Abstract
Introduction
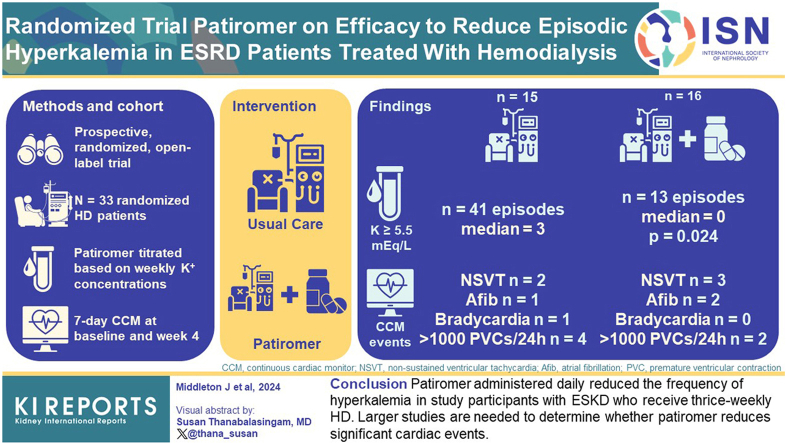
Individuals with end-stage kidney disease (ESKD) maintained on hemodialysis (HD) carry a high risk of cardiac arrhythmias. This risk is heightened by episodic hyperkalemia. The purpose of the study was to investigate whether patiromer administered daily reduced episodes of hyperkalemia in those with ESKD who receive HD, and to explore whether prescription of patiromer reduced the number of significant arrhythmia events.
Methods
This was a prospective, randomized, open-label trial. Eligible patients with ESKD on HD were identified. Participants were randomized 1:1 to patiromer versus usual care. Those randomized to patiromer were administered the medication daily, and the dose was titrated based on serum potassium concentrations at the start of weeks 1, 2, and 3. All participants received 7-day continuous cardiac monitors at baseline and at week 4.
Results
Of the 33 participants who were randomized, 1 withdrew due to adverse symptoms, and 1 withdrew due to pregnancy, leaving 31 in our analytic cohort. The mean age of randomized participants was 56 years, 55% were male, 81% were Black, and 10% were Hispanic/Latino. In week 4, the number of episodes of serum potassium ≥ 5.5 mEq/l was 13 in the patiromer group and 41 in the control group; with median number of episodes of hyperkalemia in the patiromer group significantly lower than that of control group (0 vs. 3, P = 0.024). In week 4 continuous cardiac monitors, 6 participants had > 1000/24 h premature ventricular contractions, 5 had no sustained ventricular tachycardia (VT), 3 had atrial fibrillation, and 1 had bradycardia, with no significant differences between the groups.
Conclusion
Patiromer administered daily reduced the frequency of hyperkalemia in study participants with ESKD who receive thrice-weekly HD. Larger studies are needed to determine whether patiromer reduces significant cardiac events.
Keywords: cardiac arrhythmia, cardiovascular risk, clinical trial, hemodialysis, hyperkalemia
Graphical abstract
Patients with ESKD who are treated with conventional HD schedules often exhibit life-threatening episodes of hyperkalemia. Reduced kidney function increases extracellular potassium concentration due to the combined effects of reduced potassium excretion coupled with disturbed ion distribution in the fluid compartments.1 In most patients on HD, serum potassium concentration is measured infrequently; values that exceed 5 mEq/l are observed in more than a third of patients.2,3 However, the true incidence of hyperkalemia is likely much higher with conventional HD schedules, because transient events may be incompletely captured. Episodic hyperkalemia disturbs the electrophysiology of heart muscle and can promote cardiac arrhythmias.4 It is plausible that episodes of hyperkalemia contribute to the alarmingly high rate of sudden cardiac arrest, which is by far the most common cause of death in the HD population.5 The best method to maintain potassium homeostasis in HD is not clear. Many patients with ESKD are already prescribed a restricted diet; however, adherence to potassium-limited diet can be difficult. Use of a low dialysate potassium concentration, perhaps prompting abrupt excursions of serum potassium concentration, is associated with an increased risk of sudden cardiac death.2,6 Furthermore, patients on HD already carry a high pill burden, and it is unclear if prescribing an additional oral medication will reduce the frequency of episodic hyperkalemia.7
New oral medications are available that have the potential to reduce the occurrences of hyperkalemia in patients on HD. Patiromer is an orally administered, nonabsorbed, sodium-free, cation-exchange polymer that binds potassium and is suitable for chronic administration.8, 9, 10 This potassium binding leads to fecal potassium excretion and lowering of serum potassium concentrations in a hyperkalemic patient. The purpose of the study was to determine whether patiromer administered daily reduced episodes of hyperkalemia in patients with ESKD who received HD, and to explore whether this administration reduced the number of significant arrhythmia events.
Methods
PEARL-HD was a parallel-group randomized open-label controlled trial. The primary end point in this study was to investigate if patiromer administered orally once a day reduced the frequency of episodes of hyperkalemia in patients with ESKD who received thrice-weekly HD. Secondary end points included tolerability of patiromer and the occurrence of clinically significant cardiac arrhythmias. The protocol was approved by the Duke University Institutional Review Board. The PEARL-HD protocol was registered and analyses performed were consistent with the clinicaltrials.gov protocol (NCT03781089).
Potential participants were identified from a population of approximately 489 individuals who had ESKD and were maintained on thrice-weekly HD at 5 outpatient clinics. Eligible participants were aged at least 18 years, required HD for ≥3 months, had at least 2 measured predialysis serum potassium concentrations ≥ 5.5 mEq/l or 1 measured ≥ 6.0 mEq/l over the previous 3 months, had current use of dialysate with potassium concentration of 2.0 mEq/l, received customary dietary instruction, were considered by the treating physicians to be in otherwise stable clinical condition, and (if the patient was of childbearing potential), were willing to avoid pregnancy during the study using an acceptable birth control method. Ineligible participants had not been adherent with prescribed dialysis schedule and prescribed medications, had a life expectancy < 3 months, were currently prescribed oral potassium supplements, had severe gastrointestinal disorders, had serum potassium concentration > 7.0 mEq/l in the previous 3 months, were anticipated to get a kidney transplant within the next 3 months, were living in a facility where medications were not consistently provided, or were pregnant, breastfeeding, or considering pregnancy.
Randomization was performed using Research Electronic Data Capture, a secure, web-based software for data capture.11 Participants were assigned 1:1 to either continue usual care or to receive patiromer. Those randomized to patiromer initiated on 8.4 g/d (1 pack) given once a day with breakfast or lunch, to start at the end of week 0. The participants were instructed to take no other medications for 3 hours before or after patiromer. The patiromer dose was titrated based on serum potassium concentrations at the start of weeks 1, 2, and 3. Patiromer was increased by 8.4 g/d if K ≥ 5.5 mEq/l, decreased by 8.4 g/d if K < 4.5 mEq/l, and discontinued if K < 4 mEq/l. Participants randomized to the control arm also underwent monitoring with laboratory measurements. All patients continued to receive standard-of-care monitoring by the dialysis care team, including education on low potassium diets, with the goal of limiting potassium intake to less than 60 mEq per day. Dialysate composition was not modified during the study. The primary outcome was the total number of episodes of serum potassium ≥ 5.5 mEq/l out of 6 measurements taken during week 3 and 4 of the study. If any of the 6 measurements were missing or invalid, the proportion of episodes among the observed measurements was multiplied by 6 to get a scaled sum.
All participants had a long-term heart monitor applied to wear for 7 days in week 0 and for 7 days in week 4 (Supplementary Figure S1). The Medicomp Telepatch is an extended Holter monitor that attaches with a band-aid, does not require wires, and records cardiac rhythms for up to 7 days.12 Secondary outcomes were prespecified clinically significant cardiac arrhythmias, including sustained VT, ventricular fibrillation, asystolic cardiac arrest, no sustained VT (≥ 3 beats but < 30 s), > 3 second pause), atrial fibrillation (> 30 s), premature ventricular contractions > 500/24h or bradycardia (heart rate < 40 for 5 consecutive beats. The maximum and minimum corrected QT intervals were evaluated.13
Demographic and clinical characteristics were ascertained via review of electronic medical records and weekly interviews with participants. Laboratory values and ultrafiltration volumes were collected in weeks 3 and 4.
Statistical Methods
Sample size was determined based on comparing the mean number of hyperkalemia episodes in each group using a 2-sample t-test. We determined that 20 participants per group would have 86% power to detect a between-group mean difference of 2 episodes per participant, assuming a common SD of 2 episodes and a significance level of 0.05. Participant demographics and clinical characteristics, relevant medical history, previous medications, elements of the dialysis treatment, electrolytes, and ultrafiltration volume were summarized using frequency and percentages for categorical variables and mean and SD for continuous variables. The median number of hyperkalemia episodes were compared between 2 groups using a Mann-Whitney test, and homogeneity of the prevalence of at least 1 episode of hyperkalemia across 2 groups was tested using Fisher exact test. In sensitivity analysis, we regressed number of hyperkalemia episodes and log odds of at least 1 hyperkalemic episode, respectively, on treatment assignment and adjusted for age and sex. Secondary outcomes from the cardiac monitors were presented descriptively by groups. The proportion of patients with at least one pre-specified arrhythmia event was calculated and compared using Fisher’s exact test.
Analyses were performed from October 2023 through December 2023 using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). Analyses were intention-to-treat in that participants were analyzed according to their assigned study arm.
Results
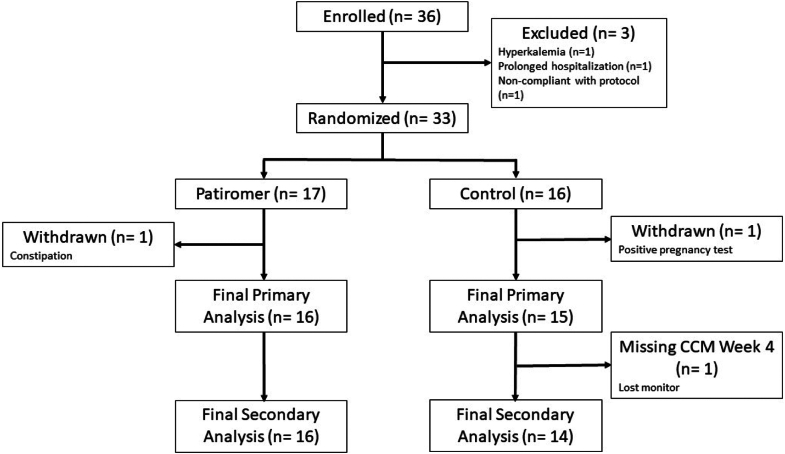
The first PEARL-HD participant was enrolled June 26, 2019, and the final participant completed the protocol March 13, 2023 (study activity was paused due to COVID restrictions in 2020). A total of 36 individuals with ESKD were enrolled, and prior to randomization, 3 were withdrawn (1 due to severe hyperkalemia, 1 due to prolonged hospitalization, and 1 due to failure to obtain a baseline electrocardiogram) (Figure 1). Of the 33 who were randomized, 1 participant in the patiromer group withdrew due to constipation, and 1 participant in the control group withdrew due to a positive pregnancy test. For the primary analysis, 31 participants were available, of whom 16 received patiromer and 15 continued usual care. One participant was missing 2 of the 6 follow-up potassium assessments, 8 participants were missing 1 of them, and the remaining had all 6 assessments. All 16 participants receiving patiromer and 14 participants in usual care were included in the secondary analysis, because 1 participant was missing heart monitor data at week 4 (Figure 1).
Figure 1.
CONSORT diagram for PEARL-HD trial. CCM, continuous cardiac monitor.
The mean age of randomized participants was 56 years, 55% were male, 81% were Black, 10% were Hispanic/Latino, and mean HD vintage was 7 years (Table 1). All participants had baseline normal sinus rhythm and normal corrected QT intervals (Table 2).
Table 1.
Characteristics of PEARL-HD participants
| Characteristics | Patiromer |
Control |
Overall |
|---|---|---|---|
| (n = 16) | (n = 15) | (N = 31) | |
| Age, yr | 54.1 (10.7) | 58.5 (11.1) | 56.2 (11.0) |
| Sex | |||
| Female | 5 (31.3%) | 9 (60.0%) | 14 (45.2%) |
| Male | 11 (68.8%) | 6 (40.0%) | 17 (54.8%) |
| Ethnicity | |||
| Hispanic or Latino | 2 (12.5%) | 1 (6.7%) | 3 (9.7%) |
| Not Hispanic or Latino | 14 (87.5%) | 14 (93.3%) | 28 (90.3%) |
| Race | |||
| White | 4 (25.0%) | 2 (13.3%) | 6 (19.4%) |
| Black/African American | 12 (75.0%) | 13 (86.7%) | 25 (80.6%) |
| Primary underlying renal diagnosis | |||
| Glomerular Disease | 1 (6.3%) | 0 (0%) | 1 (3.2%) |
| Polycystic kidney disease | 3 (18.8%) | 1 (6.7%) | 4 (12.9%) |
| Diabetic nephropathy | 5 (31.3%) | 5 (33.3%) | 10 (32.3%) |
| Hypertensive nephrosclerosis | 2 (12.5%) | 7 (46.7%) | 9 (29.0%) |
| Other | 5 (31.3%) | 2 (13.3%) | 7 (22.6%) |
| Time since dialysis, yr | 6.88 (7.26) | 4.53 (5.74) | 5.74 (6.57) |
| Prior kidney transplant | 2 (12.5%) | 1 (6.7%) | 3 (9.7%) |
| ACEI or ARB | 0 (0%) | 3 (20.0%) | 3 (9.7%) |
| Prior potassium binder medication | 2 (12.5%) | 0 (0%) | 2 (6.5%) |
| Beta blocker | 8 (50.0%) | 8 (53.3%) | 16 (51.6%) |
| Any antiarrhythmic medication | 2 (12.5%) | 1 (6.7%) | 3 (9.7%) |
| Known coronary artery disease | 3 (18.8%) | 5 (33.3%) | 8 (25.8%) |
| Known heart failure | 4 (25.0%) | 1 (6.7%) | 5 (16.1%) |
| History atrial fibrillation or atrial flutter | 5 (31.3%) | 2 (13.3%) | 7 (22.6%) |
| History of ventricular arrhythmia | 0 (0%) | 1 (6.7%) | 1 (3.2%) |
| History of stroke or TIA | 4 (25.0%) | 0 (0%) | 4 (12.9%) |
| Hypertension | 13 (81.3%) | 15 (100%) | 28 (90.3%) |
| Hyperlipidemia | 2 (12.5%) | 4 (26.7%) | 6 (19.4%) |
| Smoking status | |||
| Never | 8 (50.0%) | 11 (73.3%) | 19 (61.3%) |
| Former | 5 (31.3%) | 4 (26.7%) | 9 (29.0%) |
| Current | 3 (18.8%) | 0 (0%) | 3 (9.7%) |
| Vascular access type | |||
| Tunneled catheter | 1 (6.3%) | 1 (6.7%) | 2 (6.5%) |
| AV fistula | 5 (31.3%) | 6 (40.0%) | 11 (35.5%) |
| AV graft | 2 (12.5%) | 2 (13.3%) | 4 (12.9%) |
| Sodium (mEql/l) | 139 (2.66) | 140 (3.89) | 139 (3.28) |
| Potassium (mEq/l) | 5.50 (0.640) | 5.83 (0.483) | 5.66 (0.584) |
| Carbon dioxide (CO2) (mmol/l) | 18.9 (2.89) | 18.1 (1.79) | 18.6 (2.43) |
| Blood urea nitrogen (BUN) (mg/dl) | 55.9 (31.3) | 65.4 (22.6) | 60.3 (27.6) |
| Creatinine (mg/dl) | 10.8 (2.03) | 11.7 (2.15) | 11.2 (2.10) |
| Phosphorus (mg/dl) | 6.34 (2.69) | 6.62 (1.68) | 6.48 (2.20) |
| Albumin (g/dl) | 3.99 (0.446) | 4.15 (0.279) | 4.07 (0.377) |
| Magnesium (mg/dl) | 2.36 (0.367) | 2.45 (0.369) | 2.40 (0.363) |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AV, arteriovenous; TIA transient ischemic attack.
Values are mean (SD) and number (percentage).
Table 2.
Baseline electrocardiogram parameters PEARL-HD participants
| Parameters | Patiromer |
Control |
Overall |
|---|---|---|---|
| (n = 16) | (n = 15) | (N = 31) | |
| Ventricular rate (bpm) | 78.9 (10.9) | 79.0 (12.0) | 78.9 (11.2) |
| PR interval (msec) | 170 (30.8) | 176 (36.5) | 173 (33.2) |
| QRS interval (msec) | 90.9 (10.8) | 98.8 (25.6) | 94.7 (19.5) |
| QT interval (msec) | 407 (40.6) | 402 (37.4) | 405 (38.5) |
| QTc (msec) | 462 (26.4) | 457 (22.0) | 460 (24.1) |
bpm, beats per minute; msec milliseconds; QTc, QT interval corrected for ventricular rate.
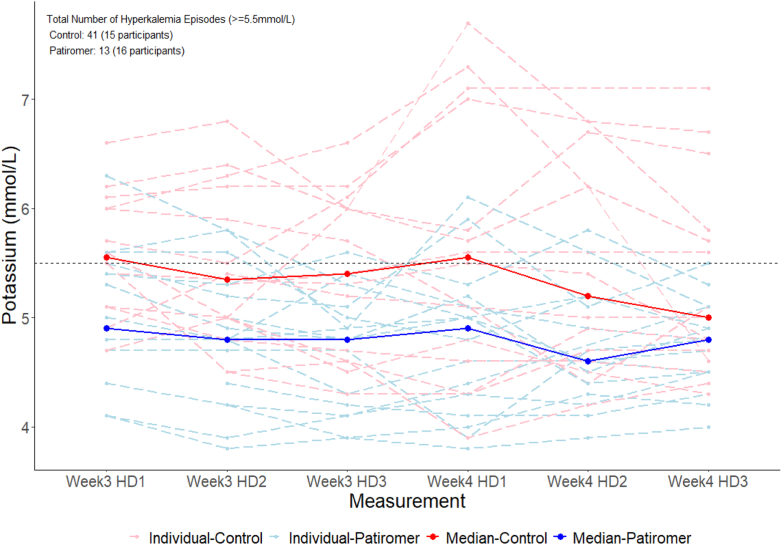
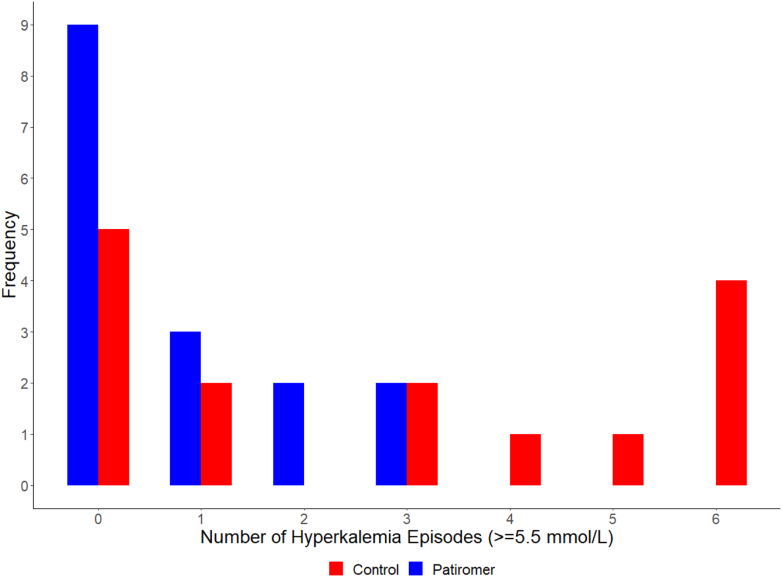
The median dose of the 16 participants receiving patiromer was 8.4 g in week 3; 6 titrated off, 2 remained at the initial dose of 8.4 g, 5 received 16.8 g, and 3 received 25.2 g. In week 4, the number of episodes of serum potassium ≥ 5.5 mEq/l was 13 in the patiromer group and 41 in the control group (Figure 2). Participants in the patiromer group had lower median number of hyperkalemia episodes per participant compared to those in the control group (0 vs. 3, P = 0.024) and were less likely to have at least 1 episode of hyperkalemia (31.2% vs. 66.7%, P = 0.076, Figure 3). These differences between patiromer and the control were consistent in sensitivity analyses adjusted for age and sex (mean difference in number of episodes: −1.98, 95% confidence interval: −3.62 to −0.35; odds ratio of at least hyperkalemia episode: 0.11, 95% confidence interval: 0.01–0.65). Additional electrolyte concentrations and ultrafiltration volumes in study participants, for weeks 3 and 4, are displayed in Supplementary Figure S3.
Figure 2.
Serum potassium concentrations, week 3 and week 4. Temporal trends in serum potassium values; dashed lines, individual values; bold solid lines, median values; red, control group; blue, patiromer group. HD, hemodialysis session.
Figure 3.
Distribution of hyperkalemia episodes. Red, control group; blue, patiromer group.
Arrhythmia events for study participants detected by continuous cardiac monitors were present in both groups at week 4 (Table 3). Six participants had >1000/24 h premature ventricular contractions, 5 had nonsustained VT events, 3 had atrial fibrillation, and 1 had bradycardia events, which were similar to the findings in week 0 (Supplementary Table S1). Because QT interval can be affected by excursions of serum potassium concentration, we also examined corrected QT intervals (QTc) in study participants. Across all participants, the mean QTc Max and QTc Min in week 4 were 0.434 seconds and 0.341 seconds, respectively (Table 3).
Table 3.
Continuous cardiac monitor results, week 4
| Patiromer (n = 16) | Control (n = 14) | Overall (N = 30) | P-value | |
|---|---|---|---|---|
| PVCs (per 24 h) | 0.36 | |||
| > 1000 | 2 (12.5%) | 4 (28.6%) | 6 (20.0%) | |
| <1000, > 500 | 1 (6.3%) | 0 (0%) | 1 (3.3%) | |
| <500 | 13 (81.3%) | 9 (64.3%) | 22 (73.3%) | |
| No sustained VT | 3 (18.8%) | 2 (14.3%) | 5 (16.7%) | >0.99 |
| Atrial fibrillation | 2 (12.5%) | 1 (7.1%) | 3 (10.0%) | >0.99 |
| Bradycardia | 0 (0%) | 1 (7.1%) | 1 (3.3%) | 0.46 |
| Pause | 0 (0%) | 0 (0%) | 0 (0%) | >0.99 |
| Any arrhythmia | 5 (31.3%) | 6 (42.9%) | 11 (36.7%) | 0.71 |
| QTc max (sec) | 0.444 (0.079) | 0.422 (0.024) | 0.434 (0.059) | 0.57 |
| QTc min (sec) | 0.330 (0.052) | 0.353 (0.034) | 0.341 (0.045) | 0.12 |
PVC, premature ventricular contraction; QTc, QT interval corrected for ventricular rate; VT ventricular tachycardia.
Nine adverse events occurred among participants in the patiromer group and 6 occurred among those in the control group. Of these, 4 events were considered probably or possibly related to study procedures and interventions, including paresthesia, constipation, severe heart burn, and skin sensitivity to Holter patch. (See Supplementary Table S2).
Discussion
In this randomized prospective trial, we successfully allocated 33 individuals with ESKD and treated with thrice-weekly HD to receive oral patiromer versus usual care. After dose titration, participants who received patiromer had lower median number of hyperkalemic events compared to those randomized to receive usual care. With prolonged cardiac monitors done at the end of the protocol, the study did not demonstrate a significant difference in occurrences of cardiac arrhythmias or of QT intervals between the 2 study groups.
Patients who are maintained on HD are often exposed to extremes of serum potassium concentration and risks of cardiovascular events. Maintaining serum potassium balance is critical to create normal serum concentrations and a stable cellular transmembrane potential.4 Maintaining a resting membrane potential of about −85 mV permits normal function of excitable tissues, particularly of skeletal muscle and heart.4 Abrupt changes in extracellular potassium concentrations and the resultant shifts in membrane potential can result in muscle paralysis and fatal arrhythmias. In patients who have ESKD, the ability to maintain potassium balance is diminished. Limiting increases in serum potassium concentration is primarily achieved with dietary potassium restriction and dialysis clearance. In a patient maintained on a conventional, 3 times/wk HD regimen, potassium intake is recommended to be limited to about 60 mEq/d (420 mEq/wk). Removal of potassium with HD typically is about 70 to 100 mEq per treatment (210–300 mEq/wk for thrice-weekly schedules).14 In a study of serum potassium values obtained within a large US dialysis organization, approximately 20% of all serum potassium measurements were ≥5.5 mEq/l, and about 12.5% of measurements were ≥6.0, whereas potassium <4.0 accounted for only 9% of all measurements.3 In a study of 2134 patients on HD, a predialysis serum potassium level of 5.1 mEq/l was associated with the lowest risk of peridialytic sudden cardiac arrest, and the risk progressively increased as the potassium deviated from 5.1 mEq/l.6 In a different cohort of 81,013 patients with HD, predialysis serum potassium concentrations between 4.6 and 5.3 mEq/l were associated with the lowest incidence of all-cause mortality, again with progressive increase in risk outside of this range.15 A multinational survey of 55,183 patients on HD in the Dialysis Outcomes and Practice Patterns Study showed that the lowest risk of death was observed among patients with serum potassium levels between 4 and 5.5 mEq/l and a significant increased risk of death and arrhythmia outcomes with serum potassium values ≥5.6 mEq/l.16 The aim of the patiromer titration used in PEARL-HD was to achieve serum potassium values between 4 and 5.5 mEq/l.
Nevertheless, the ideal method to evade episodes of hyperkalemia in patients with ESKD remains unclear. Potential hazards arise by use of low potassium dialysate and reducing serum potassium levels too rapidly during HD treatments.17 A lack of consensus exists on the ideal dialysate potassium concentration. Recent data from the Dialysis Outcomes and Practice Patterns Study reported the prevalence of prescription of potassium dialysate <2 mEq/l from as low as 3% in the US to as high as 62% in Spain.16 Multiple large retrospective studies investigated associations between dialysate potassium levels, serum potassium levels, and risk of sudden death, cardiac events, and all-cause mortality. In general, large cohort studies identified increased risks of sudden cardiac death associated with the use of low potassium dialysate < 2 mEq/l.2,16 The risks associated with low potassium dialysate are principally seen among patients with low to normal predialysis serum potassium. No long-term, prospective, controlled studies have been completed to examine the effect of low potassium dialysate on hard clinical outcomes; however, several short-term trials employed cardiac monitors to measure subclinical arrhythmic events such as ventricular ectopy, premature ventricular complexes, and changes in electrocardiographic conduction parameters.18 Some studies observed higher rates of ventricular ectopy and QTc interval prolongation associated with exposure to potassium dialysates of 0 or 1 mEq/l compared to higher potassium dialysate concentrations.19, 20, 21 In summary, circumstantial evidence suggests hazards of using a low potassium dialysate <2 mEq/l, and the evidence for risk is strongest for patients with serum potassium levels <5 mEq/l. Whether lower potassium dialysate is appropriate or potentially beneficial for patients with higher serum potassium levels is unclear.
Alternatively, hyperkalemia in patients with ESKD can potentially be managed with oral medications which can bind potassium. Sodium polystyrene sulfonate has been available since the 1950s to treat high serum potassium levels, and it is approved for treatment of hyperkalemia by the US Food and Drug Administration.22 However, patients experience adverse gastrointestinal symptoms with sodium polystyrene sulfonate, and it is associated with a risk of colonic necrosis.23 In addition, a significant sodium load can occur with sodium polystyrene sulfonate, and this can be a particular concern in patients with ESKD.24
Fishbane and colleagues reported results with a different oral potassium binder, sodium zirconium cyclosilicate, in a randomized, prospective trial in the HD population.25 In this trial, after 8 weeks of treatment, 41.2% of the patients on HD who received sodium zirconium cyclosilicate on non dialysis days had normalization of serum potassium levels after the long interdialytic interval, compared to only 1% of study participants who received placebo. This study did not include cardiac monitoring; however, there was no observed difference in serious adverse events between the treatment groups. Patiromer has also been used in patients who have ESKD.26,27 One advantage of patiromer is that it has the potential to bind phosphorus in the gastrointestinal tract, possibly limiting patient exposure to hyperphosphatemia27
Several studies used cardiac event monitors to document clinically significant arrhythmias that occur in patients with ESKD. In the MiD study, implantable loop recorders were placed in HD participants, and the study documented atrial fibrillation in 40% of patients, bradycardia/asystole in 29% of patients, and VT in 1.5% of patients.28,29 These results are similar to a study of implantable loop recorders in patients on dialysis showing a high rate of atrial fibrillation and bradycardia/asystole.30 Our group previously documented an association of witnessed sudden cardiac death in HD clinics with extremes of serum potassium levels.6 One trial is currently evaluating whether simultaneous increase in dialysate potassium concentration and use of interdialytic potassium binders will reduce the risk for cardiac arrhythmias (NCT05535920). However, clear-cut linkage of cardiac arrhythmias with serum potassium concentrations is limited.
Limitations of PEARL-HD warrant mentioned. First, neither the participants nor the study team were blinded to the intervention (due to the lack of availability of a matching placebo), potentially introducing bias in estimated between-group differences. Second, the protocol was a short duration and had limited enrollment, which can yield unreliable effect estimates due to small sample variability. Our findings in this study may not be replicated in a trial with larger sample size. Third, the Holter monitors provided detailed rhythm information; however, the devices were not implanted and often 7-day records were incomplete. Fourth, due to the limited sample size of the study, adjustments were not feasible for potential confounders such as nutritional status or residual renal function. Finally, we did not measure post dialysis serum potassium concentration and thus could not determine whether posttreatment hypokalemia occurred. Despite these limitations, strengths of the current study include broad inclusion criteria, which can result in more generalizable inference, the prospective, randomized protocol which can eliminate confounding in treatment effects, the high frequency of potassium measurements, and the novel use of prolonged Holter cardiac monitoring as opposed to event monitoring.
The PEARL-HD study was designed to investigate whether prescription of an oral potassium binder would decrease the occurrences of hyperkalemia in the vulnerable HD population. The results indicated that patiromer can reduce the frequency of these episodes. The cardiac monitor results did not demonstrate that the effect of serum potassium was associated with reduced frequency of significant cardiac arrhythmias. Knowledge gained from PEARL-HD can be applied to determine whether patiromer reduces significant cardiac events in patients with ESKD.
Disclosure
JPM reports the following: Novo Nordisk (end point adjudication), AstraZeneca (clinical trial, Advisory Board), and NIH/NIDDK (Data Safety and Monitoring Board). JPD reports the following: Acutus (Data Safety and Monitoring Board), Syneos (Data Safety and Monitoring Board), Affera (Events Committee), Biosense (Events Committee), Cordis (Events Committee), NHLBI (Events Committee), University of Rochester (Events Committee), Phillips (Events Committee), Boston Scientific (Steering Committee), and Medtronic (Advisory Board). All the other authors declared no competing interests.
Acknowledgments
David Spiegel provided valued input on the study concept. Jeanette Rutledge, Anastacia Bohannon, Robin Gilliam, and Cynthia Redd provided clinical support for the trial. This study was supported by a research grant from Relypsa.
Footnotes
Figure S1. Schematic of the PEARL-HD Protocol. HD1 HD2 HD3 first, second and third hemodialysis treatment of the week. K times that serum potassium measurements performed. CCM, continuous cardiac monitor period.
Table S1. Continuous cardiac monitors study participants, week 0. PVC, premature ventricular contraction; VT ventricular tachycardia.
Table S2. Adverse events of PEARL-HD participants.
Table S3. Electrolyte concentrations and ultrafiltration volumes in study participants, weeks 3/4.
Checklist.
Supplementary Material
Figure S1. Schematic of the PEARL-HD Protocol. Table S1. Continuous cardiac monitors study participants, week 0. Table S2. Adverse events of PEARL-HD participants. Table S3. Electrolyte concentrations and ultrafiltration volumes in study participants, weeks 3/4. Checklist.
References
- 1.Agar B.U., Culleton B.F., Fluck R., Leypoldt J.K. Potassium kinetics during hemodialysis. Hemodial Int Int Symp Home. 2015;19:23–32. doi: 10.1111/hdi.12195. [DOI] [PubMed] [Google Scholar]
- 2.Pun P.H., Middleton J.P. Dialysate potassium, dialysate magnesium, and hemodialysis risk. J Am Soc Nephrol. 2017;28:3441–3451. doi: 10.1681/ASN.2017060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunelli S.M., Du Mond C., Oestreicher N., Rakov V., Spiegel D.M. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis. 2017;70:21–29. doi: 10.1053/j.ajkd.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Weiss J.N., Qu Z., Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saran R., Robinson B., Abbott K.C., et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2018;71:A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pun P.H., Lehrich R.W., Honeycutt E.F., Herzog C.A., Middleton J.P. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre C., McQuillan R., Bell C., Battistella M. Targeted Deprescribing in an outpatient hemodialysis unit: A quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70:611–618. doi: 10.1053/j.ajkd.2017.02.374. [DOI] [PubMed] [Google Scholar]
- 8.Sterns R.H., Grieff M., Bernstein P.L. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89:546–554. doi: 10.1016/j.kint.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Pitt B., Bakris G.L., Bushinsky D.A., et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17:1057–1065. doi: 10.1002/ejhf.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S., Dey J.K., Sen S., Mukherjee R. Efficacy and safety of patiromer in hyperkalemia: a systematic review and meta-analysis. J Pharm Pract. 2018;31:6–17. doi: 10.1177/0897190017692921. [DOI] [PubMed] [Google Scholar]
- 11.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ReactDx Cardiac monitoring solutions for medical professionals. https://www.reactdx.com/medical-professionals/
- 13.Kawataki M., Kashima T., Toda H., Tanaka H. Relation between QT interval and heart rate. Applications and limitations of Bazett’s formula. J Electrocardiol. 1984;17:371–375. doi: 10.1016/s0022-0736(84)80074-6. [DOI] [PubMed] [Google Scholar]
- 14.Sanghavi S., Whiting S., Uribarri J. Potassium balance in dialysis patients. Semin Dial. 2013;26:597–603. doi: 10.1111/sdi.12123. [DOI] [PubMed] [Google Scholar]
- 15.Kovesdy C.P., Regidor D.L., Mehrotra R., et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 16.Karaboyas A., Zee J., Brunelli S.M., et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2017;69:266–277. doi: 10.1053/j.ajkd.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker B., Moledina D.G. We use dialysate potassium levels that are too low in hemodialysis. Semin Dial. 2016;29:300–302. doi: 10.1111/sdi.12495. [DOI] [PubMed] [Google Scholar]
- 18.Attia Z.I., DeSimone C.V., Dillon J.J., et al. Novel bloodless potassium determination using a signal-processed single-lead ECG. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou S., McElroy P.A., Nootens J., Beach M. Safety and efficacy of low-potassium dialysate. Am J Kidney Dis. 1989;13:137–143. doi: 10.1016/s0272-6386(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 20.Santoro A., Mancini E., Gaggi R., et al. Electrophysiological response to dialysis: the role of dialysate potassium content and profiling. Contrib Nephrol. 2005;149:295–305. doi: 10.1159/000085691. [DOI] [PubMed] [Google Scholar]
- 21.Santoro A., Mancini E., London G., et al. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc. 2008;23:1415–1421. doi: 10.1093/ndt/gfm730. [DOI] [PubMed] [Google Scholar]
- 22.Johnson K., Cazee C., Gutch C., Ogden D. Sodium polystyrene sulfonate resin candy for control of potassium in chronic dialysis patients. Clin Nephrol. 1976;5:266–268. [PubMed] [Google Scholar]
- 23.Yuan C.M., Nee R., Little D.J., Abbott K.C. Incidence of sodium polystyrene sulfonate-associated colonic necrosis. Am J Med. 2013;126 doi: 10.1016/j.amjmed.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Sterns R.H., Rojas M., Bernstein P., Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733–735. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- 25.Fishbane S., Ford M., Fukagawa M., et al. A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium Zirconium cyclosilicate for Reducing the Incidence of predialysis hyperkalemia. J Am Soc Nephrol. 2019;30:1723–1733. doi: 10.1681/ASN.2019050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaques D.A., Stucker F., Ernandez T., et al. Comparative efficacy of patiromer and sodium polystyrene sulfonate on potassium levels in chronic haemodialysis patients: a randomized crossover trial. Clin Kidney J. 2022;15:1908–1914. doi: 10.1093/ckj/sfac129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bushinsky D.A., Budden J.J., Kalra P.A., Yuan J., Quinn C.M., Epstein M. Patiromer treatment in patients with CKD, hyperkalemia, and hyperphosphatemia: a post hoc analysis of 3 clinical trials. Am J Kidney Dis. 2023;82:97–104. doi: 10.1053/j.ajkd.2023.01.444. [DOI] [PubMed] [Google Scholar]
- 28.Roy-Chaudhury P., Tumlin J.A., Koplan B.A., et al. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;93:941–951. doi: 10.1016/j.kint.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Tumlin J.A., Roy-Chaudhury P., Koplan B.A., et al. Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol. 2019;20:80. doi: 10.1186/s12882-019-1212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong M.C.G., Kalman J.M., Pedagogos E., et al. Bradycardia and asystole is the predominant mechanism of sudden cardiac death in patients with chronic kidney disease. J Am Coll Cardiol. 2015;65:1263–1265. doi: 10.1016/j.jacc.2014.12.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Schematic of the PEARL-HD Protocol. Table S1. Continuous cardiac monitors study participants, week 0. Table S2. Adverse events of PEARL-HD participants. Table S3. Electrolyte concentrations and ultrafiltration volumes in study participants, weeks 3/4. Checklist.