Abstract
Objective
Randomized data support transplantation of hearts from donors after circulatory death. This may lead to a sizeable increase in the donor pool. Regional variations in donors after circulatory death heart use were examined to help elucidate barriers to donor pool expansion.
Methods
The United Network for Organ Sharing deceased donor dataset was queried for adult (age ≥ 18 years) donors after circulatory death donors of at least 1 organ between January 2020 and December 2023. Donors were stratified by the extent their respective cardiac allografts progressed through the donation process. United Network for Organ Sharing region-level use rates and annual trends were assessed.
Results
Of 17,239 adult donors after circulatory death donors who donated at least 1 organ for transplant during the study period, 1196 (9.4%) were heart donors. Regional donors after circulatory death heart donor pursuit rates ranged from 97% to 100%, consent attainment rates from 94% to 99%, and heart recovery rates from 5% to 10%. The transplantation rate of recovered organs ranged from 90% to 97%. Multivariable logistic regression demonstrated United Network for Organ Sharing region to be independently associated with donors after circulatory death heart use after controlling for baseline differences in donor risk.
Conclusions
Transplantation of donors after circulatory death heart allografts has increased in the United States since 2020, but the overall number of hearts procured and transplanted from donors after circulatory death donors remains low. The operational barriers to transplantation of donors after circulatory death hearts require further investigation. Further, significant regional variation exists regarding rates of progression of donors after circulatory death hearts through the donation process. Sharing of successful practices among Organ Procurement Organizations and transplant centers will facilitate maximal use of this new donor pool.
Key Words: donation after circulatory death, donor pool, heart transplantation, regional variation
Regional variation in DCD donor heart use.
Central Message.
Significant regional variation exists regarding rates of DCD heart use. Sharing of successful practices among OPOs and transplant centers will help to maximally use this new donor pool.
Perspective.
Only 14% of heart transplants in the United States in 2023 were performed using allografts from DCD donors. We identified significant regional variation in rates of DCD donor heart use. Sharing of successful practices among OPOs and transplant centers as well as an improved ability to predict which donors will progress to cardiac death and which allografts will be more prone to PGD will help to maximally use this new donor pool.
The demand for heart transplantation continues to outpace the supply of donor allografts. Given this supply demand mismatch as well as the encouraging early experience from multiple international sites, donation after circulatory death (DCD) heart transplantation was introduced in the United States in 2019 to expand the available donor pool.1, 2, 3 Recently published randomized data have further supported the safety and efficacy of transplanting hearts from DCD donors, with early short-term recipient outcomes comparable to those associated with donation after brain death (DBD) donors.4 These findings have been recapitulated in multiple single- and multicenter retrospective studies.5,6
Based on the United Kingdom experience with DCD heart transplantation as well as an examination of the historical US donor pool, it was estimated that the adoption of DCD heart transplantation may ultimately lead to an increase in US heart transplant activities by 25% to 30%.7,8 Although DCD heart transplantation has increased significantly in the United States since 2019, in 2023 it represented approximately 14% of heart transplants performed nationally.9 We aimed to (1) examine regional variations in DCD heart use to help elucidate barriers to donor pool expansion and (2) identify whether regional differences exist in DCD heart use independent of variations in baseline donor risk.
Material and Methods
Data Source
The United Network for Organ Sharing (UNOS) Organ Procurement and Transplantation Network provided Standard Transplant Analysis and Research files containing deidentified transplant data from October 1987 to December 2023. The database includes prospectively collected donor and recipient data for all organ transplants performed in the United States during this period.
Study Population
The UNOS registry was queried for all adult (age ≥ 18 years) DCD donors from January 2020 to December 2023 with at least 1 organ recovered for transplant. This time period was selected because it coincides with the adoption of DCD heart transplantation in the United States. Donors with missing heart disposition in the registry were excluded.
Data Analysis
Descriptive analysis of DCD donor characteristics was performed, stratified by heart donation status. Because information on the reperfusion method has not been collected by the UNOS, direct procurement and perfusion DCD organs were distinguished from those procured using normothermic regional perfusion (NRP) using the length of time from circulatory standstill to crossclamp for cold cardioplegia administration, as previously described.10 Direct procurement and perfusion was defined as a donor death to crossclamp time of 30 minutes or less, and NRP was defined as a death to crossclamp time greater than 30 minutes.
Data are presented as median (interquartile range) for continuous variables and percent (count) for categorical variables, unless otherwise specified. Unadjusted comparisons between cohorts were performed using the Wilcoxon rank-sum test for continuous variables and the Pearson chi-square test or Fisher exact test for categorical variables, as appropriate. Donors were also stratified by the degree their respective cardiac allografts progressed through the donation process (donor pursuit, consent attainment, heart recovery, and transplantation). UNOS region-level donor center heart use rates were assessed, as well as annual trends during the study period. To determine whether regional variation in heart donor use was specific to DCD donors or reflected overall heart donor use, DCD heart use rates were indexed against DBD use rates. Adjusted logistic regression was used to identify donor factors independently associated with heart use and to examine the association between donor center UNOS region and heart use independent of baseline risk. Covariates were selected a priori based on available clinically relevant variables within the dataset. Continuous variables were modeled using restricted cubic splines and then subsequently transformed to piecewise linear splines for ease of interpretation in the multivariable model.
All statistical analyses were performed using R version 4.3.0 (R Foundation for Statistical Computing). This analysis was deemed exempt by the Duke University Institutional Review Board.
Results
Donor Characteristics
In total, 17,239 DCD donors met inclusion criteria, among whom 1196 (9.4%) were heart donors. Baseline donor demographic and clinical characteristics are presented in Table 1, stratified by heart donation status. DCD heart donors were more likely male and younger, and had a lower median body mass index (BMI) compared with nonheart DCD donors (P < .05). Heart donors were also significantly less likely to have diabetes, hypertension, or a smoking history (P < .05). Heart donors were less likely to have a cerebrovascular/stroke cause of death but were more likely to have died of head trauma (P < .05). Finally, heart donors were more likely to have also been concomitant lung, kidney, and liver donors and were significantly more likely to have undergone NRP (P < .05).
Table 1.
Donation after circulatory death donor characteristics
| Characteristic | Nonheart donor |
Heart donor |
P value |
|---|---|---|---|
| (n = 16,043) | (n = 1196) | ||
| Male sex | 10,329 (64.4%) | 1009 (84.4%) | <.001 |
| Donor age (median y, IQR) | 50 (39-58) | 31 (25-37) | <.001 |
| Donor BMI (median kg/m2, IQR) | 29.0 (24.6-34.5) | 26.9 (23.8-31.1) | <.001 |
| Donor race/ethnicity | .08 | ||
| White | 12,453 (77.7%) | 919 (76.9%) | |
| Black | 1490 (9.3%) | 109 (9.1%) | |
| Hispanic | 521 (3.2%) | 28 (2.3%) | |
| Other | 521 (3.2%) | 28 (2.3%) | |
| Donor history | |||
| Cigarette use | 4806 (30.0%) | 127 (10.6%) | <.001 |
| Cocaine use | 3346 (20.9%) | 282 (23.6%) | .028 |
| Alcohol abuse | 4836 (30.1%) | 330 (27.6%) | .068 |
| Diabetes | 2834 (17.7%) | 38 (3.2%) | <.001 |
| Hypertension | 7277 (45.4%) | 157 (13.1%) | <.001 |
| Cancer | 681 (4.2%) | 16 (1.3%) | <.001 |
| Donor creatinine (median mg/dL, IQR) | 0.8 (0.6-1.4) | 0.8 (0.6-1.0) | <.001 |
| Donor bilirubin (median mg/dL, IQR) | 0.6 (0.4-1.0) | 0.6 (0.4-0.9) | .91 |
| LVEF (median %, IQR) | 60 (50-65) | 63 (60-66) | <.001 |
| Donor cause of death | <.001 | ||
| Anoxia | 8391 (52.3%) | 580 (48.5%) | |
| Cerebrovascular/stroke | 3497 (21.8%) | 85 (7.1%) | |
| Head trauma | 2807 (17.5%) | 488 (40.8%) | |
| CNS tumor | 26 (0.2%) | 4 (0.3%) | |
| Other | 1322 (8.2%) | 39 (3.3%) | |
| ABO blood type | <.001 | ||
| A | 6332 (39.5%) | 358 (29.9%) | |
| B | 1706 (10.6%) | 100 (8.4%) | |
| AB | 586 (3.7%) | 6 (0.5%) | |
| O | 7411 (46.2%) | 732 (61.2%) | |
| NRP | 423 (2.6%) | 367 (30.7%) | <.001 |
| Lung donor | 673 (4.2%) | 189 (15.8%) | <.001 |
| Kidney donor | 11,423 (71.2%) | 1131 (94.6%) | <.001 |
| Liver donor | 3522 (22.0%) | 706 (59.0%) | <.001 |
IQR, Interquartile range; BMI, body mass index; LVEF, left ventricular ejection fraction; CNS, central nervous system; NRP, normothermic regional perfusion.
Donor Disposition
The degree to which DCD donors progressed through the heart donation process, stratified by UNOS region, is presented in Table 2. Rates of donor pursuit, defined as an Organ Procurement Organization (OPO) requesting consent for heart donation, ranged from 97% to 100%. Consent for heart donation was attained for 94% to 99% of donors for whom consent was requested. When consent was attained, heart recovery occurred in 5% to 10% of cases. Transplant rates for recovered donor hearts ranged from 90% to 97%.
Table 2.
Donation after circulatory death donor progression through the heart donation process
| UNOS region | Donor pursuit | Consent attainment | Heart recovery | Transplantation |
|---|---|---|---|---|
| 1 | 97.2% | 98.5% | 8.9% | 92.6% |
| 2 | 99.9% | 94.6% | 5.2% | 92.0% |
| 3 | 100.0% | 98.2% | 7.1% | 90.3% |
| 4 | 99.8% | 99.0% | 9.3% | 92.1% |
| 5 | 99.9% | 97.7% | 7.6% | 95.2% |
| 6 | 100.0% | 99.0% | 7.3% | 89.8% |
| 7 | 99.7% | 98.6% | 6.3% | 90.1% |
| 8 | 99.0% | 97.9% | 8.4% | 96.9% |
| 9 | 98.6% | 94.0% | 9.2% | 93.7% |
| 10 | 99.8% | 97.9% | 6.7% | 95.9% |
| 11 | 98.8% | 96.5% | 9.7% | 91.1% |
UNOS, United Network for Organ Sharing.
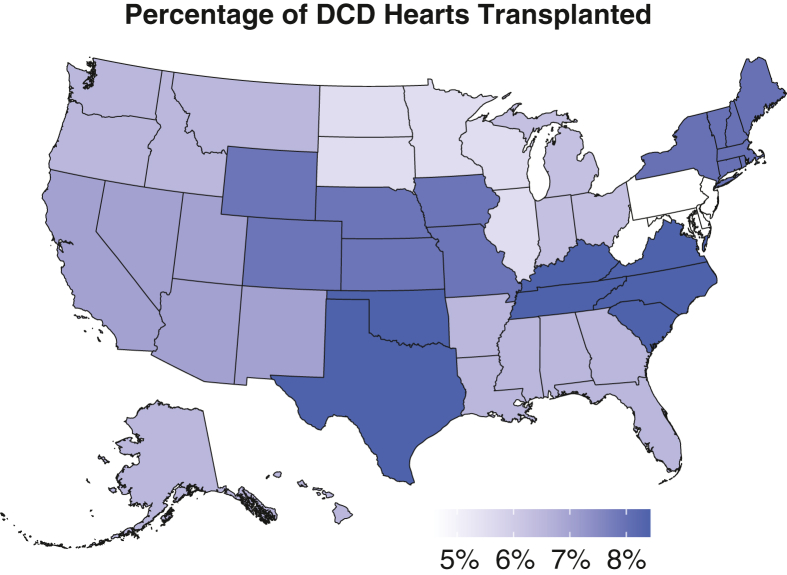
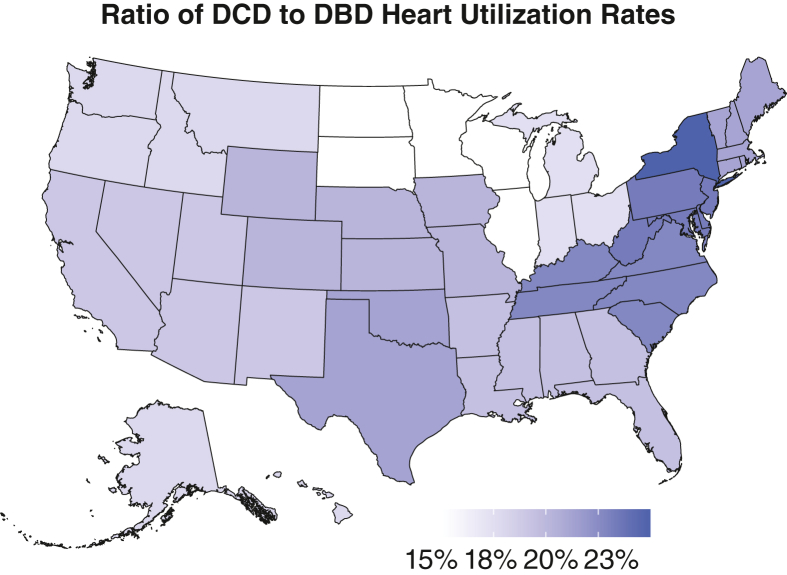
The regional variability in overall DCD heart transplant rates during the study period is illustrated in Figure 1. Donor centers in UNOS Regions 4 and 11 had the highest rates of DCD heart use (>8.4% of all DCD donors), whereas Region 2 had the lowest use rate (∼4.6%). The ratio of DCD to DBD donor heart use rates for each UNOS region is presented in Figure 2, which ranged from approximately 15% to 25%.
Figure 1.
Regional variation in DCD donor heart use rates from 2020 to 2023. DCD, Donation after circulatory death.
Figure 2.
Regional variation in ratio of DBD to donation after DCD donor heart use rates from 2020 to 2023. DCD, Donation after circulatory death; DBD, donation after brain death.
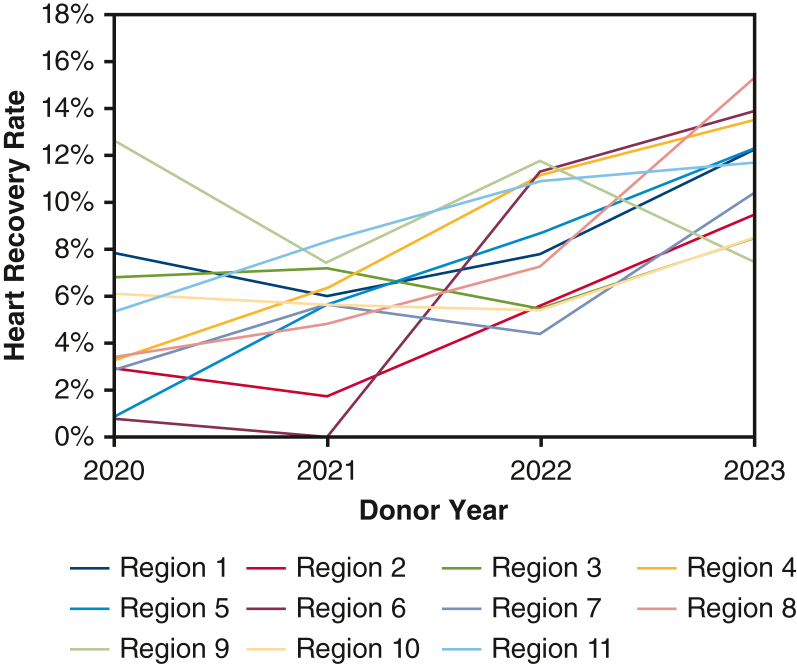
Figure 3 contains the annual trend of DCD heart recovery rates among donors in whom consent for heart donation was obtained, stratified by region. For most UNOS regions, the rate of heart recovery increased from 2020 to 2023, although for many regions there was significant year-to-year variability.
Figure 3.
Annual DCD heart recover rates from 2020 to 2023 stratified by UNOS region.
Adjusted Analysis
The multivariable logistic regression model for heart use is presented in Table 3. Donor factors independently associated with heart transplantation included male sex (odds ratio [OR], 2.81, 95% CI, 2.38-3.33) and increasing BMI less than 25 (OR, 1.11 per unit, 95% CI, 1.07-1.16). Factors associated with a lower likelihood of transplantation included cigarette use (OR, 0.74, 95% CI, 0.60-0.91), hypertension (OR, 0.61, 95% CI, 0.50-0.74), diabetes (OR, 0.45, 95% CI, 0.32-0.64), increasing age (OR, 0.82 per 5 years under 40, 95% CI, 0.78-0.87; OR, 0.36 per 5 years above 40, 95% CI, 0.30-0.42), and increasing BMI above 25 (OR, 0.88 per unit, 95% CI, 0.84-0.92). After controlling for these baseline risk factors, donor UNOS region remained a significant independent risk factor for heart use (P < .05).
Table 3.
Multivariable logistic regression model for donation after circulatory death donor heart use for transplantation
| Predictor | Odds ratio | 95% CI |
P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Donor characteristic | ||||
| Male sex | 2.81 | 2.38 | 3.33 | <.001 |
| Cigarette use | 0.74 | 0.60 | 0.91 | .005 |
| Hypertension | 0.61 | 0.50 | 0.74 | <.001 |
| Diabetes | 0.45 | 0.32 | 0.64 | <.001 |
| Age <40 (per 5 y) | 0.82 | 0.78 | 0.87 | <.001 |
| Age >40 (per 5 y) | 0.36 | 0.30 | 0.42 | <.001 |
| BMI <25 (per unit) | 1.11 | 1.07 | 1.16 | <.001 |
| BMI >25 (per unit) | 0.88 | 0.84 | 0.92 | <.001 |
| UNOS region (reference: 1) | ||||
| 2 | 0.44 | 0.29 | 0.65 | <.001 |
| 3 | 0.64 | 0.44 | 0.93 | .019 |
| 4 | 0.94 | 0.65 | 1.37 | .746 |
| 5 | 0.81 | 0.57 | 1.17 | .259 |
| 6 | 0.53 | 0.34 | 0.84 | .007 |
| 7 | 0.59 | 0.39 | 0.90 | .014 |
| 8 | 0.83 | 0.57 | 1.21 | .331 |
| 9 | 1.04 | 0.67 | 1.61 | .858 |
| 10 | 0.90 | 0.62 | 1.30 | .562 |
| 11 | 0.90 | 0.63 | 1.30 | .582 |
BMI, Body mass index; UNOS, United Network for Organ Sharing.
Discussion
Heart transplantation continues to be constrained by the availability of donor organs. To address this limitation, the heart transplant community has engaged in a multipronged effort to expand the potential donor pool through the use of extended criteria donors, longer warm ischemic times, ex vivo organ perfusion devices, and most recently through use of DCD donors.11, 12, 13, 14 Early international reports, as well as a recently published US randomized clinical trial, have demonstrated recipient outcomes comparable to those of DBD heart donors.4,6,7 Despite these promising results, DCD heart allografts remain underused. In this retrospective analysis of the UNOS heart transplant registry, we sought to identify regional variations in DCD heart allograft use through the various stages of the donation process and to assess whether these variations exist independent of baseline donor risk.
This analysis demonstrated that there were consistently high rates of DCD donor pursuit and consent attainment for heart donation, above 97% and 94%, respectively, across all UNOS regions during the study period. This is in comparison to an earlier analysis by Halpern and colleagues15 focused primarily on lung transplantation from 2011 to 2018, which noted a 94% lung consent attainment rate and concluded that DCD status was associated with significant risk aversion among OPOs. This difference may reflect growing OPO national experience with DCD donors or be a result of changing OPO behaviors in response to the 2021 Centers of Medicare and Medicaid Services new Final Rule on OPO performance.16
Although donor pursuit and consent attainment rates were universally high, DCD heart recovery rates demonstrated more regional variation and on average remained below 10% in all regions during the first 3 full years of US DCD heart transplantation. This is in comparison with an approximately 25% to 35% heart recovery rate for DBD donors.17 When examined annually, there was a clear trend toward significantly increased DCD heart recovery rates in 2023 compared with 2020, and possibly decreased regional variation, reflecting gradual adoption of DCD heart transplantation nationally during this time. In all but 1 region, recovery rates in 2023 were higher than in 2020. The 2023 recovery rates in some regions were double those in others, suggesting that significant geographic differences remain with respect to DCD heart organ offer acceptance practices.
The observation that consent rates remained high, recovery rates were low, but transplantation of recovered organs was high suggests that the variability in use is being driven at least in part by transplant center uncertainty regarding progression of the DCD donor to asystole and cardiac death within an appropriate timeframe. As the practice of DCD heart transplant moves from investigation to standard care, the maximal safe warm ischemic time is still to be defined. Currently, significant variability exists regarding the management of potential DCD donors around the time of withdrawal of care. Anesthesia practices, acceptable agonal and stand-off times, and the ability to use NRP all vary from donor hospital to hospital. Identifying opportunities for standardization of DCD donor management to accurately assess neurological status is key to changing these low percentages of acceptance and heart recovery. This is particularly important given the financial costs associated with nonrecovery of donor organs, especially when using third-party procurement services. It has also been well documented that DCD heart transplantation is associated with increased rates of primary graft dysfunction (PGD).18 The ability to more accurately predict which allografts will experience PGD may help increase DCD heart recovery rates as adoption of DCD heart transplantation is supported by a desire to expand the pool but limited by hesitation over the potentially increased risk of negative recipient outcomes.
Multivariable adjusted logistic regression identified multiple DCD donor characteristics independently associated with heart use. Several of these factors including sex, cigarette use, diabetes, and increasing age have been previously described as predictive of DBD donor heart nonuse as well.17 The UNOS region remained significantly associated with DCD donor heart use after adjusting for these donor characteristics suggesting that there are significant differences in DCD heart use geographically independent of variations in donor risk. The geographic variability observed in the ratio of DCD to DBD heart use rates also suggests that DCD regional heart use practices are independent from DBD practices.
Study Limitations
As a retrospective review of national registry data, this analysis has several limitations. First, although we did demonstrate significant regional variability in donor center DCD heart use rates, we did not have the granularity available to ascertain the underlying cause of these differences. The data did, however, reflect high rates of DCD donor pursuit and heart transplant consent attainment suggesting that regional differences in heart recovery rates are likely a result of variable transplant center rather than OPO practices. Of note, the registry only captures potential donors that were pursued by local OPOs and thus the size of the larger pool of patients for whom care was withdrawn but not pursued for organ donation cannot be ascertained. Second, DCD heart transplantation was first introduced in the United States in late 2019, at the height of the COVID-19 pandemic. Thus, some of the variability in DCD heart use rates nationally, especially early in the study period, may have been in at least in part the result of the variable impact of the pandemic on transplant activities in different healthcare systems. Because donors and recipients are often located in different UNOS regions, especially since the 2018 allocation system change, donor organ use in 1 region may not correlate exactly with transplant practices by recipient centers in that same region.19 High-volume transplant centers likely significantly influence use rates in surrounding regions; therefore, regional DCD heart use rates reflect the complex interplay between local and mid-distance transplant center practices. A future analysis focusing on the association between implanting center characteristics and DCD heart transplantation rates would help to further elucidate variations in practice and identify strategies for better donor pool use.
Conclusions
The volume of DCD heart transplantation has increased in the United States since 2020, but this pool of donor allografts likely remains underused. Significant regional variation exists regarding rates of progression of DCD hearts through the donation process. The operational barriers to transplantation of DCD hearts need to be investigated and resources applied where necessary. Sharing of successful practices among OPOs and transplant centers, standardizing donor management and assessment criteria, as well as improving our ability to predict which donors will progress to cardiac death within a timely fashion and which allografts will be more prone to PGD will help to maximally use this new donor pool.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/regional-variation-in-donation-7175.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Chew H.C., Iyer A., Connellan M., et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol. 2019;73(12):1447–1459. doi: 10.1016/j.jacc.2018.12.067. [DOI] [PubMed] [Google Scholar]
- 2.Messer S., Page A., Axell R., et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2017;36(12):1311–1318. doi: 10.1016/j.healun.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Messer S., Cernic S., Page A., et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2020;39(12):1463–1475. doi: 10.1016/j.healun.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Schroder J.N., Patel C.B., DeVore A.D., et al. Transplantation outcomes with donor hearts after circulatory death. N Engl J Med. 2023;388(23):2121–2131. doi: 10.1056/NEJMoa2212438. [DOI] [PubMed] [Google Scholar]
- 5.Kwon J.H., Ghannam A.D., Shorbaji K., et al. Early outcomes of heart transplantation using donation after circulatory death donors in the United States. Circ Heart Fail. 2022;15(12) doi: 10.1161/CIRCHEARTFAILURE.122.009844. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqi H.K., Trahanas J., Xu M., et al. Outcomes of heart transplant donation after circulatory death. J Am Coll Cardiol. 2023;82(15):1512–1520. doi: 10.1016/j.jacc.2023.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Messer S., Rushton S., Simmonds L., et al. A national pilot of donation after circulatory death (DCD) heart transplantation within the United Kingdom. J Heart Lung Transplant. 2023;42(8):1120–1130. doi: 10.1016/j.healun.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Jawitz O.K., Raman V., DeVore A.D., et al. Increasing the United States heart transplant donor pool with donation after circulatory death. J Thorac Cardiovasc Surg. 2020;159(5):e307–e309. doi: 10.1016/j.jtcvs.2019.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National data - OPTN. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/
- 10.Kwon J.H., Usry B., Hashmi Z.A., et al. Donor utilization in heart transplant with donation after circulatory death in the United States. Am J Transplant. 2024;24(1):70–78. doi: 10.1016/j.ajt.2023.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Samsky M.D., Patel C.B., Owen A., et al. Ten-year experience with extended criteria cardiac transplantation. Circ Heart Fail. 2013;6(6):1230–1238. doi: 10.1161/CIRCHEARTFAILURE.113.000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilic A., Hickey G., Mathier M., et al. Outcomes of adult heart transplantation using hepatitis C-positive donors. J Am Heart Assoc. 2020;9(2) doi: 10.1161/JAHA.119.014495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder J.N., Patel C.B., DeVore A.D., et al. Increasing utilization of extended criteria donor hearts for transplantation: the OCS Heart EXPAND Trial. JACC Heart Fail. 2024;12(3):438–447. doi: 10.1016/j.jchf.2023.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Jawitz O.K., Devore A.D., Patel C.B., Bryner B.S., Schroder J.N. EXPANDing the Donor Pool: quantifying the potential impact of a portable organ-care system for expanded criteria heart donation. J Card Fail. 2021;27(12):1462–1465. doi: 10.1016/j.cardfail.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Halpern S.E., McConnell A., Peskoe S.B., et al. A three-tier system for evaluation of organ procurement organizations’ willingness to pursue and utilize nonideal donor lungs. Am J Transplant. 2021;21(3):1269–1277. doi: 10.1111/ajt.16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organ procurement organizations conditions for coverage: revisions to Outcome measure requirements for organ procurement organizations. Fed Regist. 2021;86(20):7814. [Google Scholar]
- 17.Trivedi J.R., Cheng A., Gallo M., Schumer E.M., Massey H.T., Slaughter M.S. Predictors of donor heart utilization for transplantation in United States. Ann Thorac Surg. 2017;103(6):1900–1906. doi: 10.1016/j.athoracsur.2016.08.101. [DOI] [PubMed] [Google Scholar]
- 18.Ayer A., Truby L.K., Schroder J.N., et al. Improved outcomes in severe primary graft dysfunction after heart transplantation following donation after circulatory death compared with donation after brain death. J Card Fail. 2023;29(1):67–75. doi: 10.1016/j.cardfail.2022.10.429. [DOI] [PubMed] [Google Scholar]
- 19.Ganapathi A.M., Lampert B.C., Mokadam N.A., et al. Allocation changes in heart transplantation: what has really changed? J Thorac Cardiovasc Surg. 2023;165(2):724–733.e7. doi: 10.1016/j.jtcvs.2021.03.031. [DOI] [PubMed] [Google Scholar]