they cannot always reach very small vessels, particularly if complex anastomoses have formed4,6. Moreover, coils are not always effective in patients affected by coagulopathy as their mechanism of action depends on the status of hemostasis4. In contrast, NBCA is not limited by those factors as it can be injected via very-small-caliber micro catheters and reach very small vessels to block the vessels independently of the status of hemostasis7. However, there is concern about the risk of reflux during the injection and of nontarget embolization with potential splanchnic or visceral ischemic complications8,9.
Thus, the choice of the optimal embolic agent for TAE for GIB is still debatable10in the absence of published guidelines11. Furthermore, randomized controlled trials (RCTs) comparing embolic agents would be difficult in patients with GIB because such patients require emergency treatment12. Therefore, a systematic review and meta-analysis integrating NBCA and coil data on TAE for GIB would not only provide an overview of TAE for GIB, but also allow subgroup analyses of NBCA and coils to assess each embolic agent.
Although there have been two systematic reviews and meta-analyses on TAE with only NBCA for GIB7,10, no systematic review and meta-analysis integrating TAE with NBCA and coils as the primary embolic agent for GIB has been published. Thus, our objective was to conduct a systematic review and meta-analysis to evaluate the efficacy and safety of TAE with NBCA or coils as the primary embolic agent for GIB as the primary embolic agent.
Materials and methods
We used guidelines from the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) to compile this report. The protocol was registered in PROSPERO (CRD42023458652).
Search strategy
With the assistance of librarians, PubMed, the Cochrane Library, and Embase data resource were systematically searched for articles published from January 2000 to August 2023 using relevant Medical Subject Heading (MeSH) terms and keywords (Supplement Table S1).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) articles written in English; (2) availability of full-text articles; (3) RCTs and observational studies; and (4) articles reporting data and results of the use of TAE with either NBCA or coils as the primary embolic agent for acute nonvariceal GIB. The exclusion criteria were as follows: (1) case reports; (2) review articles; (3) letters and editorials; (4) preclinical studies; (5) articles with a sample size of < 5 cases; (6) articles with no extractable data; (7) articles reporting mixed results from different techniques or results from other techniques; and (8) articles with data included in subsequent articles or duplicate reports.
Outcomes and data extraction
The primary outcome was the clinical success rate. The following secondary outcomes were also investigated: technical success rate, 30-day rebleeding rate, major complication rate, and 30-day mortality rate.
For each study, two reviewers (T. M. and J. S.) independently retrieved information on the outcomes and study characteristics, including the first author, publication year, study country, study design, patient characteristics, and bleeding location (UGIB or LGIB). For all articles, the following data were extracted separately for both UGIB and LGIB: NBCA-lipiodol ratio; number of coils; type of coils (pushable or detachable coils); technical success, which was defined as the complete cessation of contrast extravasation or blood flow from the bleeding artery; clinical success, which was defined as no rebleeding, surgical resection as a result of major complication or GIB-related mortality within 30 days of TAE (if patients died of any cause unrelated to GIB within 30 days, they were excluded from the analysis of the clinical success.); 30-day rebleeding; major complications; and 30-day mortality. The third reviewer (R. Y.) was consulted if any discrepancies arose in the two reviewers’ findings. The complications were classified in accordance with the classification system of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE), i.e., from grade 1 (no complication) to grade 6 (death)13. Grades 3–6 were defined as major complications.
Risk of bias assessment in the included studies
The quality of the included studies was assessed using version 2 of the Cochrane Collaboration tool for RCTs (RoB2)14and the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS)15.
Analysis of subgroups
Subgroup analyses were conducted to compare NBCA and coils if there were > 10 articles selected for each outcome.
Statistical analysis
The statistical technique chosen for this study was a single-arm meta-analysis, incorporating subgroup analysis to compare embolic agents. Where comparative studies were available, single-arm data were extracted from those studies and included in the meta-analysis. As considerable between-study heterogeneity was anticipated in the primary outcome, a random-effects model was used to pool the effect sizes. The restricted maximum likelihood estimator was used to calculate the heterogeneity variance τ2. The Knapp-Hartung adjustment was used to calculate the confidence interval around the pooled effect. With a forest plot to display the outcome data from the respective studies, the following heterogeneity measures were assessed: τ2, I², and Q-statistics. For the I² statistic, values of < 25% were defined as low heterogeneity, 25–50% as moderate, and > 50% as high heterogeneity. A meta-regression analysis was conducted to identify the source of inter-study heterogeneity when I² was greater than 50%. For the meta-regression analysis, a value of p < 0.05 was used to identify the source of heterogeneity. Funnel plots and Egger’s test were used to analyze publication bias; values of p< 0.05 were considered statistically significant in Egger’s test. Egger’s test and meta-regression analysis were performed if at least four articles were selected for each meta-analysis. Sensitivity analyses were conducted by excluding articles with < 10 cases. The same method was also used for the analysis of the secondary outcomes. Descriptive and basic statistics were analyzed using Microsoft Excel (version16.66.1). The meta package of R software (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) was used for the meta-analyses. Quality assessment plots were produced using risk-of-bias visualization ‘robvis’16. For the summary of RoBANS, studies were considered to have an overall low risk of bias if they were classified as low risk in all six domains. Studies were considered to have an unclear risk of bias if at least one domain was rated as unclear risk (but no domains were rated as high risk) and as high risk if at least one domain was rated as high risk.
Results
Search results
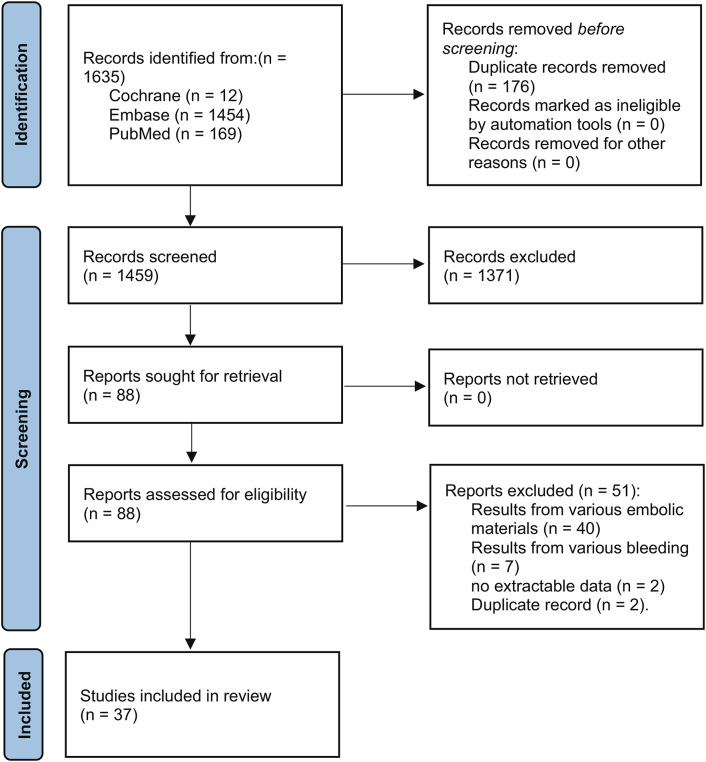
At first, literature searches identified a total of 1635 records. However, 1459 studies were left to be screened after eliminating duplicates. Following the evaluation of titles and abstracts, 1371 studies were eliminated, leaving 88 of these records to undergo a full-text review. Finally, 51 articles were excluded by applying the eligibility criteria, leaving 37 articles for systematic review and meta-analysis of the efficacy and safety of TAE for GIB with NBCA or coils4–6,8,11,12,17–47 (Fig. 1).
Fig. 1.
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram of the article selection process.
Risk-of-bias assessment
As none of the included articles had reported RCTs, none were evaluated in RoB2. Thirty two of the included articles were on single-arm retrospective studies, four articles were on retrospective case–control studies, and one article was on a single-arm prospective study. The selected articles were assessed as high (n = 34), unclear (n = 2), and low (n = 1) by RoBANS (Fig. 2).
Fig. 2.
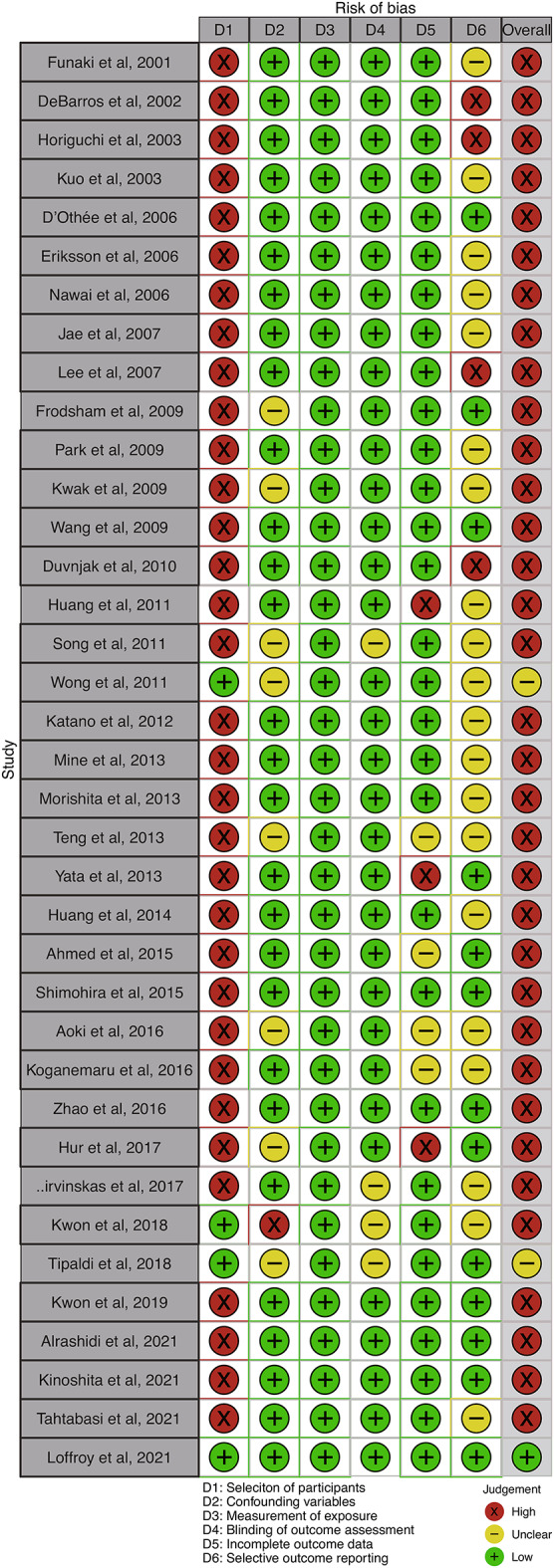
Risk-of-bias assessment. Risk-of-bias assessments were performed using the Risk-of-Bias Assessment Tool for Nonrandomized Studies (RoBANS). Green (+) indicates low risk of bias; yellow (-) indicates unclear risk of bias; and red (×) indicates high risk of bias. For the summary of risk of bias, studies were considered to have an overall low risk of bias (green +) if they were classified as low risk in all six domains. Studies were considered to have an unclear risk of bias (yellow -) if at least one domain was rated as unclear risk (but no domains were rated as high risk) and as high risk (red ×) if at least one domain was rated as high risk.
Characteristics of the included studies
The 37 selected articles involved a total of 989 cases (Tables 1 and 2). The average-weighted mean age of the subjects was 67 years. There were 67% males and 33% females in the 33 articles. Nineteen articles dealt with UGIB4,6,8,11,22,24,27,28,30–34,37,40,43,44,46,47, 16 articles with LGIB5,17–21,23,25,26,29,35,38,39,41,42,45, and two articles12,36with both. Of the 21 articles that addressed UGIB, 13 articles6,8,11,12,24,27,33,34,36,37,40,43,46reported the use of NBCA and 8 articles4,22,28,30–32,44,47of coils as the primary embolic agent. Of the 13 articles on the use of NBCA for UGIB, the NBCA-lipiodol ratio ranged from 20 to 50% in 12 articles6,8,11,12,24,27,33,36,37,40,43,46, while the most diluted NBCA-lipiodol ratio was 17% in the remaining article34. Of the eight articles on the use of coils for nonvariceal UGIB, three articles involved pushable coils22,31,47, three articles pushable or detachable coils4,32,44, while the remaining two articles did not clarify the nature of the coils28,30. The number of coils used was not stated in any of the eight articles. Of the 13 articles reporting the use of NBCA, eight articles described coagulopathy6,11,34,36,37,40,43,46. Of the 8 articles reporting the use of coils, one article described coagulopathy4. Of the 18 articles that addressed LGIB, five articles reported the use of NBCA12,25,29,36,42, 12 articles of coil5,17–21,23,26,35,38,39,41, and one article of coil or NBCA45as the primary embolic agent. The NBCA-lipiodol ratio ranged from 20 to 50% in three articles on the use of NBCA for nonvariceal LGIB12,42,45. However, the most diluted NBCA-lipiodol ratio was 10% and 17%, respectively, in the other two papers on NBCA29,36; the NBCA-lipiodol ratio was unknown in the remaining article25. Of 13 articles reporting the use of coils for UGIB, nine articles were on pushable coils17–21,23,26,35,45, one article on pushable or detachable coils38, and the remaining three articles on detachable coils5,39,41. The average-weighted mean number of coils used was 3.5 in the 5 articles in which the average number of coils could be verified19,21,23,39,41. Of 13 articles on coils, two articles described coagulopathy5,41, while three of six articles on NBCA described coagulopathy25,36,42. One article was unclear as to the number of patients in the NBCA and coil groups who had developed coagulopathy45.
Table 1.
Characteristics of the included studies on transcatheter arterial embolization for upper gastrointestinal bleeding.
| Author | Study type | Study period | Country | No. of patients | Sex (M/F) | Mean age | Primary embolic agent | NBCA-lipiodol ratio (%) | No. of coils | Type of coils | Coagulopathy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eriksson et al., 200622 | R | 2003–2005 | Sweden | 10 of 13 | 5/5 | 75 | Coil | NA | NR | Pushable | NR |
| Jae et al., 20078 | P | 1999–2002 | Korea | 32 of 32 | 28/4 | 59 | NBCA | 25 to 50 | NA | NA | NR |
| Lee et al., 200724 | R | 2004–2005 | Taiwan | 16 of 16 | 11/5 | 68 | NBCA | 20 to 30 | NA | NA | NR |
| Park et al., 200927 | R | 2000–2008 | Korea | 5 of 5 | 4/1 | 57 | NBCA | 20 to 50 | NA | NA | NR |
| Wang et al., 20096 | R | 2004–2009 | China | 20 of 20 | 13/7 | 63 | NBCA | 25 to 50 | NR | NA | 8 of 17 |
| Duvnjak et al., 201028 | R | 2007–2009 | Denmark | 40 of 40 | 26/14 | 67 | Coil | NA | NR | NR | NR |
| Song et al., 201130 | R | 2006–2007 | Korea | 12 of 16 | 7/5 | 58 | Coil | NA | NR | NR | NR |
| Wong et al., 201131 | RC | 2000–2009 | China | 32 of 88 | 21/11 | 73 | Coil | NA | NR | Pushable | NR |
| Katano et al., 201232 | R | 2004–2010 | Japan | 15 of 15 | 12/3 | 62 | Coil | NA | NR | Detachable or pushable | NR |
| Mine et al., 201333 | R | 2006–2012 | Japan | 21 of 21 | 17/4 | 66 | NBCA | 20 to 50 | NA | NA | NR |
| Morishita et al., 201334 | R | 2006–2011 | Japan | 15 of 15 | 12/3 | 68 | NBCA | 17 to 40 | NA | NA | 3 of 15 |
| Yata et al., 201336 | R | 2005–2012 | Japan | 16 of 37 | 8/8 | 69 | NBCA | 25 to 50 | NA | NA | 1 of 16 |
| Huang et al., 201437 | R | 2008–2012 | Taiwan | 49 of 49 | 31/18 | 67 | NBCA | 20 to 50 | NA | NA | 16 of 49 |
| Aoki et al., 201640 | R | 2008–2014 | Japan | 5 of 5 | 5/0 | 71 | NBCA | 25 to 40 | NA | NA | 4 of 5 |
| Hur et al., 201743 | R | 2006–2015 | Korea | 152 of 152 | 109/43 | 66 | NBCA | 25 to 33 | NA | NA | 53 of 152 |
| Širvinskas et al., 201744 | R | 2013–2015 | Lithuania | 36 of 36 | 27/9 | 66 | Coil | NA | NR | Detachable or pushable | NR |
| Kwon et al., 201812 | R | 2007–2016 | Korea | 15 of 46 | NR | NR | NBCA | 20 to 50 | NA | NA | NR |
| Tipaldi et al., 20184 | R | 2011–2017 | Italy | 41 of 71 | NR | 60 | Coil | NA | NR | Detachable or pushable | 9 of 41 |
| Alrashidi et al., 202146 | RC | 2004–2020 | Saudi Arabia | 5 of 9 | 5/0 | 64 | NBCA | 25 | NA | NA | 1 of 5 |
| Tahtabasi et al., 202147 | R | 2018–2019 | Turkey | 6 of 18 | 6/0 | 53 | Coil | NA | NR | Pushable | NR |
| Loffroy et al., 202111 | R | 2008–2019 | France | 78 of 148 | 52/26 | 72 | NBCA | 25 to 50 | NA | NA | 24 of 78 |
NA, not applicable; NBCA, n-butyl cyanoacrylate; No., number; NR, not reported; R, retrospective; RC, retrospective comparative.
Table 2.
Characteristics of the included studies on transcatheter arterial embolization for lower gastrointestinal bleeding.
| Author | Study type | Study period | Country | No. of patients | Sex (M/F) | Mean age | Primary embolic agent | NBCA-lipiodol ratio (%) | No. of coils (Mean) |
Type of coils | Coagulopathy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Funaki et al., 200117 | R | 1995–2000 | US | 27 of 27 | 16/11 | 72 | Coil | NA | NR | Pushable | NR |
| DeBarros et al., 200218 | R | 1993–1999 | US | 16 of 27 | 10/6 | 69 | Coil | NA | NR | Pushable | NR |
| Horiguchi et al., 200319 | R | NR | Japan | 6 of 14 | 4/2 | 65 | Coil | NA | 4–5 (4.2) | Pushable | NR |
| Kuo et al., 200320 | R | 1992–2002 | US | 22 of 22 | 11/11 | 62 | Coil | NA | NR | Pushable | NR |
| D’Othée et al., 200621 | R | 1997–2004 | US | 19 of 19 | 13/6 | 70 | Coil | NA | 2–6 (3) | Pushable | NR |
| Nawai et al., 200623 | R | 2002–2003 | Malaysia | 6 of 6 | 2/4 | 74 | Coil | NA | 2–12 (7.2) | Pushable | NR |
| Frodsham et al., 200925 | R | 2005–2009 | US | 14 of 14 | 9/5 | 77 | NBCA | NR | NA | NA | 5 of 14 |
| Kwak et al., 200926 | R | 2003–2007 | Korea | 17 of 36 | 13/4 | 65 | Coil | NA | NR | Pushable | NR |
| Huang et al., 201129 | R | 2006–2008 | Taiwan | 27 of 27 | 22/5 | 63 | NBCA | 10 to 40 | NA | NA | NR |
| Teng et al., 201335 | R | 1997–2009 | Korea | 26 of 26 | 19/7 | 69 | Coil | NA | NR | Pushable | NR |
| Yata et al., 201336 | R | 2005–2012 | Japan | 21 of 37 | 16/5 | 67 | NBCA | 17 to 50 | NA | NA | 10 of 21 |
| Ahmed et al., 201538 | R | 2002–2012 | US | 38 of 39 | NR | 60 | Coil | NA | NR | Detachable or pushable | NR |
| Shimohira et al., 201539 | R | NR | Japan | 5 of 5 | 2/3 | 76 | Coil | NA | 1–4 (2.4) | Detachable | NR |
| Koganemaru et al., 201641 | R | 2010–2014 | Japan | 5 of 5 | 4/1 | 59 | Coil | NA | 1–2 (1.6) | Detachable | 2 of 5 |
| Zhao et al., 201642 | R | 2013–2015 | China | 7 of 7 | 2/5 | 70 | NBCA | 25 to 33 | NA | NA | 0 of 7 |
| Kwon et al., 201812 | R | 2007–2016 | Korea | 6 of 46 | NR | NR | NBCA | 20 to 50 | NA | NA | NR |
| Kwon et al., 201945 | RC | 2005–2017 | Korea | 81 of 127 | NR | NR | NBCA | 25 to 50 | NA | NA | NR |
| Kwon et al., 201945 | RC | 2005–2017 | Korea | 8 of 127 | NR | NR | Coil | NA | NR | Pushable | NR |
| Kinoshita et al., 20215 | R | 2013–2019 | Japan | 17 of 17 | 15/2 | 69 | Coil | NA | 2–8 | Detachable | 1 of 17 |
NA, not applicable; NBCA, n-butyl cyanoacrylate; No., number; NR, not reported; R, retrospective; RC, retrospective comparative.
Analysis of primary and secondary outcomes
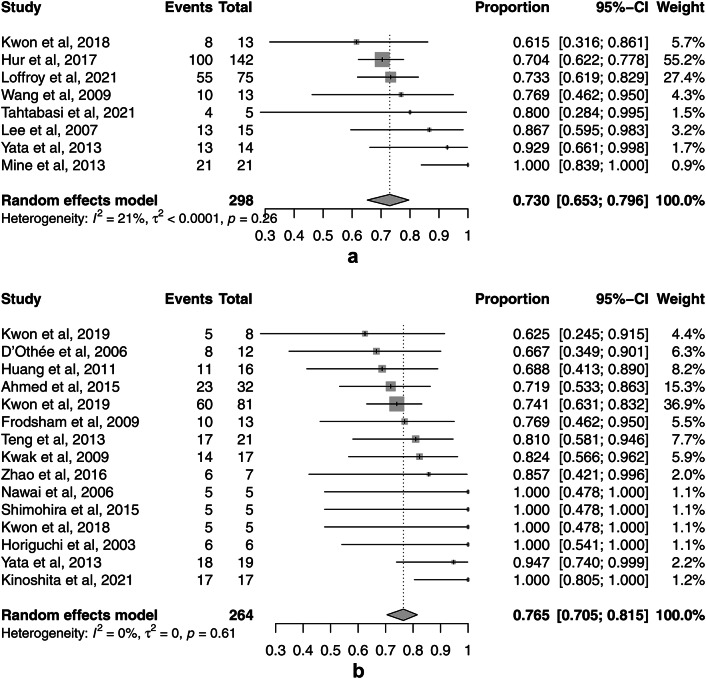
The pooled clinical success rates of TAE for UGIB and LGIB, which were the primary outcomes, were 73.0% (95% CI, 65.3–79.6%, I2 = 21.5%) (Fig. 3a) and 76.5% (95% CI, 70.5–81.5%, I2 = 0%) (Fig. 3b), respectively. There was significant publication bias for the clinical success rates of TAE for UGIB (p = 0.043) and LGIB (p = 0.006) in Egger’s test and the funnel plots (Supplement Fig. S1).
Fig. 3.
Forest plots of the pooled clinical success rates of transcatheter arterial embolization (TAE) for nonvariceal upper gastrointestinal bleeding (UGIB) and lower gastrointestinal bleeding (LGIB). (a) The forest plot of the pooled clinical success rate of TAE for UGIB. (b) The forest plot of the pooled clinical success rate of TAE for LGIB.
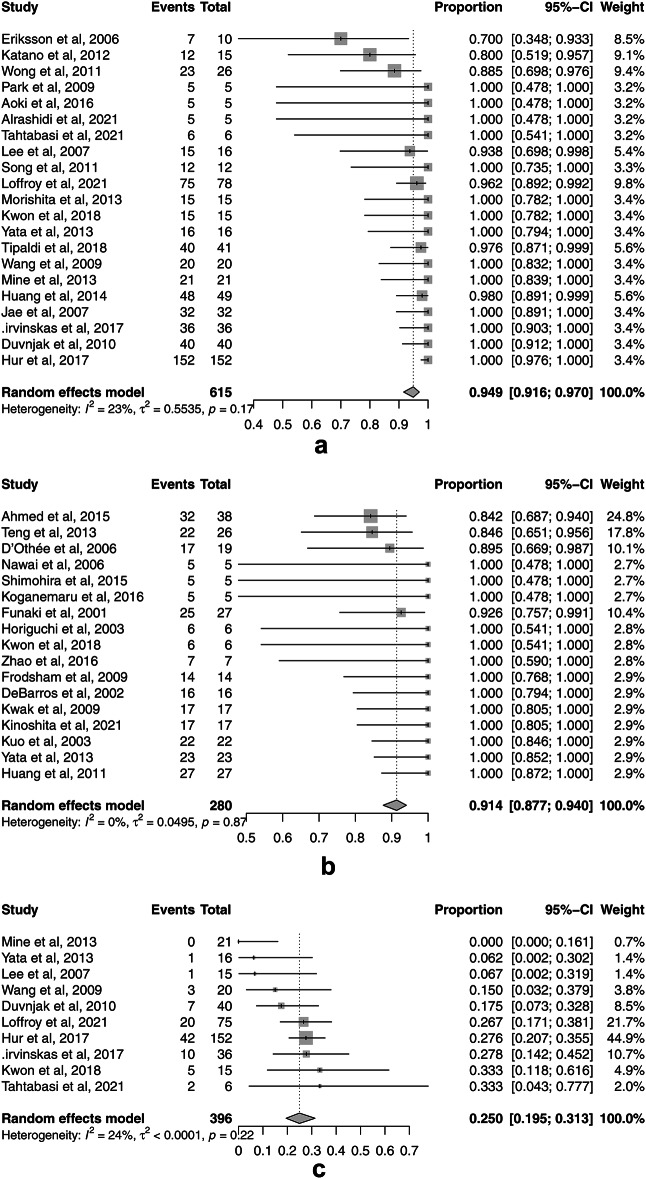
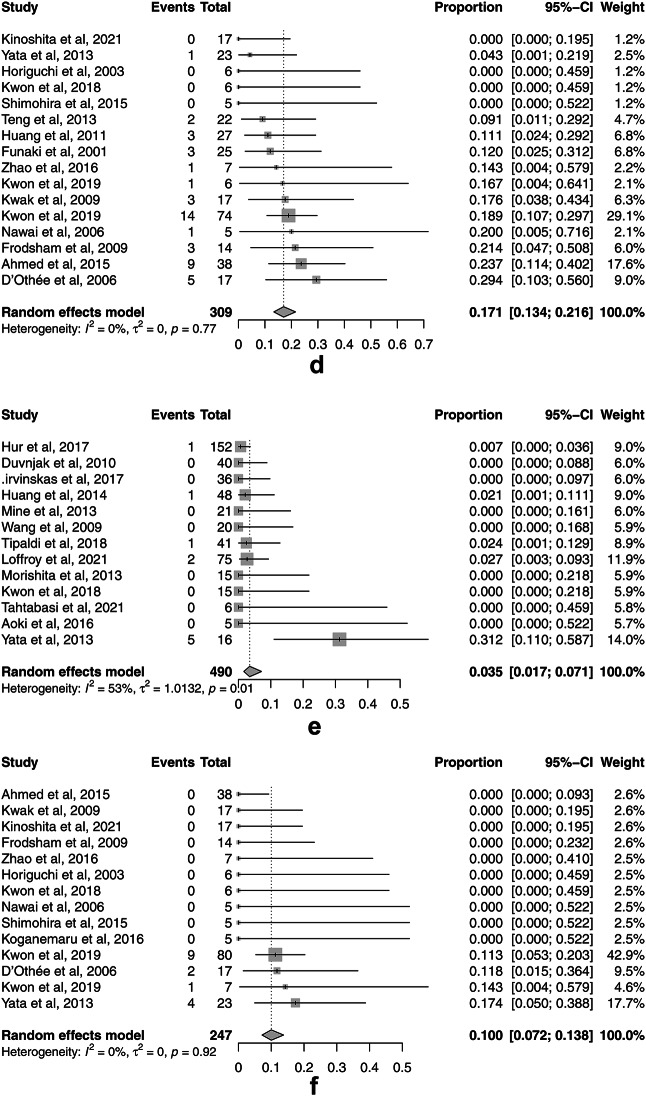
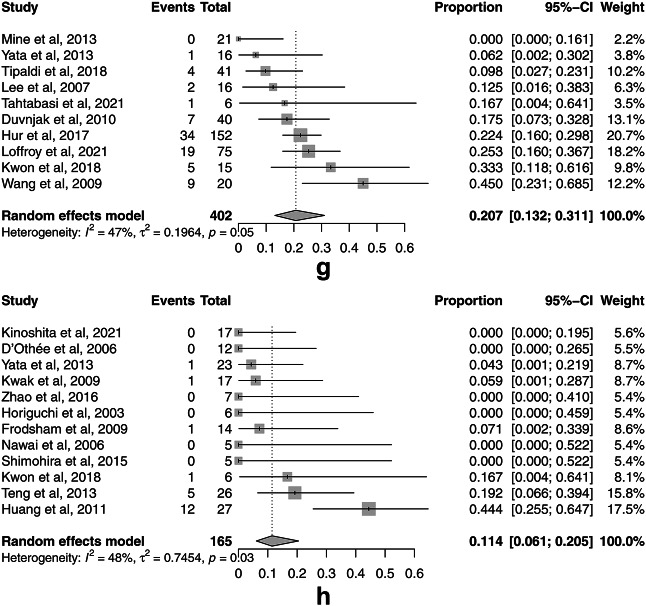
The rates for the secondary outcomes of TAE for UGIB and LGIB were as follows: pooled technical success 94.9% (95% CI, 91.6–97.0%, I2 = 23.1%) (Fig. 4a) and 91.4% (95% CI, 87.7–94.0%, I2 = 0%) (Fig. 4b); 30-day rebleeding 25.0% (95% CI, 19.5–31.3%, I2 = 24.4%) (Fig. 4c) and 17.1% (95% CI, 13.4–21.6%, I2 = 0%) (Fig. 4d); major complications 3.5% (95% CI, 1.7–7.1%, I2 = 52.8%) (Fig. 4e) and 10.0% (95% CI, 7.2–13.7%, I2 = 0%) (Fig. 4f); and 30-day overall mortality 20.7% (95% CI, 13.2–31.1%, I2 = 46.7%) (Fig. 4g) and 11.5% (95% CI, 6.1–20.5%, I2 = 48.2%) (Fig. 4h), respectively. There was no significant publication bias for only 30-day overall mortality for UGIB assessed by Egger’s test (p = 0.199) and the funnel plots (Supplement Fig. S2g). There was significant publication bias for technical success rates for UGIB (p = 0.006) and LGIB (p < 0.001), 30-day rebleeding for UGIB (p = 0.022) and LGIB (p = 0.008), major complications for UGIB (p = 0.023) and LGIB (p = 0.010), and 30-day overall mortality for LGIB (p < 0.001) as assessed by Egger’s test and the funnel plots (Supplement Fig. S2a-f, h).
Fig. 4.
Forest plots of the pooled secondary outcomes of transcatheter arterial embolization (TAE) for nonvariceal upper gastrointestinal bleeding (UGIB) and lower gastrointestinal bleeding (LGIB). (a) The forest plot of the pooled technical success rate of TAE for UGIB. (b) The forest plot of the pooled technical success rate of TAE for LGIB. (c) The forest plot of the pooled 30-day rebleeding rate of TAE for UGIB. (d) The forest plot of the pooled 30-day rebleeding rate of TAE for LGIB. (e) The forest plot of the pooled major complication rate of TAE for UGIB. (f) The forest plot of the pooled major complication rate of TAE for LGIB. (g) The forest plot of the pooled 30-day overall mortality rate of TAE for UGIB. (h) The forest plot of the pooled 30-day overall mortality rate of TAE for LGIB.
As I² was > 50% for the major complications of TAE for UGIB, meta-regression analysis was conducted. There was no evidence that the publication year or the study region (Asia or the other region) influenced the values of the major complication rates of TAE for UGIB (Table 3). The major complication rates of TAE for UGIB were only influenced by the sample size (p = 0.028) (Table 3).
Table 3.
Meta regression analysis of major complication rates of TAE for nonvariceal UGIB.
| R 2 | 95% CI | p-value | |
|---|---|---|---|
| Publication year | 0% | -0.261 to 0.178 | 0.682 |
| Sample size | 36.4% | -0.035 to -0.002 | 0.028 |
| Country (Asia or the other) | 0% | -2.225 to 1.167 | 0.507 |
CI, Confidence interval; TAE, transcatheter arterial embolization; UGIB, upper gastrointestinal bleeding.
Subgroup analysis
Subgroup analysis could be performed for all outcomes except for the clinical success rates for UGIB (Table 4, Supplement Fig. S3). The subgroup analysis showed a significant difference only for the technical success rates of LGIB between NBCA and coils (p < 0.001) (Table 5, Supplement Fig. S4).
Table 4.
Subgroup analyses of TAE for nonvariceal UGIB.
| Proportion | 95%CI | I2 | p subgroup | |
|---|---|---|---|---|
| Technical success rate (k = 21) | 0.060 | |||
| Coils (k = 8) | 0.918 | 0.789–0.971 | 44.7% | |
| NBCA (k = 13) | 0.967 | 0.949–0.979 | 0% | |
| 30-day rebleeding rate (k = 10) | 0.759 | |||
| Coils (k = 3) | 0.237 | 0.105–0.451 | 0% | |
| NBCA (k = 7) | 0.253 | 0.179–0.345 | 42.0% | |
| Major complication rate (k = 13) | 0.358 | |||
| Coils (k = 4) | 0.023 | 0.007–0.072 | 0% | |
| NBCA (k = 9) | 0.039 | 0.014–0.103 | 64.2% | |
| 30-day overall mortality rate (k = 10) | 0.054 | |||
| Coils (k = 3) | 0.143 | 0.059–0.305 | 0% | |
| NBCA (k = 7) | 0.244 | 0.146–0.378 | 50.7% |
CI, confidence interval; NBCA, n-butyl cyanoacrylate; TAE, transcatheter arterial embolization; UGIB, upper gastrointestinal bleeding.
Table 5.
Subgroup analyses of TAE for nonvariceal LGIB.
| Proportion | 95%CI | I2 | p subgroup | |
|---|---|---|---|---|
| Clinical success rate (k = 15) | 0.851 | |||
| Coils (k = 9) | 0.770 | 0.667–0.849 | 0% | |
| NBCA (k = 6) | 0.760 | 0.657–0.840 | 0% | |
| Technical success rate (k = 17) | < 0.001 | |||
| Coils (k = 12) | 0.896 | 0.850–0.930 | 0% | |
| NBCA (k = 5) | 0.965 | 0.925–0.984 | 0% | |
| 30-day rebleeding rate (k = 16) | 0.619 | |||
| Coils (k = 10) | 0.180 | 0.124–0.254 | 0% | |
| NBCA (k = 6) | 0.161 | 0.104–0.241 | 0% | |
| Major complication rate (k = 14) | 0.113 | |||
| Coils (k = 9) | 0.072 | 0.039–0.127 | 0% | |
| NBCA (k = 5) | 0.117 | 0.071–0.185 | 0% | |
| 30-day overall mortality rate (k = 12) | 0.499 | |||
| Coils (k = 7) | 0.098 | 0.049–0.188 | 0% | |
| NBCA (k = 5) | 0.145 | 0.033–0.455 | 67.0% |
CI, confidence interval; LGIB, lower gastrointestinal bleeding; NBCA, n-butyl cyanoacrylate; TAE, transcatheter arterial embolization.
Sensitivity analysis
The data of the sensitivity analyses largely overlap with those of the primary analyses, and subgroup analyses showed similar results, indicating the robustness of the findings (Supplement Table S2–5).
Discussion
This systematic review and meta-analysis investigated the efficacy and safety of TAE with NBCA or coils as the primary embolic agent for GIB and explored the differences between NBCA and coils in subgroup analyses. Our present findings may promote the understanding of TAE for GIB and act as a decision-making reference in clinical practice.
Regarding literature collection, previous systematic reviews of TAE for GIB with NBCA had collected articles since 1980 10 or 1990 7. Given the changes in technology, including improvements in digital subtraction angiography, catheter systems, and microcatheters, this systematic review and meta-analysis focused on articles published since 2000. Moreover, article collection was done to be as up-to-date as possible, that is, through August 2023 because there have been several new articles on TAE for GIB5,11,46,47after the publication of previous systematic reviews7,10.
There was significant publication bias in all outcomes except for 30-day overall mortality for UGIB in this study. This could be attributed to the tendency for “positive” results to be published, whereas “negative” results are often rejected or not even submitted.
Owing to the presence of rich anastomoses between adjacent arteries in the upper gastrointestinal tract, TAE of both feeding arteries and potential collateral channels is required for effective hemostasis8. TAE with NBCA or coils as the primary embolic agent for UGIB was found to be safe and effective in this systematic review and meta-analysis. Because there were only eight articles on the clinical success rate for UGIB in this meta-analysis, subgroup analysis comparing NBCA and coils was not conducted. Seven of the eight articles on the clinical success rates of TAE for UGIB reported the use of NBCA as the primary embolic agent. These results suggest that NBCA is the preferred primary embolic agent for TAE for UGIB in current clinical practice. A recent retrospective study with a relatively large sample size reported that NBCA as the primary agent allowed for faster and better clinical success than other embolic agents when used for TAE to safely stop refractory peptic ulcer bleeding11. However, the subgroup analyses showed no significant differences between NBCA and coils for all secondary outcomes of TAE for UGIB in this study. Caution should be exercised in interpreting these results as these subgroup analyses may lack the statistical power necessary to detect subgroup differences.
Because some of the articles were unclear on the mean age and sex ratio, meta-regression analyses could not be conducted for these factors on major complications of TAE for UGIB. The finding that the major complication rates of TAE for UGIB was influenced only by sample size (p = 0.028) may refer to the observed correlation between the volume of cases treated in TAE for UGIB and the outcomes for the patients, which indicates a volume–outcome relationship.
The vascular network in the lower gastrointestinal tract is smaller than that in the upper gastrointestinal tract45. Therefore, one of the major complications of TAE for LGIB is bowel infarction. Although the major complication rates of LGIB (10.0% (95% CI, 7.2–13.7%)) were higher than those of UGIB (3.5% (95% CI, 1.7–7.1%,)), TAE with NBCA or coils as the primary embolic agent for LGIB was found to be safe and effective in this systematic review and meta-analysis. There were no significant differences in the clinical success rates, 30-day rebleeding rates, major complication rates, and 30-day overall mortality rates between TAE with coils and NBCA for LGIB in the subgroup analyses. Animal studies have shown that bowel ischemia or infarction can be prevented by limiting NBCA embolization to three or fewer vasa recta48. However, caution is necessary to avoid inadvertent embolization of non-target vessels and extensive embolization, which may occur when an excessively large volume of NBCA is used, or when the mixture is injected at an inappropriate rate9,25. In contrast, the subgroup analysis showed a significant difference in the technical success rates of LGIB between NBCA and coils (p= 0.0009). However, recently, the development of smaller microcatheters with various detachable coils has made superselective embolization possible, improving the technical success rate of TAE with coils for LGIB5,39,41. Therefore, whether coils or NBCA should be the first choice in TAE for LGIB may require further study.
Out of 14 articles describing coagulopathy, coils and NBCA were used as the primary embolic agents in three and 11 articles, respectively. These results suggest that coil embolization does not always stop bleeding in patients with coagulopathy. However, the shorter procedural time associated with the use of NBCA than with microcoil embolization is particularly valuable in patients with life-threatening bleeding49,50. This is important in cases of massive bleeding that require urgent hemostasis. Therefore, if coagulopathy has been apparent in advance, or if hemostasis is determined to be necessary in a short procedural time, the use of NBCA should be considered for TAE for UGIB and LGIB. Naturally, to achieve hemostasis with TAE using NBCA as the primary embolic agent for UGIB or LGIB, sufficient training is required and a learning curve should be considered to minimize possible ischemic complications8,9,25,51.
Several limitations should be mentioned when interpreting the results. First, most of the included articles had a retrospective design and a high overall risk of bias. Second, unfortunately, only four comparative studies were identified, which precluded comparative meta-analyses. Thus, the focus of the study was on obtaining single-arm pooled estimates and performing subgroup analyses. Third, the study period is broad, and there may be considerable differences in clinical and technical practices. Despite these limitations, we believe that this systematic review and meta-analysis provides useful information for the current clinical utility of TAE for GIB.
In conclusion, the systematic review and meta-analysis presented here indicate that TAE with NBCA or coils as the primary embolic agent is safe and effective for both nonvariceal UGIB and LGIB.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by T. M., M. O., J. S. and R. Y. Data analyses and preparing figures were performed by T. M. and T. H. The first draft of the manuscript was written by T. M. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
All generated or analyzed data during this study have been included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable. This study did not involve human participants.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peery, A. F. et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 143, 1179–1187e1173. 10.1053/j.gastro.2012.08.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hastings, G. S. Angiographic localization and transcatheter treatment of gastrointestinal bleeding. Radiographics. 20, 1160–1168. 10.1148/radiographics.20.4.g00jl361160 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Loffroy, R. et al. When all else fails - radiological management of severe gastrointestinal bleeding. Best Pract. Res. Clin. Gastroenterol.42–4310.1016/j.bpg.2019.04.005 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Tipaldi, M. A. et al. Trans arterial embolization of non-variceal Upper gastrointestinal bleeding: is the Use of Ethylene–Vinyl Alcohol Copolymer as Safe as coils? Cardiovasc. Interv. Radiol.41, 1340–1345. 10.1007/s00270-018-1981-5 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita, M. et al. Ultraselective transcatheter arterial embolization with small-sized microcoils for acute lower gastrointestinal bleeding. CVIR Endovascular. 410.1186/s42155-021-00215-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, Y. J. et al. N-butyl cyanoacrylate embolization for control of nonvariceal gastric bleeding: a 4.5-year retrospective study of treatment and outcome. Chin. J. Radiol.34, 85–92 (2009). [Google Scholar]
- 7.Kim, P. H., Tsauo, J., Shin, J. H. & Yun, S. C. Transcatheter arterial embolization of gastrointestinal bleeding with N-Butyl cyanoacrylate: a systematic review and Meta-analysis of Safety and Efficacy. J. Vasc. Interv. Radiol.28, 522–531e525. 10.1016/j.jvir.2016.12.1220 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Jae, H. J., Chung, J. W., Jung, A. Y., Lee, W. & Park, J. H. Transcatheter arterial embolization of nonvariceal upper gastrointestinal bleeding with N-butyl cyanoacrylate. Korean J. Radiol.8, 48–56. 10.3348/kjr.2007.8.1.48 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo, H. J. et al. Clinical outcome of transcatheter arterial embolization with N-butyl-2-cyanoacrylate for control of acute gastrointestinal tract bleeding. Am. J. Roentgenol.204, 662–668. 10.2214/AJR.14.12683 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Chevallier, O. et al. Efficacy, safety and outcomes of transcatheter arterial embolization with N-butyl cyanoacrylate glue for non-variceal gastrointestinal bleeding: a systematic review and meta-analysis. Diagn. Interv. Imaging. 102, 479–487. 10.1016/j.diii.2021.03.004 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Loffroy, R. et al. Ten-year experience with arterial embolization for peptic ulcer bleeding: N-butyl cyanoacrylate glue versus other embolic agents. Eur. Radiol.31, 3015–3026. 10.1007/s00330-020-07427-y (2021). [DOI] [PubMed] [Google Scholar]
- 12.Kwon, J. H. & Han, Y. H. Efficacy and safety of superselective trans-catheter arterial embolization of upper and lower gastrointestinal bleeding using N-butyl-2-cyanoacrylate. Emerg. Radiol.25, 111–120. 10.1007/s10140-017-1552-0 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Filippiadis, D. K. et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc. Interv. Radiol.40, 1141–1146. 10.1007/s00270-017-1703-4 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 366, l4898. 10.1136/bmj.l4898 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Kim, S. Y. et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol.66, 408–414. 10.1016/j.jclinepi.2012.09.016 (2013). [DOI] [PubMed] [Google Scholar]
- 16.McGuinness, L. A. & Higgins, J. P. T. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods. 12, 55–61. 10.1002/jrsm.1411 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Funaki, B. et al. Superselective microcoil embolization of colonic hemorrhage. Am. J. Roentgenol.177, 829–836. 10.2214/ajr.177.4.1770829 (2001). [DOI] [PubMed] [Google Scholar]
- 18.DeBarros, J. et al. The changing paradigm for the treatment of colonic hemorrhage: superselective angiographic embolization. Dis. Colon Rectum. 45, 802–808. 10.1007/s10350-004-6301-2 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Horiguchi, J. et al. Morphologic and histopathologic changes in the bowel after super-selective transcatheter embolization for focal lower gastrointestinal hemorrhage. Acta Radiol. (Stockholm Sweden: 1987). 44, 334–339 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Kuo, W. T. et al. Superselective Microcoil Embolization for the treatment of Lower Gastrointestinal Hemorrhage. J. Vasc. Interv. Radiol.14, 1503–1509. 10.1097/01.RVI.0000099780.23569.E6 (2003). [DOI] [PubMed] [Google Scholar]
- 21.D’Othée, B. J., Surapaneni, P., Rabkin, D., Nasser, I. & Clouse, M. Microcoil embolization for acute lower gastrointestinal bleeding. Cardiovasc. Interv. Radiol.29, 49–58. 10.1007/s00270-004-0301-4 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Eriksson, L. G., Sundbom, M., Gustavsson, S. & Nyman, R. Endoscopic marking with a metallic clip facilitates transcatheter arterial embolization in upper peptic ulcer bleeding. J. Vasc. Interv. Radiol.17, 959–964. 10.1097/01.RVI.0000223719.79371.46 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Nawawi, O., Young, N. & So, S. Superselective coil embolization in gastrointestinal haemorrhage: early experience. Australas. Radiol.50, 21–26. 10.1111/j.1440-1673.2005.01525.x (2006). [DOI] [PubMed] [Google Scholar]
- 24.Lee, C. W. et al. Transcatheter arterial embolization of Acute Upper gastrointestinal tract bleeding with N-Butyl-2-Cyanoacrylate. J. Vasc. Interv. Radiol.18, 209–216. 10.1016/j.jvir.2006.12.003 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Frodsham, A., Berkmen, T., Ananian, C. & Fung, A. Initial experience using N-butyl cyanoacrylate for embolization of Lower Gastrointestinal Hemorrhage. J. Vasc. Interv. Radiol.20, 1312–1319. 10.1016/j.jvir.2009.06.031 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Kwak, H. S., Han, Y. M. & Lee, S. T. The clinical outcomes of transcatheter microcoil embolization in patients with active lower gastrointestinal bleeding in the small bowel. Korean J. Radiol.10, 391–397. 10.3348/kjr.2009.10.4.391 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, J. H., Kim, H. C., Chung, J. W., Jae, H. J. & Park, J. H. Transcatheter arterial embolization of arterial esophageal bleeding with the use of N-butyl cyanoacrylate. Korean J. Radiol.10, 361–365. 10.3348/kjr.2009.10.4.361 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duvnjak, S. & Andersen, P. E. The effect of transcatheter arterial embolisation for nonvariceal upper gastrointestinal bleeding. Dan. Med. Bull.57, 1–4 (2010). [PubMed] [Google Scholar]
- 29.Huang, C. C. et al. N-butyl cyanoacrylate embolization as the primary treatment of acute hemodynamically unstable lower gastrointestinal hemorrhage. J. Vasc. Interv. Radiol.22, 1594–1599. 10.1016/j.jvir.2011.07.018 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Song, J. S., Kwak, H. S. & Chung, G. H. Nonvariceal upper gastrointestinal bleeding: the usefulness of rotational angiography after endoscopic marking with a metallic clip. Korean J. Radiol.12, 473–480. 10.3348/kjr.2011.12.4.473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong, T. C. L. et al. A comparison of angiographic embolization with surgery after failed endoscopic hemostasis to bleeding peptic ulcers. Gastrointest. Endosc.73, 900–908. 10.1016/j.gie.2010.11.024 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Katano, T. et al. The efficacy of transcatheter arterial embolization as the first-choice treatment after failure of endoscopic hemostasis and endoscopic treatment resistance factors. Dig. Endoscopy. 24, 364–369. 10.1111/j.1443-1661.2012.01285.x (2012). [DOI] [PubMed] [Google Scholar]
- 33.Mine, T. et al. Glue embolization for gastroduodenal ulcer bleeding: contribution to hemodynamics and healing process. Acta Radiol.54, 934–938. 10.1177/0284185113484644 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Morishita, H. et al. Transcatheter arterial embolization with N-Butyl cyanoacrylate for acute life-threatening gastroduodenal bleeding uncontrolled by endoscopic hemostasis. J. Vasc. Interv. Radiol.24, 432–438. 10.1016/j.jvir.2012.12.017 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Teng, H. C. et al. The efficacy and long-term outcome of microcoil embolotherapy for acute lower gastrointestinal bleeding. Korean J. Radiol.14, 259–268. 10.3348/kjr.2013.14.2.259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yata, S. et al. Transcatheter arterial embolization of acute arterial bleeding in the upper and lower gastrointestinal tract with N-Butyl-2-cyanoacrylate. J. Vasc. Interv. Radiol.24, 422–431. 10.1016/j.jvir.2012.11.024 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Huang, Y. S., Chang, C. C., Liou, J. M., Jaw, F. S. & Liu, K. L. Transcatheter arterial embolization with n-butyl cyanoacrylate for nonvariceal upper gastrointestinal bleeding in hemodynamically unstable patients: results and predictors of clinical outcomes. J. Vasc. Interv. Radiol.25, 1850–1857. 10.1016/j.jvir.2014.08.005 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Ahmed, O., Jilani, D., Sheth, S., Giger, M. & Funaki, B. Long-term results of microcoil embolization for colonic haemorrhage: how common is rebleeding? Br. J. Radiol.8810.1259/bjr.20150203 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimohira, M., Hashizume, T., Ohta, K., Honda, J. & Shibamoto, Y. Triaxial transarterial embolization for lower gastrointestinal bleeding: a retrospective case series. Minim. Invasive Ther. Allied Technol.24, 119–122. 10.3109/13645706.2014.951656 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Aoki, M., Tokue, H., Koyama, Y., Tsushima, Y. & Oshima, K. Transcatheter arterial embolization with N-butyl cyanoacrylate for arterial esophageal bleeding in esophageal cancer patients. World J. Surg. Oncol.1410.1186/s12957-016-0803-y (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koganemaru, M. et al. Ultraselective embolization using a 1.7-Fr catheter and soft bare coil for small intestinal bleeding. Minim. Invasive Ther. Allied Technol.25, 345–350. 10.1080/13645706.2016.1192553 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Zhao, Y., Li, G., Yu, X. & Xie, P. Evaluation of Superselective Transcatheter Arterial Embolization with n-Butyl Cyanoacrylate in Treating Lower Gastrointestinal Bleeding: A Retrospective Study on Seven Cases. Gastroenterology Research and Practice doi: (2016). 10.1155/2016/8384349 (2016). [DOI] [PMC free article] [PubMed]
- 43.Hur, S. et al. Superselective embolization for arterial Upper gastrointestinal bleeding using N-Butyl cyanoacrylate: a single-center experience in 152 patients. J. Vasc. Interv. Radiol.28, 1673–1680. 10.1016/j.jvir.2017.07.027 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Širvinskas, A., Smolskas, E., Mikelis, K., Brimienė, V. & Brimas, G. Transcatheter arterial embolization for upper gastrointestinal tract bleeding. Wideochirurgia I Inne Techniki Maloinwazyjne. 12, 385–393. 10.5114/wiitm.2017.72319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon, J. H. et al. Transcatheter arterial embolisation for acute lower gastrointestinal haemorrhage: a single-centre study. Eur. Radiol.29, 57–67. 10.1007/s00330-018-5587-8 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Alrashidi, I. et al. Efficacy and safety of transcatheter arterial embolization for active arterial esophageal bleeding: a single-center experience. Diagn. Interventional Radiol.27, 519–523. 10.5152/dir.2021.20253 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahtabasi, M. & Kolu, M. The efficacy and clinical outcomes of transarterial embolization in acute massive upper gastrointestinal bleeding: a single-center experience. Marmara Med. J.34, 180–188. 10.5472/marumj.944254 (2021). [Google Scholar]
- 48.Ikoma, A. et al. Ischemic effects of transcatheter arterial embolization with N-butyl cyanoacrylate-lipiodol on the colon in a swine model. Cardiovasc. Intervent Radiol.33, 1009–1015. 10.1007/s00270-010-9867-1 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Toyoda, H. et al. Estimation of usefulness of N-butyl-2-cyanoacrylate-lipiodol mixture in transcatheter arterial embolization for urgent control of life-threatening massive bleeding from gastric or duodenal ulcer. J. Gastroenterol. Hepatol.11, 252–258. 10.1111/j.1440-1746.1996.tb00071.x (1996). [DOI] [PubMed] [Google Scholar]
- 50.Yonemitsu, T. et al. Evaluation of transcatheter arterial embolization with gelatin sponge particles, microcoils, and n-butyl cyanoacrylate for acute arterial bleeding in a coagulopathic condition. J. Vasc Interv Radiol.20, 1176–1187. 10.1016/j.jvir.2009.06.005 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Hur, S., Jae, H. J., Lee, M., Kim, H. C. & Chung, J. W. Safety and efficacy of transcatheter arterial embolization for lower gastrointestinal bleeding: a single-center experience with 112 patients. J. Vasc. Interv. Radiol.25, 10–19. 10.1016/j.jvir.2013.09.012 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All generated or analyzed data during this study have been included in this published article.