Abstract
Objectives
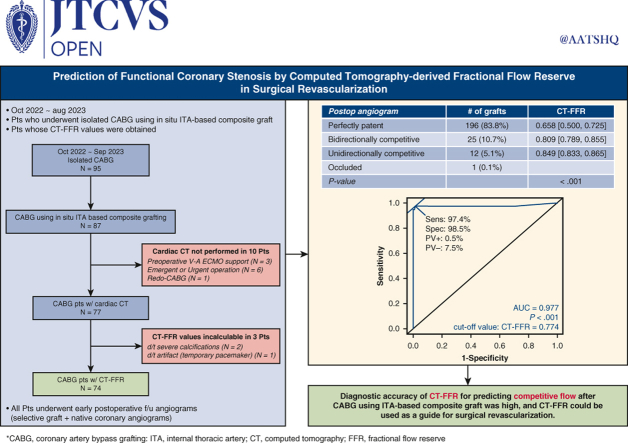
The aims of this study were (1) to compare computed tomography–derived fractional flow reserve (CT-FFR) values with graft patency and (2) to establish the cut-off value of CT-FFR for predicting competitive graft flow after coronary artery bypass grafting (CABG).
Methods
Of the 77 patients who underwent isolated CABG with an in situ internal thoracic artery (ITA)-based composite graft and who were also evaluated by preoperative cardiac CT, CT-FFR values were obtained in 74 patients. Early postoperative angiograms were performed in all 74 patients. Angiograms were performed to evaluate the grafts as well as the native coronary arteries to find any competitive flow present. Postoperative angiographic findings of graft flow were categorized as perfectly patent, bidirectionally competitive, unidirectionally competitive, and occluded. Receiver operating characteristic curve analysis of preoperative CT-FFR values for predicting postoperative angiographic competition was performed, and cutoff values of CT-FFR and area under the curve were identified.
Results
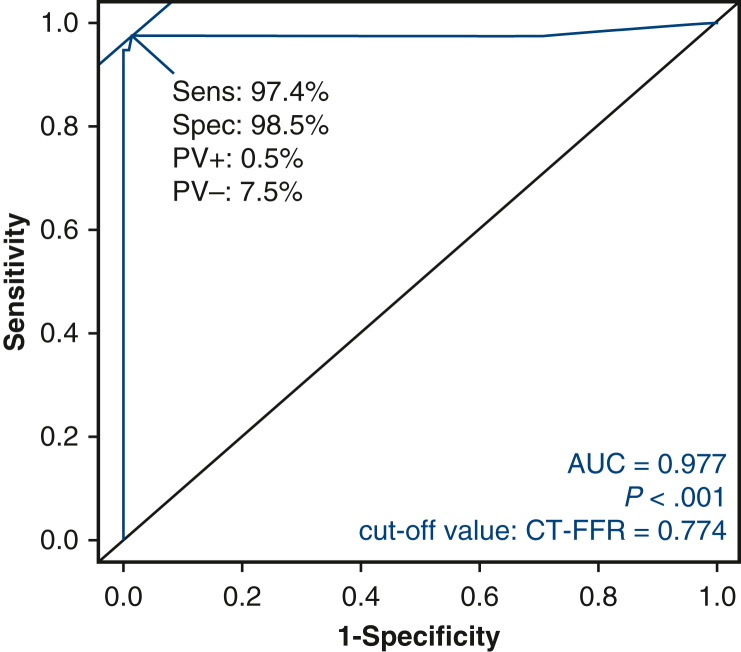
In total, 234 anastomoses were performed in 74 patients (median 3 [interquartile range, 2, 4] anastomoses per patient). Postoperative (median 1 [interquartile range, 1, 2] day) angiograms showed that 196 (83.8%) anastomoses were perfectly patent, 25 (10.7%) anastomoses were bidirectionally competitive, 12 (5.1%) anastomoses were unidirectionally competitive, and 1 (0.4%) anastomosis was occluded. Median CT-FFR values of the coronary arteries with perfectly patent, bidirectionally competitive, and unidirectionally competitive grafts were 0.658 (interquartile range, 0.500, 0.725), 0.809 (interquartile range, 0.789, 0.855), and 0.849 (interquartile range, 0.833, 0.865), respectively. The cutoff value of CT-FFR predicting competitive graft flow was 0.774 (sensitivity, 97.4%; specificity, 98.5% [area under the curve 0.977; P < .001]).
Conclusions
The diagnostic accuracy of CT-FFR for predicting competitive graft flow after CABG was high, and CT-FFR could be used as a guide for predicting functional coronary artery stenosis in surgical revascularization.
Key Words: coronary artery bypass grafts, CABG, FFR, CT-FFR
Graphical Abstract
CT-FFR could be used in prediction of a competitive flow after CABG.
Central Message.
The diagnostic accuracy of CT-FFR for predicting competitive graft flow after CABG is high, and it could be used for predicting a functional coronary stenosis in patients undergoing CABG.
Perspective.
CT-FFR could provide surgeons with more information of patients undergoing CABG, and it could be used as a guide for surgical revascularization for predicting functional stenosis and thereby maintaining long-term graft patency.
Revascularization of diseased stenotic coronary arteries in patients undergoing coronary artery bypass grafting (CABG) is commonly determined on the basis of the anatomic severity of coronary artery stenosis shown on invasive coronary angiography (ICA). Although ICA has been the gold standard for the diagnosis of coronary artery disease (CAD), it does not provide information on the functional assessment of stenosis severity. The 2018 European Guidelines for myocardial revascularization recommended fractional flow reserve (FFR) measurement to assess the hemodynamic relevance of angiographically intermediate-grade stenosis when evidence of ischemia is not available (Class I, Level of Evidence A).1 However, the clinical implementation of FFR-informed treatment decision-making is relatively limited because FFR measurement during ICA is invasive and associated with potential complications related to coronary vessel instrumentation.2 The FFR value derived from standard coronary computed tomography (CT) angiographic data sets by using advances in computational fluid dynamics (CFD) to calculate the pressure drop across a stenosis has been validated as a reliable method for noninvasive detection of lesion-specific ischemia compared with invasive FFR.3, 4, 5
When the target coronary artery stenosis is less than severe and is not functionally significant in patients undergoing CABG, an equilibrium is formed between the native coronary artery flow and the bypass graft flow at the anastomosis, and competitive flow may develop in the bypass graft.6,7 Development of competitive flow is frequently observed on early postoperative angiograms in patients who have received a composite graft on the basis of the in situ internal thoracic artery (ITA),7,8 and competitive graft flow shown on early postoperative angiograms may suggest the target coronary artery stenosis not being functionally significant.
The aims of this study were (1) to compare preoperative CT-derived FFR (CT-FFR) values with early postoperative graft patency and (2) to establish the cut-off value of CT-FFR for predicting competitive graft flow shown on early angiograms after CABG using the ITA-based composite graft.
Methods
The institutional review board reviewed the study protocol and approved the present study as a minimal-risk retrospective study (approval number MJH 2023-10-023, approval date: November 15, 2023) that did not require individual patient consent on the basis of the institutional guidelines for waiving consent.
Patients and Preoperative CT Evaluation
Of the 87 patients who underwent isolated CABG using an ITA-based composite graft between October 2022 and September 2023, 74 patients who had preoperative CT-FFR values were included in the present study (Table 1). Patients who were scheduled to receive elective CABG underwent thoracoabdominal CT angiography as a preoperative evaluation protocol to assess their vascular status from the neck to femoral vessels because of their high atherosclerotic steno-occlusive risk.9 A third-generation dual-source CT scanner (SOMATOM FORCE; Siemens Healthineers) was used to obtain measurements. Cardiac CT angiography was performed simultaneously without additional contrast material in the following scan order: precontrast thoracoabdominal CT, calcium-scoring precontrast cardiac CT, postcontrast cardiac CT, and postcontrast thoracoabdominal CT angiographies. We informed the patients about the additional radiation and also explained the possible benefits of hemodynamic assessment of lesion severity on the basis of CT-FFR. Informed consent regarding the CT angiographies was obtained from all study patients. Nine patients did not undergo CT angiography because of an urgent or emergency condition precluding CT evaluation, and 1 patient who underwent redo-CABG did not undergo preoperative cardiac CT angiography. Of the 77 patients who underwent cardiac CT angiography, 2 patients whose CT-FFR values could not be calculated as the result of severe calcification and 1 patient whose CT-FFR value could not be calculated because of an artifact (presence of a temporary pacemaker) were excluded (Figure 1). Patients were managed by the use of N-acetylcysteine and hydration to reduce the incidence of contrast medium-induced nephropathy.
Table 1.
Preoperative characteristics and risk factors of the study patients
| Female | 12 (16.2%) |
| Age, y | 66.7 ± 10.9 |
| Body mass index, kg/m2 | 24.1 (22.6, 27.0) |
| Smoking status | |
| Never smoker | 15 (20.3%) |
| Former smoker | 36 (48.6%) |
| Current smoker | 23 (31.1%) |
| Hypertension | 59 (79.7%) |
| Diabetes mellitus | 43 (58.1%) |
| Dyslipidemia | 54 (73.0%) |
| History of stroke | 19 (25.7%) |
| Chronic renal failure | 10 (13.5%) |
| Chronic obstructive pulmonary disease | 5 (6.8%) |
| Atrial fibrillation | 8 (10.8%) |
| Peripheral vascular disease | 22 (29.7%) |
| History of percutaneous coronary intervention | 17 (23.0%) |
| Left ventricular dysfunction (ejection fraction ≤35%) | 16 (21.6%) |
| Preoperative diagnosis | |
| Stable angina | 20 (27.0%) |
| Unstable angina | 23 (31.1%) |
| Postinfarction angina | 29 (39.2%) |
| Acute myocardial infarction | 2 (2.7%) |
| Number of diseased vessels | |
| 3-vessel disease | 49 (66.2%) |
| 2-vessel disease | 15 (20.3%) |
| 1-vessel disease | 10 (13.5%) |
| Left main disease | 9 (12.2%) |
| EuroSCORE II | 1.4 (0.9, 3.5) |
| Risk of mortality (STS score) | 1.3 (0.5, 2.5) |
| SYNTAX score | 40.6 ± 16.5 |
EuroSCORE, European System for Cardiac Operative Risk Evaluation; STS, Society of Thoracic Surgeons.
Figure 1.
Flow diagram. CABG, Coronary artery bypass grafting; ITA, internal thoracic artery; CT, computed tomography; V-A ECMO, venoarterial extracorporeal membrane oxygenation; FFR, fractional flow reserve.
Calculation of CT-FFR Values
Preoperative cardiac CT results of the patients were sent to AiMEDiC as a Digital Imaging and Communications in Medicine file. CT-FFR values were obtained by software (HeartMed+; AiMEDiC) that extracts a 3-dimensional model of coronary arteries using CT images and computes FFR values throughout the entire coronary arteries using CFD to calculate the pressure drop across a stenosis. The computational method is determined on the basis of the modeling approach to compute patient-specific FFR and uses CFD for the 3-dimensional, patient-specific geometry of coronary arteries coupled with the lumped parameter model for the micro- and venous compartment of the coronary vascular system.5 Of 77 patients who underwent preoperative cardiac CT angiography, CT-FFR values could not be calculated from preoperative CT angiography as the result of severe calcification or pacemaker artifact in 3 patients (Figure 1). The postoperative angiographic evaluation results were kept blind to AiMEDiC.
Operative Procedures
The ITA was harvested using a semi-skeletonization technique, in which the whole length of ITA along with accompanying veins and surrounding tissue were mobilized from the endothoracic fascia. The saphenous vein (SV) harvested with a no-touch (NT) technique was used commonly as the second graft of choice. The SV from a lower leg was preferred to the upper leg SV to decrease the possibility of size mismatch with native coronary arteries or ITA. The SV harvest was initiated after systemic heparinization during harvest of the ITA. It was performed using an open technique with 2 or 3 interrupted skin incisions along the SV course, with 1 to 2 cm of intervening skin bridges. The NT SV pedicle was harvested along with an approximately 3-mm margin of adjacent adipose tissues on both sides of the SV and thin layers of adherent connective tissues posteriorly, using an electrothermal bipolar-activated device (Ligasure; Medtronic) to minimize thermal injury. Immediately after the harvest and with no pharmacologic treatment, the reversed SV was anastomosed to the side of the ITA when constructing a composite graft.
Off-pump CABG was performed in all the patients. When the left ITA-based SV Y-composite graft was constructed, the left anterior descending coronary artery territory commonly was revascularized first by using the left ITA. The left circumflex coronary artery territory was then revascularized using the SV as a composite graft, followed by the right coronary artery territory. A sequential anastomotic technique was used for complete revascularization when more than 2 coronary arterial anastomoses were needed. All diseased left coronary artery territories with ≥70% stenosis diameter and right coronary artery territories with ≥90% stenosis shown on ICA were considered for revascularization. Transit-time flow measurement (Medi-Stim AS) to verify the anastomosis status was performed after each anastomosis and just before the pericardial closure. All patients received preoperative aspirin therapy (100 mg daily) until the day of surgery and resumed postoperatively as soon as possible (usually postoperative day 1). Clopidogrel (75 mg daily) was added simultaneously to the aspirin for 1 year postoperatively. If the patient had a high blood level of low-density lipoprotein cholesterol (>70 mg/dL), statin therapy was initiated and maintained postoperatively.
Postoperative Angiographic Evaluation
Early postoperative follow-up angiograms were performed as a standard postoperative evaluation strategy for patients undergoing CABG and included selective graft and native coronary angiograms. Patients who refused angiographic evaluation, or had aggravated renal function impairment, were excluded from the angiographic follow-up; however, patients receiving renal-replacement therapy were included in the angiographic follow-up. Competitive graft flow was frequently seen in examination of early postoperative angiograms of patients who received an ITA-based composite graft. Postoperative angiographic findings of graft flow were categorized as perfectly patent, bidirectionally competitive, unidirectionally competitive, and occluded.8,10 A perfectly patent graft flow was defined as excellent graft flow with unimpaired run-off. A bidirectionally competitive graft flow was defined as graft and grafted coronary artery flow clearly opacified by graft angiography and well-visualized graft flow by native coronary angiography. A unidirectionally competitive graft flow was defined as graft and grafted coronary artery flow not opacified by graft angiography but well-visualized graft flow by native coronary angiography. An occluded graft flow was defined as graft and grafted coronary artery flow not opacified by graft angiography and unvisualized graft flow by native coronary angiography (Figure 2).
Figure 2.
I. Perfectly patent SV composite graft on the basis of the left ITA on early postoperative angiogram in a 55-year-old-man. The left ITA was anastomosed to the LAD (black thick arrows), and the SV graft was anastomosed to the diagonal (black arrowheads), first OM (white arrowheads), and PD (white thick arrows) arteries in a sequential manner. All the grafts showed excellent graft flow with unimpaired run-off. II. A and B, Schematic figure of bidirectionally competitive graft flow. Graft and grafted coronary artery flow are clearly opacified by graft angiography (red arrow and arrowhead), and graft flow is also visualized by native coronary angiography (blue arrow and arrowhead). C and D, Bidirectionally competitive SV composite graft on the basis of the left ITA on early postoperative angiogram in a 68-year-old-man. The left ITA was anastomosed to the LAD, and the SV graft was anastomosed to the RI (black thin arrows), OM, and PL (white thin arrows) arteries in a sequential manner. C, The SV graft anastomosed to the RI and grafted RI flow were clearly opacified by graft angiography.D, The RI flow and the SV graft distal to the RI anastomosis were also well visualized by native left coronary angiography. III. A and B, Schematic figure of unidirectionally competitive graft flow. Graft and grafted coronary artery flow are not opacified by graft angiography (red arrow and arrowhead), but graft flow is visualized by native coronary angiography (blue arrow and arrowhead). C and D, Unidirectionally competitive SV composite graft on the basis of the left ITA on early postoperative angiogram in a 75-year-old-man. The left ITA was anastomosed to the LAD, and the SV graft was anastomosed to the OM and PD arteries in a sequential manner. C, The SV graft anastomosed to the PDA and grafted PDA flow were not opacified by graft angiography. D, The PDA flow and the retrograde SV graft from the PDA were well visualized by native right coronary angiography. SV, Saphenous vein; ITA, internal thoracic artery; LAD, left anterior descending; OM, obtuse marginal; PD, posterior descending; RI, ramus intermedius; PL, posterolateral.
Statistical Analyses
Statistical analysis was performed with R software, version 4.2.2 (R Foundation for Statistical Computing). Continuous data are expressed as the mean ± standard deviation for normally distributed variables or as medians (interquartile ranges) for nonnormally distributed variables, according to the Shapiro-Wilk test; and categoric data were expressed as counts (percentages). One-way analysis of variance with Tukey honestly significant difference post hoc tests were performed to compare preoperative CT-FFR values in coronary arteries, which showed perfectly patent, bidirectionally competitive, and unidirectionally competitive graft flow. Receiver operating characteristic curve analysis of preoperative CT-FFR values for predicting postoperative angiographic competitive graft flow was performed, and cutoff values of preoperative CT-FFR values and area under the curve were identified (Epi package and pROC package).
Results
Operative Data
The CABG procedures were performed using in situ left ITA-based SV composite grafting (n = 71), in situ right ITA-based SV composite grafting (n = 2), and in situ left ITA-based right ITA composite grafting (n = 1). A total of 234 anastomoses were performed in 74 patients (median 3 [2, 4] anastomoses per patient).
Postoperative Angiographic Results
Postoperative (median 1 [1, 2] day) angiograms were performed in all study patients and showed 196 (83.8%) perfectly patent anastomoses, 37 (15.8%) competitive anastomoses (25 [10.7%] bidirectionally competitive and 12 [5.1%] unidirectionally competitive), and 1 (0.4%) occluded anastomosis. Median CT-FFR values of the coronary arteries with perfectly patent, bidirectionally competitive, and unidirectionally competitive graft flow were 0.658 [0.500, 0.725], 0.809 [0.789, 0.855], and 0.849 [0.833, 0.865], respectively. The CT-FFR values among the 3 groups (perfectly patent, bidirectionally competitive, and unidirectionally competitive graft flow) were significantly different (P < .001), and post hoc analysis revealed that the differences were statistically significant between the perfectly patent and bidirectionally competitive grafts, and between the perfectly patent and unidirectionally competitive grafts (Figure 3). The cutoff value of CT-FFR in predicting competitive graft flow was 0.774 (sensitivity, 97.4%; specificity, 98.5%) (area under the curve = 0.977; P < .001) (Figure 4).
Figure 3.
Tukey honestly significant difference post hoc test after the analysis of variance of preoperative computed tomography–derived fractional flow reserve values among the perfectly patent, bidirectionally competitive, and unidirectionally competitive grafts.
Figure 4.
Receiver operating characteristic curve of preoperative computed tomography–derived fractional flow reserve (CT-FFR) values in predicting graft flow in early postoperative angiography. PV, Predictive valve; AUC, area under the curve.
Discussion
The present study demonstrated 2 main findings. First, preoperative CT-FFR values were significantly lower in the coronary arteries with perfectly patent grafts than in those with competitive grafts after CABG using the ITA-based composite graft. Second, the diagnostic accuracy of preoperative CT-FFR values for predicting competitive graft flow was high, with a cutoff value of 0.774.
The role of CABG is to relieve a patient's anginal symptoms and to improve survival. In order to accomplish this, CABG should be performed for those coronary arteries that are associated with functionally or hemodynamically significant stenoses. When the target coronary artery stenosis is not functionally significant, competitive flow may develop in the bypass graft and negatively affect the graft patency.6,7 However, ICA, the gold standard for the diagnosis of CAD, does not provide information on the functional or hemodynamic assessment of lesion severity. Currently, invasive pressure-derived FFR is the established reference standard for the functional assessment of CAD, and the FFR threshold of 0.80 is accepted for defining hemodynamically relevant lesions.1,11, 12, 13 In the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) study, FFR-guided percutaneous coronary intervention (PCI; using a cut-off ≤0.80 to indicate requirement for PCI) reduced major adverse cardiac events at 12 months with a lower number of stented arteries and less resource use compared with angiography-guided PCI.11,14 FFR-guided CABG was associated with a lower number of graft anastomoses, greater graft patency rate at 3 years, and a significant reduction in the rate of overall death or myocardial infarction at 6-year follow-up as compared with angiography-guided CABG surgery.15,16 In the Impact of Preoperative FFR on Arterial Bypass Graft Function (IMPAG) study, authors found a significant association between the preoperative FFR measurement of the target vessel and the anastomotic functionality at 6 months postoperatively, with a cut-off of 0.78, and suggested integration of FFR measurement into the preoperative diagnostic workup before CABG leads to improved anastomotic graft function.17 However, the clinical implementation of FFR-informed treatment decision-making is relatively limited because of its invasiveness, potential procedural complications, additional use of a hyperemic agent, duration of the procedure, limitation in small vessel disease, and cost.2,18
The FFR derived from standard coronary CT angiographic data sets (CT-FFR) by using advanced computational analytic approaches enables combined anatomic and hemodynamic assessment of a coronary lesion by a single noninvasive test and has been validated as a reliable method for the noninvasive detection of lesion-specific ischemia in comparison with invasive FFR.3,4,19,20 A recent meta-analysis that included CT-FFR data from 11 prospective studies and 12 retrospective studies demonstrated a pooled sensitivity and specificity of 0.85 (95% confidence interval, 0.82-0.87) and 0.81 (95% confidence interval, 0.76-0.85) to detect lesion-specific ischemia with invasive FFR as the reference standard.21 In addition to the noninvasive and cost-effective advantages, CT-FFR has an additional advantage in that the values are provided throughout the coronary arterial tree from the ostium to the distal coronary artery. Because surgical revascularization is commonly performed in the distal coronary arteries, CT-FFR values would be beneficial to physiological assessment before CABG. However, the optimal cutoff value of CT-FFR and diagnostic algorithm for decision-making by CT-FFR in clinical practice have not yet been defined. Previous studies on CT-FFR have adopted the same cutoff value as the invasive FFR, ≤0.80 for detecting hemodynamically significant ischemia.21 One recent study showed that CT-FFR overestimated hemodynamic significance as compared with invasive FFR and suggested CT-FFR ≤0.75 might be a better cutoff value for detecting hemodynamically significant ischemia than CT-FFR ≤0.80.22
In the present study, the calculation of CT-FFR was performed by software (HeartMed+; AiMEDiC). The calculation of CT-FFR was determined on the basis of vessel length of coronary arteries, which requires less computational time and excludes the possibility of error from the segmentation of left ventricular muscle, compared with previous efforts, determined on the basis of heart muscle volume and scaling law to calculate CT-FFR.5 The present study included patients who underwent isolated CABG using an ITA-based composite graft. A CABG strategy using a composite graft on the basis of the in situ ITA has advantages such as avoiding aortic manipulation and allowing efficient conduit use; however, composite grafts are susceptible to the effect of flow competition with the native coronary artery when used for a less than severely stenosed target vessel.8 In a previous study, which included 806 patients who had received NT SV Y-composite grafts on the basis of the left ITA and who were evaluated by early postoperative angiography, unidirectional competitive graft flow was observed in 102 distal anastomoses (102 of 3039 [3.4%]) of 94 patients (94 of 806 [11.7%]).8 The present study analyzed preoperative CT-FFR values to determine whether they are useful in predicting the competitive flow of the bypass graft shown on early postoperative angiograms, and CT-FFR >0.774 predicted competitive graft flow with a sensitivity of 97.4% and a specificity of 98.5%.
The surgeon will be able to select the appropriate anastomosis site on the basis of the CT-FFR values of coronary artery, and we look forward to the CT-FFR providing surgeons with valuable information and transforming clinical practice in the future.
Study Limitations
The present study has limitations that must be recognized. First, this was a retrospective observational study from a single institution, and the number of the study patients was relatively small. Second, the majority of patients underwent CABG procedures using an ITA-based SV composite graft, and this might be a limitation to extrapolate the results of the present study to those who underwent CABG procedures using a total arterial composite grafts or aortocoronary vein grafts. Third, the unit of analysis was an anastomosis and graft configuration or type of anastomoses (sequential vs individual) was not considered in the analysis. Fourth, long-term patency of the graft and its relation to the CT-FFR was not analyzed, and further study is warranted. Fifth, a potential hazard, such as contrast medium-induced nephropathy, should be considered while performing CT angiography, although none of the study patients developed contrast medium-induced nephropathy. Patients were managed by the use of N-acetylcysteine and hydration to reduce the incidence of contrast medium-induced nephropathy, and patients with renal dysfunction were managed in cooperation with the nephrologists.
Conclusions
In conclusion, the diagnostic accuracy of CT-FFR for predicting competitive flow after CABG using an ITA-based composite graft was high, and CT-FFR could be used as a guide for surgical revascularization for predicting functional stenosis and thereby maintaining long-term graft patency (Figure 5).
Figure 5.
Diagnostic accuracy of CT-FFR for predicting competitive flow after CABG using an ITA-based composite graft was high, and CT-FFR could be used as a guide for surgical revascularization. CABG, Coronary artery bypass grafting; ITA, internal thoracic artery; CT, computed tomography; FFR, fractional flow reserve; PV, predictive valve.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Neumann F.-J., Sousa-Uva M., Ahlsson A., et al. ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 2.Pothineni N.V., Shah N.N., Rochlani Y., et al. US trends in inpatient utilization of fractional flow reserve and percutaneous coronary intervention. J Am Coll Cardiol. 2016;67(6):732–733. doi: 10.1016/j.jacc.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 3.Koo B.-K., Erglis A., Doh J.-H., et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms: results from the prospective multicenter DISCOVER-FLOW(diagnosis of ischemia-causing stenoses obtained via noninvasive fractional flow reserve) study. J Am Coll Cardiol. 2011;58(19):1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 4.Norgaard B.L., Leipsic J., Gaur S., et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease. The NXT trial (Analysis of coronary blood flow using CT angiography: next steps) J Am Coll Cardiol. 2014;63(12):1145–1155. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 5.Chung J.-H., Lee K.E., Nam C.-W., et al. Diagnostic performance of a novel method for fractional flow reserve from noninvasive computed tomography angiography (NOVEL-FLOW study) Am J Cardiol. 2017;120(3):362–368. doi: 10.1016/j.amjcard.2017.04.057. [DOI] [PubMed] [Google Scholar]
- 6.Sabik J.F., III, Lytle B.W., Blackstone E.H., Khan M., Houghtaling P.L., Cosgrove D.M. Does competitive flow reduce internal thoracic artery graft patency? Ann Thorac Surg. 2003;76(5):1490–1497. doi: 10.1016/s0003-4975(03)01022-1. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima H., Kobayashi J., Toda K., et al. Angiographic evaluation of flow distribution in sequential and composite arterial grafts for three vessel disease. Eur J Cardiothorac Surg. 2012;41(4):763–768. doi: 10.1093/ejcts/ezr057. [DOI] [PubMed] [Google Scholar]
- 8.Kim M.-S., Hwang S.W., Kim K.-B. Competitive flow in vein composite grafts based on the left internal thoracic artery: early and 1-year angiographic analyses. Semin Thorac Cardiovasc Surg. 2023;35(3):483–492. doi: 10.1053/j.semtcvs.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Na K.J., Choi J.W., Hwang H.Y., Kim K.-B. Usefulness of thoraco-abdominal computed tomography angiography in coronary artery bypass patients. Eur J Cardiothorac Surg. 2018;54(6):1110–1115. doi: 10.1093/ejcts/ezy235. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.H., Oh H.C., Choi J.W., Hwang H.Y., Kim K.-B. No-touch saphenous vein harvesting may improve further the patency of saphenous vein composite grafts: early outcomes and 1-year angiographic results. Ann Thorac Surg. 2017;103(5):1489–1497. doi: 10.1016/j.athoracsur.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Tonino P.A.L., De Bruyne B., Pijls N.H.J., et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 12.De Bruyne B., Fearon W.F., Pijls N.H.J., et al. Fractional flow-reserve guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 13.Adjedj J., De Bruyne B., Flore V., et al. Significance of intermediate values of fractional flow reserve in patients with coronary artery disease. Circulation. 2016;133(5):502–508. doi: 10.1161/CIRCULATIONAHA.115.018747. [DOI] [PubMed] [Google Scholar]
- 14.Sels J.-W.E.M., Tonino P.A.L., Siebert U., et al. Fractional flow reserve in unstable angina and non-ST-segment elevation myocardial infarction: experience from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) study. JACC Cardiovasc Interv. 2011;4(11):1183–1189. doi: 10.1016/j.jcin.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Toth G., De Bruyne B., Casselman F., et al. Fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circulation. 2013;128(13):1405–1411. doi: 10.1161/CIRCULATIONAHA.113.002740. [DOI] [PubMed] [Google Scholar]
- 16.Fournier S., Toth G.G., De Bruyne B., et al. Six-year follow-up of fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circ Cardiovasc Interv. 2018;11(6) doi: 10.1161/CIRCINTERVENTIONS.117.006368. [DOI] [PubMed] [Google Scholar]
- 17.Glineur D., Grau J.B., Etienne P.-Y., et al. Impact of preoperative fractional flow reserve on arterial bypass graft anastomotic function: the IMPAG trial. Eur Heart J. 2019;40(29):2421–2428. doi: 10.1093/eurheartj/ehz329. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi R., Budoff M.J. Noninvasive FFR derived from coronary CT angiography in the management of coronary artery disease: technology and clinical update. Vasc Health Risk Manag. 2016;12:269–278. doi: 10.2147/VHRM.S79632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakazato R., Park H.-B., Berman D.S., et al. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ Cardiovasc Imaging. 2013;6(6):881–889. doi: 10.1161/CIRCIMAGING.113.000297. [DOI] [PubMed] [Google Scholar]
- 20.Coenen A., Kim Y.-H., Kruk M., et al. Diagnostic accuracy of a machine-learning approach to coronary computed tomographic angiography-based fractional flow reserve: result from the MACHINE Consortium. Circ Cardiovasc Imaging. 2018;11(6) doi: 10.1161/CIRCIMAGING.117.007217. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y., Mao M., Xiang R., et al. Diagnostic performance of computed tomography-based fraction flow reserve in identifying myocardial ischemia caused by coronary artery stenosis: a meta-analysis. Hellenic J Cardiol. 2022;63:1–7. doi: 10.1016/j.hjc.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura-Nakano Y., Kawaji T., Shiomi H., et al. Optimal cutoff value of fractional flow reserve derived from coronary computed tomography angiography for predicting hemodynamically significant coronary artery disease. Circ Cardiovasc Imaging. 2019;12(8) doi: 10.1161/CIRCIMAGING.119.008905. [DOI] [PubMed] [Google Scholar]