Abstract
Introduction
Data on age-related differences in rejection rates, infectious episodes, and tacrolimus exposure in pediatric kidney transplant recipients (pKTRs) on a tacrolimus-based immunosuppressive regimen are scarce.
Methods
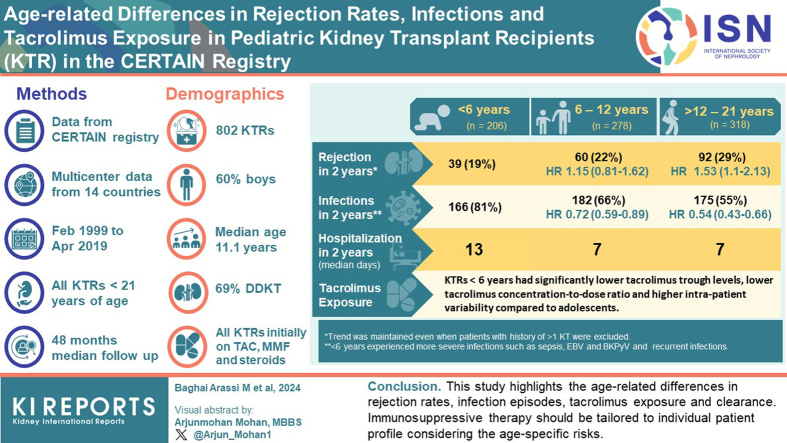
We performed a large-scale analysis of 802 pKTRs from the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) registry from 40 centers in 14 countries. The inclusion criteria were a tacrolimus-based immunosuppressive regimen and at least 2 years of follow-up. The patient population was divided into 3 age groups (infants and young children aged <6 years, school-aged children 6–12 years, and adolescents aged >12 years) to assess age-related differences in outcome.
Results
Median follow-up was 48 months (interquartile range [IQR], 36–72). Within the first 2 years posttransplant, infants, and young children had a significantly higher incidence of infections (80.6% vs. 55.0% in adolescents, P < 0.001) and a significantly higher number of cumulative hospital days (median 13 days vs. 7 days in adolescents, P < 0.001). Adolescents had a significantly higher rate of biopsy-proven acute rejection episodes in the first-year posttransplant (21.7%) than infants and young children (12.6%, P = 0.007). Infants and young children had significantly lower tacrolimus trough levels, lower tacrolimus concentration-to-dose (C/D) ratios as an approximation for higher tacrolimus clearance, and higher tacrolimus interpatient variability (TacIPV) (all P < 0.01) than adolescents.
Conclusion
This is the largest study to date in European pKTRs on a tacrolimus-based immunosuppressive regimen, and it shows important age-related differences in rejection rates, infection episodes, as well as tacrolimus exposure and clearance. This data suggests that immunosuppressive therapy in pKTRs should be tailored and personalized according to the age-specific risk profiles of this heterogeneous patient population. The data may serve as a benchmark for future studies with novel immunosuppressive drugs.
Keywords: allograft rejection, hospitalization, infection, pediatric kidney transplantation, tacrolimus
Graphical abstract
Kidney transplantation is the preferred option for the treatment of kidney failure. However, allograft rejection, infection, and graft dysfunction challenge patient and graft survival. Whereas large multicenter outcomes studies exist in adult transplant cohorts,1,2 there is a paucity of similar studies in the field of pediatric kidney transplantation, because robust and generalizable outcomes studies in pediatric cohorts are difficult to achieve due to the small number of pediatric patients requiring kidney transplantation. However, there is an urgent need for large, multicenter studies that analyze age-related differences in outcomes in this heterogeneous patient population. These data may support clinical decision making and thereby improve patient care.
Benchmarks play a key role in filling this data gap. They provide a framework for assessing the effectiveness of treatment protocols and help to evaluate and improve current practices. Whereas benchmark studies of North American and Chinese pediatric cohorts are available,3,4 similar studies are lacking in Europe. The CERTAIN registry provides a unique tool to address this need. It currently includes 95 pediatric kidney transplant centers from 26 European countries and data from 3930 pediatric patients. CERTAIN is a platform for conducting high quality research due to its detailed records and thorough follow-up. It is equipped to analyze long-term outcomes and trends in pediatric kidney transplantation.
In this study, we used the CERTAIN registry to fill the gap in age-related outcome data for pKTRs treated with a tacrolimus-based immunosuppressive regimen. Tacrolimus was chosen because of its widespread use and its status as the leading immunosuppressive agent, a position it is expected to maintain for the next decade. In addition, this choice allows for a more uniform patient cohort, while still representing the vast majority of pKTRs. Our analysis included data from 802 children at 40 centers in 14 countries over a 20-year period. This research provides critical insight into the impact of age on kidney transplant outcomes and highlights the unique challenges faced by each age group.
Methods
Study Design and Patient Population
This is a retrospective, multicenter, longitudinal cohort study using data from the CERTAIN registry, including patients who underwent kidney transplantation between February 1999 and April 2019. The CERTAIN registry provides a detailed data collection that allows an in-depth characterization of specific patient cohorts. It collects detailed longitudinal clinical and laboratory data and applies rigorous validity checking procedures (http://www.certain-registry.eu/). The time points of data collection and the corresponding time intervals are as follows: baseline (pretransplant); at months 1, 3, 6, 9, and 12; and every 6 months thereafter. The CERTAIN dataset allows for detailed and flexible documentation of the posttransplant follow-up through continuous entry of any number of relevant data (e.g., laboratory values and drug therapy). Specific case report forms collect detailed and accurate information on relevant data and events of pKTRs in the peri transplant and posttransplant course. There are 2 datasets: the minimum required dataset and the extended dataset. The minimum required dataset is mandatory for all participating centers. The extended data set provides a deeper insight into the clinical course and treatment of patients by documenting additional items, some of which are predefined and some of which can be defined by the participating center. For this study, only the minimum required dataset was used, and no specific electronic case report forms were used, thereby maximizing the number of patients included in the analysis.
The CERTAIN web application (accessible via http://www.certain-registry.eu/RegApp) has an automatic and manual data validation functionality. During data entry, the data record are automatically validated against predefined plausibility ranges. In addition, the system has an integrated manual quality assurance process. First, documented data must be approved locally at the site; second, a data quality manager at the registry headquarters randomly checks data for plausibility. Only data that pass this quality assurance process are added to the research database. These functions are available anytime, anywhere, and require only a standard web browser and internet access.
Participation in the CERTAIN registry is approved by the ethics committee at each center. Informed consent was obtained from the parents or legal guardians prior to enrollment, with assent from patients when appropriate for their age. All procedures and immunosuppressive regimens were performed according to local institutional protocols. Anthropometric, clinical, and biochemical data were collected as part of a routine follow-up at each center. The study was conducted in accordance with the Declaration of Helsinki and the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The study was designed, analyzed, and reported according to the STROBE guidelines (https://www.strobe-statement.org/, Supplementary Methods).
Inclusion and Exclusion Criteria
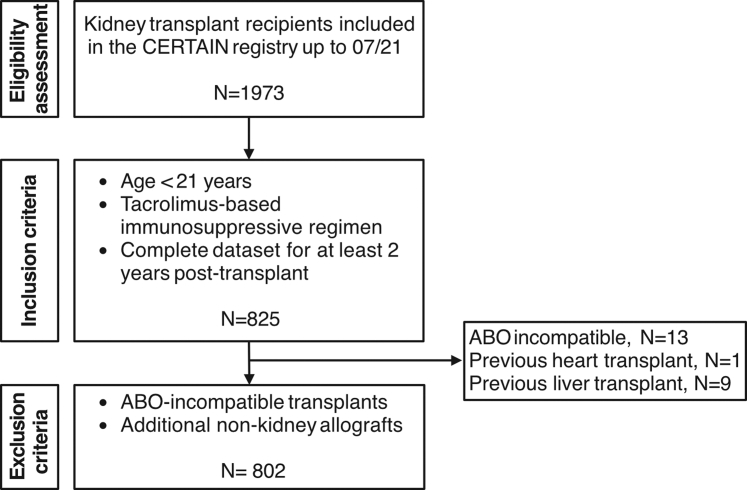
The CERTAIN registry offers participation to all patients aged <21 years5 undergoing kidney transplantation, provided that their caregivers and/or the patients themselves give informed consent when appropriate for their age, without imposing registry-specific exclusion criteria. This approach ensures broad eligibility and a true representation of the transplant population. In this study we included kidney allograft recipients who were aged 21 years or younger at the time of transplantation and were maintained on a tacrolimus-based immunosuppressive regimen throughout the first 2 years posttransplant. Eligibility required a complete minimum data set for at least 2 years posttransplant. Patients with ABO incompatible transplants or those who received additional nonkidney allografts were excluded. In Figure 1, we provide an overview of the patient inclusion and exclusion criteria.
Figure 1.
Flow diagram of patient inclusion and exclusion. Patient flow diagram showing the inclusion and exclusion criteria applied to the CERTAIN registry data to generate the cohort for the current study. Of the 1973 patients included in the CERTAIN registry as of July 2021, 1472 had ≥2 years of follow-up. Among these, only 825 had a complete minimum dataset, including tacrolimus exposure monitoring. Due to the retrospective nature of this study and the limitations of the registry data, it remains unclear whether patients with incomplete or missing tacrolimus monitoring data were on a non-tacrolimus regimen or if their data were simply incomplete, leading to their exclusion. CERTAIN, Cooperative European Pediatric Renal Transplant Initiative Research Network.
Outcome Events
Study outcomes included infection, rejection, graft dysfunction, diabetes mellitus, death, and cumulative hospital days. Because graft loss is a rare event in pKTRs, the outcome measure we used was a death-censored composite end point named allograft dysfunction, which is defined as either graft loss, or estimated glomerular filtration rate (eGFR) ≤ 30 ml/min per 1.73 m2, or a ≥50% decline from baseline eGFR at month 3 posttransplant. eGFR values > 120 ml/min per 1.73 m2 at 3 months posttransplant were set at 120 ml/min per 1.73m2. The eGFR was calculated based on the modified Schwartz formula for patients aged < 18 years or Chronic Kidney Disease Epidemiology Collaboration CKD-EPI formula if aged ≥ 18 years. Rejections were classified as such if they were confirmed by biopsy and received antirejection therapy regardless of whether the biopsy was performed for surveillance or for cause. They were further categorized based on graft histopathology using the most recent Banff classification in effect at the time of rejection (1997–2019). Rejections were further grouped into biopsy-proven acute rejections, which included all acute episodes of T cell-mediated rejection and antibody-mediated rejection; and biopsy-proven rejections, which included all acute and chronic episodes of T cell-mediated rejection and antibody-mediated rejection. Infections were identified based on the treating physician's determination of an episode of infection, using a binary survey of infection without any detailed specification. For cytomegalovirus (CMV), data on organ involvement and CMV disease were also available. There was no additional study-specific protocol for the identification of infections. Episodes of rejection and infection were categorized according to their timing, distinguishing between those occurring in the first or second year posttransplant. Recurrent infections and rejections were defined as > 1 event during follow-up.
Tacrolimus Exposure Quantification
Tacrolimus exposure was quantified using whole blood trough levels as documented in the CERTAIN database. Trough level data were categorized into early (months 1–3), mid (months 6–12), and late (months 18–24) posttransplant periods. Tacrolimus target levels were determined by center-specific protocols and individual patient risk profiles. To assess TacIPV, a minimum of 3 tacrolimus trough level measurements per patient during the 6 to 12 months posttransplant period was required. TacIPV was assessed using 2 algorithms: the observed coefficient of variation, calculated as (SD ÷ mean) × 100 and expressed as a percentage,6,7 and the observed mean absolute deviation, formulated as ([Xmean − X1] + [Xmean − X2] + ... + [Xmean − Xn] ÷ n) ÷ Xmean, where X is the tacrolimus trough level in whole blood.6,8,9 In addition, the tacrolimus concentration/dose (C/D) ratio was determined from tacrolimus trough levels and the corresponding daily doses. The C/D ratio, expressed as ng/ml/g, is reported throughout the text without units. C/D ratio data were stratified by early (mean C/D ratio of months 1 and 3), late (mean C/D ratio of months 6, 9, and 12), and total (mean C/D ratio of months 1–12) posttransplant periods. To account for differences in body size in this pediatric patient population, C/D ratio data were corrected for body surface area (BSA) (C/D ratioBSA = tacrolimus blood trough level in ng/ml ÷ [daily tacrolimus dose in mg ÷ BSA in m2]) and for body weight (C/D ratioBW = tacrolimus blood trough level in ng/ml ÷ [daily tacrolimus dose in mg ÷ body weight in kg]). To increase comparability with adult C/D ratios, BSA-corrected C/D ratio was additionally adjusted for a representative adult BSA of 1.73 m2 (C/D ratioBSA-Adult = tacrolimus blood trough level in ng/ml ÷ [daily tacrolimus dose in mg ÷ BSA in m2 × 1.73).
Statistical Analysis
Continuous variables were described by mean and SD or median and IQR, as appropriate; and categorical variables were described by absolute and relative frequencies. Comparisons between the 3 age subgroups were made using 1-way analysis of variance for continuous variables and chi-square for categorical variables. The subgroup of patients aged < 2 years (n = 18) was grouped into the youngest age group of patients aged <6 years because the small sample size did not allow separate statistical analysis. Post hoc tests were corrected using the Benjamini-Hochberg correction. Time-to-event variables were described using the Kaplan-Meier estimator, and comparisons were made using log-rank tests. Univariable and multivariable regression models were used to identify risk factors. Linear regression models were used for cumulative hospital days (in relation to follow-up time), and Cox regression analysis was used for time-to-event outcomes. Variables with P values < 0.1 in the subgroup analyses and univariable analyses were included in the multivariable analyses after medical plausibility screening. Model estimates were reported with corresponding 95% confidence intervals (CIs) for each measure (i.e., hazard ratios [HRs], inverse HRs, and regression estimates). To assess the influence of a potential era effect on patient outcomes while maintaining an even distribution of patient numbers across groups, the data were categorized into 2 different time periods of kidney transplantation: August 1999 to July 2014 (era 1, n = 402) and August 2014 to April 2019 (era 2, n = 400). These periods were chosen based on the median year to create equally sized cohorts for analysis. All analyses were performed in R Studio version > 4.2.0. Missing values were very rare, and no imputation of missing values was performed. In this exploratory analysis, all P values should be interpreted in a descriptive sense without a confirmatory value. A P value < 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 802 patients, 323 girls (40.3%) and 479 boys (59.7%), who underwent kidney transplantation between 1999 and 2019 from 40 different study centers were included (Table 1, Supplementary Table S1). The median age at transplantation was 11.1 (IQR, 6.85–15.3) years. Majority of patients (91.4%) were of Caucasian descent, and a minority of patients of African (3.1%) or Asian (5.4%) descent. In majority of patients, the primary kidney disease was congenital anomalies of the kidney and urinary tract (39.4%), followed by hereditary cystic or congenital diseases (25.3%) and primary glomerular diseases (17.6%). Sixty-nine percent of patients received a deceased donor kidney. All patients received tacrolimus, mycophenolate mofetil, and glucocorticoids as initial immunosuppressive regimen. Forty-one percent of patients received additional induction therapy with either basiliximab (34.2%), antithymocyte globulin (5.4%), rituximab (2.0%), or daclizumab (0.7%). The median follow-up time was 48 (IQR, 36–72) months posttransplant. The patient cohort was stratified into 3 age groups: < 6 years (n = 206, infants and young children), 6 to 12 years (n = 278, school-aged children), > 12 years (n = 318, adolescents). Age groups differed significantly with respect to sex, follow-up time, preemptive transplantation, primary kidney disease, induction therapy, previous kidney transplants, immunosuppressive therapy at year 1, and tacrolimus formulation. Baseline clinical and demographic characteristics are shown in Table 1.
Table 1.
Baseline clinical and demographic characteristics
| Characteristics | <6 yrs (n = 206) | 6–12 yrs (n = 278) | >12 yrs (n = 318) | Total (N = 802) | P value |
|---|---|---|---|---|---|
| Sex | 0.031 | ||||
| Female | 67 (32.5%) | 119 (42.8%) | 137 (43.1%) | 323 (40.3%) | |
| Male | 139 (67.5%) | 159 (57.2%) | 181 (56.9%) | 479 (59.7%) | |
| Age at transplantation | < 0.001 | ||||
| Median (IQR) | 4.92 (3.72–5.94) | 10.16 (8.50–11.3) | 16.09 (14.7–17.8) | 11.1 (6.85–15.3) | |
| Transplant era | 0.667 | ||||
| 08/1999-07/2014 | 108 (52.4%) | 140 (50.4%) | 154 (48.4%) | 402 (50.1%) | |
| 08/2014-04/2019 | 98 (47.6%) | 138 (49.6%) | 164 (51.6%) | 400 (49.9%) | |
| Donor type | 0.854 | ||||
| Deceased donor | 144 (69.9%) | 188 (67.6%) | 220 (69.2%) | 552 (68.8%) | |
| Living donor | 62 (30.1%) | 90 (32.4%) | 98 (30.8%) | 250 (31.2%) | |
| Follow-up time (months) | < 0.001 | ||||
| Median (IQR) | 54 (36, 84) | 54 (36, 78) | 48 (30, 60) | 48 (36, 72) | |
| Preemptive transplantation | 0.007 | ||||
| Yes | 28 (13.6%) | 68 (24.5%) | 75 (23.6%) | 171 (21.3%) | |
| Primary kidney disease | 0.002 | ||||
| CAKUT | 87 (42.2%) | 111 (39.9%) | 118 (37.1%) | 316 (39.4%) | |
| Cystic/hereditary/congenital diseases | 65 (31.6%) | 67 (24.1%) | 71 (22.3%) | 203 (25.3%) | |
| Primary glomerular disease | 32 (15.5%) | 45 (16.2%) | 64 (20.1%) | 141 (17.6%) | |
| Secondary glomerular disease/vasculitis | 6 (2.88%) | 25 (8.99%) | 18 (5.7%) | 49 (6.11%) | |
| Interstitial nephritis/pyelonephritis | 0 (0.00%) | 2 (0.72%) | 7 (2.2%) | 9 (1.12%) | |
| Neoplasms/tumors | 1 (0.49%) | 3 (1.08%) | 2 (0.6%) | 6 (0.75%) | |
| Unknown | 7 (3.40%) | 16 (5.76%) | 33 (10.4%) | 56 (6.98%) | |
| Other | 8 (3.88%) | 9 (3.24%) | 5 (1.6%) | 22 (2.74%) | |
| Number of HLA mismatches | |||||
| Overall | 0.353 | ||||
| Mean (SD) | 3.08 (1.49) | 2.92 (1.38) | 2.92 (1.38) | 2.96 (1.41) | |
| HLA-A | 0.136 | ||||
| Mean (SD) | 0.94 (0.66) | 0.94 (0.70) | 0.84 (0.68) | 0.90 (0.68) | |
| HLA-B | 0.128 | ||||
| Mean (SD) | 1.17 (0.71) | 1.05 (0.66) | 1.12 (0.63) | 1.11 (0.66) | |
| HLA-DR | 0.664 | ||||
| Mean (SD) | 0.98 (0.69) | 0.92 (0.66) | 0.96 (0.63) | 0.95 (0.66) | |
| Induction therapy | 0.007 | ||||
| Yes | 67 (32.5%) | 129 (46.4%) | 136 (42.8%) | 332 (41.4%) | |
| Previous kidney transplants | 0.005 | ||||
| Yes | 14 (6.80%) | 28 (10.1%) | 50 (15.7%) | 92 (11.5%) | |
| Cold ischemia time (hours, median (IQR)) | 0.359 | ||||
| Living donor | 2.50 (1.64–3.00) | 2.42 (1.94–3.25) | 2.50 (2.02–3.44) | 2.50 (2.00–3.25) | |
| Deceased donor | 14.1 (10.9–16.4) | 13.0 (10.2–16.0) | 13.9 (10.0–16.7) | 13.7 (10.2–16.4) | |
| Tacrolimus tradename | < 0.001 | ||||
| Prograf | 143 (69.7%) | 252 (91.0%) | 299 (94.6%) | 694 (87.0%) | |
| Modigraf | 61 (29.8%) | 12 (4.3%) | 5 (1.6%) | 78 (9.8%) | |
| Advagraf | 1 (0.49%) | 13 (4.7%) | 12 (3.8%) | 26 (3.3%) | |
| Immunosuppressive therapy at year 1 | |||||
| Tacrolimus | 206 (100%) | 278 (100%) | 318 (100%) | 802 (100%) | |
| MMF | 128 (62.1%) | 218 (78.4%) | 278 (87.4%) | 624 (77.8%) | < 0.001 |
| Azathioprine | 31 (15.1%) | 26 (9.25%) | 17 (5.35%) | 74 (9.2%) | 0.003 |
| Glucocorticoids | 160 (77.7%) | 220 (79.1%) | 284 (89.3) | 664 (82.8%) | 0.001 |
| Sirolimus | 2 (0.97%) | 1 (0.35%) | 2 (0.63%) | 5 (0.6%) | 0.861 |
| Everolimus | 13 (6.31%) | 9 (3.24%) | 12 (3.77%) | 34 (4.2%) | 0.406 |
CAKUT, congenital anomalies of the kidney and urogenital tract; HLA, human leukocyte antigen; IQR, interquartile range; MMF, mycophenolate mofetil.
Biopsy-Proven Rejections
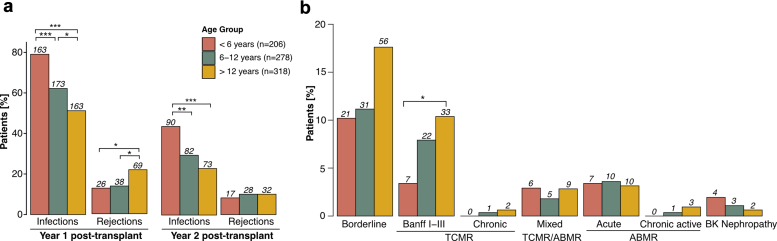
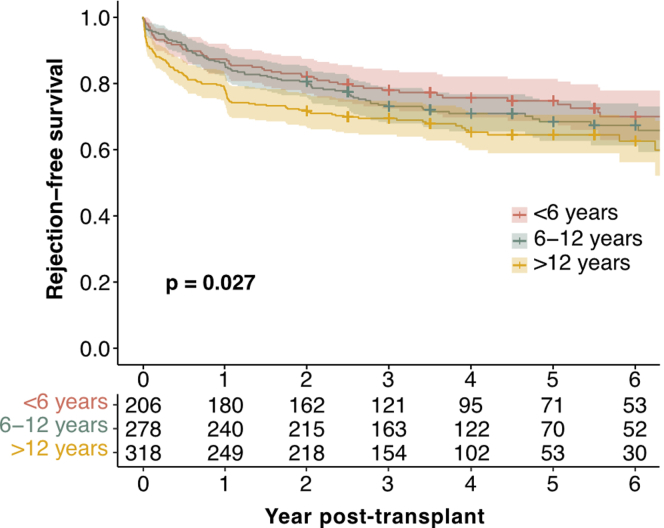
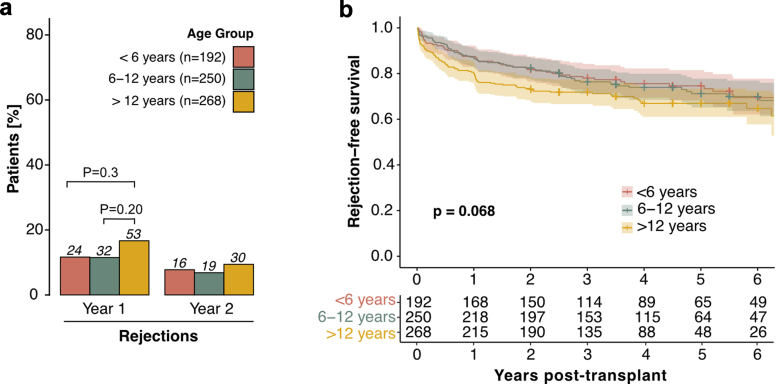
Biopsy-proven rejection episodes were the second most common event within the first 2 years after transplantation, occurring in 23.8% of patients. There was no statistically significant difference between the number of rejection episodes in living donor recipients (26.0%) and deceased donor recipients (22.8%, P = 0.56). The prevalence of rejection differed significantly by age, with the highest rate in adolescents (28.9% vs. 18.9% in infants and young children, P = 0.018). When stratified by year, most rejections occurred in the first-year posttransplant (Figure 2a), with adolescents experiencing significantly more rejections (P = 0.035) than infants and young children, and school-aged children (P = 0.043). Notably, adolescents experienced a higher rate of recurrent rejections (10.4%) than school-aged children (4.7%) or infants and young children (4.9%, P = 0.042). In addition, in the Kaplan-Meier analysis, the rejection-free survival was significantly higher (P = 0.027) in infants and young children than in adolescents throughout the entire follow-up period (Figure 3). This trend was maintained (P = 0.068), when patients with a history of more than 1 kidney transplant were excluded (n = 92, Table 1, Figure 4). Rejection episodes were further categorized based on graft histopathology using the most recent Banff classification in effect at the time of rejection (1997–2019, Figure 2b). In Table 2, we provide an overview of rejection subtypes stratified by age group and year 1 and 2 posttransplant. Borderline rejection was the most common finding, followed by T cell-mediated rejection; the latter was significantly more frequent in adolescents (P = 0.015) than in infants and young children (Figure 2b, Table 2). Multivariable Cox regression analysis revealed an increased risk of rejection in adolescents (HR, 1.53; 95% CI, 1.10–2.13; P = 0.01, Table 3). Overall rejection rates were comparable between transplant era 1 and 2. The number of rejection episodes in the subgroup of patients aged < 2 years (n = 18), grouped in the infants and young children age group, within the first 2 years is shown in Supplementary Table S3.
Figure 2.
Age-stratified outcome parameters in the first 2 years posttransplant. (a) Incidence of first episodes of rejection and infection in the first 2 years posttransplant, stratified by age group and year posttransplant. The first event of each patient in each year is considered for analysis. (b) Histopathology of allograft rejection graded according to Banff classification in the first 2 years posttransplant, stratified by age group. Color coding represents different age groups. Benjamini-Hochberg-corrected statistical significance values are indicated by asterisks as follows: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. ABMR, antibody-mediated rejection; BK, BK polyomavirus; TCMR, T cell-mediated rejection.
Figure 3.
Rejection-free survival stratified by age group. Rejection-free survival: Kaplan-Meier curves illustrating the probability of rejection-free survival over the entire follow-up period posttransplant, with age groups distinguished by color coding. Log-rank P values from the Kaplan-Meier analysis are provided to indicate the statistical significance of differences between age groups.
Figure 4.
Biopsy-proven rejection episodes in patients without previous kidney transplants stratified by age group. (a) Incidence of rejection in the first 2 years posttransplant, stratified by age group and year posttransplant. The first event of each patient in each year is considered for analysis. Benjamini-Hochberg adjusted P values are reported. (b) Rejection-free survival: Kaplan-Meier curves illustrating the probability of rejection-free survival over the entire follow-up period posttransplant, with age groups distinguished by color coding. Log-rank P values from the Kaplan-Meier analysis are provided to indicate the statistical significance of differences between age groups.
Table 2.
Kidney allograft histopathology graded according to Banff classification
|
Graft Histopathology |
Year 1 posttransplant |
Year 2 posttransplant |
||||
|---|---|---|---|---|---|---|
| < 6 yrs (n = 206) | 6–12 yrs (n = 278) | > 12 yrs (n = 318) | < 6 yrs (n = 206) | 6–12 yrs (n = 278) | > 12 yrs (n = 318) | |
| Borderline | 19 (9.2) | 20 (7.2) | 38 (11.9) | 7 (3.4) | 12 (4.3) | 19 (6.0) |
| Banff I-III TCMR | 4 (1.9)a | 11 (4.0)a | 25 (7.9) | 3 (1.5) | 11 (4.0) | 7 (2.2) |
| Chronic TCMR | 0 (0) | 1 (0.4) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) |
| Mixed ABMR/TCMR | 3 (1.5) | 5 (1.8) | 6 (1.9) | 3 (1.5) | 1 (0.4) | 4 (1.3) |
| Acute ABMR | 5 (2.4) | 6 (1.8) | 7 (2.2) | 2 (1.0) | 3 (1.1) | 2 (0.6) |
| Chronic ABMR | 0 (0) | 1 (0.4) | 2 (0.6) | 0 (0) | 0 (0) | 1 (0.3) |
| BKPyV nephropathy | 2 (1.0) | 1 (0.4) | 0 (0) | 2 (1.0) | 2 (0.7) | 2 (0.6) |
| Sum BPAR | 31 (15.0) | 42 (15.1) | 76 (23.9) | 15 (7.28) | 27 (9.71) | 32 (10.1) |
| Sum BPR | 31 (15.0) | 44 (15.8) | 79 (24.8) | 15 (7.28) | 27 (9.71) | 33 (10.4) |
ABMR, antibody-mediated rejection; BKPyV, BK polyomavirus; BPAR, biopsy-proven acute rejections (including borderline); BPR, biopsy-proven rejections; TCMR, T cell-mediated rejection.
Data are represented as n (%).
Significant differences (P < 0.05) at the respective time point posttransplant are indicated by superscript; data without common superscript are significantly different.
Table 3.
Risk factor analysis for outcome events
| Outcome events | Unadjusted HR/Estimate (95% CI) | P value | Adjusted HR/Estimate (95% CI) | P value |
|---|---|---|---|---|
| Infections | ||||
| Age group 6–12 yrs | 0.73 (0.6–0.9) | 0.002 | 0.72 (0.59–0.89) | 0.002 |
| Age group > 12 yrs | 0.54 (0.44–0.66) | <0.001 | 0.54 (0.43–0.66) | <0.001 |
| Male sex | 1.09 (0.93–1.29) | 0.29 | 1.04 (0.88–1.24) | 0.65 |
| Preemptive transplantation | 0.9 (0.74–1.1) | 0.31 | 0.90 (0.73–1.01) | 0.33 |
| CAKUT | 1.12 (0.94–1.30) | 0.22 | 1.09 (0.92–1.30) | 0.3 |
| HLA-mismatches | 0.95 (0.90–1.10) | 0.09 | 0.94 (0.89–1.00) | 0.04 |
| Induction therapy | 0.98 (0.83–1.15) | 0.78 | 1.03 (0.88–1.22) | 0.7 |
| Rejections | ||||
| Age group 6–12 yrs | 1.162 (0.83–1.63) | 0.383 | 1.15 (0.81–1.62) | 0.43 |
| Age group > 12 yrs | 1.47 (1.05–2.05) | 0.012 | 1.53 (1.10–2.13) | 0.01 |
| Male sex | 1.13 (0.88–1.46) | 0.17 | 1.22 (0.94–1.59) | 0.14 |
| Preemptive transplantation | 1.08 (0.8–1.45) | 0.63 | 1.04 (0.77–1.41) | 0.79 |
| CAKUT | 0.92 (0.71–1.19) | 0.51 | 0.87 (0.66–1.13) | 0.29 |
| HLA-mismatches | 1.01 (0.92–1.10) | 0.88 | 1.01 (0.77–1.41) | 0.79 |
| Induction therapy | 1.2 (0.94–1.54) | 0.15 | 1.15 (0.9–1.49) | 0.27 |
| Hospitalization days | ||||
| Age group 6–12 yrs | −2.17 (−3.85 to −0.49) | 0.01 | −2.12 (−3.85 to −0.39) | 0.02 |
| Age group > 12 yrs | −2.5 (−4.13 to −0.87) | 0.003 | −2.41 (−4.09 to −0.73) | 0.005 |
| Male sex | 0.80 (−0.52 to 2.12) | 0.23 | 0.68 (−0.70 to 2.07) | 0.33 |
| Preemptive transplantation | −1.34 (−2.92 to 0.24) | 0.1 | −1.04 (−2.68 to 0.6) | 0.21 |
| CAKUT | 0.46 (−0.87 to 1.79) | 0.5 | 0.23 (−1.17 to 1.63) | 0.74 |
| HLA-mismatches | 0.345 (−0.12 to 0.81) | 0.14 | 0.28 (−0.19 to 0.74) | 0.25 |
| Induction therapy | 0.35 (−0.97 to 1.67) | 0.6 | 0.37 (−0.97 to 1.7) | 0.59 |
CAKUT, congenital anomalies of the kidney and urogenital tract; CI, confidence interval; HLA, human leukocyte antigen; HR, hazard ratio.
Infections
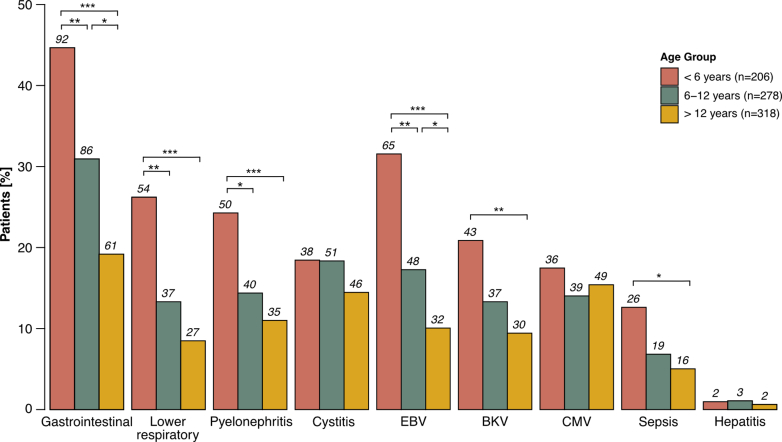
During the first 2 years posttransplant, infections were the most common outcome event, with 65.2% of patients experiencing at least 1 infectious episode. Infants and young children had the highest incidence of infections (80.6%), followed by school-aged children (65.5%) and adolescents (55.0%, P < 0.001, Figure 2a). Infants and young children had the highest incidence of recurrent infections (66.5% vs. 33.6% in adolescents, P < 0.001). This pattern was consistent for both the 1-year and the 2-year posttransplant periods (both P < 0.001). Infection rates were comparable between transplant era 1 and 2. When categorizing infections by type (Figure 5, Supplementary Table S2), gastroenteritis was the most common, closely followed by lower respiratory tract infections and pyelonephritis (all P < 0.001 for infants and young children vs. adolescents). Notably, infants and young children experienced more severe infections such as sepsis (P = 0.011) and other relevant infections such as Epstein-Barr virus (P < 0.001) and BK polyomavirus (P = 0.002). CMV infections did not differ significantly between age groups. In majority of events (92% in infants and young children, 92% in school-aged children, 83% in adolescents), CMV infection was only CMV viremia as determined by nuclear acid testing or pp65 antigenemia, without CMV syndrome or organ-invasive disease. Multivariable Cox regression analysis showed a significantly higher risk of infection in infants and young children (inverse HR, 1.85; 95% CI, 1.51–2.33; P < 0.001) than in adolescents (Table 3) and school-aged children (inverse HR, 1.39; 95% CI, 1.12–1.69; P = 0.002). The number of infections in the subgroup of patients aged < 2 years (n = 18), grouped in the infants and young children age group, within the first 2 years is shown in Supplementary Table S3.
Figure 5.
Types of infections observed within the first 2 years posttransplant. Distribution of infection types observed within the first 2 years posttransplant, stratified by age group. Color coding represents different age groups. Benjamini-Hochberg-corrected statistical significance values are indicated by asterisks as follows: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. BKV, BK polyoma virus; CMV, cytomegalovirus; EBV, Epstein-Barr virus.
Graft Dysfunction, Diabetes Mellitus, Death
The overall incidence of graft dysfunction (2.8%), diabetes mellitus (1.7%), and death (0.1%) during the first 2 years posttransplant was low. Diabetes mellitus was significantly more common in adolescents (3.8%) than in school-aged children (0.7%) and infants and young children (0%; P = 0.002). Kaplan-Meier analysis over the entire follow-up period showed no significant age differences for graft dysfunction or death. However, infants and young children, and school-aged children had a significantly higher eGFR than adolescents at months 3, 12, and 24 posttransplant (P < 0.001, Table 4).
Table 4.
Estimated glomerular filtration rate at months 3, 12, and 24 posttransplant
| Time posttransplant | < 6 yrs (n = 206) | 6–12 yrs (n = 278) | > 12 yrs (n = 318) | P value |
|---|---|---|---|---|
| Month 3 | 88.1 (29.9)a | 82.9 (27.7)a | 67.1 (18.9) | < 0.001 |
| Month 12 | 83.2 (26.1)a | 83.1 (26.5)a | 70.0 (21.4) | < 0.001 |
| Month 24 | 79.5 (25.1)a | 79.1 (23.6)a | 66.3 (20.0) | < 0.001 |
Data are mean (SD).
Significant differences at the respective time point posttransplant is indicated by superscript; data without common superscript are significantly different.
Hospitalizations
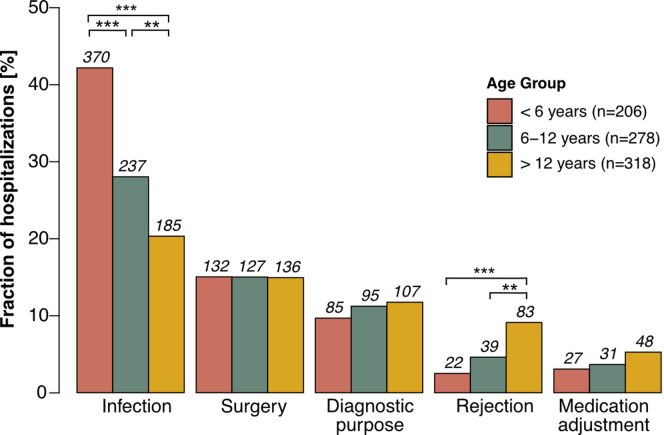
Ninety-one percent of patients experienced at least 1 hospitalization during the first 2 years posttransplant, with no significant difference between age groups (P = 0.973). The most common reason for hospitalization was infection, which was progressively more common in infants and young children, and school-aged children than in adolescents (P < 0.001, Figure 6). In addition, the total number of days spent in the hospital during the first 2 years posttransplant was significantly higher (P < 0.001) in infants and young children (median, 13 days; IQR, 4–33) than in adolescents (median, 7 days; IQR, 2–18) and school-aged children (median, 7 days; IQR, 2–21). Cumulative hospitalization days were comparable between transplant era 1 and 2. Rejection episodes were a more frequent cause of hospitalization in adolescents than in infants and young children (P < 0.001) and school-aged children (P = 0.001), even when patients with multiple kidney transplantations were excluded (P < 0.001 and P = 0.001, respectively). Multivariable linear regression analysis showed that infants and young children had a significantly higher risk of hospitalization (estimate of 2.41; 95% CI, 0.73–4.09, P = 0.005) than adolescents (Table 3) and school-aged children (2.12; 95% CI, 0.39–3.85, P = 0.02).
Figure 6.
Reasons for hospitalization in the first 2 years posttransplant. Distribution of reasons for hospitalization in the first 2 years posttransplant, stratified by age group. Color coding represents different age groups. Benjamini-Hochberg-corrected statistical significance values are indicated by asterisks as follows: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Quantification of Tacrolimus Exposure
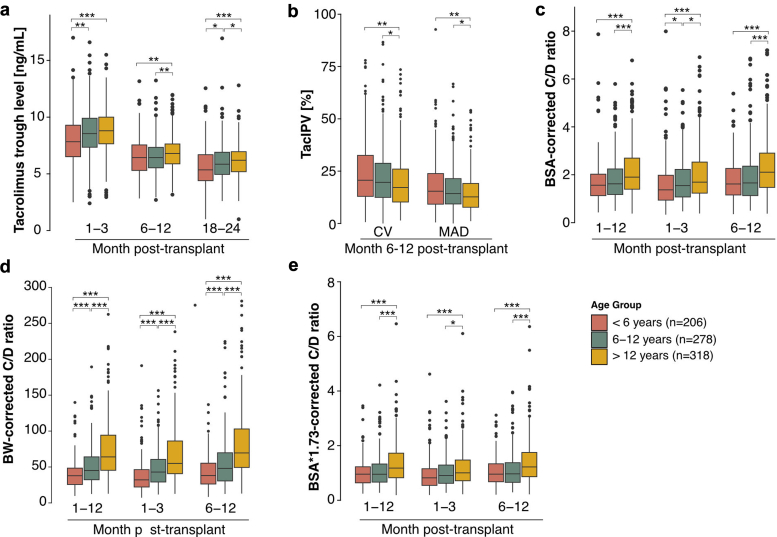
Mean tacrolimus trough levels ranged from 8.50 ± 2.13 ng/ml at month 1 to 3 posttransplant to 5.95 ± 1.62 ng/ml at months 18 to 24 posttransplant (Figure 7a). In all investigated posttransplant periods (early [months 1–3], mid [months 6–12], and late [months 18–24]), infants and young children had lower tacrolimus trough levels than school-aged children, who in turn had lower tacrolimus trough levels than adolescents (Figure 7a). These differences remained significant (P < 0.05) when patients with previous kidney transplantations were excluded. For the entire cohort, the mean TacIPV expressed as coefficient of variation was 18.9% ± 14.7% and expressed as mean absolute deviation was 13.9% ± 11.4%. For both TacIPV algorithms, adolescents had significantly lower TacIPV values than school-aged children (P < 0.03) and infants and young children (P = 0.002, Figure 7b). The mean C/D ratio corrected for BSA was 1.71 ± 1.01 (Figure 7c), 49.0 ± 35.7 corrected for body weight (Figure 7d), and 1.02 ± 0.68 corrected for BSA adjusted to 1.73 m2 (Figure 7e). The respective age-specific C/D ratios were not significantly different between the month 1 to 3 and month 6 to 12 posttransplant periods. For all 3 C/D ratio algorithms, infants and young children had significantly lower values than school-aged children, who in turn had lower values than adolescents (Figure 7c–e). None of the tacrolimus exposure parameters showed significant associations with any of the outcome events. Tacrolimus exposure in the subgroup of patients <2 years of age (N = 18), grouped in the infants and young children age group, within the first 2 years is shown in Supplementary Table S4.
Figure 7.
Tacrolimus exposure parameters stratified by age group. (a) Tacrolimus trough levels, (b) tacrolimus intrapatient variability (TacIPV) quantified by coefficient of variation (CV) and mean absolute deviation (MAD), (c) concentration/dose ratio (C/D ratio) corrected for body surface area (BSA), (d) C/D ratio corrected for body weight (BW), (e) C/D ratio corrected for BSA scaled for 1.73 m2 stratified by age and time period posttransplant. Color coding represents different age groups. Benjamini-Hochberg-corrected statistical significance values are indicated by asterisks as follows: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Discussion
This is the largest study to-date on age-related differences in rejection rates, infections, and tacrolimus exposure in pKTRs on a tacrolimus-based immunosuppressive regimen. Infants and young children had higher rates of relevant infections and more hospital days, but lower rejection rates, suggesting the need to tailor immunosuppressive strategies to their age-specific risk profile.
Our study highlights how adolescents experienced higher rates of rejection and associated hospitalizations. This pattern was statistically significant only during the first-year posttransplant and in the Kaplan-Meier analysis over the entire follow-up period. Although a similar pattern was observed during the second-year posttransplant (Figure 2a) and after exclusion of patients with previous kidney transplants, it did not reach statistical significance (P = 0.068, Figure 4). These data suggest that age and its associated psychosocial characteristics such as nonadherence may be a significant determinant of the rejection risk in pKTRs at least during the first 2 years posttransplant, but that the higher rejection risk in adolescents is also due to the higher percentage (15.7%) of patients with previous kidney transplants which may have led to sensitization to HLA antigens. Since patient enrollment began in 1999, we observed no significant era effects on rejection, infection, and hospitalization rates, which is consistent with recent findings of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) showing minimal impact of era effects on outcomes between 1997 and 2007.4
Notably, the lower percentage of patients receiving induction therapy in our cohort may be due to country-specific protocols. For example, German centers, which contributed a large number of patients, do not include induction therapy in their standard immunosuppressive protocol for patients with low or standard immunologic risk; because 2 large prospective randomized controlled trials showed that induction therapy with basiliximab in patients with low to standard immunological risk on maintenance therapy with tacrolimus in conjunction with azathioprine, or cyclosporine in conjunction with mycophenolate mofetil did not lead to a statistically significant reduction in the incidence of acute rejection episodes.10,11 This practice variation likely accounts for the lower overall percentage compared to higher rates in other regions.
Our data show that younger patients have lower tacrolimus trough levels and higher TacIPV. This contrasts with other studies suggesting higher TacIPV in adolescents due to lower adherence to the immunosuppressive medication,12, 13, 14 but is consistent with our previous single-center study15 and previous work by Prytula et al.16 showing higher TacIPV in younger patients. Although our study does not allow for causal inferences, potential factors leading to higher tacrolimus variability could be differences in metabolism,17 frequency of dose adjustments due to intercurrent infections,6,18 especially diarrhea, which is more common in infants and young children and may lead to a paradoxical increase in tacrolimus exposure, infection-related inflammation,19 and differences in gut microbiota composition between young children and adolescents.20,21 In contrast, the higher rejection rates observed in adolescents despite lower TacIPV may reflect the unique immunologic and psychosocial challenges of this age group, highlighting the need for further research to explore these complex interactions. In addition, our study is the first to report on age-related differences of the tacrolimus C/D ratio in pKTRs corrected for BSA and adjusted BSA to 1.73 m2, allowing for comparability of different C/D ratio algorithms and comparability with adult C/D ratio values. The lower C/D ratio in younger patients is consistent with the concept of a higher tacrolimus clearance in this age group, which may be due to a higher liver weight-to-body weight ratio in younger patients22 and/or age-related differences in CYP3A4 and/or CYP3A5 enzyme activity.22 This is consistent with the lower BSA-corrected and BSA-adjusted for 1.73 m2-corrected C/D ratios in our cohort compared to adult cohorts, although comparability is limited due to differences in the time period of C/D ratio quantification and concomitant immunosuppression.23,24 In contrast to a study by Naesens et al.25 on body weight-corrected C/D ratios in pKTRs, our values appear to be lower (120 ± 93.9 at month 6 posttransplant vs. 52.9 ± 42.8 during months 6–12 posttransplant).25 This difference could be due to the fact that Naesens et al.25 only included patients on a glucocorticoid-free immunosuppressive regimen, whereas 82.2% of our patients were still on glucocorticoids during the first year after transplantation. Glucocorticoids are known to increase tacrolimus clearance and thereby decrease the C/D ratio.26
The retrospective design of our study limits our ability to detect associations between tacrolimus exposure parameters and clinical outcomes, primarily due to insufficient detailed data on tacrolimus levels and corresponding doses, because this study was not designed to detect such a relationship. This highlights the importance of accurate and comprehensive monitoring of tacrolimus levels and dosages in future research to allow for meaningful analysis. Nevertheless, data in both pediatric and adult kidney transplant recipients suggest that higher TacIPV is associated with adverse graft outcomes,15,27,28 whereas the association of a lower C/D ratio with outcome has only been studied in adult kidney transplant recipients.23,24,29 However, it has to be noted that although C/D ratio and TacIPV have both shown promise as predictors of poor clinical outcomes, their integration into clinical practice is limited by the lack of prospective validation and consensus on the ideal quantification method and timing of quantification. Further research and validation in larger, multicenter adult and pediatric cohorts are needed to establish its reliability and utility in the adult and pediatric patient population.
Some additional limitations must be noted. The retrospective nature of this registry study prevents causal inferences between age, tacrolimus exposure, and transplant outcomes. In addition, the granularity of our data limited our ability to detail infections such as Epstein-Barr virus, BK polyomavirus, and CMV, because they were only recorded in binary form. However, other studies within the CERTAIN registry have addressed the issue of posttransplant opportunistic infections in more detail using study-specific electronic case report forms, which can be used as a more comprehensive reference for this research question.30, 31, 32, 33 Similarly, although occurring in only a small proportion of patients, further research is needed to elucidate the underlying causes of graft failure and death, which were not addressed in this study. In addition, this cohort may not be fully representative of all patients who underwent kidney transplantation during the study period, because only those who consented to participate in the registry and met the study inclusion criteria were included. The retrospective design of the study makes it unclear how many patients were potentially eligible but not enrolled in the registry and why they chose not to participate. Moreover, exclusion of patients with incomplete or missing tacrolimus monitoring data, who may have been on non-tacrolimus regimens or had incomplete data entries, could lead to underestimation of certain outcomes, such as new-onset diabetes mellitus, particularly among those who switched from tacrolimus to alternative regimens due to adverse events. In addition, larger centers with higher patient volumes contributed more cases, which may bias the results toward the practices and outcomes of these centers (Supplementary Table S1). These factors may affect the generalizability of the results, because the cohort included may differ from the broader transplant population. Despite these challenges, our findings support the exploration of age-specific treatment strategies to optimize outcomes and tailor immunosuppression to the unique needs of different pediatric age groups.
In conclusion, this largest study to-date of a tacrolimus-based immunosuppressive regimen in European pKTRs shows important age-related differences in rejection rates, infection episodes, and tacrolimus exposure and clearance. This data suggests that immunosuppressive therapy in pKTRs should be tailored and personalized according to the age-specific risk profiles of this heterogeneous patient population. In addition, the data represent the current standard of care for patients treated with a tacrolimus-based immunosuppression in Europe and may serve as a benchmark for future studies with novel immunosuppressive drugs, as well as a tool for communicating current therapeutic standards to patients and caregivers.
Disclosure
BT has received research grants from Novartis, Astellas, and Chiesi; and consulting fees from Bristol-Myers Squibb, CSL Behring Biotherapies for Life, Vifor, and Chiesi. All the other authors declared no competing interests.
Acknowledgments
We acknowledge funding from the Medical Faculty of Heidelberg, Germany to Maral Baghai Arassi. The authors gratefully acknowledge the funding of the CERTAIN registry by a grant from the Dietmar Hopp Foundation, the European Society for Pediatric Nephrology (ESPN), the German Society for Pediatric Nephrology (GPN), and by grants from the pharmaceutical companies Astellas, Japan, Novartis, Switzerland, and Takeda, Japan. The authors would like to thank Annette Mechler for her continuous and excellent contribution to the CERTAIN registry as well as all contributing CERTAIN study centers with special thanks to Demet Alaygut, Gema Ariceta, Martin Bald, Ortraud Beringer, Laszlo Berta, Antonia Bouts, Salim Caliskan, Henry Fehrenbach, Matthias Hansen, Michael Kaabak, Nele Kanzelmeyer, Günter Klaus, Stephen Marks, Dominik Müller, Hulya Nalcacioglu, Martin Pohl, Michael Pohl, Nikoleta Printza, Alexandr Rumyantsev, Anne-Laure Sellier-Leclerc, Thomas Simon, Oguz Söylemezoglu, Hagen Staude, and Marcus Weitz.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Table S1. Number of included patients per center.
Table S2. Infection subtypes in the first 2 years posttransplant.
Table S3. Outcome parameters in the first 2 years posttransplant for patients aged <2 years (n = 18).
Table S4. Tacrolimus exposure parameters in patients <2 years (n = 18).
STROBE Checklist.
Supplementary Material
Table S1. Number of included patients per center.
Table S2. Infection subtypes in the first 2 years posttransplant.
Table S3. Outcome parameters in the first 2 years posttransplant for patients aged <2 years (n = 18).
Table S4. Tacrolimus exposure parameters in patients <2 years (n = 18).
STROBE Checklist.
References
- 1.Astley M.E., Boenink R., Abd ElHafeez S., et al. The ERA Registry Annual Report 2020: a summary. Clin Kidney J. 2023;16:1330–1354. doi: 10.1093/ckj/sfad087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boenink R., Astley M.E., Huijben J.A., et al. The ERA Registry Annual Report 2019: summary and age comparisons. Clin Kidney J. 2022;15:452–472. doi: 10.1093/ckj/sfab273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Q., Fang X., Man X., et al. Pediatric kidney transplantation in China: an analysis from the IPNA Global Kidney Replacement Therapy Registry. Pediatr Nephrol. 2021;36:685–692. doi: 10.1007/s00467-020-04745-7. [DOI] [PubMed] [Google Scholar]
- 4.Chua A., Cramer C., Moudgil A., et al. Kidney transplant practice patterns and outcome benchmarks over 30 years: the 2018 report of the NAPRTCS. Pediatr Transplant. 2019;23 doi: 10.1111/petr.13597. [DOI] [PubMed] [Google Scholar]
- 5.Hardin A.P., Hackell J.M. Committee on practice and ambulatory medicine. Age limit of pediatrics. Pediatrics. 2017;140 doi: 10.1542/peds.2017-2151. [DOI] [PubMed] [Google Scholar]
- 6.Borra L.C.P., Roodnat J.I., Kal J.A., Mathot R.A.A., Weimar W., van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant. 2010;25:2757–2763. doi: 10.1093/ndt/gfq096. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers D.R.J. Intrapatient variability of tacrolimus exposure in solid organ transplantation: a novel marker for clinical outcome. Clin Pharmacol Ther. 2020;107:347–358. doi: 10.1002/cpt.1618. [DOI] [PubMed] [Google Scholar]
- 8.Whalen H.R., Glen J.A., Harkins V., et al. High interpatient tacrolimus variability is associated with worse outcomes in renal transplantation using a low-dose tacrolimus immunosuppressive regime. Transplantation. 2017;101:430–436. doi: 10.1097/TP.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 9.Sablik K.A., Clahsen-van Groningen M.C., Hesselink D.A., van Gelder T., Betjes M.G.H. Tacrolimus intra-patient variability is not associated with chronic active antibody mediated rejection. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offner G., Toenshoff B., Höcker B., et al. Efficacy and safety of basiliximab in pediatric renal transplant patients receiving cyclosporine, mycophenolate mofetil, and steroids. Transplantation. 2008;86:1241–1248. doi: 10.1097/TP.0b013e318188af15. [DOI] [PubMed] [Google Scholar]
- 11.Grenda R., Watson A., Vondrak K., et al. A prospective, randomized, multicenter trial of tacrolimus-based therapy with or without basiliximab in pediatric renal transplantation. Am J Transplant. 2006;6:1666–1672. doi: 10.1111/j.1600-6143.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 12.Hsiau M., Fernandez H.E., Gjertson D., Ettenger R.B., Tsai E.W. Monitoring nonadherence and acute rejection with variation in blood immunosuppressant levels in pediatric renal transplantation. Transplantation. 2011;92:918–922. doi: 10.1097/TP.0b013e31822dc34f. [DOI] [PubMed] [Google Scholar]
- 13.Rianthavorn P., Ettenger R.B. Medication non-adherence in the adolescent renal transplant recipient: a clinician’s viewpoint. Pediatr Transplant. 2005;9:398–407. doi: 10.1111/j.1399-3046.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 14.Feddersen N., Pape L., Beneke J., Brand K., Prüfe J. Adherence in pediatric renal recipients and its effect on graft outcome, a single-center, retrospective study. Pediatr Transplant. 2021;25 doi: 10.1111/petr.13922. [DOI] [PubMed] [Google Scholar]
- 15.Baghai Arassi M., Gauche L., Schmidt J., et al. Association of intraindividual tacrolimus variability with de novo donor-specific HLA antibody development and allograft rejection in pediatric kidney transplant recipients with low immunological risk. Pediatr Nephrol. 2022;37:2503–2514. doi: 10.1007/s00467-022-05426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prytula A.A., Bouts A.H., Mathot R.A.A., et al. Intra-patient variability in tacrolimus trough concentrations and renal function decline in pediatric renal transplant recipients. Pediatr Transplant. 2012;16:613–618. doi: 10.1111/j.1399-3046.2012.01727.x. [DOI] [PubMed] [Google Scholar]
- 17.Prytuła A., van Gelder T. Clinical aspects of tacrolimus use in paediatric renal transplant recipients. Pediatr Nephrol. 2019;34:31–43. doi: 10.1007/s00467-018-3892-8. [DOI] [PubMed] [Google Scholar]
- 18.Rozen-Zvi B., Schneider S., Lichtenberg S., et al. Association of the combination of time-weighted variability of tacrolimus blood level and exposure to low drug levels with graft survival after kidney transplantation. Nephrol Dial Transplant. 2017;32:gfw394. doi: 10.1093/ndt/gfw394. [DOI] [PubMed] [Google Scholar]
- 19.Bonneville E., Gautier-Veyret E., Ihl C., et al. Unexpected overdose blood concentration of tacrolimus: keep in mind the role of inflammation. Br J Clin Pharmacol. 2020;86:1888–1891. doi: 10.1111/bcp.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann M., Zimmermann-Kogadeeva M., Wegmann R., Goodman A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570:462–467. doi: 10.1038/s41586-019-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baghai Arassi M., Zeller G., Karcher N., Zimmermann M., Toenshoff B. The gut microbiome in solid organ transplantation. Pediatr Transplant. 2020;24 doi: 10.1111/petr.13866. [DOI] [PubMed] [Google Scholar]
- 22.Matalová P., Urbánek K., Anzenbacher P. Specific features of pharmacokinetics in children. Drug Metab Rev. 2016;48:70–79. doi: 10.3109/03602532.2015.1135941. [DOI] [PubMed] [Google Scholar]
- 23.Jouve T., Fonrose X., Noble J., et al. The TOMATO study (tacrolimus metabolization in kidney transplantation): impact of the concentration-dose ratio on death-censored graft survival. Transplantation. 2020;104:1263–1271. doi: 10.1097/TP.0000000000002920. [DOI] [PubMed] [Google Scholar]
- 24.Thölking G., Siats L., Fortmann C., et al. Tacrolimus concentration/dose ratio is associated with renal function after liver transplantation. Ann Transplant. 2016;21:167–179. doi: 10.12659/AOT.895898. [DOI] [PubMed] [Google Scholar]
- 25.Naesens M., Salvatierra O., Li L., Kambham N., Concepcion W., Sarwal M. Maturation of dose-corrected tacrolimus predose trough levels in pediatric kidney allograft recipients. Transplantation. 2008;85:1139–1145. doi: 10.1097/TP.0b013e31816b431a. [DOI] [PubMed] [Google Scholar]
- 26.Anglicheau D., Flamant M., Schlageter M.H., et al. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18:2409–2414. doi: 10.1093/ndt/gfg381. [DOI] [PubMed] [Google Scholar]
- 27.Shuker N., van Gelder T., Hesselink D.A. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando) 2015;29:78–84. doi: 10.1016/j.trre.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Piburn K.H., Sigurjonsdottir V.K., Indridason O.S., et al. Patterns in tacrolimus variability and association with de novo donor-specific antibody formation in pediatric kidney transplant recipients. Clin J Am Soc Nephrol. 2022;17:1194–1203. doi: 10.2215/CJN.16421221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thölking G., Fortmann C., Koch R., et al. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS One Bueno V. 2014;9 doi: 10.1371/journal.pone.0111128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fichtner A., Gauché L., Süsal C., et al. Incidence, risk factors, management strategies and outcomes of antibody-mediated rejection in pediatric kidney transplant recipients-a multicenter analysis of the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN). Research Square (Research Square) https://dooi,org/10.21203/rs.3.rs-4016549/v1 [DOI] [PubMed]
- 31.Höcker B., Schneble L., Murer L., et al. Epidemiology of and risk factors for BK polyomavirus replication and nephropathy in pediatric renal transplant recipients: an international CERTAIN registry study. Transplantation. 2019;103(6):1224–1233. doi: 10.1097/TP.0000000000002414. [DOI] [PubMed] [Google Scholar]
- 32.Höcker B., Zencke S., Pape L., et al. Impact of everolimus and low-dose cyclosporin on cytomegalovirus replication and disease in pediatric renal transplantation. Am J Transplant. 2016;16:921–929. doi: 10.1111/ajt.13649. [DOI] [PubMed] [Google Scholar]
- 33.Höcker B., Zencke S., Krupka K., et al. Cytomegalovirus infection in pediatric renal transplantation and the impact of chemoprophylaxis with (Val-)Ganciclovir. Transplantation. 2016;100:862–870. doi: 10.1097/TP.0000000000000888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.