Abstract
Introduction
The significant burden of chronic kidney disease (CKD) is not recognized as a global public health priority, although policies aimed at delaying progression to later stages are required. Therefore, there is need for a holistic disease model to inform decision making that accounts for the multidimensional impact of CKD, and the interrelated factors that modulate progression.
Methods
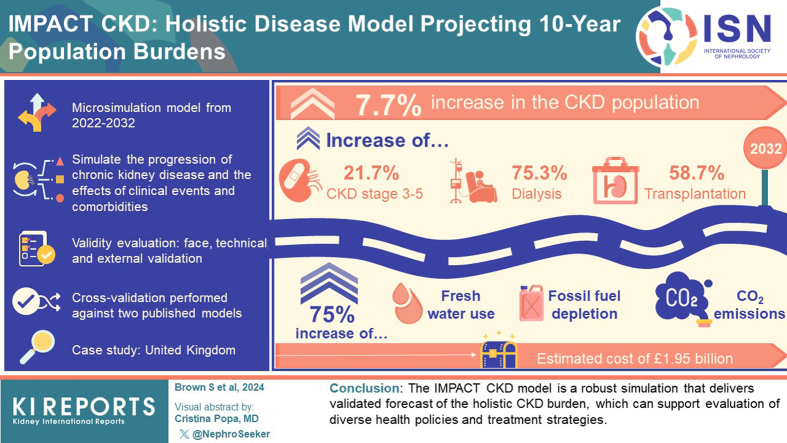
IMPACT CKD is a microsimulation model that simulates CKD progression and incorporates the effect of clinical events and comorbidities. CKD status is assigned using estimated glomerular filtration rate (eGFR) and albuminuria levels, and CKD progression is predicted by an annual eGFR decline rate. The model projects clinical, health care resource use, economic, patient, societal, and environmental burdens from 2022 to 2032. During development, face, technical, and external validity were evaluated, with calibration conducted to population data. Further, cross-validation was conducted against 2 published models. The United Kingdom (UK) was selected as the case study for validation.
Results
A 7.7% increase in the CKD population by 2032 was predicted, with increasing numbers of patients with CKD stage 3 to 5 (21.7%), dialysis (75.3%), and transplantation (58.7%). The increase of patients on renal replacement therapy (RRT) results in an increase of 75% across freshwater use, fossil fuel depletion, and CO2 emissions over the next decade, and an estimated cost of £1.95 billion in 2032. Projections reflect validated findings from other models.
Conclusion
The IMPACT CKD model is a robust simulation that delivers validated forecasts of the holistic CKD burden, which can support evaluation of diverse health policies and treatment strategies.
Keywords: chronic kidney disease, burden of disease, epidemiology, microsimulation model
Graphical abstract
CKD is a common condition with a global prevalence of approximately 13%.1 The overall burden of CKD is increasing in many countries, due to eGFR decline and increased comorbidities in aging populations.2 The health care system expends considerable resources, notably for dialysis and kidney transplant, to manage patients with kidney failure. In 2023, the overall economic burden of CKD was estimated to account for 3.2% of all UK’s annual health care system spending (∼£6.4 billion of £197 billion).3 In addition, CKD impairs the ability of patients and caregivers to participate in daily activities,4 with an estimated cost of £372 million in productivity loss in the UK for 2023.3 This value may underestimate the true burden of CKD given that many patients with CKD remain undiagnosed.5 Recent publications have associated dialysis with substantial environmental impact due to water consumption, use of single-use plastic, and high energy expenditures. Research on the environmental burden of CKD, as well as the broad societal and patient burden is limited.6
Major discrepancies exist between the reported increasing burden of CKD and global recognition of CKD as a health priority. Only 51% of governments recognized CKD as a health priority, and 34% of countries have CKD-specific policy plans.7 To effectively reduce the burden of CKD, policymakers need to evaluate the impact of national strategies on a long-term timescale, using a comprehensive viewpoint that captures the dynamic clinical, health care resource use, patient, societal, and environmental burdens.8 There is a need for an integrated framework capable of synthesizing the complex information available, while accounting for the dynamic and interrelated nature of CKD progression and treatment. Such a framework could provide insights that serve as the foundation for actionable policies. Disease modeling can serve this function, facilitating the evaluation of policy, and allowing for assessment of the effectiveness of different strategies.
A systematic literature review identified a need for individual patient simulation models with extensively validated data inputs that adequately account for heterogeneity within a population.9 IMPACT CKD is such a model, and the first designed to project the burden of CKD across many policy domains (clinical, health care resource use, economic, patient, societal, and environmental). IMPACT CKD is informed by a conceptual framework for CKD built by a team of researchers at the London School of Economics, developed in consultation with over 60 global cross-functional experts.10 The framework considers CKD care from primary preventative strategies through to secondary and tertiary care, dialysis, transplant, and end-of-life. In addition, the IMPACT CKD model incorporates recent evidence to address previous research gaps (Inside CKD8 and DISCOVER CKD11) and allows for prediction of environmental outcomes12 and patient-related financial burdens (PACE CKD13).
IMPACT CKD is intended to support population-specific evaluation of health policy decisions around system funding and efficiency; as well as CKD-specific prevention, screening, and treatment by projecting CKD burdens over a 10-year period. This paper describes the model development methodology and validation of the IMPACT CKD model using UK setting as a case study.
Methods
Model Overview
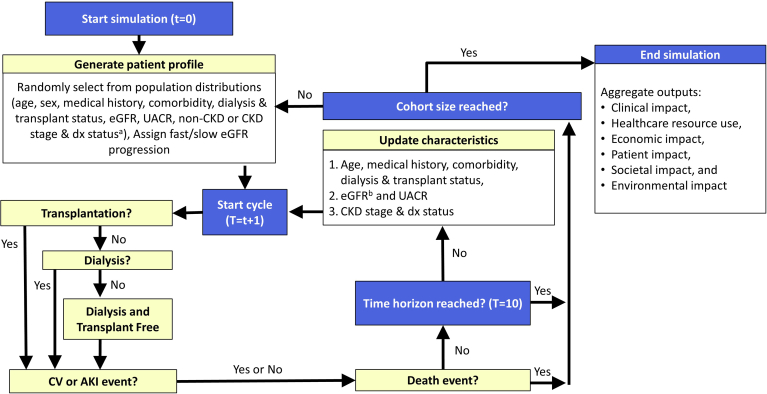
The IMPACT CKD model is a patient-level microsimulation designed to simulate CKD development and progression to estimate the clinical, health care resource use, economic, patient, societal, and environmental burdens (Figure 1). The model is composed of 3 modules as follows: (i) generation of simulated population using population-specific prevalence distributions; (ii) simulation of patient journey for 1 million individuals, for 10 years (baseline year [2022] and 10 simulated years [2023–2032]); and (iii) data aggregation and reporting extrapolated to the population of interest. The model was developed in Microsoft Excel (Version: 2208; Build: 16.0.15601.20446) with Visual Basic for Applications (VBA) programming.
Figure 1.
CKD micro-simulation flowchart. aNone, partial, or unlimited based on proportion of patients within a population. beGFR was updated based on regression equations (Supplementary Table S2). UACR was updated in each cycle a patient transitioned between age strata (i.e., increments of 10 years from age 25 onward) based on country-specific albuminuria distributions. Note: Patients could experience CV (stroke, MI, AKI, HF hospitalization) that were not modeled as disease states. Medical history includes history of stroke or MI. Comorbidities include HF, hypertension, and diabetes. AKI, acute kidney disease; CKD, chronic kidney disease; CV, cardiovascular; dx, diagnosis; eGFR, estimated glomerular filtration rate; HF, heart failure; MI, myocardial infarction; pts, patients; RRT, renal replacement therapy; UACR, albumin-creatinine ratio; t, cycle; T, time horizon.
Generate Simulated Population
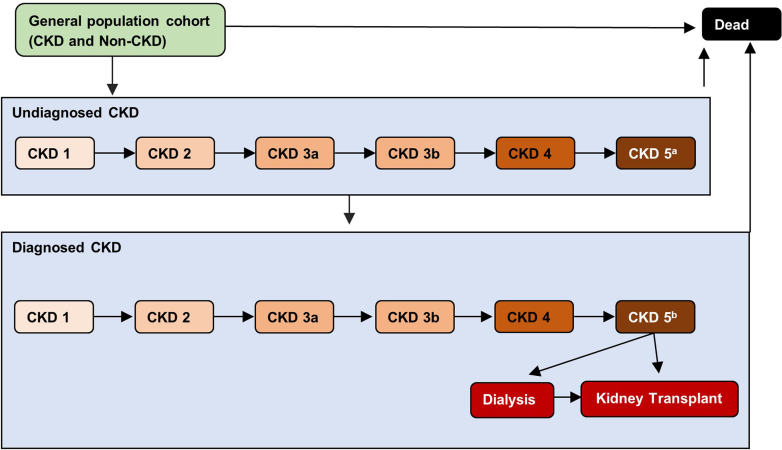
The model generates a cohort that, on average, represents the entire population of interest (including patients with and without CKD). At the baseline year, simulated individuals were randomly drawn from the observed population-specific distributions of a set of characteristics, including age, biological sex (referred to hereafter as sex), eGFR, urinary albumin-to-creatinine ratio (UACR), dialysis or transplant status, CKD diagnosis status, and access-to-care status (Table 1). Based on eGFR and UACR levels, patients were then assigned to a CKD stage or no CKD (Supplementary Table S1; Supplementary References,S1–S40).23 Patients with CKD were assigned a diagnosis probability dependent on CKD stage. This is reflected in the modeled health states, which include diagnosed and undiagnosed CKD stages (1, 2, 3a, 3b, 4, and 5), as well as dialysis, kidney transplant, non-CKD (i.e., normal kidney function), and death (Figure 2). Individuals without CKD could develop CKD in subsequent years (See Simulate Patient Journey). Comorbidities (diabetes, heart failure [HF], and hypertension), stroke history, and myocardial infraction history were randomly assigned based on age, sex, and CKD stage prevalence distributions.
Table 1.
Patient characteristics assigned during patient creation
| Patient characteristic | Assigned value | Assignment | Sources for case study |
|---|---|---|---|
| General characteristics | |||
| Agea | 0–115 yr | Drawn from age distribution of country. | UK Office for National Statistics.14 |
| Sexa | Male / Female | Prevalence by age for country. | |
| Access-to-care | None, partial, unlimited | Assigned based on proportion of population for country. | Assumed based on public health care system of UK |
| CKD-related characteristics | |||
| Undergoing dialysis | Yes / No | Prevalence by age and sex for country. | UK Renal Registry Report, 2022.15 |
| Prior kidney transplant | Yes / No | ||
| eGFR ratea | 0–200 ml/min per 1.73 m2 | Health Survey for England 2016, NHS Digital.16 | |
| UACRa | 0–1500 mg/g | Health Survey for England 2016, NHS Digital.16 | |
| CKD stage | No CKD; CKD stages 1, 2, 3a, 3b, 4, 5 | Assigned based on eGFR and UACR using KDIGO matrix. | KDIGO and NICE guidelines.17,18 |
| CKD diagnosis | Yes / No | Assigned based on diagnosis rates by CKD stage for country. | Hirst et al., 20205 |
| Comorbidities | |||
| Diabetesa | Yes / No | General population: prevalence by age and sex for country. | Health Survey for England 2019.19 |
| Hypertensiona | Yes / No | CKD: prevalence by CKD stage for country. | |
| Heart failurea | Yes / No | British Heart Foundation, 2020.20 | |
| History of CVD | |||
| History of myocardial infarctiona | Yes / No | General population: prevalence by age and sex for country. | British Heart Foundation, 2020.20 |
| History of strokea | Yes / No | CKD: prevalence by CKD stage for country. | |
| Other characteristics | |||
| Fast progressora | Yes / No | Assigned based on prevalence in patients with (i) diabetes, (ii) HF, and (iii) without diabetes or heart failure. | Go et al., 201821; George et al., 201722 |
CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HF, heart failure; KDIGO, Kidney Disease: Improving Global Outcomes; NHS, National Health Service; NICE, The National Institute for Health and Care Excellence; UACR, urinary albumin-to-creatinine ratio; UK, United Kingdom.
Included in the eGFR decline equation.
Figure 2.
Model disease states. aUndiagnosed CKD stage 5 patients excludes patients treated with RRT. bCKD stage 5 patients not on RRT are assumed to be treated with conventional care. These patients include pre-RRT patients (i.e., waiting for dialysis or transplantation) and those who decline RRT treatment. CKD, chronic kidney disease; RRT, renal replacement therapy.
All individuals (CKD and non-CKD) could also be assigned to a fast eGFR progression category reflecting a proportion of patients who may experience faster than average eGFR decline (see Simulate Patient Journey for details on eGFR decline). Fast eGFR progression was determined by comorbidity status using unique prevalence rates for individuals with the following: (i) diabetes; (ii) HF; and (iii) without diabetes or HF, in a hierarchical manner. Individuals with both diabetes and HF were assigned using the prevalence rate for patients with diabetes. All other individuals were assigned as general eGFR progressors. Although all individuals could be assigned as a fast progressor during population generation, only patients with CKD could experience faster eGFR decline during the model simulation.
Simulate Patient Journey
In each 1-year cycle, CKD progression, transplant, initiation of dialysis and clinical events could occur. Kidney transplant was subject to age and eGFR thresholds and dependent on access-to-care determined probability or the transition probability from dialysis. A cap on transplantation each year was defined based on historical data and a user-specified growth rate reflecting increasing organ availability. The probability of initiating dialysis was specified for 4 eGFR categories (<5, 5–9, 10–14, and 15–19 ml/min per 1.73 m2) and by access-to-care status (partial or unlimited). Patients who were starting dialysis received hemodialysis (clinic-based or home-based) or peritoneal dialysis based on local practice data. The probability of an eligible patient not initiating dialysis due to choice, access-to-care (dialysis availability), or clinical criteria was specified by the same 4 eGFR categories across 5 age ranges (<70, 70–74, 75–79, 80–84, and 85+ years); these patients received conventional kidney supportive care. An incident dialysis cap and growth rate were used to reflect limits on availability of dialysis.
Patients could experience transient clinical events (myocardial infraction, stroke, acute kidney injury, and hospitalization due to HF) during a cycle based on age-specific and sex-specific probabilities, with adjustment for CKD stage. An acute kidney injury event resulted in either full recovery, partial recovery, or no recovery; the latter 2 were associated with 1-time reductions in eGFR. In each cycle, patients could die due to the following causes and hierarchy: (i) transplantation or dialysis (first/subsequent year); (ii) myocardial infraction, stroke, or acute kidney injury (event year); (iii) HF (annual); (iv) CKD (by stage), and (v) general population mortality. Mortality for patients with CKD but not on RRT was assigned by applying a CKD-stage specific hazard ratio to the general population mortality probability by age and sex.
Parameters for all simulated individuals, with CKD or non-CKD, were updated at the end of each cycle and new comorbidities could be developed. Decline in eGFR for patients with CKD was informed by eGFR slope equations for general and fast eGFR progressors derived from the DISCOVER-CKD study (Supplementary Table S2).11,24 Decline in eGFR for individuals without CKD occurred at a linear rate starting at the age of 30 years (estimated based on the literature at −0.225 ml/min per 1.73 m2 per year25, 26, 27) regardless of fast progression status determined during population generation. UACR values were updated based on 10-year age categories by sex.16 The updated eGFR and UACR values informed the CKD stage update.17 If patients entered a new CKD stage, diagnosis status was reassessed. Across all health states, patients were assumed to receive lifestyle and medical interventions for CKD and comorbidities as per routine clinical practice in the population. Changes in treatment interventions and associated treatment effect can be adjusted and will be considered in future analyses of policy interventions.
Data Aggregation and Reporting
Projected epidemiological and clinical outputs for the 1-million simulated individuals, including prevalence of CKD and number of patients with CKD, clinical events, comorbidities, RRT, and mortality, were aggregated and then extrapolated to the population of interest considering births and immigration in the population without CKD. Based on these outputs, the model further projected health-system resource burden, patient impact, societal, economic, and environmental burdens (Table 2). Resource use was health state specific but was not associated with costs to avoid double counting. In addition to costs associated with each pre-RRT CKD state, transplantation (procedure and maintenance), or dialysis (hemodialysis or peritoneal) health state, costs for clinical events, CKD treatment, and CKD screening could be included in the total costs. From the patient perspective, financial well-being (as measured by Financial Well-being Survey and Financial Toxicity Survey) together with quality-adjusted life-years (patient relevant preference measurement) were assessed and reported by CKD stage. From a societal perspective, a set of productivity-related measures were projected for both patients with CKD and their caregivers by CKD stage and RRT status. A proportion of patients with CKD were assigned a caregiver, by CKD stage and RRT modality, which allowed for estimation of lost income (related to lost productivity due to CKD), missed workdays, lost gross domestic product, and lost full-time equivalents in both patients with CKD and caregivers. Environmental burden (i.e., freshwater consumption, fossil fuel depletion, and overall carbon footprint) was calculated by applying the CKD stage specific inputs to the number of diagnosed patients within each stage and aggregating for all patients with CKD. All key model assumptions are included in Supplementary Table S3.
Table 2.
IMPACT CKD key model outputs
| Domain | Output |
|---|---|
| Clinical impact | Percent changes in CKD subpopulations. |
| Number of patients with CKD by stage. | |
| Prevalence of CKD by stage. | |
| Number of patients with comorbidities (e.g., diabetes, hypertension, heart failure). | |
| Proportion of diagnosed and undiagnosed patients with CKD. | |
| Number of patients undergoing dialysis. | |
| Number of patients undergoing transplant. | |
| Annualized cardiovascular cases by CKD stage (i.e., myocardial infarction, stroke, hospitalization due to heart failure). | |
| Total cardiovascular cases by type of CVD. | |
| Annualized AKI cases by CKD stage. | |
| Annual mortality by CKD stage. | |
| Health care resource use | Number of ER visits in patients with CKD-by-CKD stage, and RRT status. |
| Number of hospitalizations in patients with CKD-by-CKD stage, and RRT status. | |
| Number of critical care visits in patients with CKD-by-CKD stage, and RRT status. | |
| Number of outpatient visits in patients with CKD-by-CKD stage, and RRT status. | |
| Economic impact | Costs associated with diagnosed CKD, dialysis, and transplant by CKD stage. |
| Costs associated with clinical events. | |
| Patient impact | QALYs (patient relevant preference management) per patient with CKD. |
| Financial well-being (using CFPB scores) and financial burden (using FACIT-Cost score) of diagnosed patients with CKD and patients without CKD. | |
| Cumulative lost income for patients with CKD and caregivers by CKD stage, and RRT status. | |
| Societal impact | Cumulative missed workdays for patients with diagnosed CKD and caregivers by CKD stage, and RRT status. |
| Cumulative lost GDP for patients with diagnosed CKD and caregivers by patient presenteeism/absenteeism, caregiver absenteeism. | |
| Cumulative lost FTEs for patients with diagnosed CKD and caregivers by patient presenteeism/absenteeism, caregiver absenteeism. | |
| Environmental impact | Freshwater consumption among patients with diagnosed CKD by CKD stage, and RRT status. |
| Fossil fuel depletion among patients with diagnosed CKD by CKD stage, and RRT status. | |
| Carbon production among patients with diagnosed CKD by CKD stage, and RRT status. |
AKI, acute kidney injury; CKD, chronic kidney disease; CFPB, Consumer Financial Protection Bureau; CVD, cardiovascular disease; ER, emergency room; FACIT, Functional Assessment of Chronic Illness Therapy; FTE, full-time equivalent; GDP, gross domestic product; RRT, renal replacement therapy; QALY, quality-adjusted life year.
Model Calibration and Validation
In complex disease simulation models, validation and calibration of input variables are used to ensure model outputs are aligned with known population data, and to reduce the impact of data, structural, or analytic uncertainty in accordance with guidelines (Supplementary Table S4).28, 29, 30, 31 The need for validation and calibration stems from several types of error that are common to all simulation models of complex diseases (See Validation and Calibration for IMPACT CKD Model in the Supplementary Materials).
Four validation steps were undertaken, starting with testing the face validity of the model structure with clinical experts in 8 countries (including the UK) during the development phase followed by technical validation (i.e., independent review and extreme value testing), and external predictive validation to ensure that the results align with historical literature values. Two cross-validation exercises were then conducted on selected outputs for 5-year projections versus the Inside CKD model,8 and for 10-year projections versus the UK Kidney Research model.3 Both comparator models used similar model states and many of the same datasets as inputs. Prior to cross-validation, calibration of select model inputs for the UK case study was conducted within a plausible range to align model outcomes with the validation targets using an iterative process (more details in the Supplementary Material). Due to the COVID-19 related disruption of health care services, the model was preferentially calibrated to data outside the 2020 to 2022 period.
Input Data for UK Analysis
Bibliographic searching in PubMed and UK-specific renal databases was supplemented by online data searches and consultation with clinical, policy, and health economic experts (Supplementary Table S5 to S35). When UK-specific data was unavailable, proxy data from other countries or expert opinion was utilized. It was assumed that the incidence of kidney transplantation would grow at 1% (aligning with population growth, reflecting increased organ availability) and dialysis capacity would grow at 3% per year (reflecting historical trends). Data aggregation for environmental burden was derived from a recent life-cycle assessment performed in the UK, which estimated the annual environmental impacts by CKD stage.12 Patient burden (financial well-being and burden) was informed by the PACE-CKD study, which included a noninterventional survey that presented evidence on the burden of CKD for patients compared to the general population.13
Results (Case Study–UK)
Calibration
The external validity of the model was tested through comparison of specific model outputs to the historical literature values. After calibration, the IMPACT CKD model projections were aligned with known data for dialysis, transplantation, clinical events, and CKD distribution parameters (Table 3). Most notably, the number of patients with new dialysis and transplantation was consistent with the UK Renal Registry,15 and National Health Services Blood and Transplant33 respectively, and the growth rate for dialysis was consistent with historical renal registry data.15 The model was also aligned to an extensive range of validation targets for the UK (Supplementary Table S36).
Table 3.
Model values and validation targets for UK analyses
| Parameter | Validation target (per million or as %) | Calibrated model output (per million or as %) | Validation source |
|---|---|---|---|
| Baseline characteristics | |||
| % Female in total population | 50.60 | 50.59 | UK ONS32 |
| Average age of total population (yr) | 40.30 | 40.61 | |
| CKD population in UK | |||
| Proportion of CKD stage 1–2 in UK population | 7.7% | 7.0% | NHS 201616 |
| Proportion of CKD Stage 3–5 in UK population | 5.1% | 5.2% | |
| RRT | |||
| Median age for patients starting dialysis or getting transplant (yr) | 63.70 | 66.01 | UK Renal Registry 202215 |
| Mean eGFR at start of dialysis | 6.90 | 6.83 | |
| Number of new transplants/yr (per million) | 47.9 | 49 | NHS Blood and Transplant; 3,207 (209/2020)33 |
| Number of new dialysis/yr (per million) | 131 | 122 | UK Renal Registry 202215 |
| AKI | |||
| AKI events in population (CKD and non-CKD) (per million) | 5300 to 20,700 | 16,498 | UK Kidney Association34 |
| % AKI events in patients with CKD | 32.4% | 40.85% | Argyropoulos 201935 |
| 55% | Abdalrahim 202036 | ||
| AKI deaths/yr for population (CKD and non-CKD) (per million) | 1074 to 3582 | 2803 | NHS England37; 100,000 deaths per year (range: 72k–240k) |
| Events | |||
| Average stroke events/yr in population (per million) | 1040–1500 | 1067 | Lee et al., 2011;38 Stroke Association39 |
| Average MIs/yr in population (per million) | 1492 | 1580 | BHF 2022;20 100,000/yr |
| Mortality | |||
| Mortality (%) in population (CKD and non-CKD) over 10 yrs | 1.04% | 1.06% | UK ONS32 |
AKI, acute kidney foundation; BHF, British Heart Foundation; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HD, hemodialysis; HHF, hypotensive heart foundation; HSE, Health Survey of England; MI, myocardial infarction; NHS, National Health Service; ONS, Office for National Statistics; PD, peritoneal dialysis; RRT, renal replacement therapy.
UK Case Study Results From 2022 to 2032
In brief, the CKD population in the UK was projected to increase at a faster rate than the general UK population (7.7% vs. 5.2%, respectively; Supplementary Table S37). The number of patients with CKD stage 3 to 5, on dialysis, and having transplantation were projected to grow 21.7%, 75.3%, and 58.7%, respectively. It was projected that in 2032, CKD-related health care costs will represent 2.3% of the UK health care budget, with stage 3 CKD accounting for an annual cost of £1.8 billion (42% of all CKD cases) and dialysis accounting for an annual cost of £1.5 billion (48% of prevalent RRT). The increase in patients with CKD was projected to result in 365 million missed workdays (an equivalent 1.8 million lost full-time equivalents over the next decade), and lost patient income of £49 billion. The substantial increase in patients on RRT drives an average increase of 75% across freshwater use, fossil fuel depletion, and CO2 emissions over the next decade, with 82% to 89% attributable to dialysis alone.
Cross-Validation
Cross-validation of the IMPACT CKD versus the recently developed Inside CKD8 and UK Kidney Research3 models (Table 4) found similar trends for growth in the population with CKD and burden of illness. IMPACT CKD estimated a total prevalence of 8.3 million patients with CKD which fell between the other models ranging from 7.2 million3 to 9.2 million (Inside CKD; 2022). All models predicted increases in the number of patients with CKD, with IMPACT CKD predicting a 10-year increase of 7.7%, compared to 5.8% for the UK Kidney Research model. The Inside CKD model provided a 5-year growth rate of 1.1%. IMPACT CKD estimated 43% of patients with CKD in 2022 to have stage 3 to 5 (4.7 million in stage 1–2, 3.5 million in stage 3–5), aligning with the UK Kidney Research model projecting 45% of patients with CKD in stage 3–5 (3.9 million in stage 1–2, 3.25 million in stage 3–5) and the Health Survey of England 2016 data16 for UK CKD stage distribution. Inside CKD used a baseline proportion of CKD 3 to 5 of 60% based on adjustments to the National Health Services16 dataset. Over 10 years, IMPACT CKD predicted an increase in stage 3 to 5 CKD of 21.7% (from 3.5–4.3 million), which was similar to the 20.0% growth (from 3.25–3.9 million) predicted by the UK Kidney Research model. Inside CKD predicted a reduction in the proportion of patients with CKD at stages 3 to 5; however, this may be due to large difference in the proportion of patients in CKD stages 3 to 5 at baseline.
Table 4.
Cross-validation of IMPACT CKD to published models
| Source | Estimated prevalence (2022) | Projected prevalence (2023) | Projected prevalence (2027) | Projected prevalence (2032) | Projected prevalence (2033) |
|---|---|---|---|---|---|
| Prevalence of CKDa (%) | |||||
| IMPACT CKDb | 8.3 M (42% CKD 3–5) | 8.4 M (44% CKD 3–5) | 8.7 M (46% CKD 3–5) | 8.9 M (48% CKD 3–5) | NA |
| UK Kidney Research 2023c | NA | 7.2 M (45% CKD 3–5) | NA | NA | 7.6 M (51% CKD 3–5) |
| Inside CKDd | 9.2 M (60% CKD 3–5) | NA | 9.3 M (59% CKD 3–5) | NA | NA |
| Prevalence of patients on dialysis | |||||
| IMPACT CKDb | 33,098 | 36,850 | 51,523 | 58,022 | NA |
| UK Kidney Research 2023c,e | 32,792 | 33,310 | Constrained: 30,334 Unconstrained: 55,873 |
Constrained: 33,838 Unconstrained: 119,359 |
Constrained: 33,845 Unconstrained: 142,920 |
| Inside CKDd | 28,963 | 29,265 | 35,290 | NA | NA |
| Annual number of new kidney transplants | |||||
| IMPACT CKDb | NA | 3149 | 3216 | 3350 | NA |
| UK Kidney Research 2023c,e | 2879 | 2976 | Constrained: 3171 Unconstrained: 5139 |
Constrained: 3484 Unconstrained: 10,084 |
Constrained: 3615 Unconstrained: 11,665 |
| Prevalence of kidney transplant recipients | |||||
| IMPACT CKDb | 40,267 | 42,478 | 52,461 | 63,918 | NA |
| Inside CKDd | 42,587 | 42,462 | 43,189 | NA | NA |
| Annual prevalence of overall RRT (total prevalence of dialysis and new kidney transplant) | |||||
| IMPACT CKDb | 33,098 | 39,999 | 54,739 | 61,372 | NA |
| UK Kidney Research 2023c,e | 32,800 | 33,310 | Constrained: 34,900 Unconstrained: 61,000 |
Constrained: 37,300 Unconstrained: 129,400 |
Constrained: 37,460 Unconstrained: 154,585 |
| Prevalence of overall RRT patients (Total prevalence of dialysis and total prevalence of kidney transplant) | |||||
| IMPACT CKDb | 73,365 | 79,328 | 103,984 | 121,940 | NA |
| Inside CKDd | 71,549 | 71,728 | 78,479 | NA | NA |
CKD, chronic kidney disease; M, million; NA, not available; RRT, renal replacement therapy; UK, United Kingdom.
Prevalence of CKD includes Stages 1-5 and those on RRT.
The IMPACT CKD model projected the prevalence of CKD in the UK from 2022 to 2032.
The Kidney Research UK projected the prevalence of CKD in the UK from 2023 to 2032. In the absence of explicitly reported values, data was extracted from figures using DigitizeIt software (version 2.5.3, I. Bormann, Germany).
The Inside CKD model projected the prevalence of CKD in the UK from 2022 to 2027.
The constrained approach for projecting RRT burden reflected historic trends in numbers for a maximized (at capacity) health system. The unconstrained approach using historical transition probabilities predicted exponential increases in the number of adults needing RRT.
IMPACT CKD was aligned with the UK Kidney Research unconstrained projections for the number of patients on dialysis at year 5 and within the range of unconstrained and constrained projections for patients receiving a new transplant at year 10. In both models, 10-year growth was driven by assumptions regarding the rate of kidney transplantation. IMPACT CKD used current incident rates of transplantation and dialysis per million population with growth in incident transplantation consistent with growth in the general population (to model change in organ availability), and growth in incident dialysis consistent with changes in historical rates. In contrast, the UK Kidney Research model assumed constrained or unconstrained growth in transplantation and dialysis. The true number of transplants and new dialysis cases in future years will be contingent on the supply of organs and access to dialysis, and as such is uncertain. However, the growth in dialysis patients projected by the IMPACT CKD model (18% over 8 years) is supported by historical data on dialysis growth from the UK Renal Registry (15% over 8 years). Inside CKD reported lower rates of growth of dialysis and transplantation.
Discussion
Despite the large disease burden and poor patient outcomes of CKD, there is an apparent lack of policy prioritization globally.7 Guided by a conceptual framework supported by a large group of experts in CKD care,10 the IMPACT CKD model represents a validated microsimulation tool designed to help policy makers understand the holistic burden of CKD, and determine the benefits of interventions across a range of outcomes. The IMPACT CKD model has several strengths. As a patient-level microsimulation, it captures the heterogeneity in a population and provides the broadest range of disease outcomes in any CKD simulation model allowing for estimation of the holistic burden of CKD. Further, the model incorporates factors that cause rapid progression of CKD using data from DISCOVER CKD to better capture disease progression.11,24 The model is flexible, allowing adaptation to any patient population to test the impact of a wide range of health policies aimed at improving population health, or altering CKD diagnosis and treatment.3,8,9
The model is similar in structure to several published CKD models and was cross-validated against 2 recent models in a UK setting.3,8 Commonalities with available models include cross-sectional sampling of a country population, and incorporation of CKD stages defined by eGFR and UACR. Unique features include consideration of subpopulations of a country without access to health care services; user-modifiable growth rates in population-level RRT capacities; and the consideration of the large proportion of patients with stage 5 CKD who do not proceed to RRT due to difficulties with access to dialysis facilities, patient choice to receive conservative care, or due to organ availability.
The IMPACT CKD projections reflect findings from other recent models in terms of high CKD prevalence and burden. The results for the UK validate the recent findings from the UK Kidney Research3 report which predicts substantial increases in CKD burden over the next decade, in excess of what would be expected due to UK population growth. The results of the present analysis underscore how the aging UK population with high comorbidity rates is expected to dramatically increase the burden of CKD for patients, the health care system, and society. This increased burden was predicted due to a growing CKD population and higher rates of progression to later stages of CKD with worse outcomes and greater costs. Notably, these projected increases assume the persistence of present care standards over the model time horizon but could be mitigated by interventions that delay disease progression (such as early detection, and improved treatments) in the future.
As illustrated with the UK case study, the IMPACT CKD model has the potential to generate critical information to directly inform medical practice. With the projected increase in the number of patients with CKD, a significant increase is anticipated in the number of general physician visits, which will impact the organization and resources of primary care. Armed with knowledge of the anticipated extent of this increase, policymakers in the UK could devise and implement strategies to properly prepare primary care physicians and equip them with training on early diagnosis and treatment of CKD. Furthermore, the increased number of patients with stage 3 to 5 CKD will result in a higher demand for specialized care, as well as an increased need for RRT, leading to a greater requirement for specialized staff (e.g., nephrologists, nurses, pharmacists, etc.), dialysis slots, and other resources (e.g., drivers, facilities, caregivers, etc.). Therefore, building efficient and effective interfaces between primary and secondary care will be a crucial component to ensure that secondary care services are not overwhelmed with the increased clinical burden of CKD. To this end, UK policymakers could further utilize the model to support resource management due to improved understanding of CKD’s impact on resource use and health care costs. Beyond the clinical and economic implications, the model highlights the urgency of prioritizing CKD policies to benefit patients, society, and the environment to reduce hundreds of millions of missed workdays and billions of pound sterling in lost patient income. Policies that aim to reduce CKD progression to later stages would also support National Health Services initiatives to reach zero emissions by 2045.40 Overall, the model presents a comprehensive picture of CKD care, with consideration of the multidimensional impacts which supports population-specific, evidence-based, and data-driven policy development and evaluation.
The IMPACT CKD model shares limitations common to all simulation models, including the need for substantial amounts of population-level data, which is often unavailable. In the case of missing data for the present analysis, proxies were used from other countries, leading to increased uncertainty. Similarly, the model structure reflects the best available knowledge of CKD to date; however, gaps in knowledge of the disease may introduce uncertainty. For example, this lack of comprehensive data precluded consideration of the interdependence of patient characteristics that would inform a correlation matrix for all patient characteristics. To understand the impact of uncertainty when using the model, extensive sensitivity and scenario analyses should be conducted and presented. Considering that CKD progression is based on eGFR regression equations for patients with CKD, and eGFR decline for the general population, these are candidates for sensitivity analyses in future work.
Although knowledge gaps regarding the CKD disease process and some data inputs persist, policymakers and health care providers need to make decisions regarding CKD prevention, screening, and treatment. The IMPACT CKD model will support this evidence-based decision making by integrating data across domains to project the overall, long-term, population-level burden of CKD. The IMPACT CKD model could be used to evaluate and quantify the benefits of new CKD policies in terms of their clinical, health system related, economic, patient-centric, societal, and environmental impacts.
The IMPACT CKD model is a robust simulation tool, adept at providing comprehensive insights across various aspects of CKD—clinical, health care resource use, economic, patient-centric, societal, and environmental. This model not only delivers forecasts of the overall burden of CKD, but also enables the evaluation of diverse health policies and treatment strategies. As evidenced by the UK analysis, an aging population with high comorbidity rates contributes to the growing CKD burden, underscoring the urgent need for strategic action. Anticipating a 21.7% increase in patients with late-stage CKD in the UK over the next decade, the health care system must proactively accommodate escalating demands for specialized care. To mitigate these demands, early identification and proactive management of CKD must be prioritized. Therefore, the IMPACT CKD model serves as an evidence-based decision-making tool which may help to shape the future of CKD management and intervention.
Disclosure
SB, HG, and SP and are employees of EVERSANA; AstraZeneca contracted EVERSANA to complete this study. CJW is an employee of London School of Economics; AstraZeneca contracted London School of Economics for this study. JJGS and JC are employees of AstraZeneca. AFM and DCW received honoraria for participating in consultant meetings for AstraZeneca.
Acknowledgments
The authors would like to thank Anthony Zara, Bo Ren Long, and Daniel Grima from EVERSANA; Kyra Obolensky and Naveen Rao from AstraZeneca; and Isaac Bencomo-Bermudez and George Wharton from the London School of Economics for their support. The authors would also like to thank Dr. Petra Sandow, Dr. Francisco Brotons-Munto, Dr. Neil Skolnik, Dr. Ming-hui Zhao, Prof. Steve Chadban, and Prof. Janwillem W.H. Kocks. This work received funding from AstraZeneca.
Data Availability Statement
All data are included in the manuscript and/or supporting materials.
Author Contributions
SB, HG, SP, JJGS, and JC were involved in the conception and design of the study. All the authors were involved in the analysis of data and all the authors contributed to the interpretation of data. In addition, all the authors agree to be accountable for all aspects of the work.
Footnotes
Validation and calibration for IMPACT CKD Model.
Model Inputs.
Validation and calibration targets.
UK case study results.
Supplementary References.
Table S1. CKD stage as defined by eGFR and UACR.
Table S2. eGFR equations for CKD progressors.
Table S3. Key model assumptions.
Table S4. Validation targets and input parameters subject to calibration.
Table S5. Kidney transplantation model inputs.
Table S6. Dialysis model inputs—percentage of eligible patients by eGFR.
Table S7. Dialysis model inputs—percentage of eligible patients that receive conventional kidney supportive care by age and eGFR.
Table S8. Event risk—annual probabilities of myocardial infarction for the general UK population by age and sex.
Table S9. Event risk—annual probabilities of stroke/TIA for the general UK population by age and sex.
Table S10. Event risk—annual probabilities of acute kidney injury for the general UK population by age.
Table S11. Event risk—annual probabilities of HF resulting in hospitalization for the general UK population by age.
Table S12. Event risk—CKD-specific relative risk of myocardial infarction by CKD stage and age.
Table S13. Event risk—CKD-specific hazard ratios for incidence of stroke by eGFR and UACR status.
Table S14. Event risk—CKD-specific relative risk of acute kidney injury by CKD stage and age.
Table S15. Event risk—CKD-specific rate ratios of hospitalization due to HF by CKD category.
Table S16. Event risk—outcomes of acute kidney injury.
Table S17. Relative risk of all-cause mortality by CKD stage.
Table S18. Mortality—probability of patient survival by transplant type.
Table S19. Dialysis mortality—probability of patient mortality by age.
Table S20. Mortality—probability of patient mortality from clinical events.
Table S21. Mortality rates (qx), males and females, UK, 2018–2020.
Table S22. Comorbidities risk—annual probabilities of T2DM for the general UK population by age.
Table S23. Comorbidities risk—annual probabilities of hypertension for the general UK population by age and sex.
Table S24. Comorbidities risk—annual probabilities of HF for the general UK population by age and sex.
Table S25. Comorbidities risk—CKD-specific relative risk of T2DM by CKD stage and age.
Table S26. Comorbidities risk—CKD-specific relative risk of HF by CKD stage and age.
Table S27. Comorbidities risk—CKD-specific relative risk of hypertension by CKD stage and age.
Table S28. Annual resource burden associated with CKD.
Table S29. Annual per patient costs associated with treatment of CKD by stage and treatment modality.
Table S30. Health state utility inputs and event-related disutility.
Table S31. Absenteeism and presenteeism in patients with CKD.
Table S32. Absenteeism in caregivers of patients with CKD.
Table S33. Productivity inputs.
Table S34. Annual per patient environmental burden associated with CKD.
Table S35. Lifestyle and financial burden associated with CKD.
Table S36. Targets for model calibration and external validation.
Table S37. IMPACT CKD predictions for change from baseline (2022) to 2032 for clinical, economic, patient, societal, and environmental burdens.
Supplementary Material
Validation and calibration for IMPACT CKD Model. Model Inputs. Validation and calibration targets. UK case study results. Supplementary References. Table S1. CKD stage as defined by eGFR and UACR. Table S2. eGFR equations for CKD progressors. Table S3. Key model assumptions. Table S4. Validation targets and input parameters subject to calibration. Table S5. Kidney transplantation model inputs. Table S6. Dialysis model inputs—percentage of eligible patients by eGFR. Table S7. Dialysis model inputs—percentage of eligible patients that receive conventional kidney supportive care by age and eGFR. Table S8. Event risk—annual probabilities of myocardial infarction for the general UK population by age and sex. Table S9. Event risk—annual probabilities of stroke/TIA for the general UK population by age and sex. Table S10. Event risk—annual probabilities of acute kidney injury for the general UK population by age. Table S11. Event risk—annual probabilities of HF resulting in hospitalization for the general UK population by age. Table S12. Event risk—CKD-specific relative risk of myocardial infarction by CKD stage and age. Table S13. Event risk—CKD-specific hazard ratios for incidence of stroke by eGFR and UACR status. Table S14. Event risk—CKD-specific relative risk of acute kidney injury by CKD stage and age. Table S15. Event risk—CKD-specific rate ratios of hospitalization due to HF by CKD category. Table S16. Event risk—outcomes of acute kidney injury. Table S17. Relative risk of all-cause mortality by CKD stage. Table S18. Mortality—probability of patient survival by transplant type. Table S19. Dialysis mortality—probability of patient mortality by age. Table S20. Mortality—probability of patient mortality from clinical events. Table S21. Mortality rates (qx), males and females, UK, 2018–2020. Table S22. Comorbidities risk—annual probabilities of T2DM for the general UK population by age. Table S23. Comorbidities risk—annual probabilities of hypertension for the general UK population by age and sex. Table S24. Comorbidities risk—annual probabilities of HF for the general UK population by age and sex. Table S25. Comorbidities risk—CKD-specific relative risk of T2DM by CKD stage and age. Table S26. Comorbidities risk—CKD-specific relative risk of HF by CKD stage and age. Table S27. Comorbidities risk—CKD-specific relative risk of hypertension by CKD stage and age. Table S28. Annual resource burden associated with CKD. Table S29. Annual per patient costs associated with treatment of CKD by stage and treatment modality. Table S30. Health state utility inputs and event-related disutility. Table S31. Absenteeism and presenteeism in patients with CKD. Table S32. Absenteeism in caregivers of patients with CKD. Table S33. Productivity inputs. Table S34. Annual per patient environmental burden associated with CKD. Table S35. Lifestyle and financial burden associated with CKD. Table S36. Targets for model calibration and external validation. Table S37. IMPACT CKD predictions for change from baseline (2022) to 2032 for clinical, economic, patient, societal, and environmental burdens.
References
- 1.Hill N.R., Fatoba S.T., Oke J.L., et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lv J.C., Zhang L.X. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Research UK . 2023. Kidney disease: a UK public health emergency. The health economics of kidney disease to 2033.https://www.kidneyresearchuk.org/wp-content/uploads/2023/06/Economics-of-Kidney-Disease-full-report_accessible.pdf [Google Scholar]
- 4.Michalopoulos S.N., Gauthier-Loiselle M., Aigbogun M.S., et al. Patient and care partner burden in CKD patients with and without anemia: a US-based survey. Kidney Med. 2022;4 doi: 10.1016/j.xkme.2022.100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirst J.A., Hill N., O’Callaghan C.A., et al. Prevalence of chronic kidney disease in the community using data from OxRen: a UK population-based cohort study. Br J Gen Pract. 2020;70:e285–e293. doi: 10.3399/bjgp20X708245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stigant C.E., Rajan T., Barraclough K.A., Miller F.A. The necessity of environmentally sustainable kidney care. Can J Kidney Health Dis. 2023;10 doi: 10.1177/20543581231166484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuen B.L., Bello A.K., Levin A., et al. National health policies and strategies for addressing chronic kidney disease: data from the International Society of Nephrology Global Kidney Health Atlas. PLOS Glob Public Health. 2023;3 doi: 10.1371/journal.pgph.0001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangri N., Chadban S., Cabrera C., Retat L., Sanchez J.J.G. Projecting the epidemiological and economic impact of chronic kidney disease using patient-level microsimulation modelling: rationale and methods of inside CKD. Adv Ther. 2023;40:265–281. doi: 10.1007/s12325-022-02353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugrue D.M., Ward T., Rai S., McEwan P., van Haalen H.G.M. Economic modelling of chronic kidney disease: A systematic literature review to inform conceptual model design. Pharmacoeconomics. 2019;37:1451–1468. doi: 10.1007/s40273-019-00835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bermudez I.B., Webber C.J., Sanchez J.J.G., et al. The health, socioeconomic and environmental impact of CKD in the UK: building a conceptual framework. Nephrol Dial Transplant. 2023;4167:38. doi: 10.1093/ndt/gfad063c_4167. [DOI] [Google Scholar]
- 11.Heerspink H, Nolan S, Carrero JJ, et al. Clinical outcomes in patients with CKD and rapid or non-rapid eGFR decline: a report from the DISCOVER CKD retrospective cohort. Adv Ther. 2024;41:3264–3277. doi: 10.1007/s12325-024-02913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoccali C., Barraclough K., Eckelman M., et al. The environmental impact of chronic kidney disease internationally: results of a life cycle assessment. Nephrol Dial Transplant. 2023;2695:38. doi: 10.1093/ndt/gfad063c_2695. [DOI] [Google Scholar]
- 13.Esposito C., Chadban S., Rangaswami J., et al. PACE-CKD: health-related quality of life of patients with CKD and caregivers: results from a us survey. Nephrol Dial Transplant. 2023;3990:38. doi: 10.1093/ndt/gfad063c_3990. [DOI] [Google Scholar]
- 14.Office for National Statistics Mid-year population estimates, UK, June 2020. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2020#age-structure-of-the-uk-population
- 15.UK Renal Registry UK Renal Registry 24th Annual Report, Bristol, UK. 2022. https://ukkidney.org/sites/renal.org/files/publication/file-attachments/24th_UKRR_ANNUAL_REPORT_BOOK%20version%203%20-%20edited%202024.pdf
- 16.NHS Digital; 2016. Health Survey for England. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2016#highlights
- 17.Improving Global Outcomes CKD Work Group, KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 18.NICE. Overview Chronic kidney disease: assessment and management. https://www.nice.org.uk/guidance/ng203
- 19.NHS Digital; 2019. Health Survey for England. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019#data-sets
- 20.British Heart Foundation Heart & Circulatory Disease Statistics 2020. 2020. https://www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2020
- 21.Go A.S., Yang J., Tan T.C., et al. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 2018;19:146. doi: 10.1186/s12882-018-0942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George L.K., Koshy S.K.G., Molnar M.Z., et al. Heart failure increases the risk of adverse renal outcomes in patients with normal kidney function. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;3 https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf [Google Scholar]
- 24.Pecoits-Filho R., James G., Carrero J.J., et al. Methods and rationale of the DISCOVER CKD global observational study. Clin Kidney J. 2021;14:1570–1578. doi: 10.1093/ckj/sfab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waas T., Schulz A., Lotz J., et al. Distribution of estimated glomerular filtration rate and determinants of its age dependent loss in a German population-based study. Sci Rep. 2021;11 doi: 10.1038/s41598-021-89442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksen B.O., Palsson R., Ebert N., et al. GFR in healthy aging: an individual participant data meta-analysis of iohexol clearance in European population-based cohorts. J Am Soc Nephrol. 2020;31:1602–1615. doi: 10.1681/ASN.2020020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein J.R., Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17:302–307. doi: 10.1053/j.ackd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briggs A.H., Weinstein M.C., Fenwick E.A., et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Mak. 2012;32:722–732. doi: 10.1177/0272989X12458348. [DOI] [PubMed] [Google Scholar]
- 29.Eddy D.M., Hollingworth W., Caro J.J., et al. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Med Decis Making. 2012;32:733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 30.Goldie S.J., Grima D., Kohli M., Wright T.C., Weinstein M., Franco E. A comprehensive natural history model of HPV infection and cervical cancer to estimate the clinical impact of a prophylactic HPV-16/18 vaccine. Int J Cancer. 2003;106:896–904. doi: 10.1002/ijc.11334. [DOI] [PubMed] [Google Scholar]
- 31.Karnon J., Vanni T. Calibrating models in economic evaluation: a comparison of alternative measures of goodness of fit, parameter search strategies and convergence criteria. Pharmacoeconomics. 2011;29:51–62. doi: 10.2165/11584610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Office for National Statistics . Office for National Statistics; 2021. National life tables–life expectancy in the UK: 2018 to 2020.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2018to2020#:∼:text=In%20the%20UK%20the%20median,estimates%20for%202015%20to%202017 [Google Scholar]
- 33.NHS Blood and Transplant Annual Report on Kidney Transplantation. NHS Blood and Transplant. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/29222/kidney-annual-report-2021-22update.pdf
- 34.Acute kidney injury (AKI) in England - a report on the nationwide collection of AKI warning test scores from 2018. The UK Kidney Association. https://ukkidney.org/audit-research/publications-presentations/report/acute-kidney-injury-aki-england-report-nationwide
- 35.Argyropoulos A., Townley S., Upton P.M., Dickinson S., Pollard A.S. Identifying on admission patients likely to develop acute kidney injury in hospital. BMC Nephrol. 2019;20:56. doi: 10.1186/s12882-019-1237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdalrahim M.S., Khalil A.A., Alramly M., Alshlool K.N., Abed M.A., Moser D.K. Pre-existing chronic kidney disease and acute kidney injury among critically ill patients. Heart Lung. 2020;49:626–629. doi: 10.1016/j.hrtlng.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Factsheet: Implementation of NICE Guideline on Acute Kidney Injury (AKI) 2014. https://www.england.nhs.uk/wp-content/uploads/2014/02/rm-fs-10-4.pdf
- 38.Lee S., Shafe A.C., Cowie M.R. UK stroke incidence, mortality and cardiovascular risk management 1999-2008: time-trend analysis from the General Practice Research Database. BMJ Open. 2011;1 doi: 10.1136/bmjopen-2011-000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stroke Association Stroke statistics; 2023. https://www.stroke.org.uk/what-is-stroke/stroke-statistics
- 40.McGeoch L., Hardie T., Coxon C., Cameron G. Net zero care: what will it take? https://www.health.org.uk/publications/long-reads/net-zero-care-what-will-it-take#:∼:text=The%20NHS%20in%20England%20has,estates%2C%20energy%20and%20supply%20chains
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation and calibration for IMPACT CKD Model. Model Inputs. Validation and calibration targets. UK case study results. Supplementary References. Table S1. CKD stage as defined by eGFR and UACR. Table S2. eGFR equations for CKD progressors. Table S3. Key model assumptions. Table S4. Validation targets and input parameters subject to calibration. Table S5. Kidney transplantation model inputs. Table S6. Dialysis model inputs—percentage of eligible patients by eGFR. Table S7. Dialysis model inputs—percentage of eligible patients that receive conventional kidney supportive care by age and eGFR. Table S8. Event risk—annual probabilities of myocardial infarction for the general UK population by age and sex. Table S9. Event risk—annual probabilities of stroke/TIA for the general UK population by age and sex. Table S10. Event risk—annual probabilities of acute kidney injury for the general UK population by age. Table S11. Event risk—annual probabilities of HF resulting in hospitalization for the general UK population by age. Table S12. Event risk—CKD-specific relative risk of myocardial infarction by CKD stage and age. Table S13. Event risk—CKD-specific hazard ratios for incidence of stroke by eGFR and UACR status. Table S14. Event risk—CKD-specific relative risk of acute kidney injury by CKD stage and age. Table S15. Event risk—CKD-specific rate ratios of hospitalization due to HF by CKD category. Table S16. Event risk—outcomes of acute kidney injury. Table S17. Relative risk of all-cause mortality by CKD stage. Table S18. Mortality—probability of patient survival by transplant type. Table S19. Dialysis mortality—probability of patient mortality by age. Table S20. Mortality—probability of patient mortality from clinical events. Table S21. Mortality rates (qx), males and females, UK, 2018–2020. Table S22. Comorbidities risk—annual probabilities of T2DM for the general UK population by age. Table S23. Comorbidities risk—annual probabilities of hypertension for the general UK population by age and sex. Table S24. Comorbidities risk—annual probabilities of HF for the general UK population by age and sex. Table S25. Comorbidities risk—CKD-specific relative risk of T2DM by CKD stage and age. Table S26. Comorbidities risk—CKD-specific relative risk of HF by CKD stage and age. Table S27. Comorbidities risk—CKD-specific relative risk of hypertension by CKD stage and age. Table S28. Annual resource burden associated with CKD. Table S29. Annual per patient costs associated with treatment of CKD by stage and treatment modality. Table S30. Health state utility inputs and event-related disutility. Table S31. Absenteeism and presenteeism in patients with CKD. Table S32. Absenteeism in caregivers of patients with CKD. Table S33. Productivity inputs. Table S34. Annual per patient environmental burden associated with CKD. Table S35. Lifestyle and financial burden associated with CKD. Table S36. Targets for model calibration and external validation. Table S37. IMPACT CKD predictions for change from baseline (2022) to 2032 for clinical, economic, patient, societal, and environmental burdens.
Data Availability Statement
All data are included in the manuscript and/or supporting materials.