Abstract
Background
Uterine fibroids are the most common non‐malignant growths in women of childbearing age. They are associated with heavy menstrual bleeding and subfertility. Herbal preparations are commonly used as alternatives to surgical procedures.
Objectives
To evaluate the effectiveness and safety of Chinese herbal medicine for treatment of uterine fibroids.
Search methods
The authors with the guidance of the Trials Search Coordinator searched the following electronic databases: the Trials Registers of the Cochrane Menstrual Disorders and Subfertility Group and the Cochrane Complementary Medicine Field, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 4), MEDLINE, EMBASE, the Chinese Biomedical Database, the Traditional Chinese Medical Literature Analysis and Retrieval System (TCMLARS), AMED, and LILACS. The searches were up to 11 September 2012.
Selection criteria
Randomised controlled trials comparing herbal preparations with no intervention, placebo, medical treatment, or surgical procedures in women with uterine fibroids. We included trials of herbal preparations with or without conventional therapy.
Data collection and analysis
Two review authors collected data independently. We assessed trial risk of bias according to our methodological criteria. We presented dichotomous data as risk ratios (RR) and continuous outcomes as mean differences (MD), both with 95% confidence intervals (CI).
Main results
We included 21 randomised trials (involving 2222 women) and the majority of them had unclear or high risk of bias. There were several different herbal preparations used within the included trials. The average treatment duration was three to six months. The primary outcome of uterine fibroid related symptoms was not reported in any of the included trials. The majority of the trials reported fibroid volume and size of the uterus.
Compared with mifepristone, Tripterygium wilfordii extract was associated with a greater reduction in the fibroid volume (MD ‐23.03 cm3, 95% CI ‐28.39 to ‐17.67; 2 trials) and in uterine size (MD ‐51.25 cm3, 95% CI ‐77.70 to ‐24.80; 2 trials). There was no evidence of a significant difference between Nona Roguy herbal product and gonadotropin‐releasing hormone (GnRH) agonist on the average fibroid volume or the uterine size. The combination of Guizhi Fuling formula and mifepristone was associated with a greater reduction in the fibroid volume (‐1.72 [‐2.42, ‐1.02] 7 trials) and in uterine size (MD ‐31.63 [95% CI ‐54.58, ‐8.68] 3 trials)) compared with mifepristone alone. Only 13/21 trials reported on adverse events and no serious adverse effects from herbal preparations were reported.
Authors' conclusions
Current evidence does not support or refute the use of herbal preparations for treatment of uterine fibroids due to insufficient studies with large sample sizes and of high quality. Further high quality trials evaluating clinically relevant outcomes are warranted.
Keywords: Female; Humans; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Gonadotropin‐Releasing Hormone; Gonadotropin‐Releasing Hormone/therapeutic use; Hormone Antagonists; Hormone Antagonists/therapeutic use; Leiomyoma; Leiomyoma/drug therapy; Leiomyoma/pathology; Mifepristone; Mifepristone/therapeutic use; Phytotherapy; Phytotherapy/methods; Plant Preparations; Plant Preparations/adverse effects; Plant Preparations/therapeutic use; Randomized Controlled Trials as Topic; Uterine Neoplasms; Uterine Neoplasms/drug therapy; Uterine Neoplasms/pathology
Plain language summary
Herbal preparations for the treatment of women with uterine fibroids
Uterine fibroids are benign (non‐cancerous) growths in the uterus. They are the most common type of growth found in a woman's pelvis, being present in about one in four or five women older than 35 years. Although many women with fibroids are not aware of them, the growths may cause symptoms or problems due to their size, number, or location. Common symptoms can include longer or more frequent menstrual periods, heavy bleeding, menstrual pain, pressure in the lower abdomen, infertility, or miscarriages. Women with these symptoms may require treatment. Fibroids can be treated with surgery, such as myomectomy (removal of the fibroids while leaving the uterus in place) or hysterectomy (removal of the uterus). Another approach is uterine artery embolization, by which the blood vessels to the uterus are blocked. Drugs such as gonadotropin‐releasing hormone (GnRH) agonists may be used to shrink fibroids and to control bleeding.
Herbal preparations are commonly used alternatives to drug treatment, surgery, or both. This systematic review included 21 randomised clinical trials involving 2222 women with uterine fibroids. There is no evidence on the effectiveness of herbal preparations for symptom relief as no trials evaluated this properly. Compared with conventional medication, one herbal preparation, Tripterygium wilfordii, may have a more beneficial effect in reducing the volume of uterine fibroids. Another five herbal medicines appeared to be similar to conventional medication in reducing the volume of fibroids. The herbal medicine Guizhi Fuling formula showed a significantly greater effect in reducing the volume of the fibroids when combined with mifepristone versus mifepristone alone. However, these clinical trials were small in terms of the number of participants and the trial quality was low. Thirteen out of 21 included trials reported on adverse effects of herbal preparations and found some minor problems such as stomach discomfort, nausea, hot flushes, and poor appetite although no serious adverse effects were identified. The effect of herbal preparations for uterine fibroids is therefore not confirmed in this review and needs to be studied in large, good quality trials.
Summary of findings
Summary of findings for the main comparison. Herbal preparations versus placebo.
| Herbal preparations versus placebo for uterine fibroids | ||
| Patient or population: Women with uterine fibroids Intervention: Herbal preparations versus placebo | ||
| Outcomes | Quality of the evidence (GRADE) | Comments |
| Change in fibroid related symptoms | No evidence available | Only one study made this comparison and did not report this outcome |
| Adverse events | One double blind, placebo‐controlled, randomised trial | Only one study made this comparison and did not report clinically relevant adverse events. Results of lab tests were reported.1 |
| 1When Guihong turtle shell was compared with placebo there was one case in the herbal group who developed abnormal serum blood urea nitrogen (BUN) and creatinine levels after three months treatment, but no case occurred in the placebo group. Liver function was monitored and no participant developed abnormal levels of serum alanine transaminase (ALT) or aspartate aminotransferase (AST) levels | ||
Summary of findings 2. Herbal preparations versus medication.
| Herbal preparations versus medication for uterine fibroids | ||
| Patient or population: Women with uterine fibroids Intervention: Herbal preparations versus medication | ||
| Outcomes | Quality of the evidence (GRADE) | Comments |
| Change in fibroid related symptoms | No evidence available | Seven studies made this comparison and did not report this outcome |
| Adverse events | Low | Seven studies made this comparison and did not report clinically relevant adverse events such as subject undertaking surgery, or occurrence of complications. Results of lab tests were reported.1 |
| Average volume of fibroids (cm3) | Low | 5/7 trials demonstrated no significant difference between herbal preparations and medications. 2/7 showed Tripterygium wilfordii more effective in reducing average volume of fibroids compared with mifepristone. (Analysis 1.1) |
| Average size of uterus (cm3) | Low | Six trials reported this outcome. Tripterygium wilfordii showed significantly better effect than mifepristone in two trials. Huoxue Huayu Ruanjian Sanjie, Huoxue Sanjie decoction, and Xiaozheng decoction was less effective than mifepristone. There was no significant difference between Nona Roguy and GnRHa. (Analysis 1.2) |
| 15/7 trials in this category reported outcome of adverse effects (Table 5) and the reported adverse events included amenorrhoea, menopausal symptoms, gastrointestinal discomfort from herbal treatment group. No serious adverse events such as death or disability were reported. Liver function was monitored and no participant developed abnormal levels of serum alanine transaminase (ALT) or aspartate aminotransferase (AST) levels | ||
1.1. Analysis.

Comparison 1 Herbal preparations versus medication, Outcome 1 Average volume of uterine fibroids (cm3).
1.2. Analysis.

Comparison 1 Herbal preparations versus medication, Outcome 2 Average size of uterus (cm3).
2. Adverse events of herbal medicines reported in the included trials.
| Herbs | Formulation | No. of cases | Study ID |
| Gongliuqing capsule | capsule | Adverse effect was not observed during the treatment period and no case had abnormal liver and kidney function. | Liu LY 2010 |
| Huoxue Huayu Ruanjian Sanjie | decoction | Four out of 18 participants developed gastroenterologic discomfort, and no case had amenorrhoea or menopausal symptoms. All participants (12) developed amenorrhoea, 10 had menopausal symptoms, and 10 had gastroenterologic discomfort. | Yan LQ 2000 |
| Huoxue Sanjie Tang | decoction | In herbal treatment group, one case (1.7%) had stomach discomfort and one case had increased serum ALT level, but became normal after six months. | Lu JX 2007 |
| Guizhi Fuling Tang | decoction | Some patients reported adverse effects, such as mild nausea, loss of appetite, somnolence, hot flush and hyposexuality but no data were provided. There was no case with abnormal liver, kidney, haematological or hypokalaemic findings. | Liu Y 2009 |
| Guihong turtle shell wan | pill | One patient in herbal treatment group developed abnormal level of serum BUN and creatinine. | Ma R 2010 |
| Guizhi Fuling capsule | capsule | Few women from both groups had mild hot flushes, poor appetite, dry vagina, and upper abdomen discomfort. These symptoms disappeared at one to two weeks after quit the drug. | Deng XL 2010 |
| Guizhi Fuling capsule | capsule | Two cases had mild abdomen discomfort, and the incidence rate of adverse effects was 8%. | Dong M 2011 |
| Guizhi Fuling capsule | capsule | The reported adverse effects from herbal treatment group had mild hot flush (one case), mild skin itching (one case), stomach discomfort (two cases). There was no significant difference between herbal group and control group for the incidence rate. No participant developed liver of kidney dysfunction. | Mao CX 2012 |
| Guizhi Fuling wan | pill | In this trial, Guizhi Fuling pills were used along with mifepristone and compared with mifepristone alone. All participants developed amenorrhoea in both groups. Four out of 33 in experimental group and 12 out of 33 in mifepristone group developed nausea, anorexia, drowsiness. | Mao XG 2012 |
| Tripterygium glycosides | tablet | 22 out of 62 in herbal treatment group developed amenorrhoea, 16 complained about discomfort in stomach; among 22 women with amenorrhoea, two had hot flushes, fatigue, palpitation, but were able to continue the treatment. 62 (100%) in mifepristone group developed amenorrhoea, and 46 out of 62 developed nausea, fatigue, palpitation, hot flushes, drowsiness. There was no abnormal liver function, kidney function and haematological examination. | Fu WJ 2005 |
| Tripterygium glycosides | tablet | 18 out of 32 in herbal group developed amenorrhoea, and 25 out of 32 developed stomach discomfort, hot flushes, fatigue, palpitation; while all participants in mifepristone group developed amenorrhoea, and 22 out of 30 had nausea, fatigue, palpitation, drowsiness. There was no case with abnormal liver, kidney, lipids or hypoglycemia findings. | Wen Q 2005 |
| Lenge Xiaozheng Tang | decoction | Few participants developed nausea and dizziness in both groups. No person had abnormal liver, kidney, or haematological examination. | Zhu FH 2006 |
Summary of findings 3. Herbal preparations plus medication versus medication.
| Herbal preparations versus medication for uterine fibroids | ||
| Patient or population: Women with uterine fibroids Intervention: Herbal preparations plus medication versus medication | ||
| Outcomes | Quality of the evidence (GRADE) | Comments |
| Change in fibroid related symptoms | No evidence available | Thirteen trials made this comparison and did not report this outcome |
| Adverse events | Low | Thirteen trials made this comparison and did not report clinically relevant adverse events such as subject undertaking surgery, or occurrence of complications. Results of lab tests were reported.1 |
| Average volume of maximum fibroids (cm3) | Low | Compared with mifepristone, Guizhi Fuling formula plus mifepristone showed significantly better effect on reducing average volume of maximum fibroids from seven trials. Guizhi Fuling capsule and Leuprolide acetate was more effective than mifepristone in one trial. Gongliuqing capsule plus mifepristone was more effective than mifepristone in one trial. Lenge Xiaozheng Tang plus mifepristone showed no significant difference compared with mifepristone in one trial. (Analysis 2.1) |
| Average volume of total multiple fibroids (cm3) | Low | Three trials reported this outcome. Guizhi Fuling capsule plus mifepristone showed better effect compared to mifepristone in two trials. Another trial showed better effect of Jiliu Tang plus mifepristone than mifepristone alone. (Analysis 2.2) |
| Average size of uterus (cm3) | Low | Four trials reported this outcome. The results from three trials showed significantly beneficial effect of Guizhi Fuling capsule plus mifepristone compared to mifepristone. Another trial showed better effect of Jiliu Tang plus mifepristone compared to mifepristone. (Analysis 2.3) |
| 18/13 trials in this category reported outcome of adverse effects (Table 5) and the reported adverse events included amenorrhoea, menopausal symptoms such as hot flushes, palpitation; gastrointestinal discomfort, itching, from herbal treatment group. No serious adverse events such as death or disability were reported. Liver function was monitored and no participant developed abnormal levels of serum alanine transaminase (ALT) or aspartate aminotransferase (AST) levels | ||
2.1. Analysis.

Comparison 2 Herbal preparations plus medication versus medication, Outcome 1 Average volume of maximum fibroids (cm3).
2.2. Analysis.

Comparison 2 Herbal preparations plus medication versus medication, Outcome 2 Average volume of total multiple fibroids (cm3).
2.3. Analysis.

Comparison 2 Herbal preparations plus medication versus medication, Outcome 3 Average size of uterus (cm3).
Background
Description of the condition
Uterine fibroids are the most common, non‐cancerous uterine growths in women of childbearing age. Alternative names are uterine leiomyomata, fibromyoma, myoma, or fibroids. The lifetime risk of fibroids in a woman over the age of 45 years has been estimated to be more than 60%, including symptomatic and non‐symptomatic conditions (Okolo 2008). Around 30% of women of childbearing age have clinically symptomatic uterine fibroids (Newbold 2000; Stewart 2001). Common symptoms may include heavy or painful periods; prolonged menstrual periods; bleeding between periods; pelvic pain or low back pain; 'fullness' in the lower abdomen, with or without urinary or rectal symptoms due to compression; and reproductive problems, such as infertility, multiple miscarriages, or early onset of labour during pregnancy. Many women with uterine fibroids do not have any symptoms. A recent investigation of 21,479 women across eight countries showed that the prevalence of fibroids was from 9.4% (UK) to 17.8% (Italy) in the age population of 40 to 49 years (Zimmermann 2012). Uterine fibroids constitute the main reason for hysterectomies to be carried out, based on data between 1990 and 1997 in the United States (Farquhar 2002).
Uterine fibroids are growths of muscular and fibrous cells within, or attached to, the wall of the uterus. According to the location of the growth, they can be categorised as submucosal when they grow just underneath the uterine lining, intramural when they are in between the muscles of the uterus, and subserosal when they are on the outside of the uterus. Fibroids may grow as a single tumour or in clusters. A single fibroid can be less than one inch in size or can grow to eight inches or more. A group of fibroids can also vary in size. The cause of uterine fibroids remains unknown, however genetic, hormonal, immunological, and environmental factors may play a role in starting the growth of fibroids, or in continuing that growth (Munro 2011). Several risk factors for uterine fibroids have been identified. African‐American women are at three‐ to five‐times greater risk than white women. Women who are overweight or obese for their height (based on body mass index (BMI)) are also at slightly higher risk than women who are average in weight for their height. Women who have given birth appear to be at lower risk (Marshall 1997).
Recommended treatment for uterine fibroids depends on the severity of symptoms, the woman's age, pregnancy status, desire for future pregnancies, general health, and the characteristics of the fibroids (Stewart 2001). If a woman shows no symptoms, or the fibroids are small, she may not need any treatment. If a woman has serious symptoms or pain, medical therapy can be used to relieve symptoms. Such treatment may include gonadotropin‐releasing hormone agonists (GnRHa) (Lethaby 2001); synthetic steroids with antiprogesterone activity, such as mifepristone, to slow or stop the growth of fibroids (Tristan 2012); and the use of progesterone and its derivatives for short‐term treatment of bleeding and for inhibiting the fibroids' growth (Grigorieva 2003; Maruo 2004).
Surgical therapy is considered to be an effective treatment and includes myomectomy to remove only the fibroids and leave the healthy uterus, or hysterectomy to remove the entire uterus (Falcone 2002; Griffiths 2006). Another accepted treatment is uterine artery embolization (UAE), which is used to block off the blood supply to the uterus and so make the fibroids shrink (Gupta 2012; McLucas 2001; Tranquart 2002; Watson 2002). However, few women with uterine fibroids prefer surgery and women may seek less invasive options, such as pain medication, medical therapy, or other alternative therapies.
Description of the intervention
Among alternative therapies, herbal treatments for fibroids are used in several medical traditions and countries (Fugh‐Berman 2004). For example, in China the use of traditional Chinese herbal medicines for treating uterine fibroids is a common clinical practice. In this review, herbal preparations are defined as any formulation of medicinal herbs including extracts, raw herbs, or herbal decoctions prescribed by practitioners. These could include herbal products such as Chinese proprietary medicine or self‐prepared herbal decoctions. In Chinese medicine, herbal medicine has been used for many years for different diseases or conditions. For example, the herbal medicine Guizhi Fuling formula has been described in historical classics in ancient China for treatment of women's symptoms, and it is still used in China (Li J 2008). However, there are huge variations in the herbal preparations used, which will depend on the practitioners themselves and on the individualised treatment of different women.
How the intervention might work
According to the theory of Chinese medicine, practitioners recognise uterine fibroids as a condition of imbalance between yin and yang in the body (in allopathic terms, disturbances of the endocrine system and blood circulation). Therefore, it is important that the practitioners make a diagnosis based on the symptoms and signs from observing the tongue and taking the pulse, and this practice is called 'pattern differentiation' (Chinese medicine diagnosis). The practitioners prescribe a herbal formula according to the pattern of the syndrome (in Chinese, Zheng). Clinical studies from the Chinese literature show that Chinese herbal preparations might relieve symptoms and shrink the fibroid tumours without significant adverse effects (Huang 2003; Xiong 2002). One of the commonly used herbal medicines is Guizhi Fuling formula, and basic studies showed that Guizhi Fuling formula might work on fibroids by promoting qi flow and blood circulation, immune regulation, and softening and resolving hard lumps (Ji 2011; Li J 2008; Sang 2004). However, the exact mechanisms of the therapeutic effect are not fully understood.
Why it is important to do this review
Is the practice of using herbs for fibroids supported by well‐designed clinical evidence? We aim to review the clinical research studies systemically and inform practice by presenting comprehensive, critically appraised evidence.
Objectives
The primary objective was to evaluate the effectiveness and safety of Chinese herbal medicine for treatment of uterine fibroids.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials were eligible for inclusion, regardless of blinding, publication status, or language. We planned to include cross‐over randomised trials but to use only the data from the first phase. We excluded quasi‐randomised trials or 'randomised' trials with false methods for random allocation of participants, or where a trial was not stated to be randomised.
Types of participants
Women with uterine fibroids diagnosed by clinical symptoms and physical signs,and confirmed by ultrasound scanning, computed tomography (CT), magnetic resonance imaging (MRI), or a combination of more than one of these procedures. We planned to include women with fibroid related symptoms and palpable uterine fibroids, without confirmation by imaging technology, and to compare these in subgroup analyses. We also planned to include women without any symptoms who were found to have uterine fibroids during routine gynaecological examination, which were confirmed by imaging techniques.
Types of interventions
Experimental interventions included Chinese patented herbal medicines, other patented herbal products pertaining to different traditional medicines, extracts of a single herb or a compound of herbs, or other individualised herbal remedies. We did not limit the administration or formulation of herbal preparations, such as capsule, tablet, granule, decoction, or injection. The control interventions included no treatment, placebo, medical therapy, or surgical procedures.
Types of outcome measures
Primary outcomes
Uterine fibroid related symptoms such as heavy, irregular, or prolonged menstrual periods; bleeding between periods; pelvic or low back pain; and low abdominal pressure symptoms such as frequent or urgent urination, or constipation. Symptoms could be measured by either patient reporting or an instrument, regardless of blinding.
Adverse effects of herbal preparations.
Secondary outcomes
3. Number of women undertaking surgery (myomectomy, hysterectomy, embolization) due to failure of medical prevention or management of the above symptoms.
4. Incidence of complications including anaemia, infertility, miscarriage, premature labour and delivery, abnormal fetal position.
5. Quality of life (measured by a validated scale or instrument).
6. Number and size of the fibroids, the volume of the uterus, or both.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for published and unpublished randomised trials of herbal medicine, without language restriction and in consultation with the Mentrual Disorders and Subfertility Group (MDSG) Trials Search Coordinator:
Trials Registers of the Cochrane MDSG and the Cochrane Complementary Medicine Field;
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 9);
MEDLINE (1966 to September 2012), EMBASE (1998 to September 2012), AMED, and LILACS (www.bireme.br/bvs/I/ibd.htm) from their date of inception onwards.
The following five major Chinese biomedical databases were searched:
1. Chinese Biomedical Literature database (CBM) (http://www.imicams.ac.cn/);
2. Chinese Medical Current Content (CMCC) (http://www.cmcc.org.cn);
3. China National Knowledge Infrastructure (CNKI‐CAJ) (www.cnki.net);
4. VIP information/Chinese Scientific Journals Database (CSJD‐VIP) (http://dx3.cqvip.com/);
5. WanFang database/Chinese Medicine Premier (hppt://www.wanfangdata.com.cn/).
We used the search terms: uterine fibroids, hysteromyoma, uterine leiomyomata, fibromyoma, myoma; and combined with traditional medicine, alternative medicine, plant extracts, medicinal plants, non‐prescription drugs, herbs, complementary medicine, Chinese medicine, phytodrug or phytopharmaceutical. We had no restriction on publication type. The detailed search strategies are listed in Appendix 1 and Appendix 2.
Searching other resources
We checked the reference lists of identified randomised controlled trials and review articles in order to find further trials not identified by the electronic searches.
We searched for ongoing trials through the National Research Register and the website www.controlled‐trials.com.
We also checked the 'grey' literature, including unpublished conference proceedings or abstract books, and contacted pharmaceutical companies which produce herbal medicines for uterine fibroids to identify unpublished trials.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, conducted by H Yang, the full texts of all potentially eligible studies were retrieved. Two review authors (JP Liu and H Yang) independently selected the trials to be included in the review according to the prespecified selection criteria. Any disagreements were resolved by discussion. Y Xia confirmed the randomisation through phone calls to Chinese trialists.
Data extraction and management
Two review authors (JP Liu and H Yang) independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the authors. Any disagreements were resolved by discussion or by a third review author (Y Xia). Papers not in Chinese, English, Japanese, or Italian were translated with the help of the Cochrane Menstrual Disorders and Subfertility Group. We extracted the following characteristics and data from each included trial: primary author, study setting, methodology, age, gender, and ethnicity of participants, number of participants randomised and analysed, participant inclusion and exclusion criteria, symptoms and methods for measurement, the diagnostic criteria, type of herb or herbs, quality of the products, route of delivery, dosage and duration of intervention, details of the comparison regime, duration of follow up, reasons for and number that dropped out or were lost during follow up, outcome measures (end of treatment and at follow up), and number and type of adverse events.
We sought data on the number of participants with each outcome by allocated treatment group, irrespective of compliance or follow up, to allow an intention‐to‐treat analysis. For three‐arm trials, the data from the control group would be split in half so that half of the participants and half of the events would be used in each comparison.
Assessment of risk of bias in included studies
Two review authors (JP Liu and H Yang) independently assessed the included studies for risk of bias using the Cochrane risk of bias assessment tool (www.cochrane‐handbook.org) to assess: selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other bias. Disagreements were resolved by discussion or by a third review author (F Cardini). We described all judgements fully and presented the conclusions in the 'Risk of bias' table.
Generation of the allocation sequence
Low risk of bias: if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice may also be considered as low risk if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Allocation concealment
Low risk of bias: if the allocation of participants involved a central independent unit, on‐site locked computer, identical appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes. Envelopes should be serially numbered, sealed, and opaque. However, this information is rarely provided, indicating an increased risk of bias.
Unclear risk of bias: if the trial was described as randomised but the method used to conceal the allocation was not described, or the sealed envelopes were not described as opaque.
High risk of bias: if the allocation sequence was known to the investigators who assigned participants, or if the study was quasi‐randomised.
Blinding (or masks)
Low risk of bias: double blinding, if the trial was described as double blind and both the participant and physician were blinded, or participant and outcome assessor. Unclear risk of bias: single blinding, if the participants, or physicians, or outcome assessors were blinded. High risk of bias: open‐label, if blinding was not applied.
Incomplete data reporting
Low risk of bias: if the dropout numbers were low (e.g. less than 20%) and they were evenly distributed among different groups, or if it was specified that there were no withdrawals or losses to follow up. Unclear risk of bias: if the report gave the impression that there had been no withdrawals or losses to follow up but this was not specifically stated. High risk of bias: if the number of, or reasons for, withdrawals or losses to follow up were not described.
Selective reporting bias
Selective reporting is a type of reporting bias that affects the internal validity of an individual study. It refers to the selective reporting of some outcomes (for example positive outcomes) and the failure to report others (for example adverse events). If the trial protocols were not available, we would compare the outcome measures in the method section with the actual reported outcomes in the results for the assessment of selective reporting bias.
Other bias
We considered baseline comparability as an important factor for other bias. If baseline data were comparable, the study would be at low risk of other bias. Otherwise, no information or insufficient information would be considered as either high or unclear risk of bias.
Measures of treatment effect
We presented dichotomous data as risk ratios (RR) and continuous outcomes as mean differences (MD), both with 95% confidence intervals (CI). If similar outcomes were reported on different scales (for example change in weight) we would calculate the standardised mean difference (SMD) with 95% CI.
Unit of analysis issues
The primary analysis would be per woman randomised. Only first‐phase data from cross‐over trials would be included.
Dealing with missing data
We would perform analyses by intention to treat where possible and attempt to obtain missing data from the original trialists. For dichotomous outcomes, participants with incomplete or missing data were to be included in a sensitivity analysis by counting them as treatment failures to explore the possible effect of loss to follow up on the findings ('worst‐case' scenario). For continuous data, we took a 'carry forward' approach, in which we used the last observed patient data, if available, as the missing data to conduct data analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We would assess statistical heterogeneity by the I2 statistic. An I2 measurement greater than 50% would be taken to indicate substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
If there were 10 or more studies in an analysis, we would use a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) (Egger 1997; Vickers 1998).
Data synthesis
If the studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons: 1. herbal medicine versus no treatment;
2. herbal medicine versus placebo;
3. herbal medicine versus pharmacological treatment;
4. herbal medicines versus a surgical procedure; or
5. herbal medicines plus conventional therapy versus conventional therapy.
Furthermore, if a combined analysis showed significant heterogeneity (defined as P < 0.1 for the heterogeneity test), we would use a random‐effects model for the analysis.
Subgroup analysis and investigation of heterogeneity
If a sufficient number of randomised trials was identified and data were available, we would have performed subgroup analyses according to symptoms (presence or absence of), diagnosis with or without imaging confirmation, and the location of uterine fibroids (submucosal, intramural, or subserosal fibroids). Whenever there was significant heterogeneity we used a random‐effects model and investigated heterogeneity in both the clinical characteristics and methodological differences between studies. We would carry out subgroup analyses in Review Manager 5.1.7 (RevMan 2012) to see if any differences were explained by differences between the studies.
Sensitivity analysis
If a sufficient number of randomised trials were identified for the same interventions, we would conduct sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility of studies and analysis. These analyses would include consideration of whether the review conclusions would have differed if:
1. eligibility was restricted to studies without high risk of bias;
2. alternative imputation strategies had been implemented;
3. the summary effect measure was odds ratio rather than risk ratio.
Overall quality of the body of evidence: summary of findings table
Summary of findings tables were generated using GRADEPRO software. These tables evaluated the overall quality of the body of evidence for the main review outcomes using GRADE criteria (study limitations (that is risk of bias), consistency of effect, imprecision, indirectness, and publication bias). Judgements about evidence quality (high, moderate, or low) were justified, documented, and incorporated into reporting of results for each outcome.
We intend to complete an update of the review every 24 months.
Results
Description of studies
Results of the search
Our initial electronic searches identified 984 citations, with a further 35 from additional handsearches. After reading titles and abstracts we excluded 733 of these because they were either duplicates, non‐clinical studies, review articles, case reports, case series, or had study objectives different from this review. A total of 158 references published in Chinese or in English were retrieved for further assessment. We excluded 93 of these studies because they did not meet our inclusion criteria. We contacted the trial authors of 13 trials to confirm the randomisation methods and missing data, and this allowed us to exclude, by phone calls, 136 trials that had claimed to be 'randomised'. This was due to inadequate randomisation methods or failure to provide required data (Figure 1).
1.

Study flow diagram.
In the previous version of the review, only two trials were included. Our updated searches in September 2012 identified 132 trials, and 19 randomised trials were eligible to be included taking the total number of included trials to 21.
Included studies
Study design and setting
We were able to include 21 parallel‐group, randomised controlled trials (RCTs) involving 2222 participants in this review (Deng XL 2010; Dong M 2011; Fu WJ 2005; Gu HH 2011; Hazlina 2005; Lai XL 2010; Liu Y 2009; Liu LY 2010; Lu JX 2007; Lu HJ 2010; Luo SQ 2010; Ma R 2010; Mao CX 2012; Mao XG 2012; Ni XP 2012; Wang XR 2011; Wen Q 2005; Wu JH 2011; Wu YF 2011; Yan LQ 2000; Zhu FH 2006). These RCTs reported random allocation of participants with uterine fibroids to herbal medicines or placebo, mifepristone, or GnRH agonist. Twelve trials compared the herbal medicine Guizhi Fuling formula plus medication with medication alone (including eight trials of Guizhi Fuling capsule plus mifepristone versus mifepristone, one trial of Guizhi Fuling capsule plus leuprolide versus mifepristone, one trial of Gongliuqing capsule plus mifepristone versus mifepristone, one trial of Lenge Xiaozheng Tang plus mifepristone versus mifepristone, and one trial of Jiliu Tang plus mifepristone versus mifepristone). The 21 RCTs are listed in the table 'Characteristics of included studies'. Twenty trials were published in Chinese and one trial in English. No trial had a pre‐trial sample size estimation (power calculation) or was presented as a multicentre trial.
Participants
A total of 2222 women with uterine fibroids were randomised into herbal treatment (n = 1118) or control (n = 1104). Twenty trials were conducted in China and one trial in Malaysia. The 21 trials included women of childbearing age with uterine fibroids, diagnosed through routine gynaecological examination and confirmed by B‐mode ultrasound (most commonly used diagnostic method for uterine fibroids, which can show a clear two (or three) dimensional image of the size and location of uterine fibroids). As available outcome data were limited, we could not perform prespecified subgroup analyses, that is of symptom type or location of fibroids.
Interventions
Ten herbal preparations were tested in the 21 trials (Table 4). The controls were placebo (one trial), pharmaceutical medicines including mifepristone and GnRH agonist (eight trials). Twelve trials tested herbal medicine plus medication versus medication (including four herbal medicines: Guizhi Fuling capsule, Qingliuqing capsule, Lenge Xiaozheng Tang, and Jiliu Tang). The average treatment duration was 3.6 months (ranging from three to six months).
1. Compositions of herbal preparations in 22 randomised trials.
| Name of herbal drugs | Composition | Formulation | Study ID |
| Herbal product Nona Roguy | Cassia angustifolia, Parkia roxburghii, Zingiber officinale, Trachyspermum ammi, Glycyrrhiza glabra, Usnea barbata, Curcuma domestica, Gastrochilus pandurata, Eryngium foetidum, Citrus Hystrix. | semi‐liquid | Hazlina 2005 |
| Huoxue Sanjie Tang | Herbal formula composed of 11 herbs: Radix Angelicae Sinensis 20 g, Herba Leonuri 20 g, Raidix Paeoniae Alba 15 g, Spina Gleditsiae 15 g, Radix Salviae Miltiorrhiae 15 g, Rhizoma Cyperi 12 g, Rhizoma Sparganii 12 g, Rhizoma Curcumae 12 g, Squama Manitis 12 g, Portulaca grandiflora 12 g, Concha Ostreae 10 g | decoction | Lu JX 2007 |
| Guizhi Fuling capsule | Herbal formula composed of 5 herbs: Ramulus Cinnamomi Cassiae, Poriae Cocos, Semen Persicae, Radix Paeoniae Lactiflorae, Cortex Moutan Radicis. The dosage was not reported. | capsule | Deng XL 2010; Dong M 2011; Gu HH 2011; Lu HJ 2010; Mao CX 2012; Mao XG 2012; Wang XR 2011; Wu JH 2011; Wu YF 2011 |
| Guizhi Fuling Tang |
Herbal formula composed of 12 herbs: Ramulus Cinnamomi 15g, Smilax china L 20g, Cortexmoutan 15g, Semen Persicae 10g, Radix Platycodi 15g, Angelic Diels 10g, Rhizomaatractylodi smacrocephala 10g, Radix Bupleuri 10g, Sparganium stoloniferum Buch.‐Ham 10g, Curcuma aeruginosa Roxb 10g, Rhizoma Cyperi 10g, Carnis Ostreae 30g | decoction | Liu Y 2009 |
| Guihong turtle shell pill | Herbal formula composed of 12 herbs: Ramulus Cinnamomi 6g, Smilax china L 9g, CarthamustinctoriusL 9g, Cortexmoutan 9g, Curcuma aeruginosa Roxb 9g, Semen Persicae 9g, Carapax Trionycis15g, Sargassum fusiforme 9g, Laminaria japonica Aiesch 9g, Taraxacum mongolicum Hand‐Mazz 9g, Rhizoma Cyperi 9g, Spica prunellae Vulgari 15g |
pill | Ma R 2010 |
| Jiliu Tang | Practitioner prescribed formula composed of 14 herbs: raw Radices Rehmanniae 20g, Cornus officinalis 20g, Rhizoma Dioscoreae 20g, Rhizoma Alismatis 15g, Poria cocos 15g, turtle shell 30g, tortoise plastron 20g, oyster shell 30g, Malayan pangolin 10g, Rhizoma Dioscoreae Bulbiferae 10g, Bulbus Fritillariae Thunbergii 15g, Rhizoma Sparganii 10g, Leech 10g, Glycyrrhiza uralensis 6g. | decoction | Luo SQ 2010 |
| Tripterygium wilfordii Hook | Extracted glycosides from Chinese herb Tripterygium wilfordii (Lei Gong Teng) | tablet | Fu WJ 2005; Wen Q 2005 |
| Xiaozheng Tang |
Herbal formula composed of 12 herbs: Scutellaria barbata D.Don 15g, Hedyotis diffusa Willd 15g, Sparganium stoloniferum Buch.‐Ham 10g, Curcuma aeruginosa Roxb 10g, Boswellia carterii 4g, Commiphora molmol/Commiphora myrrha 4g, Semen Citri Reticulatae Citrus tangerina 10g, Spina Gleditsiae 15g, Sargassum fusiforme 30g, Carnis Ostreae 30g, Salviae Chinesnsis Benth 15g, Litchi chinensis Sonn 10g | decoction | Ni XP 2012 |
| Gong Liu Qing capsule | Herbal formula composed of 11 herbs: prepared Radix et Rhizoma Rhei, Eupolyphaga sinensis Walker, Leech, semenpersicae, cattail pollen, Fructus Aurantii Immaturus, Concha Ostreae, Radices Rehmanniae, Radix Paeoniae Alba, Glycyrrhiza uralensis; manufactured by Chengdu Zhonghui Pharmaceutical Company | capsule | Lai XL 2010; Liu LY 2010 |
| Huoxue Huayu Ruanjian Sanjie formula | Practitioner prescribed herbal formula composed of 10 herbs: Codonopsis pilosula, Parasitic loranthus, Radix Polygoni Multiflori, Radix Achyranthis Bidentatae, Ramuli Euonymi, Semen Impatientis, Prunella Spike, Prepared Turtle Shell, Concha Arcae, Raw Oysters. No dosage reported. | decoction | Yan LQ 2000 |
| Lenge Xiaozheng Tang | Practitioner prescribed herbal formula composed of 10 herbs: Angelica sinensis 10g, Bighead Atractylodes Rhizome 10g, Radix Paeoniae Rubra 15g, Radix Bupleuri 10g, Poria Cocos 20g, Rhizoma Sparganii 10g, Curcuma Zedoary 10g, Rhizoma Cyperi 10g, Rhizoma Pleionis 15g, Raw Oyster 30g. | decoction | Zhu FH 2006 |
Outcomes
No trial reported the primary outcome for effectiveness, that is uterine fibroid related symptoms measured by a validated instrument. Thirteen of 21 trials reported the outcome of adverse events in relation to herbal medicines (Table 5). Among secondary outcomes, no trials reported the need for surgical treatment, quality of life, or incidence of complications such as infertility. The outcomes reported were volume of the fibroids or size of the uterus. The volume of fibroids or the size of the uterus was measured by B‐mode ultrasound. However, the method of calculating volume was different in some of the trials. Some studies reported the average volume of the fibroids by calculating the maximum fibroid size in each woman, while others reported the average volume by calculating the totality of multiple fibroids. Three trials reported follow up after the completion of treatment, ranging from three to six months.
Excluded studies
The reasons for exclusion of 137 studies are listed in the table 'Characteristics of excluded studies'.
Risk of bias in included studies
We had made phone calls to the authors of included 'randomised' trials to confirm the randomisation methods and enquire about missing information. This led to us excluding some 'randomised' trials we planned to include. In general, the included trials had high or unclear risk of bias, and therefore, they were evaluated as low methodological quality (Figure 2; Figure 3).
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
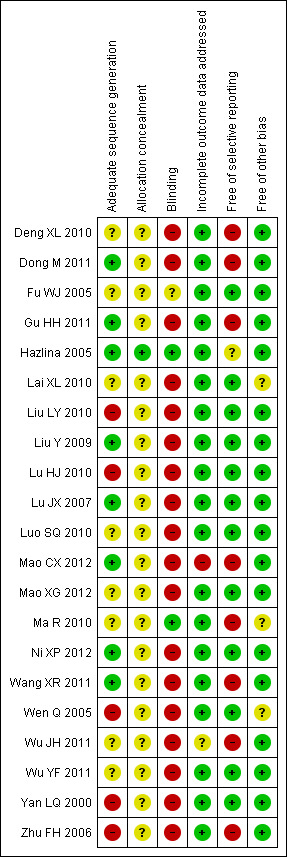
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Our contact with trial authors by phone resulted in our exclusion of eight trials that we originally planned to include (see Characteristics of excluded studies). The main reasons were inadequate methods for allocation of participants, such as alternate allocation, or the data for this review were not available and the authors failed to provide them. We were not able to perform meaningful sensitivity or funnel plot analysis due to the limited number of trials.
Allocation
Eight out of 21 included trials reported generation of allocation sequence. However, only one trial described adequate allocation concealment (Hazlina 2005). The other trials did not report the method for allocation concealment.
Blinding
Two trials applied blinding (Hazlina 2005; Ma R 2010), including one trial using blinding of participants and personnel (Ma R 2010) and one trial using blinding of the outcome assessor (Hazlina 2005).
Incomplete outcome data
All included trials analysed all or most (> 95%) women randomised and we judged the majority of trials to be at low risk of bias. However, due to the non‐availability of raw data from the missing participants for continuous data, we were not able to do an intention‐to‐treat analysis.
Selective reporting
As we were not able to get access to trial protocols for any of the 21 trials, we made our judgement by comparing the outcome measures mentioned in the method section with the reporting in results: 13/21 trials reported all outcome measures described in the methods and, therefore, were evaluated as at low risk of bias; 7/21 partially reported the outcomes in the results, and were evaluated as at high risk of bias.
Other potential sources of bias
All trials reported baseline comparability between the two groups, and were considered to be at low risk of bias. We found no potential sources of within‐study bias in the included trials.
Effects of interventions
See: Table 1; Table 2; Table 3
1. Herbal medicine versus placebo
No trial in this category reported the primary outcome, that is fibroids related symptoms, using validated methods. One trial tested the herbal medicine Guihong turtle shell pill against placebo for three‐month treatment of 136 women with uterine fibroids (Ma R 2010). The participants were diagnosed as having qi stagnation and blood stasis, without an indication for surgery.
Since this trial evaluated non‐relevant outcomes such as disappearance or shrinkage of uterine fibroids, we were not able to evaluate the efficacy of herbal medicine in this comparison.
In the Ma R 2010 study, there was one case in the herbal group who developed abnormal serum blood urea nitrogen (BUN) and creatinine levels after three months of treatment, which might represent potential impairment to kidney function. No case with abnormal kidney function occurred in the placebo group. Liver function was monitored and no trial participant developed abnormal levels of serum alanine transaminase (ALT) or aspartate aminotransferase (AST).
2. Herbal medicine versus medication
No trial in this category reported the primary outcome, that is fibroids related symptoms, using validated methods. Seven trials compared herbal medicines versus medication (Fu WJ 2005; Hazlina 2005; Lai XL 2010; Lu JX 2007; Ni XP 2012; Wen Q 2005; Yan LQ 2000). Six different herbal preparations were tested: Gongliuqing capsule, Nona Roguy herbal product, Huoxue Huayu Ruanjian Sanjie, Huoxue Sanjie decoction, Tripterygium wilfordii, and Xiaozheng decoction. Since no two trials tested the same herbal medicine except for Tripterygium wilfordii, a meta‐analysis was performed for Tripterygium wilfordii compared with mifepristone on the average volume of uterine fibroids.
2.1 Average volume of uterine fibroids
Among four herbal medicines compared to mifepristone, there was no significant difference between herbal medicine and mifepristone in the average volume of uterine fibroids (Analysis 1.1). The tested herbal medicines were Gongliuqing capsule (Lai XL 2010), Huoxue Huayu Ruanjian Sanjie (Yan LQ 2000), Huoxue Sanjie decoction (Lu JX 2007), and Xiaozheng decoction (Ni XP 2012). Similarly, there was no significant difference between the Nona Roguy herbal product and GnRH agonist in average volume of uterine fibroids (Hazlina 2005). However, herbal extracts of Tripterygium wilfordii showed a significantly better effect than mifepristone in the average volume of uterine fibroids (MD ‐23.03 cm3, 95% CI ‐28.39 to ‐17.67) from two trials (Fu WJ 2005; Wen Q 2005).
2.2 Average size of uterus
Six trials reported on the size of the uterus after three to six months of treatment. Extracts of Tripterygium wilfordii showed significantly better effect than mifepristone in reducing the size of the uterus (MD ‐51.25 cm3, 95% CI ‐77.7 to ‐24.8; 2 trials) (Fu WJ 2005; Wen Q 2005). However, three other herbal medicines were less effective than mifepristone, including Huoxue Huayu Ruanjian Sanjie, Huoxue Sanjie decoction, and Xiaozheng decoction, for the average size of the uterus (Analysis 1.2).
There was no significant difference between the herbal preparation Nona Roguy and the GnRH agonist regarding uterus size (Hazlina 2005).
2.3 Adverse effects
Five trials in this category reported on adverse effects (Table 5) and the reported adverse effects included amenorrhoea, menopausal symptoms, gastrointestinal discomfort in the herbal treatment group. However, similar adverse effects were reported in the control group. No serious adverse events such as death or disability were reported.
3. Herbal medicine plus medical treatment versus medical treatment
No trial in this category reported the primary outcome, that is fibroids related symptoms, using validated methods. Thirteen randomised trials tested herbal medicine plus medication against medication alone. Nine trials compared the herbal medicine Guizhi Fuling formula plus mifepristone versus mifepristone (Deng XL 2010; Gu HH 2011; Liu Y 2009; Lu HJ 2010; Mao CX 2012; Mao XG 2012; Wang XR 2011; Wu JH 2011; Wu YF 2011). One trial tested Guizhi Fuling capsule plus leuprolide acetate against mifepristone (Dong M 2011). Guizhi Fuling formula was taken in either capsule or decoction form. Three other herbal medicines were tested with mifepristone against mifepristone alone, including Gongliuqing capsule (Liu LY 2010), Lenge Xiaozheng Tang (Zhu FH 2006), and Jiliu Tang (Luo SQ 2010).
3.1 Average volume of maximum fibroids
Compared with mifepristone alone, Guizhi Fuling formula combined with mifepristone showed a significantly better effect in reducing the average volume of maximum fibroids (MD ‐1.72 cm3, 95% CI ‐2.42 to ‐1.02; 7 trials) using the random‐effects model (Analysis 2.1).
The combination therapy of Guizhi Fuling capsule and leuprolide acetate was more effective than mifepristone alone in one trial (Dong M 2011). Similarly, Gongliuqing capsule plus mifepristone was more effective than mifepristone alone in one trial (Liu LY 2010). In another trial, Lenge Xiaozheng Tang plus mifepristone showed no significant difference compared to mifepristone alone in one trial (Zhu FH 2006).
3.2 Average volume of total multiple fibroids
Three trials measured and reported the average volume of the total multiple fibroids (Liu Y 2009; Lu HJ 2010; Luo SQ 2010). Guizhi Fuling capsule plus mifepristone showed a better effect in reducing the average volume of total multiple fibroids (MD ‐16.58 cm3, 95% CI ‐20.30 to ‐12.86; 2 trials) compared to mifepristone (Analysis 2.2). Another trial showed a better effect of Jiliu Tang plus mifepristone in reducing the average volume of total multiple fibroids than mifepristone alone (MD ‐16.30 cm3, ‐18.97 to ‐13.63) (Luo SQ 2010).
3.3 Average size of uterus
Four trials in this category reported on the average size of the uterus after treatment (Deng XL 2010; Liu Y 2009; Luo SQ 2010; Wu JH 2011). A pooled analysis of data from three trials showed a significant beneficial effect of Guizhi Fuling capsule plus mifepristone in reducing the average size of uterus compared to mifepristone alone (MD ‐31.63 cm3, 95% CI ‐54.58 to ‐8.68) using the random‐effects model. There was significant heterogeneity for this analysis with an I2 of 90%, which might be due to the relatively large size of the uterus from one trial (Liu Y 2009). Another trial showed a better effect of Jiliu Tang plus mifepristone in reducing the average size of the uterus (MD ‐47.80 cm3, ‐55.68 to ‐39.92) (Luo SQ 2010).
3.4 Adverse effects
Eight trials in this category reported on the adverse effects in relation to the herbal treatments (Deng XL 2010; Dong M 2011; Liu Y 2009; Liu LY 2010; Mao CX 2012; Mao XG 2012; Wu JH 2011; Zhu FH 2006) (Table 5). The adverse effects included gastrointestinal discomfort, itching, and hot flushes. Similar adverse effects were reported in the control group. No serious adverse events from herbal preparations were reported.
4. Other analyses
Our specified sensitivity analyses, subgroup analyses, and test for publication bias were unable to be performed due to significant heterogeneity of the herbal interventions and the limited number of trials under each comparison.
Although no trial reported on the primary outcome of symptoms, we summarised the major findings in the summary of findings tables (Table 1; Table 2; Table 3).
Discussion
Summary of main results
This systematic review included 21 randomised trials of herbal preparations for the treatment of uterine fibroids. The majority of the herbal preparations were tested in single trials. Only the herbal medicines Guizhi Fuling formula (either capsule or decoction) and Tripterygium wilfordii were tested in two or more trials, with or without mifepristone against mifepristone alone. One trial with good quality was published in English (Hazlina 2005) and the other trials, published in Chinese, were of low quality. No trials reported menstrual symptoms related to fibroids, quality of life, incidence of complications, or the need for a surgical procedure. Most of the trials reported the volume of fibroids or size of the uterus, or both. The trials in this review showed a similar effect of herbal preparations combined with medication in terms of reduced volume of uterine fibroids. However, due to the small sample of the trials and methodological flaws in the majority of the trials, any indicated benefit is not conclusive. Further large and rigorous trials are needed.
Overall completeness and applicability of evidence
The included studies tested 10 different herbal medicines with conventional therapy, including mifepristone or GnRHa. In general, there was no significant difference between herbs and medical treatment for the reduced volume of fibroids. However, the lack of statistical significant difference does not mean equal effectiveness as none of the trials were designed as equivalence or non‐inferiority trials and the sample size was no more than 100 in each arm in the majority of the trials.
With regard to the conventional medical treatments used in the included studies, the efficacy of mifepristone for reducing fibroid volume has not been firmly established (Tristan 2012). A Cochrane systematic review demonstrated that mifepristone reduced heavy menstrual bleeding and improved fibroid‐specific quality of life (Tristan 2012). Unfortunately, none of the trials in our review reported these outcomes and we don't know whether herbal medicines can be helpful in relieving fibroid related symptoms or not.
The evidence from this review is not sufficiently convincing to support a clinical recommendation due to the following aspects of the trials.
There is a lack of evidence on the clinical effect of individual herbal preparations for menstrual symptom improvement in uterine fibroids. In clinical practice, Chinese herbal medicine is used mainly for symptom improvement, but this was not confirmed from the included trials due to the lack of a validated measurement, or reporting of symptoms or quality of life. Almost all reported outcomes were surrogate outcomes and may not reflect the clinical effectiveness. Comparisons with placebo are needed, as there is no clear evidence of the efficacy of the comparators used in the trials.
Although Chinese herbal extracts of Tripterygium wilfordii showed a promising effect compared with mifepristone, the findings are not confirmed as we only had two small trials that were of poor quality and uncertain evidence on safety. The Chinese herbal medicine Guizhi Fuling showed a promising benefit when combined with mifepristone versus mifepristone alone. However, the findings need to be verified in large, rigorous trials.
The trials reported outcomes by the end of treatment or at short‐term follow up. For those women with asymptomatic fibroids or mild symptoms, the use of herbal therapies is intended to prevent fibroid growth or to manage the mild symptoms. For this (wide) subgroup of women the main outcome is the avoidance of surgical treatment, measured through long‐term follow up. Future trialists are encouraged to adopt this outcome, as women may simply reach their menopause without needing surgery. In addition, reproductive outcomes related to uterine fibroids, such as the relationship between submucosal, intramural, or subserosal fibroids and pregnancy rates, miscarriage, and malpresentation, should be addressed in future trials (Klatsky 2008).
Reporting of adverse events in relation to herbal preparations was not sufficient in the included trials, and one trial suggested potential kidney function impairment after three‐month herbal treatment. Therefore, the safety of herbal medicine is still undetermined.
Quality of the evidence
This systematic review has several methodological limitations. Firstly, there is a lack of high quality trials and we had to exclude some of the trials that claimed to be randomised because of an unexplainable skew in the distribution of participants among the compared groups or an inadequate method for sequence generation for randomisation, which means they were highly prone to selection bias (Liu J 2002).
Secondly, trials did not report use of double blinding (except for one trial), which may be related to performance and detection bias (Moher 1998; Schulz 1995).
Thirdly, the trials had a small sample size. Although some data analyses did not demonstrate a statistically significant difference between herbal medicines and conventional medicine, the results are likely to have been underpowered. Therefore, the size of the trials may mean that the analyses may not establish with confidence that the two interventions have equivalent effects.
Fourth, the trials failed to report clinically useful outcomes such as symptoms or quality of life, which may suggest evidence of selective reporting bias. We could not differentiate the participants with symptoms from those without symptoms in the included studies. It is difficult to justify the herbal medicine treatment as some women without symptoms may not need any treatment. Therefore, we suggest that future trials should measure and report clinical symptoms as one of the major outcomes.
The above limitations mean that potential bias may have been present in the selection of participants, administration of treatment, and assessment of outcomes in the primary studies. Methodologically less rigorous trials show significantly larger intervention effects than more robust trials (Egger 2003; Kjaergard 2001; Moher 1998; Schulz 1995). An empirical study has shown that Chinese trials are significantly affected by publication bias (Vickers 1998). When interpreting the present findings, publication bias should be taken into consideration accordingly.
In summary, the findings of this review should be interpreted with caution due to the small sample sizes, low methodological quality in the majority of the 21 trials, and the limited number of trials included for each individual herbal preparation.
Potential biases in the review process
Although we conducted comprehensive searches in both English and Chinese databases, we may have missed some studies published in the non‐English or non‐Chinese literature, such as in the Japanese or Korean language. Second, we endeavoured to contact trial authors to clarify the methods for randomisation and obtain missing data, but the response was not satisfactory and leaves some trials with unclear randomisation. This may cause selection bias, and may not reflect the whole picture in using herbal medicine for the treatment of uterine fibroids.
Agreements and disagreements with other studies or reviews
As far as we know, there is no other systematic review or meta‐analysis published on the same topic. We also could not identify any large, multicentre trials for a comparison of our findings with other types of evidence.
Authors' conclusions
Implications for practice.
Current evidence does not support the use of herbal preparations for treatment of uterine fibroids. There is no conclusive evidence of benefit due to a limited number of trials conducted for individual herbal preparations, the methodological quality of the primary studies, and their insufficient power to meet robust conclusions.
Implications for research.
Further well‐designed, randomised, double blind, placebo‐controlled trials are needed to evaluate herbal preparations for uterine fibroids. To improve quality, trials needs to use appropriate allocation concealment; blinding of participants, researchers and outcome assessors; and clarify the number of participants randomised and the number analysed. Clinically relevant outcomes, such as symptoms, quality of life, infertility, and anaemia, should be addressed and measured using validated patient‐reported instruments. Potentially promising herbal preparations require further trials with large samples. As in this systematic review, the quality of herbal medicines was not reported in detail and for future trials it is important to investigate herbal medicines according to a set of criteria which include a preparation consistent with the description in the pharmacopoeia, chemical standardisation, biological assays, animal models, and clinical testing (Yuan 2000). It will be necessary to improve the description of the herbal medicines being tested, for example plant species, geographical origin, harvest season, preparation procedures, and the quality of the products. Furthermore, future trials should pay more attention to the adverse effects of herbal medicines, especially for long‐term use. Adverse events should be fully recorded and reported. Finally, trial reports should follow international standards, such as the CONSORT statement (http://www.consort‐statement.org/), and the trial protocol should be registered and accessible.
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2013 | New search has been performed | 19 randomised trials included: (Deng XL 2010; Dong M 2011; Fu WJ 2005; Gu HH 2011; Lai XL 2010; Liu Y 2009; Liu LY 2010; Lu HJ 2010; Luo SQ 2010; Ma R 2010; Mao CX 2012; Mao XG 2012; Ni XP 2012; Wang XR 2011; Wen Q 2005; Wu JH 2011; Wu YF 2011; Yan LQ 2000; Zhu FH 2006, making the total number of studies 21 in this review. No changes to conclusions. |
| 12 October 2012 | New citation required but conclusions have not changed | The new studies added did not lead to any change in conclusions. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 16 April 2008 | Amended | Converted to new review format. |
| 14 April 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors thank the Cochrane Menstrual Disorders and Subfertility Group (MDSG) for their expertise and editorial input. We would like to specifically thank the Trials Search Coordinator of the MDSG, Marian Showell, for her help with the literature searches. We thank Dr Nik Hazlina, Nik Hussain for providing us with additional data from their study. We also thank Ms Nini Chen for helping with validating data extraction and analyses in the updating of the review.
This work was funded by the Grant Number 2011ZX09302‐006‐01(5) and 101207007 from the Ministry of Science and Technology of China. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Jianping Liu's work was supported by the Programme of Innovative Research Team Project (2011‐CXTD‐09) of Beijing University of Chinese Medicine, and the "111" Project (B08006).
Appendices
Appendix 1. Detailed search strategies for English literature
MDSG keywords Sept 2012
Keywords CONTAINS "fibroid" or "Leiomyoma" or "myoma" or "myomas" or "myomata" or "uterine fibroids" or "uterine leiomyomas" or "uterine myoma" or "uterine myomas" or "Uterine Neoplasms" or "fibroids" or Title CONTAINS "fibroid" or "Leiomyoma" or "myoma" or "myomas" or "myomata" or "uterine fibroids" or "uterine leiomyomas" or "uterine myoma" or "uterine myomas" or "Uterine Neoplasms" or "fibroids"
AND
Keywords CONTAINS "Chinese herbal medicine" or "chinese herbal preparations" or "Chinese herbal remedy" or "Chinese traditional medicine" or "plant extracts" or "herbal preparations" or "herbal remedy", "herbal supplement" or "herbal supplements" or Title CONTAINS "Chinese herbal medicine" or "chinese herbal preparations" or "Chinese herbal remedy" or "Chinese traditional medicine" or "plant extracts" or "herbal preparations" or "herbal remedy", "herbal supplement" or "herbal supplements"
AMED 1985 to Sept 2012
1 traditional medicine$.tw. (5881)
2 exp plant extracts/ or exp drugs, chinese herbal/ (15672)
3 chinese herb$.tw. (1492)
4 plant extract$.tw. (9931)
5 chinese medicine$.tw. (1026)
6 exp Plants, Medicinal/ (14154)
7 (Plant$ adj2 Medicin$).tw. (13318)
8 herb$.tw. (9770)
9 exp Phytotherapy/ (1049)
10 Phytotherap$.tw. (1346)
11 alternative medicine$.tw. (1201)
12 exp ethnopharmacology/ or exp remedies/ or exp traditional medicine chinese/ (4761)
13 exp herbal drugs/ (6344)
14 or/1‐13 (26465)
15 exp uterine neoplasms/ (20)
16 (uterine adj5 neoplasm$).tw. (28)
17 fibroid$.tw. (23)
18 (fibroma$ or leiomyom$).tw. (48)
19 (myoma$ or hysteromyom$).tw. (15)
20 fibroid$.tw. (23)
21 or/15‐20 (87)
22 14 and 21 (17)
23 from 22 keep 1‐17 (17)
CENTRAL Issue 4, 2012
1 exp Fibroma/ (1)
2 fibroma$.tw. (16)
3 leiomyom$.tw. (148)
4 exp Myoma/ (7)
5 myoma$.tw. (150)
6 hysteromyom$.tw. (7)
7 fibroma$.tw. (16)
8 fibroid$.tw. (153)
9 exp Leiomyoma/ (247)
10 or/1‐9 (442)
11 exp medicine, traditional/ or exp medicine, oriental traditional/ (351)
12 traditional medicine$.tw. (69)
13 exp plant extracts/ or exp drugs, chinese herbal/ (2840)
14 chinese herb$.tw. (331)
15 plant extract$.tw. (72)
16 chinese medicine$.tw. (428)
17 exp Plants, Medicinal/ (742)
18 (Plant$ adj2 Medicin$).tw. (35)
19 herb$.tw. (1117)
20 exp Phytotherapy/ (1566)
21 Phytotherap$.tw. (48)
22 alternative medicine$.tw. (69)
23 or/11‐22 (4822)
24 10 and 23 (7)
25 from 24 keep 1‐7 (7)
CINAHL 1982 to Sept 2012
1 exp Fibroma/ (0)
2 fibroma$.tw. (184)
3 leiomyom$.tw. (177)
4 exp Myoma/ (36)
5 myoma$.tw. (72)
6 hysteromyom$.tw. (0)
7 fibroma$.tw. (184)
8 fibroid$.tw. (297)
9 exp Leiomyoma/ (569)
10 or/1‐9 (879)
11 exp medicine, traditional/ or exp medicine, oriental traditional/ (10482)
12 traditional medicine$.tw. (294)
13 exp plant extracts/ or exp drugs, chinese herbal/ (2691)
14 chinese herb$.tw. (313)
15 plant extract$.tw. (123)
16 chinese medicine$.tw. (678)
17 exp Plants, Medicinal/ (13658)
18 (Plant$ adj2 Medicin$).tw. (236)
19 herb$.tw. (4109)
20 exp Phytotherapy/ (3397)
21 Phytotherap$.tw. (93)
22 alternative medicine$.tw. (2179)
23 or/11‐22 (26022)
24 10 and 23 (14)
25 exp clinical trials/ (57427)
26 Clinical trial.pt. (29998)
27 (clinic$ adj trial$1).tw. (13150)
28 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$3 or mask$3)).tw. (7801)
29 Randomi?ed control$ trial$.tw. (11197)
30 Random assignment/ (17445)
31 Random$ allocat$.tw. (1211)
32 Placebo$.tw. (10846)
33 Placebos/ (4145)
34 Quantitative studies/ (3735)
35 Allocat$ random$.tw. (73)
36 or/25‐35 (79254)
37 24 and 36 (2)
38 from 37 keep 1‐2 (2)
EMBASE to Sept 2012
1 exp traditional medicine/ or exp chinese medicine/ or exp herbal medicine/ or exp oriental medicine/ (21306)
2 exp Plant Extract/ (57548)
3 exp Medicinal Plant/ (40872)
4 (traditional adj2 medicin$).tw. (6936)
5 chinese herb$.tw. (2504)
6 plant extract$.tw. (2889)
7 chinese medicine.tw. (3872)
8 (herbal adj2 medicin$).tw. (3621)
9 (oriental adj2 medicine).tw. (354)
10 (Medicin$ adj2 Plant$).tw. (4699)
11 herb$.tw. (27378)
12 exp Phytotherapy/ (3672)
13 Phytotherap$.tw. (1297)
14 alternative medicine$.tw. (2950)
15 or/1‐14 (109922)
16 exp benign uterus tumor/ or exp leiomyoma/ or exp uterus myoma/ (8988)
17 exp Fibroma/ (2830)
18 (Fibroma$ or leiomyom$).tw. (9914)
19 (myoma$ or hysteromyom$).tw. (2258)
20 fibroid$.tw. (2241)
21 or/16‐20 (17218)
22 15 and 21 (35)
23 Clinical trial/ (495185)
24 Randomized controlled trials/ (155511)
25 Random Allocation/ (25203)
26 Single‐Blind Method/ (7410)
27 Double‐Blind Method/ (68576)
28 Cross‐Over Studies/ (20046)
29 Placebos/ (111054)
30 Randomi?ed controlled trial$.tw. (28060)
31 RCT.tw. (2194)
32 Random allocation.tw. (605)
33 Randomly allocated.tw. (9592)
34 Allocated randomly.tw. (1314)
35 (allocated adj2 random).tw. (552)
36 Single blind$.tw. (7066)
37 Double blind$.tw. (81296)
38 ((treble or triple) adj blind$).tw. (127)
39 Placebo$.tw. (104327)
40 Prospective Studies/ (73142)
41 or/23‐40 (651841)
42 Case study/ (5369)
43 Case report.tw. (110903)
44 Abstract report/ or letter/ (461484)
45 or/42‐44 (575754)
46 41 not 45 (629234)
47 animal/ (18235)
48 human/ (6058876)
49 47 not 48 (14465)
50 46 not 49 (629138)
51 or/23‐50 (6253078)
52 22 and 51 (33)
53 from 52 keep 1‐33 (33)
MEDLINE 1950 to Sept 2012
1 exp Fibroma/ (9610)
2 fibroma$.tw. (7047)
3 leiomyom$.tw. (7602)
4 exp Myoma/ (1626)
5 myoma$.tw. (3317)
6 hysteromyom$.tw. (26)
7 fibroma$.tw. (7047)
8 fibroid$.tw. (2244)
9 exp Leiomyoma/ (13308)
10 or/1‐9 (29717)
11 exp medicine, traditional/ or exp medicine, oriental traditional/ (17249)
12 traditional medicine$.tw. (2357)
13 exp plant extracts/ or exp drugs, chinese herbal/ (59586)
14 chinese herb$.tw. (2614)
15 plant extract$.tw. (2601)
16 chinese medicine$.tw. (3950)
17 exp Plants, Medicinal/ (44249)
18 (Plant$ adj2 Medicin$).tw. (4189)
19 herb$.tw. (29907)
20 exp Phytotherapy/ (17380)
21 Phytotherap$.tw. (781)
22 alternative medicine$.tw. (3563)
23 or/11‐22 (132460)
24 10 and 23 (43)
25 randomised controlled trial.pt. (251334)
26 controlled clinical trial.pt. (77422)
27 randomised controlled trials as topic/ (53023)
28 random allocation/ (60395)
29 double blind method/ (96065)
30 single blind method/ (11789)
31 or/25‐30 (424467)
32 animals/ not (animals/ and humans/) (3189559)
33 31 not 32 (397756)
34 clinical trial.pt. (446433)
35 exp clinical trials as topic/ (201557)
36 (clinic$ adj25 trial$).ti,ab. (142061)
37 cross‐over studies/ (21493)
38 (crossover or cross‐over or cross over).tw. (40169)
39 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. (95360)
40 placebos/ (26962)
41 placebo$.ti,ab. (108242)
42 random$.ti,ab. (401463)
43 research design/ (51618)
44 or/34‐43 (910204)
45 44 not 32 (843399)
46 33 or 45 (865071)
47 24 and 46 (8)
48 from 47 keep 1‐8 (8)
PsycINFO 1806 to Sept 2012
1 exp Fibroma/ (0)
2 fibroma$.tw. (15)
3 leiomyom$.tw. (2)
4 exp Myoma/ (0)
5 myoma$.tw. (12)
6 hysteromyom$.tw. (1)
7 fibroma$.tw. (15)
8 fibroid$.tw. (15)
9 exp Leiomyoma/ (0)
10 or/1‐9 (44)
11 exp medicine, traditional/ or exp medicine, oriental traditional/ (0)
12 traditional medicine$.tw. (205)
13 exp plant extracts/ or exp drugs, chinese herbal/ (0)
14 chinese herb$.tw. (65)
15 plant extract$.tw. (41)
16 chinese medicine$.tw. (212)
17 exp Plants, Medicinal/ (0)
18 (Plant$ adj2 Medicin$).tw. (68)
19 herb$.tw. (3468)
20 exp Phytotherapy/ (0)
21 Phytotherap$.tw. (14)
22 alternative medicine$.tw. (790)
23 or/11‐22 (4537)
24 10 and 23 (1)
25 from 24 keep 1 (1)
Appendix 2. Search strategies for Chinese biomedical databases
Since the Chinese databases have different indexing and search functions, we listed below generic string search terms for use in different databases:
1 Zi Gong ji liu (Chinese spelling, in English 'uterine fibroids')
2 Zhong yao (Chinese materia medica)
3 Zhong cheng yao (Chinese patent medicine)
4 Zhong cao yao (Chinese herbal drug)
5 Tang yao (herbal decoction)
6 Lin chuang yan jiu (clinical studies)
7 Lin chuang shi yan (clinical trials)
Data and analyses
Comparison 1. Herbal preparations versus medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average volume of uterine fibroids (cm3) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Gongliuqing capsule versus mifepristone | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.62, 0.40] |
| 1.2 Huoxue Huayu Ruanjian Sanjie versus mifepristone | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 7.97 [‐4.24, 20.18] |
| 1.3 Huoxue Sanjie decoction versus mifepristone | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 4.98 [‐6.08, 16.04] |
| 1.4 Nona Roguy herbal product versus GnRH agonist | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 6.74 [‐27.43, 40.91] |
| 1.5 Tripterygium wilfordii versus mifepristone | 2 | 186 | Mean Difference (IV, Fixed, 95% CI) | ‐23.03 [‐28.39, ‐17.67] |
| 1.6 Xiaozheng decoction versus mifepristonee | 1 | 260 | Mean Difference (IV, Fixed, 95% CI) | 1.79 [‐2.13, 5.71] |
| 2 Average size of uterus (cm3) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Huoxue Huayu Ruanjian Sanjie versus mifepristone | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 22.76 [10.76, 34.76] |
| 2.2 Huoxue Sanjie decoction versus mifepristone | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 23.23 [17.85, 28.61] |
| 2.3 Nona Roguy herbal product versus GnRH agonist | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐23.61 [‐223.63, 176.41] |
| 2.4 xiaozheng decoction versus mifepristone | 1 | 260 | Mean Difference (IV, Random, 95% CI) | 10.22 [3.25, 17.19] |
| 2.5 Tripterygium wilfordii versus mifepristone | 2 | 186 | Mean Difference (IV, Random, 95% CI) | ‐51.25 [‐77.70, ‐24.80] |
Comparison 2. Herbal preparations plus medication versus medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average volume of maximum fibroids (cm3) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Gongliuqing capsule plus mifepristone versus mifepristone | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐5.28 [‐6.46, ‐4.10] |
| 1.2 Guizhi Fuling capsules plus mifepristone versus mifepristone | 7 | 687 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐2.42, ‐1.02] |
| 1.3 Guizhi Fuling capsule plus Leuprolide acetate versus mifepristone | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.50, 1.00] |
| 1.4 Lenge Xiaozheng Tang plus mifepristone versus mifepristone | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐3.24, 0.36] |
| 2 Average volume of total multiple fibroids (cm3) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Guizhi Fuling capsules plus mifepristone versus mifepristone | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐16.58 [‐20.30, ‐12.86] |
| 2.2 Jiliu Tang plus mifepristone versus mifepristone | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐16.30 [‐18.97, ‐13.63] |
| 3 Average size of uterus (cm3) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Guizhi Fuling capsules plus mifepristone versus mifepristone | 3 | 231 | Mean Difference (IV, Random, 95% CI) | ‐31.63 [‐54.58, ‐8.68] |
| 3.2 Jiliu Tang plus mifepristone versus mifepristone | 1 | 138 | Mean Difference (IV, Random, 95% CI) | ‐47.80 [‐55.68, ‐39.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Deng XL 2010.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: follow up for 12 months, but loss to follow up was not reported
Intention‐treat analyses: no Baseline comparability: parity, age, weight, average volume of uterine fibroids (P > 0.05) |
|
| Participants | 69 participants with uterine fibroids, The diagnosis was made by a routine gynaecological examination and type B ultrasonography. 33 in Guizhi Fuling capsule plus mifepristone group and 36 in mifepristone group Inclusion and exclusion criteria were specified |
|
| Interventions | Experimental:
Guizhi Fuling capsule, 3 capsules each time, three times per day for 90 days; mifepristone, taken from the second to the third day of menstrual cycle, 12.5mg daily for 90 days. Control: Mifepristone, taken from the second to the third day of menstrual cycle, 12.5mg daily for 90 days. |
|
| Outcomes | Fibroid volume measured by ultrasound, follicle‐stimulating hormone, luteinizing hormone, estradiol and progesterone, and adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal medicine taken as capsule while the western drug was tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | High risk | Outcome measures mentioned in the methods were reported in results |
| Free of other bias | Low risk | Baseline data reported and comparable |
Dong M 2011.
| Methods | Generation of allocation sequence: random number table
Double blinding: not mentioned
Loss to follow up: follow up was for 6 months after the end of the treatment
Intention‐treat analyses: no Baseline comparability: parity, age, disease duration, number of fibroids, average volume of uterine fibroid (P > 0.05) |
|
| Participants | 100 participants with uterine fibroids, the diagnosis was made by a routine gynaecological examination and type B ultrasonography 50 in Guizhi Fuling capsule plus leuprolide acetate group and 50 in mifepristone group Exclusion criteria: serious medical conditions, pregnancy or breast feeding women |
|
| Interventions | Experimental:
Guizhi Fuling capsule, 3 capsules each time, three times per day for 90 days; leuprolide acetate injection once a month, for a total of 3 months. Control: mifepristone, taken from the first day of menstrual cycle, 12.5mg daily for 90 days. |
|
| Outcomes | Fibroid volume measured by ultrasound, and adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number table |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal medicine taken as capsule while the western drug was tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomized and reported in outcome |
| Free of selective reporting | High risk | We could not access the trial protocol, and symptoms were not reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Fu WJ 2005.
| Methods | Generation of allocation sequence: unclear
Double blinding: not mentioned
Loss to follow up: follow up was for 6 months after the end of the treatment
Intention‐treat analyses: no Baseline comparability: age, complications, number of fibroids, average volume of uterine fibroid (P > 0.05) |
|
| Participants | 124 participants with uterine fibroids, the diagnosis was made by a routine gynaecological examination and type B ultrasonography. 62 in Tripterygium wilfordii group and 62 in mifepristone group Inclusion and exclusion criteria: specified |
|
| Interventions | Experimental:
Tripterygium wilfordii extract tablet, 40 mg daily (divided into three times, 10 mg, 10 mg, 20 mg, respectively), for 3 months. Control: mifepristone, taken from the first day of menstrual cycle, 25mg daily for 3 months. |
|
| Outcomes | Fibroid and uterus volume measured by ultrasound, and adverse effects. | |
| Notes | We had phoned the authors three times in 2009, but authors refused to provide information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Unclear risk | Not reported |
| Blinding All outcomes | Unclear risk | No information |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of participants randomised and analysed |
| Free of selective reporting | Low risk | All mentioned measurements in the methods were reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Gu HH 2011.
| Methods | Generation of allocation sequence: random number table
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: parity, age, disease duration, average volume of uterine fibroids (P > 0.05) |
|
| Participants | 134 participants with uterine fibroid, the diagnosis was made by a routine gynaecological examination and type B ultrasonography. 67 in Guizhi Fuling capsule plus mifepristone group and 67 in mifepristone group Exclusion criteria: adenomyosis of uterus, endometriosis, and gestation or suckling period women |
|
| Interventions | Experimental:
Guizhi Fuling capsule, 4 capsules each time, three times per day for 90 days; mifepristone, taken from the first day of menstrual cycle, 12.5mg daily for 90 days. Control: mifepristone, taken from the first day of menstrual cycle, 12.5mg daily for 90 days. |
|
| Outcomes | Fibroid volume measured by ultrasound, estradiol and progesterone. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number table |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal medicine taken as capsule while the western drug was tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | High risk | Trial protocol not accessed, and symptoms were not reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Hazlina 2005.
| Methods | Generation of allocation sequence: adequate
Blinding: single blind
Withdrawal or loss to follow up: 1 participant was withdrawn from the study due to hypersensitivity to GnRH agonist, and 1 participant became pregnant during the 4th visit. The 2 participants were excluded from the study. Follow up was for 6 months after the end of the treatment
Intention‐to‐treat analyses: no Baseline comparability: parity, age, level of education, symptom, duration of diagnosis, BMI, haemoglobin level, number of fibroids (P > 0.05) |
|
| Participants | 35 participants with uterine fibroids confirmed by ultrasound (18 in herbal group and 17 in GnRH group) Inclusion and exclusion criteria were specified |
|
| Interventions | Experimental:
herbal preparation was formulated and manufactured by Mustajab Industry. It contained 10 herbs, given daily orally in semi‐liquid form for a total of 6 months. Control: gonadotropin‐releasing hormone (GnRH) agonist 3.75 mg, intramuscular or subcutaneous injection once a month, for a total of 3 months. |
|
| Outcomes | Fibroid and uterine volume measured by ultrasound, serum FSH, LH and estradiol, and adverse effects. | |
| Notes | Sample size was calculated by using the Pocok's formula with 90% power of study and 95% confidence interval. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Drawing of papers containing 'A' or 'B' by participants. The operator who did the allocation was blinded |
| Allocation concealment | Low risk | Independent operator allocated the participants |
| Blinding All outcomes | Low risk | Single blind (outcome assessor) |
| Incomplete outcome data addressed All outcomes | Low risk | Number of participants with loss to follow up reported |
| Free of selective reporting | Unclear risk | Trial protocol not accessed, and symptoms were not reported in results |
| Free of other bias | Low risk | baseline data were comparable |
Lai XL 2010.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: follow up was for 3 months after the end of the treatment
Intention‐treat analyses: no Baseline comparability: parity, age, average volume of uterine fibroids (P > 0.05) |
|
| Participants | 188 participants with uterine fibroids, the diagnosis was made by a routine gynaecological examination and type B ultrasonography. 94 in Gongliuqing capsule group and 94 in mifepristone group Exclusion criteria: heart, liver, kidney and blood diseases, uterus size were all less than 3 months of pregnancy, the diameter of uterine fibroids < 60 mm, or mifepristone counter indication. |
|
| Interventions | Experimental:
Gongliuqing capsule, 3 capsules each time, three times per day for 3 months, stop use during menstrual period. Control: mifepristone, 25 mg daily, for 3 months. |
|
| Outcomes | Fibroid and uterine volume measured by ultrasound, progestin and estradiol, and adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal medicine taken as capsule while the control drug was tablet |
| Incomplete outcome data addressed All outcomes | Low risk | 86.2% participants completed follow up, and the number of participants with loss to follow up reported |
| Free of selective reporting | Low risk | Outcome measures mentioned in the methods were reported in results |
| Free of other bias | Unclear risk | insufficient information to make judgment |
Liu LY 2010.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: parity, age, kinds of uterine fibroids and average volume of uterine fibroids (P > 0.05) |
|
| Participants | 100 participants with uterine fibroids, the diagnosis was made by a routine gynaecological examination and type B ultrasonography 50 in Gongliuqing capsule plus mifepristone group and 50 in mifepristone group Exclusion criteria: malignant lesions in endometrial and endocervical; heart, liver, kidney and blood diseases; endocrinal diseases |
|
| Interventions | Experimental:
Gongliuqing capsule, 3 capsules each time, three times per day for 90 days; Mifepristone, taken from the first day of menstrual cycle, 12.5mg daily for 90 days, menstrual drug withdrawal. Control: mifepristone, taken from the first day of menstrual cycle, 12.5mg daily for 90 days, menstrual drug withdrawal. |
|
| Outcomes | Fibroid and uterine volume measured by ultrasound, and adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal medicine taken as capsule while the western drug was tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomized and reported in outcome |
| Free of selective reporting | Low risk | Outcome measures mentioned in the methods were reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Liu Y 2009.
| Methods | Generation of allocation sequence: random number table
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: parity, age, menometrorrhagia or menstruation prolonged, compression symptoms, dysmenorrhea; haemoglobin level (P>0.05) |
|
| Participants | 60 participants with uterine fibroids, the diagnosis was made by symptoms,signs by routine gynaecological examination and type B ultrasonography 30 in herbal treatment combined with mifepristone group and 30 in mifepristone group Exclusion criteria: gestation or suckling period women; heart, liver, kidney and blood diseases, endocrinal diseases |
|
| Interventions | Experimental:
Guizhi Fuling Wan (a herbal formula of 12 herbs modified according to symptoms), twice daily for 3 months; mifepristone 12.5mg daily for 3 months. Control: mifepristone, taken before sleep from the first day of menstrual cycle, 12.5mg daily for 3 months. |
|
| Outcomes | Disappearance of uterine fibroids, relapse of fibroids, fibroid volume measured by ultrasound, serum FSH, LH, estradiol and progesterone, and adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number table |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal medicine taken as decoction while the western drug was tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | Low risk | Outcome measures mentioned in the methods were reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Lu HJ 2010.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: parity, age, menometrorrhagia or menstruation prolong, disease duration, average volume of uterine fibroids (P > 0.05) |
|
| Participants | 120 patients with uterine fibroid. The diagnosis was made by symptoms,signs by routine gynaecological examination and type B ultrasonography 60 patients in herbal treatment combined with mifepristone group and 60 patients in mifepristone group Exclusion criteria: heart, liver, kidney and blood diseases; malignant lesions in endometrium, other tumors in cervix of the uterus and uterine appendages; or mifepristone counter indication |
|
| Interventions | Experimental:
Guizhi Fuling capsule (a herbal formula of 12 herbs modified according to symptoms), three times per day for 3 months; mifepristone 12.5mg daily for 3 months. Control: mifepristone, taken from the first day of menstrual cycle, 12.5mg daily for 3 months. |
|
| Outcomes | Fibroid and uterine volume measured by ultrasound. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal medicine taken as decoction while the western drug was tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | Low risk | Outcome measures mentioned in the methods were reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Lu JX 2007.
| Methods | Generation of allocation sequence: random number table
Double blinding: not mentioned
Loss to follow up: follow up for 3 months, but loss to follow up was not reported
Intention‐treat analyses: no Baseline comparability: age, disease duration (no statistical testing) |
|
| Participants | 115 participants with uterine fibroid, belonging to type of qi‐stagnancy and blood stasis by TCM diagnosis. The diagnosis was made by a routine gynaecological examination and type B ultrasonography 59 in Huoxue Sanjie decoction group and 56 in mifepristone group Exclusion criteria: heart, liver, kidney and blood diseases, endocrinal diseases, the diameter of uterine fibroids > 60 mm, or mifepristone counter indication, postmenopause women |
|
| Interventions | Experimental:
Huoxue Sanjie decoction (self‐prescribed herbal formula), twice daily for 6 months. Control: mifepristone, taken before sleep from the first or second day of menstrual cycle, 10 mg daily for 6 months. |
|
| Outcomes | Disappearance of uterine fibroids, uterine‐volume reduction, volume of uterine fibroids, and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number table |
| Allocation concealment | Unclear risk | No information about concealment |
| Blinding All outcomes | High risk | Herbal decoction compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Low risk | Same number of participants randomised and analysed |
| Free of selective reporting | Low risk | The study presented the results in accordance with the outcome measures in the method |
| Free of other bias | Low risk | Baseline data were comparable |
Luo SQ 2010.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: parity, age, average volume of uterine fibroids (P > 0.05) |
|
| Participants | 138 patients with uterine fibroid. The diagnosis was made by symptoms, signs by routine gynaecological examination and type B ultrasonography 70 patients in herbal treatment combined with mifepristone group and 60 patients in mifepristone group Exclusion criteria: malignant lesions in endometrial and endocervical; anaemia caused by blood disorders and internal medical illness |
|
| Interventions | Experimental:
Jiliu Tang decoction (self‐prescribed herbal formula ) group, twice daily for 6 months; mifepristone taken from the first day of menstrual cycle, 10 mg daily for 6 months. Control: mifepristone, taken from the first day of menstrual cycle, 10 mg daily for 6 months. |
|
| Outcomes | Disappearance of uterine fibroids, fibroid and uterine volume measured by ultrasound. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal decoction compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | Low risk | Outcome measures mentioned in the methods were reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Ma R 2010.
| Methods | Generation of allocation sequence: randomisation (not described)
Double blinding: yes
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: not reported |
|
| Participants | 136 participants with uterine fibroid, belonging to type of qi stagnation and blood stasis by TCM diagnosis. The diagnosis was made by a routine gynaecological examination and type B ultrasonography 68 in Guihong turtle shell pills group and 68 in placebo group Exclusion criteria were specified |
|
| Interventions | Experimental:
Guihong turtle shell pill, 9 g each time, twice per day for 3 months. Control: placebo, 9 g each time, twice per day for 3 months. |
|
| Outcomes | Disappearance of uterine fibroids, fibroid and uterine volume measured by ultrasound, and adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomisation method not described |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | Low risk | Two drugs are both pills, with a volume of 54g/bottle, and provided by the pharmacy department of traditional Chinese hospital affiliated to Xinjiang Medical University |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | High risk | Some of the observed outcome measures were not reported |
| Free of other bias | Unclear risk | No information for baseline comparability |
Mao CX 2012.
| Methods | Generation of allocation sequence: random number table
Double blinding: not mentioned
Loss to follow up: reported
Intention‐treat analyses: no Baseline comparability: parity, age, disease duration, type and distribution of uterine fibroids, average volume of uterine fibroids (P > 0.05) |
|
| Participants | 120 participants with uterine fibroid, belonging to type of qi stagnation and blood stasis by TCM diagnosis. The diagnosis was made by a routine gynaecological examination and type B ultrasonography 60 in Guizhi Fuling capsule plus mifepristone group and 60 in mifepristone group Exclusion criteria: heart, liver, kidney and blood diseases, endocrinal diseases, or mifepristone counter indication, postmenopause women |
|
| Interventions | Experimental: Guizhi Fuling capsule, 3 capsules each time, three times per day for 3 months except menstrual period; mifepristone taken from the first day of menstrual cycle, 12.5 mg daily for 3 months. Control: mifepristone, taken from the first day of menstrual cycle, 12.5 mg daily for 3 months. |
|
| Outcomes | Disappearance of uterine fibroids, volume of uterine fibroids, relapse of fibroids and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number table |
| Allocation concealment | Unclear risk | No information about concealment |
| Blinding All outcomes | High risk | Guizhi Fuling capsules compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | High risk | Same number of participants randomised and analysed for fibroids volume, but 8 in treatment group and 10 in control group were lost during 6 months follow up. No intention to treat analysis was applied |
| Free of selective reporting | High risk | Some of the observed outcome measures were not reported |
| Free of other bias | Low risk | Baseline data were comparable |
Mao XG 2012.
| Methods | Generation of allocation sequence: not reported
Double blinding: not mentioned
Loss to follow up: not reported
Intention‐treat analyses: no Baseline comparability: age, symptoms, average haemoglobin level (P > 0.05) |
|
| Participants | 66 participants with uterine fibroid. The diagnosis was made by a routine gynaecological examination and type B ultrasonography 33 in Guizhi Fuling capsule plus mifepristone group and 33 in mifepristone group |
|
| Interventions | Experimental: Guizhi Fuling capsule, 3 capsules each time, three times per day for 3 months; mifepristone 25mg, twice daily, for 3 months. Control: mifepristone, 25 mg, twice daily, for 3 months. |
|
| Outcomes | Disappearance of uterine fibroids, volume of uterine fibroids, relapse of fibroids and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information |
| Allocation concealment | Unclear risk | No information |
| Blinding All outcomes | High risk | Herbal capsule plus mifepristone versus mifepristone |
| Incomplete outcome data addressed All outcomes | Low risk | Same number of participants randomised and analysed |
| Free of selective reporting | Low risk | Outcome measures mentioned in the methods were reported in the results |
| Free of other bias | Low risk | Baseline data were comparable |
Ni XP 2012.
| Methods | Generation of allocation sequence: draw of lots
Double blinding: not mentioned
Loss to follow up: no participant was withdrawn from the study. Follow up was for 12 months after the end of the treatment
Intention‐treat analyses: no Baseline comparability: parity, age, disease duration; average volume of uterine fibroids, haemoglobin level (P > 0.05) |
|
| Participants | 260 participants with uterine fibroids. The diagnosis was made by a routine gynaecological examination and type B ultrasonography 130 in Xiaozheng decoction (self‐prescribed herbal formula) group and 130 in mifepristone group Exclusion criteria: gestation or suckling period women; adenomyosis of uterus, endometriosis; heart, liver, kidney and blood diseases, endocrinal diseases |
|
| Interventions | Experimental:
Xiaozheng decoction (self‐prescribed herbal formula ) group, twice daily for 3 months, menstrual drug withdrawal. Control: Mifepristonge, taken before sleep from the first to third day of menstrual cycle, 25 mg daily for 3 months. |
|
| Outcomes | Disappearance of uterine fibroids, fibroid and uterine volume measured by ultrasound, haemglobin level. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Draw lots |
| Allocation concealment | Unclear risk | No information about concealment |
| Blinding All outcomes | High risk | Herbal decoction compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Low risk | Same number of participants randomised and analysed |
| Free of selective reporting | Low risk | The study presented the results in accordance with the outcome measures in the method |
| Free of other bias | Low risk | Baseline data were comparable |
Wang XR 2011.
| Methods | Generation of allocation sequence: random number table
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: age, disease duration, and volume of uterine fibroids |
|
| Participants | 120 participants with uterine fibroid (60 in Guizhi Fuling plus mifepristone group and 60 in mifepristone group) Inclusion and exclusion criteria were specified |
|
| Interventions | Experimental:
Guizhi Fuling capsule, 4 capsules each time, three times per day for 3 months; mifepristone taken from the first day of menstrual cycle, 12.5 mg daily for 3 months. Control: mifepristone, taken from the first day of menstrual cycle, 12.5 mg daily for 3 months. |
|
| Outcomes | Fibroid volume measured by ultrasound, serum FSH, LH, estradiol and progesterone. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number table |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal decoction compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomized and reported in outcome |
| Free of selective reporting | High risk | Trial protocol not available, and outcome of symptoms was not reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Wen Q 2005.
| Methods | Generation of allocation sequence: unclear (author refused to answer)
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: age, symptom, volume of uterine fibroids and uterus |
|
| Participants | 62 participants with uterine fibroid (32 in Tripterygium wilfordii group and 30 in mifepristone group) Inclusion and exclusion criteria were specified |
|
| Interventions | Experimental:
Tripterygium wilfordii extract, 40 mg daily, divided into three times taken (10 mg, 10 mg, 20 mg, respectively), for 3 months. Control: mifepristone tablet, 25 mg daily, for 3 months. |
|
| Outcomes | Symptoms, volume of uterine fibroids and uterus, and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Not reported and the author refused to answer our phone call (13 Jan 2009) |
| Allocation concealment | Unclear risk | No information |
| Blinding All outcomes | High risk | Different administration of herbal tablet and mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of participants randomised and analysed |
| Free of selective reporting | Low risk | The observed measures were reported in the results |
| Free of other bias | Unclear risk | Insufficient information available |
Wu JH 2011.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: age, anaemia, menometrorrhagia or menstruation prolonged, compression symptoms, dysmenorrhea (P > 0.05) |
|
| Participants | 102 patients with uterine fibroid. The diagnosis was made by symptoms, signs by routine gynaecological examination and type B ultrasonography. 51 in Guizhi Fuling capsule plus mifepristone group and 51 in mifepristone group Exclusion criteria: heart, liver, kidney and blood diseases, or mifepristone counter indication |
|
| Interventions | Experimental:
Guizhi Fuling capsule, 4 capsules each time, three times per day for 3 months. Mifepristone taken from the first day of menstrual cycle, 10 mg daily for 3 months. Control: mifepristone, taken from the first day of menstrual cycle, 10 mg daily for 3 months. |
|
| Outcomes | Uterine‐volume reduction, volume of uterine fibroids, serum FSH, LH, estradiol and progesterone, and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Guizhi Fuling capsules compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Unclear risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | High risk | Trial protocol not available, and outcome of symptoms was not reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Wu YF 2011.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: not mentioned.
Intention‐treat analyses: no Baseline comparability: parity, age, menometrorrhagia or menstruation prolonged, compression symptoms (P > 0.05) |
|
| Participants | 76 participants with uterine fibroid (38 in Guizhi Fuling capsule plus mifepristone group and 38 in mifepristone group). The diagnosis was made by symptoms, signs by routine gynaecological examination and type B ultrasonography Exclusion criteria: other malignant lesions of the reproductive system, or mifepristone counter indication |
|
| Interventions | Experimental:
Guizhi Fuling capsule, 4 capsules each time, three times per day for 3 months, menstrual drug withdrawal. Mifepristone taken from the first day of menstrual cycle, 25 mg daily for 3 months. Control: mifepristone, taken from the first day of menstrual cycle, 25 mg daily for 3 months. |
|
| Outcomes | Fibroid volume measured by ultrasound, serum FSH, LH, estradiol and progesterone. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Guizhi Fuling capsules compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | Low risk | Outcome measures mentioned in the methods were reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Yan LQ 2000.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: age, disease duration, and volume of uterine fibroids (P > 0.05) |
|
| Participants | 30 patients with uterine fibroid. The diagnosis was made by symptoms, signs by routine gynaecological examination and type B ultrasonography. 18 patients in self‐prescribed herbal decoction group and 12 patients in mifepristone group Inclusion and exclusion criteria were specified |
|
| Interventions | Experimental: self‐prescribed herbal decoction group, twice daily for 3 months, menstrual drug withdrawal. Control: mifepristone, taken from the fifth day of menstrual cycle, 25 mg daily for 3 months. |
|
| Outcomes | Uterine‐volume reduction, volume of uterine fibroids, and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal decoction compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomised and reported in outcome |
| Free of selective reporting | Low risk | Outcome measures mentioned in the methods were reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
Zhu FH 2006.
| Methods | Generation of allocation sequence: not mentioned
Double blinding: not mentioned
Loss to follow up: not mentioned
Intention‐treat analyses: no Baseline comparability: age (P > 0.05) |
|
| Participants | 67 patients with uterine fibroid. The diagnosis was made by symptoms, signs by routine gynaecological examination and type B ultrasonography. 35 patients in Lenge Xiaozheng decoction (self‐prescribed herbal formula) plus mifepristone group and 32 patients in mifepristone group) Exclusion criteria: malignant lesions in endometrium, other tumours in cervix of the uterus and uterine appendages; or mifepristone counter indication |
|
| Interventions | Experimental:
Lenge Xiaozheng decoction (self‐prescribed herbal formula ) group, twice daily for 3 months; mifepristone taken from the first day of menstrual cycle, 25 mg daily for 3 months. Control: mifepristone, taken from the first day of menstrual cycle, 25 mg daily for 3 months. |
|
| Outcomes | Fibroid volume measured by ultrasound. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Not mentioned |
| Allocation concealment | Unclear risk | No information provided |
| Blinding All outcomes | High risk | Herbal decoction compared with mifepristone tablet |
| Incomplete outcome data addressed All outcomes | Low risk | The same number of patients randomized and reported in outcome |
| Free of selective reporting | High risk | Trial protocol not available, and outcome of symptoms was not reported in results |
| Free of other bias | Low risk | Baseline data were comparable |
UAE = uterine artery embolization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akase 2003 | Clinical trial using Kampo medicine to treat anaemia of patients with uterine myoma |
| An ZR 2005 | Case series of 43 patients treated by herbal medicine |
| Chen QM 2007 | Randomised trial comparing herbal preparation Rupi Anxiao capsule plus methyltestosterone with Rupi Anxiao capsule for treatment of 100 patients with hysteromyoma. The intervention and comparison did not meet the criteria for this review |
| Cuan CL 2002 | Case series of 30 patients with uterine fibroids treated by herbal medicine |
| Du WH 1993 | Case series of 40 patients with uterine fibroids treated by herbal medicine |
| Fang RD 2001 | Randomised trial comparing two different herbal preparations (Xiao Liu Yin versus Guizhi Fuling capsule) in treatment of 160 cases of uterine fibroids |
| Feng FQ 2003 | According to the telephone interview by the author, the trial was planned as randomised trial, but could not apply randomisation due to treatment preference of patients. |
| Feng X 2004 | Case series of 39 patients with uterine fibroids treated by herbal medicine |
| Fu 2003 | Randomised trial comparing two different herbal preparations (self‐prescribed herbal formula versus Guizhi Fuling capsules) for treatment of 72 cases of uterine fibroids |
| Fu P 2004 | Randomised trial comparing two different herbal preparations (Xuejie Hualiu granules versus Guizhi Fuling capsules) in 72 cases of uterine fibroids |
| Gao SM 2006 | Randomised trial comparing two different herbal preparations (self‐prescribed herbal formula versus Guizhi Fuling pills) in treatment of 60 cases of uterine fibroids. |
| Gao XL 2001 | Randomised trial comparing herbal preparation with methyltestosterone in treatment of 134 cases of uterine fibroids. The data for outcome are not available. |
| Gao YP 2000 | Randomised trial comparing herbal preparation with mifepristone in treatment of 85 cases of uterine fibroids. However, there was a significant skew distribution in the numbers of participants between the two groups (65 versus 20), which could not be explained by a proper randomisation. |
| Gu H 1992 | Case series of 35 patients with uterine fibroids treated by herbal medicine |
| Gu Y 2011 | Randomised trial comparing two herbal preparations (Si Jun Zi decoction plus Guizhi Fuling capsules) with mifepristone in treatment of 82 cases of uterine fibroids |
| Guo AQ 2000 | Randomised trial comparing herbal preparation with mifepristone for treatment of 68 patients with myoma of uterus. The data for outcome are not available. |
| Han HL 1992 | Case series of 118 patients with uterine fibroids treated by herbal medicine |
| Han MX 2002 | Case series of 32 patients with uterine fibroids treated by herbal medicine |
| He H 2003 | Randomised trial comparing herbal preparation with methyltestosterone for treatment of 68 patients with hysteromyoma. The data for outcome are not available. |
| Hu TX 2009 | Randomised trial comparing Gong Liu Qing capsules with radiofrequency ablation in treatment of 42 patients with uterine fibroids. The intervention did not meet our inclusion criteria. |
| Hu WD 2007 | Randomised trial comparing herbal preparation Shugan Xiaozheng decoction with mifepristone for treatment of 109 patients with hysteromyoma. The data for outcome are not available. |
| Hu WH 2009 | Randomised trial comparing herbal preparation Guizhi Fuling capsules with mifepristone for treatment of 115 women with hysteromyoma. There was a significant skew distribution of participants between the two groups (86 versus 39), which could not be explained by a proper randomisation. |
| Huang XX 2011 | Randomised trial comparing herbal preparation (self‐prescribed herbal formula) with mifepristone for treatment of 620 patients with hysteromyoma. The data for outcome are not available. |
| Ji CW 2004 | Randomised trial comparing two different herbal preparations (self‐prescribed herbal formula versus Bai Xiao Dan) in treatment of 300 cases of uterine fibroids. |
| Jia WH 2012 | Randomised trial comparing herbal preparation plus mifepristone with mifepristone for treatment of 95 patients with hysteromyoma. The data for outcome are not available. |
| Jiang JF 2002 | Randomised trial comparing two different herbal preparations (Eleng Xiaoliu decoction versus Guizhi Fuling capsules) in treatment of 180 cases of uterine fibroids |
| Jiang LG 2006 | Randomised trial comparing two different herbal preparations (self‐prescribed herbal formula versus Guizhi Fuling capsules) in treatment of 54 cases of uterine fibroids |
| Jiang P 2003 | Randomised trial comparing western medicine plus herbal preparation with western medicine for treatment of 72 patients with hysteromyoma. The data for outcome are not available. |
| Jiang XJ 2007 | Quasi‐randomised trial comparing mifepristone plus Guizhi Fuling capsules with mifepristone alone in 118 cases of uterine fibroids |
| Jiang Y 2003 | Case series of 186 patients with uterine fibroids treated by herbal medicine |
| Jiao JF 2011 | Randomised trial comparing herbal preparation Guizhi Fuling capsule plus mifepristone with mifepristone for treatment of 39 patients with hysteromyoma. The data for outcome are not available. |
| Jiao ML 2005 | Randomised trial comparing two different herbal preparations (Gong Ji Ning pills versus Guizhi Fuling capsules) in treatment of 108 cases of uterine fibroids |
| Kang YP 2005 | Randomised trial comparing mifepristone plus herbal preparation enema with mifepristone for treatment of 88 patients with hysteromyoma.The data for outcome are not available. |
| Lai HH 2004 | Randomised trial comparing two different herbal preparations (self‐prescribed herbal formula Pu Gui Wan versus Gong Liu Qing capsules) in treatment of 50 cases of uterine fibroids |
| Li CY 2004 | Randomised trial comparing two different herbal preparations (self‐prescribed herbal formula plus ear acupoint pressing versus Guizhi Fuling capsules) in treatment of 120 cases of uterine fibroids |
| Li DM 1998 | Randomised trial comparing two different herbal preparations (self‐prescribed Xiao Zheng Yin versus Guizhi Fuling pills) in treatment of 68 cases of uterine fibroids |
| Li FY 1993 | Case series of 40 patients with uterine fibroids treated by herbal medicine |
| Li JX 2005 | Randomised trial comparing two different herbal preparations (self‐prescribed Zigong Xiaoliu tablets versus Guizhi Fuling pills) in treatment of 125 cases of uterine fibroids |
| Li JX 2006 | Randomised trial comparing herbal preparation Guizhi Fuling capsules with mifepristone for treatment of 58 patients with hysteromyoma.The data for outcome are not available. |
| Li KY 2005 | Case‐control study of the effect of Lizhi Sanjie pill on oestrogen and progestin levels in uterine fibroids |
| Li LH 2010 | Randomised trial comparing two different herbal preparations (self‐prescribed Xiaoliu capsules versus Guizhi Fuling capsules) in treatment of 126 cases of uterine fibroids. |
| Li LZ 2003 | Randomised trial comparing two different herbal preparations (Sanjie Xiaoliu Tang versus Guizhi Fuling capsules) in treatment of 120 cases of uterine fibroids |
| Li WY 1999 | Randomised trial comparing two different herbal preparations (Xiao Zheng Wan versus Guizhi Fuling capsules) in treatment of 150 cases of uterine fibroids |
| Li WY 2002 | Randomised trial comparing two different herbal preparations (Xiao Zheng Wan versus Guizhi Fuling capsules) in treatment of 300 cases of uterine fibroids |
| Li Y 2003 | Randomised trial comparing two different herbal preparations (Danqi Huazheng capsules versus Guizhi Fuling capsules) in treatment of 240 cases of uterine fibroids |
| Li YY 2002 | Case series of 98 patients with uterine fibroids treated by herbal medicine |
| Liang XZ 2011 | Randomised trial comparing two different herbal preparations (Qizhu Xiaozheng decoction versus Guizhi Fuling capsules) in treatment of 120 cases of uterine fibroids |
| Liang YY 2006 | Case series of 42 patients with uterine fibroids treated by herbal medicine |
| Liu DX 2005 | Randomised trial comparing two different herbal preparations (Xiao Zheng Wan versus Guizhi Fuling pills) in treatment of 80 cases of uterine fibroids |
| Liu GY 2005 | Randomised trial comparing two different herbal preparations (Zhechong Siwu Tang versus Guizhi Fuling pills) in treatment of 75 cases of uterine fibroids |
| Liu JY 2002 | Randomised trial comparing mifepristone plus herbal preparation Xiaoliu pills mifepristone for treatment of 126 patients with hysteromyoma.The data for outcome are not available. |
| Liu QP 2001 | Case series of 37 patients with uterine fibroids treated by herbal medicine |
| Liu XF 2011 | Randomised trial tested Guizhi Fuling Tang plus mifepristone against mifepristone in 80 women with uterine fibroids. However, the data of relevant outcomes were not available. |
| Lu M 2007 | Randomised trial comparing two different herbal preparations (Yiqi Huayu Xiaozheng Tang versus Guizhi Fuling capsules) in treatment of 120 cases of uterine fibroids |
| Lu SQ 2000 | Randomised trial comparing two different herbal preparations (Xiaozheng Sanjie tablets versus Bai Xiao Dan) in treatment of 160 cases of uterine fibroids |
| Lu Y 2005 | Randomised trial comparing two different herbal preparations (Hua Zheng Tang versus Guizhi Fuling capsules) in treatment of 60 cases of uterine fibroids |
| Luo L 2003 | Randomised trial comparing two different herbal preparations (Qing Gong Liu capsules versus Guizhi Fuling capsules) in treatment of 165 cases of uterine fibroids |
| Lv 2007 | Randomised trial comparing two different herbal preparations (Yiqi Huayu Xiaozheng Tang versus Guizhi Fuling capsules) in treatment of 120 cases of uterine fibroids |
| Ma WX 2003 | Case series of 42 patients with uterine fibroids treated by herbal medicine |
| Man JP 2009 | Randomised trial comparing herbal preparation Huazheng Xiaoliu decoction with different medicines (ext leonuri sibirici liq, oxytocin, ergometrine, extractum ergotae liquidum, etc) in treatment of 108 cases of uterine fibroids. |
| Meng LJ 2010 | Randomised trial comparing herbal preparation Shuangju granules plus mifepristone with mifepristone for treatment of 120 patients with hysteromyoma.The data for outcome are not available. |
| Miao XL 2002 | Controlled clinical study comparing two herbal medicines for treatment of 130 cases of uterine fibroids |
| Min XL 2007 | Randomised trial comparing two different herbal preparations (Xiao Liu formula versus Guizhi Fuling capsules) in treatment of 84 cases of uterine fibroids |
| Miu XY 2003 | Randomised trial comparing two different herbal preparations (Ji Liu powder versus Guizhi Fuling decoction) in treatment of 400 cases of uterine fibroids |
| Miu XY 2007 | Duplicated publication with Miu XY 2003 |
| Mo KL 2011 | Randomised trial comparing herbal preparation Xiao Jie An capsule plus mifepristone with mifepristone for treatment of 128 patients with hysteromyoma. The data for outcome are not available. |
| Pan XH 2011 | Randomised trial comparing herbal preparation Gongliuqing capsule plus mifepristone with mifepristone for treatment of 78 patients with hysteromyoma.The data for outcome are not available. |
| Peng XJ 2007 | Randomised trial was originally planned to be included in this review, but no information about method for a proper randomisation was acquired through phone calls to the trial author (6 Jan 2009). |
| Peng YS 2010 | Randomised trial comparing two different TCM therapy (acupuncture plus self‐prescribed herbal decoction versus self‐prescribed herbal decoction) in treatment of 62 cases of uterine fibroids. |
| Qi C 2003 | Randomised trial comparing two different herbal preparations (Bushen Huoxue formula versus Guizhi Fuling capsules) in treatment of 93 cases of uterine fibroids |
| Qian L 2007 | Randomised trial comparing two different herbal preparations (Bushen Xiaoliu formula versus Guizhi Fuling capsules) in treatment of 80 cases of uterine fibroids |
| Qin WP 2010 | Randomized trial comparing different herbal medicines. |
| Qiu HN 2006 | Randomised trial comparing mifepristone plus Guizhi Fuling capsules with Guizhi Fuling capsules in treatment of 108 pre‐menopausal women with uterine fibroids. The intervention did not meet our inclusion criteria |
| Sakamoto 1992 | Case series |
| Sakamoto 1998 | Case series |
| Sang H 2004 | Clinical study comparing Kangfu Xiaozheng tablet with Guizhi Fuling pill in 120 cases of hysteromyoma |
| Sang HL 2002 | Randomised trial comparing two different herbal preparations (Kangfu Xiaozheng tablets versus Guizhi Fuling pills) in treatment of 250 cases of uterine fibroids |
| Sang HL 2003 | Randomised trial comparing two different herbal preparations (Kangfu Xiaozheng tablets versus Guizhi Fuling pills) in treatment of 220 cases of uterine fibroids |
| Shen D 2006 | Randomised trial was originally planned to include, but our contact with trial author failed to confirm a proper randomisation method through phone call (16 Jan 2009). |
| Shu S 2001 | Randomised trial comparing hormone and vitamins plus herbal preparation during menstrual period with hormone plus herbal medicine Guizhi Fuling decoction in treatment of 50 cases of uterine fibroids. The interventions did not meet our inclusion criteria |
| Song JS 2010 | Systematic review of Guizhi Fuling capsules versus western medicine for treatment of uterine fibroids. |
| Song SM 2006 | Randomised trial comparing two different herbal preparations (Guizhi Fuling capsules versus Jin Gang Teng liquid) in treatment of 252 cases of uterine fibroids |
| Su GH 2005 | Case series of 43 patients with uterine fibroids treated by herbal medicine |
| Su XC 2005 | Randomised trial comparing two different herbal preparations (Zi Bao Kang capsules versus Guizhi Fuling capsules) in treatment of 100 cases of uterine fibroids |
| Sun DJ 2007 | Case series |
| Sun L 1995 | Case series |
| Tan SY 2003 | Randomised trial comparing herbal preparation Lengzhu Xiaoliu decoction with mifepristone for treatment of 142 patients with hysteromyoma.The data for outcome are not available. |
| Tian LJ 2005 | Randomised trial comparing two different herbal preparations (Fu Liu Qing No.1 decoction versus Guizhi Fuling capsules) in treatment of 120 cases of uterine fibroids. |
| Tian Y 2011 | Randomised trial comparing herbal preparation Gongliuqing capsules plus mifepristone with mifepristone for treatment of 158 patients with hysteromyoma. Different daily dosages of mifepristone in treatment group and control group (25 mg once daily in treatment group and 12.5 mg once daily in control group). |
| Wang D 2000 | Randomised trial comparing Xiaoji pills with placebo for treatment of 62 patients with hysteromyoma.The data for outcome are not available. |
| Wang DQ 2012 | Randomised trial comparing herbal preparation Gongliuqing capsule plus mifepristone with mifepristone for treatment of 76 patients with hysteromyoma.The data for outcome are not available. |
| Wang HM 2009 | Randomised trial comparing Huoxue Huayu recipe after the therapy of uterine artery embolization (UAE) with no intervention after UAE in treatment of 60 cases with uterine myoma.The trial author refused to provide information about the randomisation method through phone call (22 Jun 2012). The study objective was to test herbal medicine as a supplement to UAE therapy. |
| Wang HZ 2010 | Randomised trial comparing herbal preparation (self‐prescribed herbal formula) with mifepristone for treatment of 104 patients with hysteromyoma. The data for outcome are not available. |
| Wang JH 2002 | Case series of 67 cases of uterine fibroids treated by herbal medicine |
| Wang JH 2007 | Randomised trial comparing two different herbal preparations (Hua Zheng Dan versus Guizhi Fuling capsules) in treatment of 110 cases of uterine fibroids |
| Wang MD 1999 | Randomised trial comparing herbal preparation with methyltestosterone in treatment of 149 cases of uterine fibroids. However, there was a significant skew distribution in the numbers of participants between the two groups (112 versus 37), which could not be explained by a proper randomisation. |
| Wang P 2005 | Randomised trial comparing a self‐prescribed herbal formula (oral and enema) plus external use of another herbal formula with the same self‐prescribed herbal formula (oral) in treatment of 123 cases of uterine fibroids |
| Wang SL 2011 | Randomised trial comparing two different herbal preparations (self‐prescribed Xiaoliu capsule versus Guizhi Fuling capsules) in treatment of 100 cases of uterine fibroids. |
| Wang YH 2006 | Randomised trial comparing two different herbal preparations (Huayu Xiaozheng decoction versus Guizhi Fuling capsules) in treatment of 80 cases of uterine fibroids |
| Wang YL 2004 | Plagiarism of another study (Liu JY 2002) |
| Wen XL 2007 | Randomised trial comparing two different herbal preparations (Xiao Liu Fang versus Guizhi Fuling capsules) in treatment of 84 cases of uterine fibroids |
| Weng SQ 2010 | Case series of 50 patients with uterine fibroids treated by Ankun tablet. |
| Wu N 2002 | Randomised trial comparing two different herbal preparations (Xiao Zheng San plus other herbs versus Xiao Zheng San only in treatment of 87 cases of uterine fibroids |
| Wu XM 2007 | Case series |
| Xiao CC 1990 | Case series of 125 patients with uterine fibroids treated by herbal medicine |
| Xiong DM 2006 | Randomised trial was originally planned to include, but our contact with trial author confirmed use of alternate allocation according to the sequence of patient admission (quasi‐randomisation) through phone call (5 Jan 2009). |
| Xu H 2005 | Case series of 38 patients with uterine fibroids treated by herbal medicine |
| Xu Y 2007 | Case series |
| Yan H 1994 | Non‐randomised controlled clinical study testing acupuncture comparing with methyltestosterone, testosterone, or Guizhi Fuling capsules in treatment of 187 cases of uterine fibroids |
| Yan Y 2001 | Quasi‐randomised trial comparing Guizhi Fuling capsules combined with mifepristone versus mifepristone alone in 68 patients with uterine fibroids |
| Yang JL 2001 | Randomised trial comparing two different herbal preparations (Gong Liu Qing capsules versus Guizhi Fuling capsules) in treatment of 300 cases of uterine fibroids |
| Yang YX 2005 | Three‐arm randomised trial comparing two different herbal preparations with mifepristone in treatment of 344 cases of uterine fibroids. However, there was a significant skew in the numbers of participants among three groups (148 versus 96 versus 100), which could not be explained by a proper randomisation. |
| Yang ZM 2011 | Randomised trial comparing two different herbal preparations (self‐prescribed Lingjia Xiaozheng decoction versus Guizhi Fuling capsules ) in treatment of 68 cases of uterine fibroids. |
| Ye JF 2001 | Randomised trial comparing two different herbal preparations (Xiao Liu formula versus Guizhi Fuling capsules) in treatment of 90 cases of uterine fibroids |
| Ye TH 2002 | Randomised trial comparing two different herbal preparations (Gong Liu Xiao capsules versus Gong Liu Qing capsules) in treatment of 90 cases of uterine fibroids |
| Yu JY 2004 | Case series of 60 patients with uterine fibroids treated by herbal medicine |
| Yu QL 2003 | Randomised trial comparing two different herbal preparations (Wanying Xiaoliu powder versus Guizhi Fuling capsules) in treatment of 60 cases of uterine fibroids |
| Yu T 2002 | Randomised trial comparing two different herbal preparations (Huashi Sanjie Tang versus Ping Xiao capsules) in treatment of 50 cases of uterine fibroids |
| Zhan YR 2007 | Randomised trial was originally planned to include, but our contact with trial author failed to confirm a proper study design through phone call (26 Dec 2008). |
| Zhang H 2005 | Randomised trial comparing herbal preparation with no intervention after transcatheter arterial embolization in treatment of 31 cases of uterine fibroids. The data for outcome are not available. |
| Zhang M 2004 | Case report |
| Zhang QM 2007 | Randomised trial comparing two different herbal preparations (Yuanshi Xiaoliu Tang versus Guizhi Fuling capsules) in treatment of 54 cases of uterine fibroids |
| Zhang WH 2011 | Randomised trial comparing herbal preparation Gong Liu Qing capsule with methyltestosterone for treatment of 34 patients with uterine myoma.The data for outcome are not available. |
| Zhang WL 2005 | Randomised trial comparing herbal medicine Huoxue Huatan Xiaozhen formula plus mifepristone with mifepristone alone, but trial author was not able to confirm the randomisation method and the suspicious outcome data in our contact by phone call (16 Jan 2009). |
| Zhang XB 2011 | Randomised trial comparing arterial embolisation plus Xiaozheng decoction (self‐prescribed herbal formula) with arterial embolisation for treatment of 68 patients, but the drugs for embolisation were not specified. |
| Zhang XW 1997 | Randomised trial comparing two different herbal preparations (Hai Kun decoction versus Guizhi Fuling pills) in treatment of 38 cases of uterine fibroids |
| Zhang Z 2006 | Case series of 62 patients with uterine fibroids treated by herbal medicine |
| Zhao LX 2003 | Randomised trial comparing Tripterygium wilfordii with mifepristone for treatment of 126 women with uterine fibroids. However, the trial author confirmed to use alternate allocation (quasi‐randomisation) through phone call (7 Jan 2009). |
| Zheng CY 2003 | Case series of 60 patients with uterine fibroids treated by herbal medicine |
| Zhong Q 2006 | Randomised trial comparing mifepristone combined with Guizhi Fuling capsules versus hormone (GnRH‐a) in treatment of 104 cases of uterine fibroids. The intervention comparison did not comply with the inclusion criteria of this review |
| Zhong XR 2010 | Randomised trial comparing herbal preparation Gong Liu Qing tablets plus mifepristone with mifepristone for treatment of 345 patients with hysteromyoma. The outcome data for our review are not available. |
| Zhou J 1997 | Randomised trial comparing herbal preparation with placebo in treatment of 100 cases of uterine fibroids.There was a significant skew distribution of participants (71 versus 29), which could not be explained by an appropriate randomisation. |
| Zhou MY 2003 | Randomised trial testing herbal medicine Dahuang Tang as an adjunctive therapy to uterine arterial embolisation (UAE) in women with uterine fibroids. The research objective is to relieve adverse effects from the UAE therapy. Our contact with trial author failed to confirm the randomisation method through phone call (13 Jan 2009). |
| Zhou YJ 2006 | Randomised trial comparing 2 different herbal preparations (Guizhi Fuling capsules versus Xuefu Zhuyu capsules) in treatment of 60 cases of uterine fibroids |
| Zhou YR 1999 | Randomised trial comparing herbal preparation with mifepristone in treatment of 80 cases of uterine fibroids.The outcome data for our review were not available. |
| Zhu JY 1997 | Randomised trial comparing different formulation of the same herbal medicine (Lichong Sanjie pills versus Lichong Sanjie decoction) in treatment of 110 cases of uterine fibroids |
| Zou DH 1993 | Case series of 93 patients with uterine fibroids treated by herbal medicine |
Contributions of authors
Jianping Liu conceived the review, wrote the protocol, performed quality assessment and data analyses, wrote and updated the review.
Hong Yang identified studies, extracted data, performed quality assessment, and analysed data.
Yun Xia contacted trial authors for confirmation of randomisation and to obtain missing data.
Francesco Cardini revised the protocol and the review.
Sources of support
Internal sources
Beijing University of Chinese Medicine, China.
National Research Centre in Complementary and Alternative Medicine (NAFKAM), Norway.
External sources
The '111' Project (B08006), China.
The Programme for Innovative Research Team (No. 2011‐CXTD‐09) of Beijing University of Chinese Medicine, China.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Deng XL 2010 {published data only}
- Zheng XL, Li CR. Clinical observation of Guizhi Fuling capsule combined mifepristone treating 33 cases of uterine myoma. Hebei Journal of Traditional Chinese Medicine 2010;32(11):1670‐1. [Google Scholar]
Dong M 2011 {published data only}
- Dong M. Clinical research of Guizhi Fuling capsule combined leuprorelin treating premenopause uterine myoma. China Healthcare Innovation 2011;6(19):69‐70. [Google Scholar]
Fu WJ 2005 {published data only}
- Fu WJ, Ni J, Zheng LJ, Wang XF. [Observation on therapeutic effects of small dosage of Tripterygium Wilfordii Hook for treatment of uterine leiomyoma]. Journal of Hebei North University (Medical Edition) 2005;22(1):46‐8. [Google Scholar]
Gu HH 2011 {published data only}
- Gu HH, Hu QY. [Effect of integrated Chinese and western medicine in treating uterine myoma]. Strait Pharmaceutical Journal 2011;23(4):126‐7. [Google Scholar]
Hazlina 2005 {published data only}
- Hazlina NNH, Pazudin MI, Nor Aliza AG, Mohsin Sahil SJ. Clinical study to compare the efficacy and adverse effects of Nona Roguy herbal formulation and gonadotrophin releasing hormone agonist (GnRH) in the treatment of uterine fibroids. International Medical Journal 2005;12(4):295‐302. [Google Scholar]
Lai XL 2010 {published data only}
- Lai XL, Zheng LZ, Ma XJ. [Gongliuqing capsule treated 94 cases of uterine myoma]. Jiangxi Journal of Traditional Chinese Medicine 2010;41(328):46‐7. [Google Scholar]
Liu LY 2010 {published data only}
- Liu LY. [Clinical research of Gongliuqing capsule combined with mifepristone for treating uterine myoma]. China Higher Medical Education 2010;23(12):140‐1. [Google Scholar]
Liu Y 2009 {published data only}
- Liu Y. [Observation of efficacy on combined therapy of mifepristone and Chinese herbal medicine on 30 cases of hysteromyoma]. World Journal of Integrated Traditional and Western Medicine 2009;4(4):268‐70. [Google Scholar]
Lu HJ 2010 {published data only}
- Lu HJ. [Curative effect observation of mifepristone combined with Guilin Fuling capsule treating uterine myoma]. Chinese Journal of Clinical Rational Drug Use 2010;3(17):49‐50. [Google Scholar]
Lu JX 2007 {published data only}
- Lu JX. [Comparison of Huoxue Sanjie decoction with mifepristone in the treatment of uterine fibroids]. Shandong Journal of Medicine and Pharmacology 2007;47(19):109‐10. [Google Scholar]
Luo SQ 2010 {published data only}
- Luo SQ. [Clinical observation of Jiliu decoction combined with mifepristone in the treatment of uterine myoma]. Journal of Emergency in Traditional Chinese Medicine 2010;19(6):943‐4. [Google Scholar]
Mao CX 2012 {published data only}
- Mao CX, Cai RR, Wang XP, Wu YL. [Curative effects on treating premenopause hysteromyoma by mifepristone with Guizhi Fuling capsule]. Chinese Archives of Traditional Chinese Medicine 2012;30(3):665‐7. [Google Scholar]
Mao XG 2012 {published data only}
- Mao XG, Fang SS. [Clinical observation of Guizhi Fuling capsule combined mifepristone for treating 33 cases of uterine myoma]. Asia‐Pacific Traditional Medicine 2012;8(4):125‐6. [Google Scholar]
Ma R 2010 {published data only}
- Ma R, Cheng HL. [Guihong turtle shell pill for treating 68 cases of Qi and blood stagnation uterine myoma]. Henan Traditional Chinese Medicine 2010;30(4):382‐3. [Google Scholar]
Ni XP 2012 {published data only}
- Ni XP, Ma DZ, Lei LH. [Clinical observation of Xiaozheng decoction for 130 cases of uterine leiomyoma]. Journal of Traditional Chinese Medicine 2012;53(7):588‐90, 594. [Google Scholar]
Wang XR 2011 {published data only}
- Wang XR. [Clinical effect of GuizhiFuling capsule combined mifepristone in the treatment of uterine myoma]. Strait Pharmaceutical Journal 2011;23(10):127‐9. [Google Scholar]
Wen Q 2005 {published data only}
- Wen Q. [Therapeutic observation on effect of Tripterygium Wilfordii Hook and mifepristone in treatment of uterine leiomyoma]. Clinical Medicine of China 2005;21(3):277‐9. [Google Scholar]
Wu JH 2011 {published data only}
- Wu JH. [Analysis of clinical effect of Guizhi Fuling capsule combined mifepristone in the treatment of uterine myoma]. Maternal and Child Health Care of China 2011;26(19):2910‐1. [Google Scholar]
Wu YF 2011 {published data only}
- Wu YF, He L. Curative effect observation of mifepristone combined Guilin Fuling capsule treating uterine myoma. Progress in Modern Biomedicine 2010;3(17):49‐50. [Google Scholar]
Yan LQ 2000 {published data only}
- Yan LQ. [Clinical study on uterine fibroids treated by traditional Chinese medicine]. Journal of Changzhi Medical College 2000;14(4):304‐5. [Google Scholar]
Zhu FH 2006 {published data only}
- Zhu FH. [35 cases of hysteromyoma treated with mifepristone and Eleng Xiaozheng Decoction]. Fujian Journal of Traditional Chinese Medicine 2006;37(3):37. [Google Scholar]
References to studies excluded from this review
Akase 2003 {published data only}
- Akase T, Onodera S, Jobo T, Matsushita R, Kaneko M, Tashiro S‐I. [A comparative study of the usefulness of Toki‐shakuyaku‐san and an oral iron preparation in the treatment of hypochromic anaemia in cases of uterine myoma]. Yakugaku Zasshi 2003;123(9):817‐24. [DOI] [PubMed] [Google Scholar]
An ZR 2005 {published data only}
- An ZR. [43 cases of uterine fibroids treated by Gong Liu Tang]. Modern Traditional Chinese Medicine 2005;25(6):30. [Google Scholar]
Chen QM 2007 {published data only}
- Chen QM, Li FS, Wang JP. [Observation of therapeutic effects of Rupi Anxiao capsules plus small dosage of methyltestosterone in treatment of hysteromyoma]. Maternal and Child Health Care of China 2007;22(11):1459‐61. [Google Scholar]
Cuan CL 2002 {published data only}
- Cuan CL, Shang HQ, Fu YP. [30 cases of uterine fibroids treated by Ding Jing Tang]. Shaanxi Journal of Traditional Chinese Medicine 2002;23(11):975‐6. [Google Scholar]
Du WH 1993 {published data only}
- Du WH. [Retentive enema and oral taking of Guizhi Fuling Wan for treatment of 40 cases of uterine fibroids]. Shandong Journal of Traditional Chinese Medicine 1993;12(2):28‐9. [Google Scholar]
Fang RD 2001 {published data only}
- Fang RD, Zhang XH, Wang QY, Lin YZ, Yang SP, Rao WN, et al. [Clinical study on the treatment of uterine leiomyoma with Xiao Liu Yin iontophoresis]. Chinese Journal of Information on Traditional Chinese Medicine 2001;12(8):47‐9. [Google Scholar]
Feng FQ 2003 {published data only}
- Feng FQ. [Therapeutic effect of treating uterine fibroids with Guizhi Fuling capsules]. Modern Practical Medicine 2003;15(6):389. [Google Scholar]
Feng X 2004 {published data only}
- Feng X. [Clinical observation of 39 cases of uterine fibroids treated by self‐prescribed Ping Liu Tang]. Journal of Hebei Traditional Chinese Medicine and Pharmacology 2004;19(2):17. [Google Scholar]
Fu 2003 {published data only}
- Fu J. Treatment of 48 cases of uterine fibroids by Yiqi Guchong and Huayu Sanjie therapy. Journal of Traditional Chinese Medicine 2003;44(9):706. [Google Scholar]
Fu P 2004 {published data only}
- Fu P, He JL, Cui L, Chen XC. [Clinical observation on effect of Xuejie Hualiu Granule in treating hysteromyoma]. Chinese Archives of Traditional Chinese Medicine 2004;22(10):1082‐4. [Google Scholar]
Gao SM 2006 {published data only}
- Gao SM, Liu HY. [Clinical observation of 40 cases of hysteromyoma treated with therapy of nourishing Qi and activating blood circulation]. Journal of Practical Traditional Chinese Internal Medicine 2006;20(2):190‐1. [Google Scholar]
Gao XL 2001 {published data only}
- Gao XL, Zhang XR. [Clinical observation of 104 cases of hysteromyoma treated by Xiao Zheng No. 1]. Shanxi Journal of Traditional Chinese Medicine 2001;17(1):17‐8. [Google Scholar]
Gao YP 2000 {published data only}
- Gao YP, Chen DF. [Clinical study on effect of Tripterygium Wilfordii Hook on uterine leiomyoma]. Chinese Journal of Obstetrics and Gynaecology 2000;35(7):430‐2. [PubMed] [Google Scholar]
Gu H 1992 {published data only}
- Gu H. [Clinical summary of 35 cases of uterine fibroids treated with traditional Chinese medicine]. Jiangsu Journal of Traditional Chinese Medicine 1992;13(12):7‐8. [Google Scholar]
Guo AQ 2000 {published data only}
- Guo AQ, Guo YL, Wang XQ. [Clinical observation on treatment of 38 cases of climacteric myoma of uterus by combined traditional Chinese and western medicine]. Journal of Heze Medical College 2000;12(1):50‐2. [Google Scholar]
Gu Y 2011 {published data only}
- Gu Y. Effect of modified Sijunzi decoction combined Guizhi Fuling capsule treating uterine myoma. Journal of Practical Traditional Chinese Medicine 2011;27(12):838‐9. [Google Scholar]
Han HL 1992 {published data only}
- Han HL, Wang GF, Yang RX. [118 cases of uterine fibroids treated by Xiao Liu Wan]. Chinese Archives of Traditional Chinese Medicine 1992;10(1):36. [Google Scholar]
Han MX 2002 {published data only}
- Han MX, Zhu ZS, Liu F. [32 cases of uterine fibroids treated by Qizhi Xiangfu Wan]. Shanxi Journal of Traditional Chinese Medicine 2002;18(1):21. [Google Scholar]
He H 2003 {published data only}
- He H. [Treatment of 38 cases of hysteromyoma by Huangqi Danshen decoction]. Journal of Guangming Traditional Chinese Medicine 2003;18(109):36. [Google Scholar]
Huang XX 2011 {published data only}
- Huang XX. Analysis of social economic benefits of treating uterine myoma with Chinese medicinal formulae. Hainan Medical Journal 2011;22(1):45‐6. [Google Scholar]
Hu TX 2009 {published data only}
- Hu TX, Shan XL, Shen SF. Comparison of curative effect of Gongliuqing capsule and radiofrequency catheter ablation in the treatment of uterine myoma. Journal of Clinical Medicine 2009;29(1):68‐70. [Google Scholar]
Hu WD 2007 {published data only}
- Hu WD. [Analysis on clinical efficacy of Shugan Xiaozheng decoction on hysteromyoma]. Modern Diagnosis and Treatment 2007;18(5):283, 320. [Google Scholar]
Hu WH 2009 {published data only}
- Hu WH, Yang M. Clinical observation of Guizhi Fuling capsule treating 115 cases of uterine myoma. China Practical Medicine 2009;4(16):30‐1. [Google Scholar]
Jiang JF 2002 {published data only}
- Jiang JF. [Observation of therapeutic effects in 60 cases of hysteromyoma treated by Eleng Xiaoliu Tang]. Shandong Journal of Traditional Chinese Medicine 2002;21(4):215. [Google Scholar]
Jiang LG 2006 {published data only}
- Jiang LG. [Clinical observation of 36 cases of hysteromyoma treated with Chinese herbal medicine compound]. Chinese Journal for Clinicians 2006;34(9):40‐1. [Google Scholar]
Jiang P 2003 {published data only}
- Jiang P, Zhao Y, Ruan YL, Han Y. [Clinical observation of 70 cases of hysteromyoma treated with integrated traditional Chinese and western medicine]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2003;12(7):707‐8. [Google Scholar]
Jiang XJ 2007 {published data only}
- Jiang XJ, He FH. [Clinical analysis of mifepristone sequential treatment with Guizhi Fuling capsule on uterine myoma]. Zhejiang Journal of Clinical Medicine 2007;9(10):1338. [Google Scholar]
Jiang Y 2003 {published data only}
- Jiang Y, Xie PS. [186 cases of uterine fibroids treated by traditional Chinese medicine]. Chinese Journal of Information on Traditional Chinese Medicine 2003;10(1):44. [Google Scholar]
Jiao JF 2011 {published data only}
- Jiao JF. [Mifepristone combined Guilin Fuling capsule treated 39 cases of uterine myoma]. Chinese Journal of Experimental Traditional Medical Formulae 2011;17(12):292‐3. [Google Scholar]
Jiao ML 2005 {published data only}
- Jiao ML, Jiao XW. [Clinical observation of 53 cases of hysteromyoma treated by Gong Ji Ning pills]. Modern Traditional Chinese Medicine 2005;3(3):49. [Google Scholar]
Jia WH 2012 {published data only}
- Jia WH. Treating 50 cases of uterine myoma with integrative medicine. Chinese Medicine Modern Distance Education of China 2012;10(2):58. [Google Scholar]
Ji CW 2004 {published data only}
- Ji CW, Li QS, Pei ZY. [Clinical observation on treating hysteromyoma by Kunyaosan capsule]. Chinese Journal of Practical Integrated Chinese and Western Medicine 2004;4(1):117. [Google Scholar]
Kang YP 2005 {published data only}
- Kang YP, Zheng SG. [46 cases of hysteromyoma at early stage treated with integrated traditional Chinese and western medicine]. Shaanxi Journal of Traditional Chinese Medicine 2005;26(5):402‐3. [Google Scholar]
Lai HH 2004 {published data only}
- Lai HH, Gao SF, Deng YM, Zhu ZH, Feng LP, Lai XF. [Clinical observation on Pugui Pill for treatment of 30 cases of hysteromyoma of stagnant heat type]. Journal of Traditional Chinese Medicine 2004;45(3):201‐3. [Google Scholar]
Liang XZ 2011 {published data only}
- Liang XZ, Zhang L. [Clinical research of Qizhuxiaozheng decoction treating uterine myoma]. Chinese Remedies & Clinics 2011;11(11):1309‐10. [Google Scholar]
Liang YY 2006 {published data only}
- Liang YY, Huang YH. [Ruanjian Sanjie granule for treatment of 42 cases of uterine fibroids]. Shaanxi Journal of Traditional Chinese Medicine 2006;27(6):677‐8. [Google Scholar]
Li CY 2004 {published data only}
- Li CY. [Clinical observation of Chinese herbal medicine plus ear acupoints pressing for the treatment of uterine fibroids with blood stasis]. Liaoning Journal of Traditional Chinese Medicine 2004;31(12):1015. [Google Scholar]
Li DM 1998 {published data only}
- Li DM, Gao M, Li HM. [Observation of therapeutic effect of self‐prescribed Xiao Zheng Yin for the treatment of hysteromyoma]. Chinese Journal of Traditional Medical Science and Technology 1998;5(2):85. [Google Scholar]
Li FY 1993 {published data only}
- Li FY. [40 cases of uterine fibroids at early stage treated by Chinese herbal medicine]. New Journal of Traditional Chinese Medicine 1993;25(10):39‐40. [Google Scholar]
Li JX 2005 {published data only}
- Li JX. [Clinical observation of 125 cases of hysteromyoma with Qi‐stagnancy and blood stasis treated with Zigong Xiaoliu tablets]. Practice on Chinese Clinical Medicines 2005;3(29):47‐8. [Google Scholar]
Li JX 2006 {published data only}
- Li JX, Zhang HZ. [Observation on therapeutic effects in hysteromyoma treated with integrated traditional Chinese and western medicine]. Journal of Changzhi Medical College 2006;20(4):300‐1. [Google Scholar]
Li KY 2005 {published data only}
- Li KY, Luo SP, Kuang ZS, Deng GP, Tao LL, Xie YH. [Effect of Lizhi Sanjie pill on levels of oestrogen and progestin of hysteromyoma patients]. Journal of Nanjing TCM University 2005;21(4):228‐30. [Google Scholar]
Li LH 2010 {published data only}
- Li LH. [Xiaoliutang decoction for treatment of 66 cases of uterine myoma]. Guangming Journal of Chinese Medicine. 2010;25(7):1228. [Google Scholar]
Li LZ 2003 {published data only}
- Li LZ, Li GH, Chen P. [Clinical observation of Sanjie Xiaoliu Decoction for treatment of hysteromyoma]. Tianjin Journal of Traditional Chinese Medicine 2003;20(5):22‐3. [Google Scholar]
Liu DX 2005 {published data only}
- Liu DX, Luo X. [Clinical observation on the results in treatment of hyperplasia of mammary glands, hysteromyoma and oviduct blocking cases with Xiaoliu pills]. China Tropical Medicine 2005;5(8):1684‐5. [Google Scholar]
Liu GY 2005 {published data only}
- Liu GY. [Clinical observation on the treatment of 45 cases of uterine myoma with Zhechong Siwu decoction]. Guiding Journal of TCM 2005;11(2):33‐4. [Google Scholar]
Liu JY 2002 {published data only}
- Liu JY, Meng FL, Zhu L, Yu B, Liu YH, Nie FH. [Treatment of 64 cases of hysteromyoma in perimenopause women with integrated traditional Chinese and western medicine]. Chinese Journal of Integrated Traditional and Western Medicine 2002;22(4):248. [Google Scholar]
Liu QP 2001 {published data only}
- Liu QP, He YN, Guo H. [Clinical observation of Guizhi Fuling Wan Jiawei for treatment of 37 cases of uterine fibroids]. Yunan Journal of Traditional Chinese Medicine and Materia Medica 2001;22(3):35‐6. [Google Scholar]
Liu XF 2011 {published data only}
- Liu XF. [Clinical observation on Chinese herbal medicine differentiation combined with western drug for treatment of uterine fibroids]. Liaoning Journal of Traditional Chinese Medicine 2011;38(6):1160‐1. [Google Scholar]
Li WY 1999 {published data only}
- Li WY, Yang GH, Jiang YH. [Clinical observation of Xiao Zheng Pill for treatment of hysteromyoma]. Hebei Journal of Traditional Chinese Medicine 1999;21(6):331‐2. [Google Scholar]
Li WY 2002 {published data only}
- Li WY. [Clinical study of treating hysteromyoma by Xiao Zheng Wan]. Chinese Journal of the Practical Chinese with Modern Medicine 2002;2(15):839‐40. [Google Scholar]
Li Y 2003 {published data only}
- Li Y, Qin SF, Wang LN. [Clinical observation of Danqi Huazheng capsule in the treatment of hysteromyoma]. Journal of Henan University of Chinese Medicine 2003;18(3):39‐40. [Google Scholar]
Li YY 2002 {published data only}
- Li YY. [Oral taking and external application of Chinese herbal medicine for treatment of 98 cases of uterine fibroids]. Hubei Journal of Traditional Chinese Medicine 2002;24(8):38‐9. [Google Scholar]
Lu M 2007 {published data only}
- Lu M. [Clinical observation of Yiqi Huayu Xiaozheng Tang for treatment of 60 cases of uterine fibroids]. Shandong Journal of Traditional Chinese Medicine 2007;26(9):604‐6. [Google Scholar]
Luo L 2003 {published data only}
- Luo L, Zhu YP. [Effect of treating 110 cases of uterine fibroids with the use of Qing Gong Liu capsules]. Chinese Journal of Traditional Medical Science and Technology 2003;10(6):382‐3. [Google Scholar]
Lu SQ 2000 {published data only}
- Lu SQ, Lin YC, Peng ZY. [Clinical observation of Xiaozheng Sanjie tablet in treating 120 cases of hysteromyoma]. Correspondence Journal of Traditional Chinese Medicine 2000;19(5):48‐9. [Google Scholar]
Lu Y 2005 {published data only}
- Lu Y, Deng ZH. [Clinical observation on the treatment of hysteromyoma with Qi‐stagnancy and blood‐stasis syndrome with Huazheng Decoction]. Journal of Anhui Traditional Chinese Medical College 2005;24(6):16‐7. [Google Scholar]
Lv 2007 {published data only}
- Lv M. [Clinical observation of 60 cases of hysteromyoma treated by Yiqi Huayu Xiaozheng Decoction]. Shandong Journal of Traditional Chinese Medicine 2007;26(9):604‐6. [Google Scholar]
Man JP 2009 {published data only}
- Man JP 2009. Huazhen Xiaoliu decoction treating 72 cases of uterine myoma. Chinese Medicine Modern Distance Education of China 2009;7(8):111. [Google Scholar]
Ma WX 2003 {published data only}
- Ma WX, Li CP. [Xiao Liu Tang for treatment of 42 cases of uterine fibroids]. Traditional Chinese Medicine Research 2003;16(4):43‐4. [Google Scholar]
Meng LJ 2010 {published data only}
- Meng LJ, Li Y. [Efficacy analysis of Shuangju granules combined with mifepristone for treating uterine myoma]. Modern Chinese journal of integrated Chinese and Western Medicine 2010;19(20):2505‐6. [Google Scholar]
Miao XL 2002 {published data only}
- Miao XL. [Clinical curative observation of Xiao Liu granule for treatment of 130 cases of uterine fibroids]. Yunan Journal of Traditional Chinese Medicine and Materia Medica 2002;23(5):9‐10. [Google Scholar]
Min XL 2007 {published data only}
- Min XL. [Treatment of 42 cases of hysteromyoma by Xiao Liu formula]. Shaanxi Journal of Traditional Chinese Medicine 2007;28(3):298‐9. [Google Scholar]
Miu XY 2003 {published data only}
- Miu XY. [Treatment of 280 cases of hysteromyoma by Ji Liu San]. Fujian Journal of Traditional Chinese Medicine 2003;34(6):7‐8. [Google Scholar]
Miu XY 2007 {published data only}
- Miu XY. [Observation on therapeutic effect on Ji Liu San and Guizhi Fuling Tang for treatment of uterine fibroids]. Guangming Journal of Chinese Medicine 2007;22(2):31‐3. [Google Scholar]
Mo KL 2011 {published data only}
- Mo KL. [Application of Xiaojie'an combined with mifepristone in treatment of hysteromyoma in perimenopause period]. Chinese Journal of Clinical Rational Drug Use 2011;4(5C):57. [Google Scholar]
Pan XH 2011 {published data only}
- Pan XH. [Mifepristone combined with Guizhi Fuling capsule for treating 39 cases of uterine myoma]. Journal of Practical Traditional Chinese Medicine 2011;27(12):851. [Google Scholar]
Peng XJ 2007 {published data only}
- Peng XJ, Gao Y, Wang K. [Clinical Study on Huaji‐Ye for treatment of uterine leiomyoma]. Shandong Journal of Traditional Chinese Medicine 2007;26(7):459‐60. [Google Scholar]
Peng YS 2010 {published data only}
- Peng YS, Qian X, Peng GJ. [Study on the theraputic effect of comprehensive treatment by Acupuncture with Chinese herbs on hysteromyoma]. China Medical Herald 2011;7(2):82‐3. [Google Scholar]
Qian L 2007 {published data only}
- Qian L, Qi C, Zhang QH. [Clinical observation of Bushen Xiaoliu recipe for 80 patients with myoma of uterus]. Journal of Practical Diagnosis and Therapy 2007;21(8):574‐6. [Google Scholar]
Qi C 2003 {published data only}
- Qi C, Qian B, Zhang QH, Huang ZH. [Clinical observation of 63 cases of hysteromyoma treated with herbal formula of nourishing kidney and activating blood circulation]. Journal of Sichuan of Traditional Chinese Medicine 2003;21(5):45‐6. [Google Scholar]
Qin WP 2010 {published data only}
- Qin WP, Peng LY, Niu YL, Zhang LJ. [Effective observation of herbal internal and external administration in the treatment of 122 cases of uterine myoma]. Hebei Journal of Traditional Chinese Medicine 2010;32(7):1005‐6. [Google Scholar]
Qiu HN 2006 {published data only}
- Qiu HN, Li FS. [Clinical observation of mifepristone combined with Guizhi Fuling capsules in perimenopause women with hysteromyoma]. Journal of Clinical Medicine in Practice 2006;10(3):103‐4. [Google Scholar]
Sakamoto 1992 {published data only}
- Sakamoto S, Yoshino H, Shirahata Y, Shimodairo K, Okamoto R. Pharmacotherapeutic effects of kuei‐chih‐fu‐ling‐wan (keishi‐bukuryo‐gan) on human uterine myomas. American Journal of Chinese Medicine 1992;20(3‐4):313‐7. [DOI] [PubMed] [Google Scholar]
Sakamoto 1998 {published data only}
- Sakamoto S, Mitamura T, Iwasawa M, Kitsunai H, Shindou K, Yagishita Y, et al. Conservative management for perimenopausal women with uterine leiomyomas using Chinese herbal medicines and synthetic analogs of gonadotropin‐releasing hormone. In Vivo 1998;12(3):333‐7. [PubMed] [Google Scholar]
Sang H 2004 {published data only}
- Sang H, Wu B. [Clinical and experimental research into treatment of hysteromyoma with promoting Qi flow and blood circulation, softening and resolving hard lump]. Journal of Traditional Chinese Medicine 2004;24(4):274‐9. [PubMed] [Google Scholar]
Sang HL 2002 {published data only}
- Sang HL, Zhang QW, Jia SH. [Treatment of 150 cases of hysteromyoma with Kangfu Xiaozheng tablets]. Shandong Journal of Traditional Chinese Medicine 2002;21(3):143‐4. [Google Scholar]
Sang HL 2003 {published data only}
- Sang HL, Wu BQ. [Clinical and experimental study on Xingqi Huoxue and Ruanjian Sanjie therapy for treatment of uterine fibroids]. Journal of Traditional Chinese Medicine 2003;44(1):41‐3. [Google Scholar]
Shen D 2006 {published data only}
- Shen D, Shen XP. [Clinical observation on hysteromyoma treated by testosterone with or without Guizhi Fuling capsules]. Chinese Modern Medicine and Clinics 2006;1(5):12‐3. [Google Scholar]
Shu S 2001 {published data only}
- Shu S, Shu C. [Clinical observation of 30 cases of hysteromyoma treated with integrated traditional Chinese and western medicine]. Journal of Luoyang Medical College 2001;19(4):308‐9. [Google Scholar]
Song JS 2010 {published data only}
- Song JS, Gao C, Xiong J, Xue XH, Zhou YF, Shang TG. [Guizhi Fuling capsule versus western medicine for the treatment of uterine myoma: a systematic review. Chinese Journal of Evidence‐Based Medicine 2010;10(12):1439‐55. [Google Scholar]
Song SM 2006 {published data only}
- Song SM, Hou YZ, Wang AF, Qian JY. [Clinical observation of 504 cases of hysteromyoma treated with Guizhi Fuling capsules]. Applied Journal of General Practice 2006;4(3):319. [Google Scholar]
Su GH 2005 {published data only}
- Su GH. [Observation on therapeutic effect of Chinese medicine for treatment of uterine fibroids]. Shanxi Journal of Traditional Chinese Medicine 2005;21(6):60. [Google Scholar]
Sun DJ 2007 {published data only}
- Sun DJ. [Observation on therapeutic effect of integrated Chinese and western medicine for treatment of 68 cases of uterine fibroids]. Asia‐Pacific Traditional Medicine 2007;3(8):55. [Google Scholar]
Sun L 1995 {published data only}
- Sun L. [38 cases of hysteromyoma treated with tumour‐resolving decoction]. Journal of Traditional Chinese Medicine 1995;15(4):273‐6. [PubMed] [Google Scholar]
Su XC 2005 {published data only}
- Su XC, Wang YL, Zheng YS, Liu CL, Yan BC, Kong JX, et al. [Summary of 50 cases of uterine fibroids treated selectively by period with Zi Bao Kang capsule]. Hunan Journal of Traditional Chinese Medicine 2005;21(1):19‐20. [Google Scholar]
Tan SY 2003 {published data only}
- Tan SY. [Therapeutic observation of hysteromyoma treated with integrated traditional Chinese and western medicine]. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine 2003;3(3):11‐2. [Google Scholar]
Tian LJ 2005 {published data only}
- Tian LJ. [Clinical observation of Fu Liu Qing No. 1 for treatment of hysteromyoma in perimenopausal women]. Journal of Sichuan of Traditional Chinese Medicine 2005;23(6):69‐70. [Google Scholar]
Tian Y 2011 {published data only}
- Tian Y. [Clinical observation of mifepristone combined with Gongliuqing capsule in the treatment of uterine myoma]. Shandong Medical Journal 2011;51(1):53‐4. [Google Scholar]
Wang D 2000 {published data only}
- Wang D, Fan LX. [Treatment of 32 cases of myoma of uterus by Xiao Ji Pills]. Shaanxi Journal of Traditional Chinese Medicine 2000;21(5):199. [Google Scholar]
Wang DQ 2012 {published data only}
- Wang DQ. [Effect of Guizhi Fuling capsule combined with mifepristone for treating 38 cases of uterine myoma]. Journal of Military Surgeon in Southwest China 2012;14(2):258. [Google Scholar]
Wang HM 2009 {published data only}
- Wang HM, Liang RN, Luo XQ. [Clinical study on hysteromyoma treated with the method of huoxuehuayu after uterine artery embolization]. Modern Medicine Journal of China 2009;11(8):1‐3. [Google Scholar]
Wang HZ 2010 {published data only}
- Wang HZ, Chang H. [Clinical observation of Guizhi Fuling capsule combined with Danggui Shaoyao‐San for treating 52 cases of uterine myoma]. Clinical Journal of Traditional Chinese Medicine 2010;22(4):322‐3. [Google Scholar]
Wang JH 2002 {published data only}
- Wang JH. [Chinese medicine for treatment of 67 cases of uterine fibroids]. Liaoning Journal of Traditional Chinese Medicine 2002;29(6):335. [Google Scholar]
Wang JH 2007 {published data only}
- Wang JH. [Clinical study on Hua Zheng Dan for treatment of uterine leiomyoma]. Liaoning Journal of Traditional Chinese Medicine 2007;34(6):782‐3. [Google Scholar]
Wang MD 1999 {published data only}
- Wang MD. [Clinical observation of 112 cases of uterine leiomyoma treated by Tripterygium Wilfordii Hook]. Journal of Zhejiang University of Traditional Chinese Medicine 1999;23(6):29. [Google Scholar]
Wang P 2005 {published data only}
- Wang P, Wang LM, Li L. [Therapeutic effect observation of 74 cases of uterine myoma treated by integration internal and external body]. Guiding Joumal of Traditional Chinese Medicine 2005;11(5):31‐3. [Google Scholar]
Wang SL 2011 {published data only}
- Wang SL. Self‐prescribed Xiaoliu Capsule treated 50 cases of uterine myoma. Chinese Journal of Traditional Medical Science and Technology 2011;18(1):77. [Google Scholar]
Wang YH 2006 {published data only}
- Wang YH, Han N. [Clinical observation of 40 cases of hysteromyoma treated by Huayu Xiaozheng decoction]. Chinese Journal of the Practical Chinese with Modern Medicine 2006;19(24):2947‐9. [Google Scholar]
Wang YL 2004 {published data only}
- Wang YL. [Clinical observation of 64 cases of hysteromyoma in perimenopause women treated with integrated traditional Chinese and western medicine]. Jilin Journal of Traditional Chinese Medicine 2004;25(1):38‐9. [Google Scholar]
Weng SQ 2010 {published data only}
- Weng SQ, Huang LH, Wang XY, Xia AJ, Li QL. [Clinical observation of Ankun tablet treating 50 cases of uterine myoma]. Information on Traditional Chinese Medicine 2010;27(3):96‐8. [Google Scholar]
Wen XL 2007 {published data only}
- Wen XL. [Xiao Liu Fang for treatment of 42 cases of uterine fibroids]. Shaanxi Journal of Traditional Chinese Medicine 2007;28(3):298‐9. [Google Scholar]
Wu N 2002 {published data only}
- Wu N. [Clinical observation on treatment of 55 cases of hysteromyoma by reinforcing kidney and regulating menstrual cycle]. Jiangsu Journal of Traditional Chinese Medicine 2002;23(6):21‐2. [Google Scholar]
Wu XM 2007 {published data only}
- Wu XM, Liu F, Liu QZ. [Observation of hysteromyoma treated by combination of traditional Chinese medicine and western medicine]. Hei Long Jiang Medical Journal 2007;31(8):610‐1. [Google Scholar]
Xiao CC 1990 {published data only}
- Xiao CC, Yang BY, Huangpu X, Guo Y, Zhang SM. [Clinical observation on Jiliu Neixiao Wan for treatment of 125 cases of uterine fibroids]. China Journal of Traditional Chinese Medicine and Pharmacy 1990;5(2):39‐41. [Google Scholar]
Xiong DM 2006 {published data only}
- Xiong DM, Zhang XL. [Clinical therapeutic observation of 68 cases of hysteromyoma treated with integrated traditional Chinese and western medicine]. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 2006;16(1):38‐40. [Google Scholar]
- Xiong DM, Zhao L, Zhang XL. [Treatment of 38 cases of uterine fibroids by Guizhi Fuling capsules]. Shaanxi Journal of Traditional Chinese Medicine 2006;27(6):679‐80. [Google Scholar]
Xu H 2005 {published data only}
- Xu H, Xu ZX. [Xiao Zheng Tang for treatment of 38 cases of uterine fibroids]. Liaoning Journal of Traditional Chinese Medicine 2005;32(10):1045. [Google Scholar]
Xu Y 2007 {published data only}
- Xu Y. [Effect observation of integrated Chinese and western medicine for treatment of uterine fibroids]. Clinical Misdiagnosis and Mistherapy 2007;20(7):60‐1. [Google Scholar]
Yang JL 2001 {published data only}
- Yang JL, Yan XP. [Clinical observation of 300 cases of hysteromyoma treated with Gong Liu Qing capsule]. Journal of Chengdu University of Traditional Chinese Medicine 2001;24(1):10‐3. [Google Scholar]
Yang YX 2005 {published data only}
- Yang YX, Huang BZ, Wang MJ, Li RR. [Clinical observation of 148 cases of hysteromyoma treated with integrated traditional Chinese and western medicine]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2005;14(24):3233‐4. [Google Scholar]
Yang ZM 2011 {published data only}
- Yang ZM. [Clinical observation of treating uterine myoma by syndrome differentiation]. Guide of China Medicine 2011;9(5):148‐9. [Google Scholar]
Yan H 1994 {published data only}
- Yan H, Wang J. [Clinical study on acupuncture for treatment of hysteromyoma]. Acupuncture Research 1994;19(2):14‐6. [PubMed] [Google Scholar]
Yan Y 2001 {published data only}
- Yan Y. [Clinical observation of Guizhi Fuling capsules combined with mifepristone for the treatment of uterine fibroids]. Acta Academiae Medicinae Xuzhou 2001;21(5):428‐9. [Google Scholar]
Ye JF 2001 {published data only}
- Ye JF, Wang YX. [Ingredient added "Tumor‐Reducing Prescription" for hysteromyoma in 45 cases]. Shanghai Journal of Traditional Chinese Medicine 2001;35(2):33‐4. [Google Scholar]
Ye TH 2002 {published data only}
- Ye TH. [Clinical observation of 60 cases of hysteromyoma treated with Gong Liu Xiao capsule]. Journal of Sichuan of Traditional Chinese Medicine 2002;20(1):53‐4. [Google Scholar]
Yu JY 2004 {published data only}
- Yu JY. [Xiao Liu Wan for treatment of 60 cases of uterine fibroids]. Nei Mongol Journal of Traditional Chinese Medicine 2004;23(4):9. [Google Scholar]
Yu QL 2003 {published data only}
- Yu QL. [Clinical observation of 30 cases of hysteromyoma treated by Wanying Xiaoliu San]. Chinese Journal of Current Clinical Medicine 2003;1(8):724‐5. [Google Scholar]
Yu T 2002 {published data only}
- Yu T, Cao SW, Zhi N. [Clinical observation of 30 cases of hysteromyoma treated by Huashi Sanjie Decoction]. Jiangxi Journal of Traditional Chinese Medicine 2002;33(5):23‐4. [Google Scholar]
Zhang H 2005 {published data only}
- Zhang H. [Chinese herbal medicine combined with transcatheter arterial embolization for treatment of 21 cases of uterine fibroids]. Shandong Journal of Traditional Chinese Medicine 2005;24(12):725‐6. [Google Scholar]
Zhang M 2004 {published data only}
- Zhang M. [Use of worms for treatment of uterine fibroids]. Liaoning Journal of Traditional Chinese Medicine 2004;31(1):61. [Google Scholar]
Zhang QM 2007 {published data only}
- Zhang QM, Chen X, Sun ZY, Yuan JL. [Clinical observation on curative effect of Yuan Xiao Liu decoction for treatment of hysteromyoma]. Liaoning Journal of Traditional Chinese Medicine 2007;34(9):1272‐3. [Google Scholar]
Zhang WH 2011 {published data only}
- Zhang WH. [Clinical observation of Guizhi Fuling capsule treating 34 cases of uterine myoma]. Seeking Medicine and Asking Drug 2011;9(10):202. [Google Scholar]
Zhang WL 2005 {published data only}
- Zhang WL, Shen J. [Clinical observation of mifepristone alone or in combination of Chinese medicine for treatment of uterine fibroids]. Chinese Journal of Clinical Medicine Research 2005;11(17):2463‐4. [Google Scholar]
Zhang XB 2011 {published data only}
- Zhang BX, Yang JH, Liu YL. [Clinical observation of interventional embolization combined with Xiaozheng Tang for treating uterine myoma]. Medical Information 2011;9(9):4542‐3. [Google Scholar]
Zhang XW 1997 {published data only}
- Zhang XW, Hao SZ. [The clinical analysis of thirty‐eight cases of hysteromyoma treated with Hai Kun decoction]. Chinese Journal of Marine Drugs 1997;16(2):43‐5. [Google Scholar]
Zhang Z 2006 {published data only}
- Zhang Z. [Jiawei Guizhi Fuling Wan for treatment of 62 cases of uterine fibroids]. Journal of Sichuan of Traditional Chinese Medicine 2006;24(1):88. [Google Scholar]
Zhan YR 2007 {published data only}
- Zhan YR. [Analysis of mifepristone and Guizhi Fuling capsule for treatment of uterine fibroids]. International Medicine and Health Guidance News 2007;13(2):63‐5. [Google Scholar]
Zhao LX 2003 {published data only}
- Zhao LX. [Treatment of 64 cases of uterine fibroids by Tripterygium Wilfordii Hook]. Chinese Journal of Integrated Traditional and Western Medicine 2003;23(10):787‐8. [Google Scholar]
Zheng CY 2003 {published data only}
- Zheng CY, Zhong QL. [Dahuang Zhechong Wan Jiawei for treatment of 60 cases of uterine fibroids]. Guangxi Journal of Traditional Chinese Medicine 2003;26(5):33. [Google Scholar]
Zhong Q 2006 {published data only}
- Zhong Q, Fen JY, Liang SX, Xu YJ. [Clinical observation of hysteromyoma in perimenopause women by conservative treatment of integrated traditional Chinese and western medicine]. Chinese Journal of Clinical Medicine Research 2006;12(7):964‐5. [Google Scholar]
Zhong XR 2010 {published data only}
- Zhong XR, Lin F, Tang GC. [Clinical observation of mifepristone combined with Gongliuqing capsule in the treatment of uterine myoma]. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine 2010;10(5):41, 43. [Google Scholar]
Zhou J 1997 {published data only}
- Zhou J, Zhu M, Li YX, Shi JR, Yuan MF, Shen ZL, et al. [Clinical and experimental study on improving cellular immunological function of uterine myoma patients by Xiaoliu tablet]. Zhongguo Zhong Xi Yi Jie He Za Zhi 1997;17(5):277‐9. [PubMed] [Google Scholar]
Zhou MY 2003 {published data only}
- Zhou MY, Wang JJ, Cao LP, Chen CL, Liu P. [Observation on therapeutic effect of Da Huang Tang combined with uterine artery embolization for treatment of uterine fibroids]. Liaoning Journal of Traditional Chinese Medicine 2003;30(7):561‐2. [Google Scholar]
Zhou YJ 2006 {published data only}
- Zhou YJ, Gao J. [Treatment of 30 cases of hysteromyoma with Guizhi Fuling capsules]. China Journal of Clinical Medicine Hygiene 2006;4(10):66‐7. [Google Scholar]
Zhou YR 1999 {published data only}
- Zhou YR, Sun FL, Wang CX. [Clinical observation of 48 cases of hysteromyoma treated with Bushen, Poyu and Huatan recipe]. Chinese Journal of Primary Medicine and Pharmacy 1999;6(5):282. [Google Scholar]
Zhu JY 1997 {published data only}
- Zhu JY, Chen M, Zhuang CS, Luo ZH. [Treatment of 80 cases of hysteromyoma with LIzhong Sanjie pills]. New Journal of Traditional Chinese Medicine 1997;29(1):34. [Google Scholar]
Zou DH 1993 {published data only}
- Zou DH. [Clinical observation on practitioner Gong Zi Fu's differentiation of syndrome for treatment of 93 cases of uterine fibroids]. Jiangxi Journal of Traditional Chinese Medicine 1993;24(6):13‐4. [Google Scholar]
Additional references
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 2003
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment 2003;7(1):1‐76. [PubMed] [Google Scholar]
Falcone 2002
- Falcone T, Bedaiwy MA. Minimally invasive management of uterine fibroids. Current Opinion of Obstetrics and Gynecology 2002;14:401‐7. [DOI] [PubMed] [Google Scholar]
Farquhar 2002
- Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990‐1997. Obstetrics and Gynecology 2002;99(2):229‐34. [DOI] [PubMed] [Google Scholar]
Fugh‐Berman 2004
- Fugh‐Berman A, Balick MJ, Kronenberg F, Ososki AL, O'Connor B, Reiff M, et al. Treatment of fibroids: the use of beets (Beta vulgaris) and molasses (Saccharum officinarum) as an herbal therapy by Dominican healers in New York City. Journal of Ethnopharmacology 2004;92(2‐3):337‐9. [DOI] [PubMed] [Google Scholar]
Griffiths 2006
- Griffiths A, D'Angelo A, Amso N. Surgical treatment of fibroids for subfertility. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003857] [DOI] [PubMed] [Google Scholar]
Grigorieva 2003
- Grigorieva V, Chen‐Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel‐releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertility and Sterility 2003;79(5):1194‐8. [DOI] [PubMed] [Google Scholar]
Gupta 2012
- Gupta JK, Sinha AS, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD005073.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Huang 2003
- Huang YY. [Research advance and prospects of traditional Chinese medicine and western medicine for treatment of uterine fibroids]. Tianjin Journal of Traditional Chinese Medicine 2003;20(6):78‐80. [Google Scholar]
Ji 2011
- Ji X, Gao J, Cai X, Lu W, Hu C, Wang Z, et al. Immunological regulation of Chinese herb Guizhi Fuling capsule on rat endometriosis model. J Ethnopharmacol 2011;134(3):624‐9. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Klatsky 2008
- Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. American Journal of Obstetrics and Gynecology 2008;198(4):357‐66. [DOI] [PubMed] [Google Scholar]
Lethaby 2001
- Lethaby A, Vollenhoven B, Sowter M. Pre‐operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000547] [DOI] [PubMed] [Google Scholar]
Li J 2008
- Li J, Jin GL. Current research status of Chinese medicine for treatment of uterine fibroids. Academic Journal of Liaoning University of Traditional Chinese Medicine 2008;10(1):73‐4. [Google Scholar]
Liu J 2002
- Liu J, Kjaergard LL, Gluud C. Misuse of randomization: a review of Chinese randomized trials of herbal medicines for chronic hepatitis B. American Journal of Chinese Medicine 2002;30(1):173‐6. [DOI] [PubMed] [Google Scholar]
Marshall 1997
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstetrics and Gynecology 1997;90(6):967‐73. [DOI] [PubMed] [Google Scholar]
Maruo 2004
- Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Human Reproduction Update 2004;10(3):207‐20. [DOI] [PubMed] [Google Scholar]
McLucas 2001
- McLucas B, Goodwin S, Adler L, Rappaport A, Reed R, Perrella R. Pregnancy following uterine fibroid embolization. International Journal of Gynaecology and Obstetrics 2001;74(1):1‐7. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Munro 2011
- Munro MG. Uterine leiomyomas, current concepts: pathogenesis, impact on reproductive health, and medical, procedural, and surgical management. Obstetrics and Gynecology Clinics of North America 2011;38(4):703‐31. [DOI] [PubMed] [Google Scholar]
Newbold 2000
- Newbold RR, DiAugustine RP, Risinger JI, Everitt JI, Walmer DK, Parrott EC, et al. Advances in uterine leiomyoma research: conference overview, summary, and future research recommendations. Environmental Health Perspectives 2000;108 Suppl 5:769‐73. [DOI] [PubMed] [Google Scholar]
Okolo 2008
- Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Practice & Research. Clinical Obstetrics & Gynaecology 2008;22(4):571‐88. [DOI] [PubMed] [Google Scholar]
Sang 2004
- Sang H. Clinical and experimental research into treatment of hysteromyoma with promoting qi flow and blood circulation, softening and resolving hard lump. Journal of Traditional Chinese Medicine 2004;24(4):274‐9. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Stewart 2001
- Stewart EA. Uterine fibroids. Lancet 2001;357:293‐8. [DOI] [PubMed] [Google Scholar]
Tranquart 2002
- Tranquart F, Brunereau L, Cottier JP, Marret H, Gallas S, Lebrun JL, et al. Prospective sonographic assessment of uterine artery embolization for the treatment of fibroids. Ultrasound in Obstetrics and Gynecology 2002;19(1):81‐7. [DOI] [PubMed] [Google Scholar]
Tristan 2012
- Tristan M, Orozco LJ, Steed A, Ramírez‐Morera A, Stone P. Mifepristone for uterine fibroids. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD007687.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19:159‐66. [DOI] [PubMed] [Google Scholar]
Watson 2002
- Watson GM, Walker WJ. Uterine artery embolization for the treatment of symptomatic fibroids in 114 women: reduction in size of the fibroids and women's views of the success of the treatment. British Journal of Obstetrics and Gynaecology 2002;109(2):129‐35. [DOI] [PubMed] [Google Scholar]
Xiong 2002
- Xiong JH, Xiong CY. [Current status of treatment of uterine fibroids by traditional Chinese medicine]. Journal of Jiangxi College of Traditional Chinese Medicine 2002;14(4):61‐3. [Google Scholar]
Yuan 2000
- Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacology and Therapeutics 2000;86:191‐8. [DOI] [PubMed] [Google Scholar]
Zimmermann 2012
- Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet‐based survey of 21,746 women. BMC Womens Health 2012;12:6. doi: 10.1186/1472‐6874‐12‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]


