Introduction
Thrombotic microangiopathy (TMA) is a severe complication of kidney transplantation. It may present as a recurrence of atypical hemolytic uremic syndrome (aHUS) or may occur de novo.1
Kidney graft outcome in patients with recurrent aHUS is poor and strongly dependent on the early initiation of anti-C5 therapy.1 However, diagnosing aHUS recurrence, as well as de novo TMA, is challenging because patients may present without hematological signs.1 A biopsy may not be feasible, particularly in patients with thrombocytopenia.
Here, we evaluated whether an ex vivo assay of serum-induced terminal complement complex (C5b-9) formation on human microvascular endothelial cells (HMEC-1), which efficiently detects complement dysregulation in nontransplanted patients with aHUS,2 could also help diagnose posttransplant recurrent aHUS. We also evaluated whether de novo posttransplant TMA is associated with endothelial complement activation, which remains a widely discussed issue,3,4 and whether the C5b-9 formation assay could support diagnosis. Due to the high risk of recurrence, the incidence of TMA in kidney grafts exceeds 36 times in patients with a pretransplant history of aHUS, compared to those with other causes of end-stage renal disease. This underscores the importance of accurately diagnosing native kidney disease.1 Unfortunately, 20% to 30% of patients on transplant waiting lists have no diagnosis.5 To address this additional issue, we investigated whether the C5b-9 assay could help to identify aHUS cases among patients with end-stage renal disease.
Results
This retrospective study included all consecutive patients we analyzed with the serum-induced C5b-9 formation assay between 2014 and 2023 and who fulfilled the inclusion criteria (Supplementary Methods). The patients’ main characteristics are presented in Table 1 (individual values are detailed in Supplementary Tables S1–S6).
Table 1.
Patient clinical and biochemical features
| Variable | Kidney transplant: aHUS |
Kidney transplant: no aHUS |
Chronic dialysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Recurrence (n = 12) | No recurrence (n = 11) | P | de novo TMA (n = 15) | no TMA (n = 7) | P | aHUS (n = 28) | no aHUS (n = 11) | P | |
| Male, % | 66.67% (n = 12) | 27% (n = 11) | ns | 73% (n = 15) | 86% (n = 7) | ns | 32% (n = 28) | 82% (n = 11) | f |
| Age, yra | 48 (26–72) (n = 12) | 43 (13–75) (n = 11) | ns | 60 (46–81) (n = 15) | 56 (36–81) (n = 7) | ns | 45 (2–74) (n = 28) | 46 (26–62) (n = 11) | ns |
| Native kidney disease | aHUS (n = 12) | aHUS (n = 11) | IgAN, n = 3; ADPKD, n = 2; DN, n = 2; Goodpasture, n = 1; pyelonephritis, n = 1; HN, n = 1; ON, n = 1; TIN, n = 1; lupus N, n = 1; PAH/CRI, n = 1 |
IgA N, n = 3; ADPKD, n = 1; FSGS, n = 1; proteinuric N, n = 1; hypertensive N, n = 1 |
aHUS (n = 28) | ADPKD, n = 4; CRI n = 2; proteinuric N, n = 1; Fabry, n = 1; MPGN, n = 1; Horse kidney, n = 1; nephroangiosclerosis, n = 1 |
|||
| Days post txa | 128 (1–9189) (n = 12)b | 1585 (7–4383) (n = 11) | ns | 5 (1–6595) (n = 15) | 730 (6–2190) (n = 7) | f | |||
| Deceased donor % | 100% (n = 10) | 100% (n = 10) | ns | 100% (n = 14) | 86% (n = 7) | ns | |||
| IS | |||||||||
| CsA/Tac | 83.33% (n = 12) | 100% (n = 9) | ns | 92.8% (n = 14) | 100% (n = 7) | ns | |||
| MMF/AZT | 66.66% (n = 12) | 100% (n = 9) | ns | 92.8% (n = 14) | 100% (n = 7) | ns | |||
| steroids | 91.67% (n = 12) | 77.8% (n = 9) | ns | 100% (n = 14) | 14.3% (n = 7) | f | |||
| sirolimus | 8.33% (n = 12) | 0% (n = 9) | ns | 0% (n = 14) | 0% (n = 7) | ns | |||
| Serum creatinine (0.55–1.25 mg/dl)c | 3.4 (1.5–9.56) (n = 12) |
1.52 (0.76–3.85) (n = 10) |
f | 5.6 (1.4–12.4) (n = 15) |
1.33 (1.03–2.29) (n = 7) |
f | 8.4 (3.2–14.9) (n = 23) |
10.36 (7.24–17.47) (n = 11) |
f |
| Hemoglobin (14–18 g/dl) | 9.4 (8.5–13.5) (n = 11) |
12.4 (6.9–15) (n = 10) |
f | 9.15 (7.5–13.6) (n = 14) |
13.4 (10.7–14.9) (n = 7) |
f | 11.5 (8–14.6) (n = 24) |
11.9 (9–13.4) (n = 11) |
ns |
| Schistocytes (% of + patients) | 100% (n = 4) | 0% (n = 1) | 100% (n = 7) | n.a. | n.a. | n.a. | |||
| Haptoglobin (30–200 mg/dl) | 40 (<1–95) (n = 11) |
n.a. | 29 (<1–97) (n = 14) |
n.a. | 91 (37–232) (n = 3) |
n.a. | |||
| LDH (266–500 IU/l) | 567 (165–1977) (n = 12) |
327 (115–372) (n = 5) |
f | 628 (317– 1790) (n = 13) |
n.a. | 397 (136–653) (n = 19) |
336.5 (257–440) (n = 8) |
ns | |
| Platelets (150–400 × 103/μl) | 107.5 (37–275) (n = 12) |
218.5 (150–440) (n = 10) |
f | 90 (36–178) (n = 15) |
200 (139–343) (n = 7) |
f | 188.5 (105–395) (n = 24) |
256 (166–359) (n = 11) |
ns |
| Serum C3 (70–152 mg/dl) | 74.5 (46–109) (n = 8) |
105 (21–125) (n = 7) |
ns | 81 (59–104) (n = 7) |
n.a. | 67 (36.4–125) (n = 18) |
103.5 (64–149) (n = 8) |
f | |
| Rare var carrier % | 36.36% (n = 11) | 36.36% (n = 11) | ns | 20% (n = 15) | n.a. | 59% (n = 27) | 0% (n = 4) | f | |
| Risk alleles (na) | 3 (0–4) (n = 10) | 1 (0–3) (n = 10) | f | 1 (0–2) (n = 15) | n.a. | 2 (0–4) (n = 24) | n.a. | ||
| Plasma sC5b-9 (140–280 ng/ml) | 242 (96–1043) (n = 5) |
485.5 (219–2092) (n = 4) |
ns | 358 (235–974) (n = 7) |
n.a. | 294 (91–1436) (n = 13) |
175 (n = 1) | ||
| C5b-9 on unstimulated HMEC-1 (50%–150%) | 184.5 (84–298) (n = 12) |
131 (78–215) (n = 11) |
f | 197 (171–378) (n = 15) |
101 (95–217) (n = 7) |
f | 137 (78–249) (n = 21) |
98 (65–115) (n = 11) |
f |
| C5b-9 on ADP-activated HMEC-1 (60%–149%) | 192 (86–365) (n = 12) |
173 (135–264) (n = 11) |
ns | 244 (201–384) (n = 15) |
109 (97–221) (n = 7) |
f | 221 (154–1219) (n = 28) |
121 (78–159) (n = 11) |
f |
| Anti-C5 response, %d | 100% (n = 3)e | 90% (n = 10)e | |||||||
Risk alleles, CFH: H3, MCP: ggaac.
Continuous variables are given as median (range). Three aHUS-recurrence patients were treated with eculizumab after serum collection and all experienced remission (complete, n = 2; hematological, n = 1). Two aHUS-no recurrence patients had received eculizumab either for prophylaxis or to treat a recurrence and had safely discontinued the drug >8 weeks before serum collection.
ADPKD, autosomal dominant polycystic kidney disease; AZT, azathioprine; CRI, chronic renal insufficiency; CsA, cyclosporin A; D, diabetic; FSGS, focal segmental glomerulosclerosis; H, hypertensive; IS, immunosuppression; MMF, mycophenolate; MPGN, membrano-proliferative-glomerulonephritis; n.a., not available; N, nephropathy; ns, not significant; O,obstructive; PAH/CRI, pulmonary arterial hypertension/chronic renal insufficiency; ST, steroids; Tac, tacrolimus; TI, tubulointerstitial.
At the time of sample collection.
Number of patients with available data are in brackets.
Normal ranges in brackets.
Patients have not been taking C5 inhibitory drugs for at least 8 weeks prior to blood drawing.
number of patients treated.
P < 0.05.
C5b-9 Assay in Posttransplant aHUS Recurrence
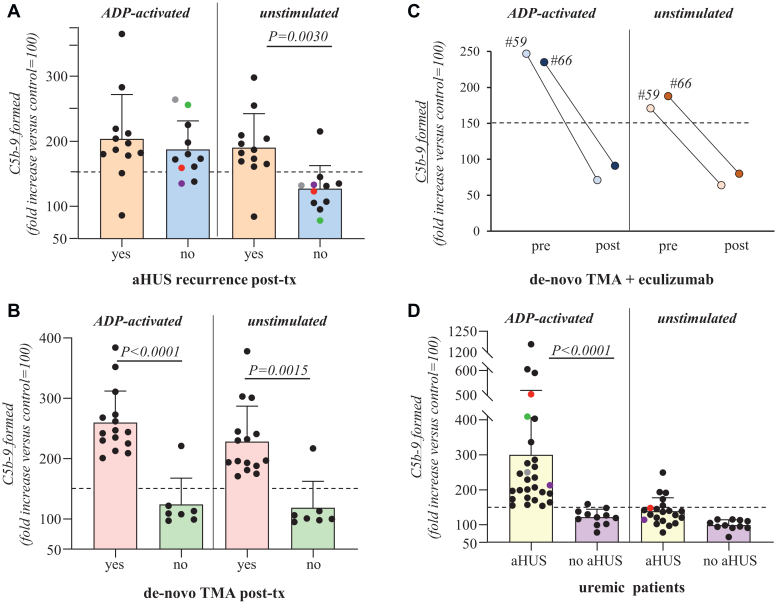
Serum from patients with aHUS after kidney transplant induced higher than normal range formation of C5b-9 on ADP-activated HMEC-1 (Supplementary Methods). Median values did not differ between patients with recurrence (aHUS-r, n = 12) and patients with stable graft function or with other causes of graft deterioration (aHUS-nr, n = 11 Figure 1a, Table 1, and Supplementary Tables S7–S8).
Figure 1.
Ex-vivo serum-induced C5b-9 formation on endothelial cells. Human microvascular endothelial cells (HMEC-1), either unstimulated or preactivated for 10 minutes with adenosine diphosphate (ADP, 10 μmol/l) were incubated for 2 hours with serum (50% in test medium; details in Supplementary Material) from: panel a, aHUS kidney transplant (tx) patients with clinical and/or histologic signs of recurrence (yes: n = 13) and aHUS kidney transplant patients with stable graft function or with other causes of graft deterioration (no: n = 11), the colored circles indicate the 4 patients who were also studied while on chronic dialysis; panel (b), kidney transplant (tx) patients presenting with de novo TMA (n = 15) and renal transplant patients with stable graft function (n = 7); panel (c), kidney transplant patients with de novo TMA studied both before (pre) and after (post) initiation of eculizumab treatment (n = 2); panel (d), chronically dialyzed patients with a history of complement-related aHUS in the native kidneys (n = 28 on ADP-activated HMEC-1; n = 21 on unstimulated HMEC-1, 7 patients were not analyzed on unstimulated cells due to limited serum volume) and chronically dialyzed patients with other causes of end-stage renal disease (n = 11 both on ADP-activated and unstimulated HMEC-1), the colored circles indicate the 4 patients who were also studied after transplantation. Of note, 5 chronically dialyzed patients with aHUS showed elevated C5b-9 formation also on unstimulated HMEC-1; of these, 1 patient had multiple relapses, 1 patient had lost 2 grafts due to recurrences, and 1 patient had clinical signs of chronic aHUS. At the end of incubation, cells were washed, fixed, and stained with rabbit antihuman complement C5b-9 complex antibody, followed by FITC-conjugated secondary antibody. An Apotome Axio Imager Z2 (Zeiss) laser microscope was used to view the fluorescent staining on the endothelial cell surface, and the HMEC-1 area covered by C5b-9 staining was calculated using automatic edge detection (Image J software) in 15 high-power fields. For each sample, the highest and the lowest values were discarded and the mean of the other 13 fields was calculated, and values were expressed as the percentage of C5b-9 deposits induced by a pool of sera from 10 healthy controls run in parallel (reference 100%). Horizontal dashed lines are upper limit of the normal range (mean ± 2 SDs) of 35 (on ADP-activated HMEC-1) and 22 (on unstimulated HMEC-1) different healthy controls. FITC, fluorescein isothiocyanate; TMA, thrombotic microangiopathy.
To evaluate whether the assay might indicate an ongoing aHUS recurrence in the graft, we analyzed the results with serum added to unstimulated HMEC-1. Excessive C5b-9 formation was observed in all patients with aHUS-r, with the exception of 1, whose serum was collected after plasma-exchange (Figure 1a, Table 1, and Supplementary Table S7).2 Conversely, only 1 out of 11 patients with aHUS-nr exhibited abnormal serum-induced C5b-9 formation on unstimulated HMEC-1 (Figure 1a, Table 1, and Supplementary Table S8). The positive and negative predictive values of the C5b-9 assay were 0.916 and 0.909, respectively (Supplementary Results).
Serum C3 and plasma sC5b-9 levels did not differ between aHUS-r and aHUS-nr (Table 1 and Supplementary Tables S7–S8).
Rare complement gene variants were identified in 4 of 11 patients with aHUS-r and in 4 of 11 with aHUS-nr and the median of aHUS risk alleles was 3 in aHUS-r and 1 in aHUS-nr (Table 1 and Supplementary Tables S7–S8).S1-S9
C5b-9 Assay in De Novo Posttransplant TMA
Elevated C5b-9 formation on both ADP-activated and unstimulated HMEC-1 was observed in all patients with active de novo TMA (Figure 1b, Table 1, and Supplementary Table S9). Plasma sC5b-9 levels were elevated in only 3 of 7 patients (Supplementary Table S9). Rare complement gene variants were identified in 3 of 15 patients. Nine of the 10 patients who received eculizumab achieved remission (complete, n = 5; hematological, n = 4; Supplementary Table S3). Patients (n = 2) showed full normalization of the C5b-9 assay after eculizumab initiation (Figure 1c). All but 1 stable transplant patient exhibited normal C5b-9 formation on both ADP-activated and unstimulated HMEC-1 (Figure 1b, Table 1, and Supplementary Table S5). The positive and negative predictive values of the C5b-9 assay were 0.937 and 1, respectively (Supplementary Results).
C5b-9 Assay in Uremic Patients
All dialyzed patients with aHUS (n = 28) versus only 1 of 11 dialyzed patients without aHUS exhibited excessive serum-induced C5b-9 formation on ADP-activated HMEC-1 (Figure 1d; Table 1; and Supplementary Tables S4, S6, and S10). Five patients with aHUS also showed elevated C5b-9 formation on unstimulated HMEC-1 (Figure 1d, Table 1, and Supplementary Table S10).
A rare gene variant was identified in 15 out of 27 patients with uremic aHUS. Abnormal serum C3 and plasma sC5b-9 levels were found in 60% of dialyzed patients with aHUS (Supplementary Table S10).
Discussion
We show that the assay of serum-induced C5b-9 formation on endothelium may help as follows: (i) diagnose aHUS posttransplant recurrence; (ii) document endothelial complement dysregulation during de novo posttransplant TMA; and (iii) identify, among dialyzed patients, who has a previous history of aHUS.
Importantly, all but 1 patient with posttransplant aHUS recurrence showed exuberant serum-induced C5b-9 formation on HMEC-1, including those with a normal circulating complement profile. This finding demonstrates that there is massive ongoing endothelial-restricted complement activation, just as we have previously observed in nontransplanted patients with aHUS.6
Duineveld et al.7 questioned the use of the C5b-9 assay in diagnosing and managing aHUS kidney transplant patients; however, their position is unconvincing, because none of the patients had aHUS recurrence. Instead, Duineveld et al.7 analyzed stable transplant patients with either a history of aHUS in the native kidneys or other primary kidney diseases, making an inappropriate comparison. The authors called for studies that evaluate the assay in aHUS transplant recipients with and without recurrence. This is in fact the design we have used, and the results, which show that elevated C5b-9 formation on unstimulated endothelium was restricted to patients with an aHUS recurrence, support further development of the assay to help in managing patients with aHUS, after transplantation.
The role of complement in the pathophysiology of de novo TMA after kidney transplantation is a matter of debate.1 The results here, which show elevated serum-induced C5b-9 formation on HMEC-1 in patients with de novo posttransplant TMA, unlike patients with stable graft function, are consistent with those of 2 recent reports that used the same test, which however either included very few patients,8 or lacked transplanted controls.3 Like the latter,3 here we found that eculizumab treatment induced normalization of hemolysis indices and improvements in graft function in most patients, and normalized serum-induced C5b-9 formation.
Finally, we provide evidence that the C5b-9 assay specifically identifies uremic patients with a previous history of aHUS, as confirmed by the fact that all dialyzed patients with aHUS but only 1 with other kidney diseases exhibited elevated serum-induced C5b-9 formation on activated HMEC-1. The fact that in some patients with aHUS on chronic hemodialysis, but in no patient with aHUS on peritoneal dialysis, the test was positive even on unstimulated HMEC-1 could reflect a state of complement activation by hemodialysis itself on a genetic background of complement dysregulation.S10 Whether peritoneal dialysis could represent a better preemptive strategy in patients with aHUS is worth investigating.
All cases of aHUS should be identified before transplant to guide the choice of treatment options and limit the risk of relapse;1 however, approximately one-fifth of patients with end-stage renal disease do not know the cause of the primary kidney disease.5 Genetic screening may be inconclusive because the failure to identify a complement gene variant does not rule out aHUS.9 Our data indicate that pretransplant evaluation of serum-induced C5b-9 formation could be a tool to help in posttransplant management and to decide about anticomplement prophylaxis.
Due to the retrospective design of this study, we could not evaluate whether the test can predict a recurrence. However, this is likely considering our previous data on aHUS recurrence in nontransplant patients.2
Efforts are also ongoing to develop new user-friendly versions of the assay to incorporate the serum-induced C5b-9 formation testing in daily clinical practice.
Disclosure
MN has received honoraria from Alexion Pharmaceuticals and Sobi for giving lectures, and for participating in advisory boards, and she has received research grants from Omeros, Gemini, Novartis and BioCryst Pharmaceuticals. All the other authors declared no competing interests.
Acknowledgments
DS is the recipient of a fellowship from Fondazione Aiuti per la Ricerca sulle Malattie Rare ARMR ONLUS (Bergamo, Italy). We would like to acknowledge the contributions of Silvia Prandini, Sara Gamba, and Miriam Rigoldi in sample and clinical data collection. The authors thank all clinicians from the other centers, and patients for their membership in and support of the International Registry of HUS/TTP (Appendix in Supplementary Material). The authors thank Kerstin Mierke for English language editing. The authors are deeply grateful for the generous support provided in memory of Mr. Rodolfo Luzzana.
Data Availability Statement
The data set is available from the corresponding author upon request.
Author Contributions
Conception and design were by MN, SA, and GR. Experimental work and acquisition and interpretation of experimental data were done by SG, SA, DS, CM, and MG. Clinical data and sample collection were done by EB, CM, ED, GC, and GLM. Writing of the original draft was done by MN, SA. Critical revision of the manuscript was done by GR and AB. All the authors revised and approved the final version of the manuscript.
Footnotes
Supplementary Methods.
Supplementary Results.
Supplementary References.
Appendix. International Registry of Recurrent and Familial HUS-TTP.
Table S1. Characteristics and clinical parameters of patients with aHUS with recurrence after kidney transplant at the time of sample collection.
Table S2. Characteristics and clinical parameters of transplanted patients with aHUS with no sign of recurrence at the time of sample collection.
Table S3. Characteristics and clinical parameters of patients with de novo TMA at the time of sample collection.
Table S4. Characteristics and clinical parameters of patients with aHUS on chronic dialysis at the time of sample collection.
Table S5. Characteristics, clinical, and complement parameters of stable renal transplant patients at the time of sample collection.
Table S6. Characteristics, clinical, and complement parameters of control dialyzed patients at the time of sample collection.
Table S7. Complement parameters and genetic characteristics of patients with aHUS with recurrence after kidney transplant at the time of sample collection.
Table S8. Complement parameters and genetic characteristics of transplanted patients with aHUS with no sign of recurrence at the time of sample collection.
Table S9. Complement parameters and genetic characteristics of patients with de novo TMA at the time of sample collection.
Table S10. Complement parameters and genetic characteristics of patients with aHUS on chronic dialysis at the time of sample collection.
Supplementary Material
Supplementary Methods. Supplementary Results. Supplementary References. Appendix. International Registry of Recurrent and Familial HUS-TTP. Table S1. Characteristics and clinical parameters of patients with aHUS with recurrence after kidney transplant at the time of sample collection. Table S2. Characteristics and clinical parameters of transplanted patients with aHUS with no sign of recurrence at the time of sample collection. Table S3. Characteristics and clinical parameters of patients with de novo TMA at the time of sample collection. Table S4. Characteristics and clinical parameters of patients with aHUS on chronic dialysis at the time of sample collection. Table S5. Characteristics, clinical, and complement parameters of stable renal transplant patients at the time of sample collection. Table S6. Characteristics, clinical, and complement parameters of control dialyzed patients at the time of sample collection. Table S7. Complement parameters and genetic characteristics of patients with aHUS with recurrence after kidney transplant at the time of sample collection. Table S8. Complement parameters and genetic characteristics of transplanted patients with aHUS with no sign of recurrence at the time of sample collection. Table S9. Complement parameters and genetic characteristics of patients with de novo TMA at the time of sample collection. Table S10. Complement parameters and genetic characteristics of patients with aHUS on chronic dialysis at the time of sample collection.
References
- 1.Imanifard Z., Liguori L., Remuzzi G. TMA in kidney transplantation. Transplantation. 2023;107:2329–2340. doi: 10.1097/TP.0000000000004585. [DOI] [PubMed] [Google Scholar]
- 2.Galbusera M., Noris M., Gastoldi S., et al. An ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am J Kidney Dis. 2019;74:56–72. doi: 10.1053/j.ajkd.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Maritati F., Corradetti V., Bini C., et al. “Eculizumab First” in the management of posttransplant thrombotic microangiopathy. Kidney Int Rep. 2024;9:982–993. doi: 10.1016/j.ekir.2024.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouwmeester R.N., Wetzels J.F.M., van de Kar N. Eculizumab in posttransplant TMA: unproven benefit: a response to Maritati et al.: “eculizumab first” in the management of posttransplant thrombotic microangiopathy. Kidney Int Rep. 2024;9:1929. doi: 10.1016/j.ekir.2024.02.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer A., Pippias M., Noordzij M., et al. The European Renal Association-European dialysis and transplant association (ERA-EDTA) registry annual report 2015: a summary. Clin Kidney J. 2018;11:108–122. doi: 10.1093/ckj/sfx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noris M., Galbusera M., Gastoldi S., et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715–1726. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duineveld C., Bouwmeester R.N., van den Heuvel L.P.W.J., van de Kar N.C.A.J., Wetzels J.F.M. Ex vivo Test of Complement dysregulation in atypical hemolytic uremic syndrome kidney transplant patients: a pilot study. Kidney Int Rep. 2023;9:145–151. doi: 10.1016/j.ekir.2023.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin M., Llorens-Cebria C., León-Román J., et al. Ex vivo C5b-9 deposition test to monitor complement activity in clinical and subclinical atypical hemolytic uremic syndrome and in transplantation-associated thrombotic microangiopathy. Kidney Int Rep. 2024;9:2227–2239. doi: 10.1016/j.ekir.2024.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noris M., Bresin E., Mele C., Remuzzi G. Genetic atypical hemolytic-uremic syndrome. GeneReviews - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK1367/ [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Supplementary Results. Supplementary References. Appendix. International Registry of Recurrent and Familial HUS-TTP. Table S1. Characteristics and clinical parameters of patients with aHUS with recurrence after kidney transplant at the time of sample collection. Table S2. Characteristics and clinical parameters of transplanted patients with aHUS with no sign of recurrence at the time of sample collection. Table S3. Characteristics and clinical parameters of patients with de novo TMA at the time of sample collection. Table S4. Characteristics and clinical parameters of patients with aHUS on chronic dialysis at the time of sample collection. Table S5. Characteristics, clinical, and complement parameters of stable renal transplant patients at the time of sample collection. Table S6. Characteristics, clinical, and complement parameters of control dialyzed patients at the time of sample collection. Table S7. Complement parameters and genetic characteristics of patients with aHUS with recurrence after kidney transplant at the time of sample collection. Table S8. Complement parameters and genetic characteristics of transplanted patients with aHUS with no sign of recurrence at the time of sample collection. Table S9. Complement parameters and genetic characteristics of patients with de novo TMA at the time of sample collection. Table S10. Complement parameters and genetic characteristics of patients with aHUS on chronic dialysis at the time of sample collection.
Data Availability Statement
The data set is available from the corresponding author upon request.