To the Editor:
Membranous nephropathy (MN) is characterized by subepithelial immune-complex deposits, consisting of antibodies to target antigens.1 The use of laser microdissection with tandem mass spectrometry led to the discovery of several novel target antigens.2 Many cases associated with these novel target antigens have distinct clinical, epidemiological, and pathological features. Two novel “putative” antigens, exostosin-1 (EXT1) and exostosin-2 (EXT2), are associated with autoimmune diseases in the majority of cases, predominantly systemic lupus erythematosus.3 Conversely, about one-third of patients with lupus MN test positive for EXT1/EXT2.4
Posttransplant MN can manifest either as de novo or recurrent MN. Approximately 57% of de novo MN cases test positive for protocadherin FAT1.5 We describe the first case of de novo EXT1/EXT2-associated post-transplant MN.
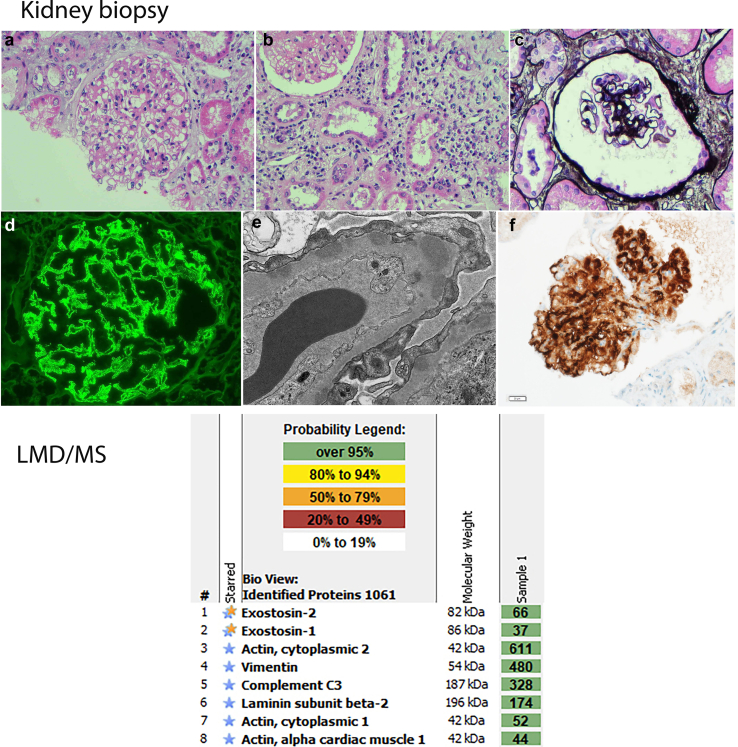
A 79-year-old male with a living-related kidney transplant for anti glomerular basement membrane glomerulonephritis presented 18 years posttransplant with acute allograft dysfunction, proteinuria, and active urinary sediment. Work-up for infection, autoimmune disease, and malignancy was negative. Renal biopsy revealed chronic active cell-mediated rejection, Banff grade IA. The presence of mild glomerulitis and mild peritubular capillaritis suggested a component of antibody-mediated rejection, although C4d-staining was negative in peritubular capillaries. Glomerular capillary wall thickening and global granular capillary wall staining for IgG, C3, C4d, kappa, and lambda suggested MN. Electron microscopy revealed subepithelial and intramembranous electron-dense deposits, confirming the diagnosis of MN. Immunostaining was negative for PLA2R but strongly positive for EXT1/EXT2. Laser microdissection with tandem mass spectrometry revealed high spectral counts for EXT1/EXT2 (Figure 1).
Figure 1.
Upper panel. Exostosin-associated MN. (a–c) Light microscopy showing mild mesangial expansion and thickened glomerular capillary walls without apparent GBM duplication, along with interstitial inflammation (a and b: hematoxylin and eosin; a: 400×, b: 200×, c: silver methenamine 400×). (d) Immunofluorescence microscopy showing granular IgG along the capillary walls (400×). (e) Electron microscopy showing subepithelial electron dense deposits. (f) Immunohistochemistry showing strong positive staining for exostosin 2 along the glomerular capillary walls (400×). Lower panel. Laser microdissection and mass spectrometry (LMD/MS), showing high spectral counts of exostosin 1 and 2 (EXT1/EXT2), and C3. Spectral counts of IgG1 are higher than of IgG4, similar to EXT1/EXT2-associated MN in native kidneys (not shown). Housekeeping proteins include actin, vimentin, and laminin. LMD/MS did not detect any other antigens typically associated with MN, including PLA2R, THSD7A, NELL1, NCAM1, CNTN1, Sema3B, protocadherin FAT1, NDNF, and PCDH7. GBM, glomerular basement membrane; MN, membranous nephropathy.
Remarkably, the presence of EXT1/EXT2 in lupus MN is associated with a better prognosis.4 It is hypothesized that abundance of EXT-1/EXT-2 may protect against harmful events, including complement activation, by promoting the synthesis of heparan sulfate, an essential component of the glomerular basement membrane. The patient presented with features of antibody-mediated rejection. Exposure of hidden antigens on the allograft podocytes may have led to subepithelial antibody deposition and immune complex formation causing de novo MN. In this scenario, secretion of EXT1/EXT2 by podocytes may represent a secondary phenomenon to minimize damage induced by antibody-mediated rejection. Alternatively, donor-specific antibody-mediated damage to podocytes may have exposed otherwise hidden EXT1/EXT2 and precipitated in situ formation of subepithelial immune complexes.
Patient Consent
There are no specific patient identifiers in the report. The biopsy was from 4 years ago and we report a finding in one case that was identified recently as part of different study (there is a valid IRB for the study).
References
- 1.Sethi S., Beck L.H., Jr., Glassock R.J., et al. Mayo Clinic consensus report on membranous nephropathy: proposal for a novel classification. Kidney Int. 2023;104:1092–1102. doi: 10.1016/j.kint.2023.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Sethi S. New ‘antigens’ in membranous nephropathy. J Am Soc Nephrol. 2021;32:268–278. doi: 10.1681/ASN.2020071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi S., Madden B.J., Debiec H., et al. Exostosin 1/exostosin 2–associated membranous nephropathy. J Am Soc Nephrol. 2019;30:1123–1136. doi: 10.1681/ASN.2018080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravindran A., Casal Moura M., Fervenza F.C., et al. Patients with membranous lupus nephritis, exostosin-positivity and exostosin-negativity represent two different phenotypes. J Am Soc Nephrol. 2021;32:695–706. doi: 10.1681/ASN.2020081181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S., Madden B., Moura M.C., et al. FAT1 is a target antigen in a subset of de novo allograft membranous nephropathy associated with antibody mediated rejection. Kidney Int. 2024;106:985–990. doi: 10.1016/j.kint.2024.08.009. [DOI] [PubMed] [Google Scholar]