Abstract
Background
Circulatory support with a catheter-based microaxial flow pump (mAFP) plays a major role in the treatment of severe cardiogenic shock. In most patients who fail to recover while on temporary mechanical circulatory support (tMCS) and who are not eligible for heart transplantation, durable left ventricular assist device (dLVAD) implantation is usually considered a reliable option. This study aimed to describe the outcome of dLVAD therapy following mAFP support and to identify predictors of mortality.
Methods
This was a retrospective analysis of data from a multicenter registry on patients who underwent dLVAD implantation following tMCS with a mAFP between January 2017 and October 2022 (n = 332) from 19 European centers.
Results
Patients were supported with an Impella 5.5 (n = 92), 5.0 (n = 153) or CP (n = 87) and were transitioned to a HeartWare HVAD (n = 128) or Heartmate 3 (n = 204) during the same period. One hundred and twenty-five patients (39.2%) also required extracorporeal life support before and/or during mAFP therapy. The 30-day and 1-year survival were 87.8% and 71.1%, respectively. The following risk factors for 1-year mortality were identified: age (odds ratio [OR], 1.02), specifically age over 55 years (OR, 1.09), body mass index >30 kg/m2 (OR, 2.2), female sex (OR for male sex, 0.43), elevated total bilirubin (OR, 1.12), and low platelet count (OR, 0.996).
Conclusions
Based on the identified risk factors, a risk score for estimating 1-year mortality was calculated to optimize patient selection for dLVAD implantation.
Key Words: cardiogenic shock, mechanical circulatory support, Impella, LVAD, outcome
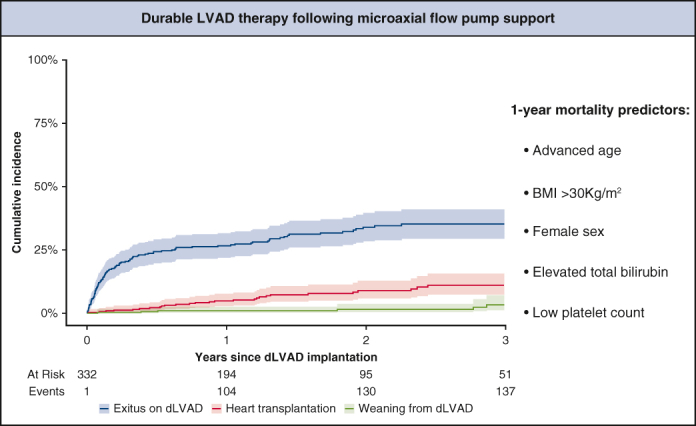
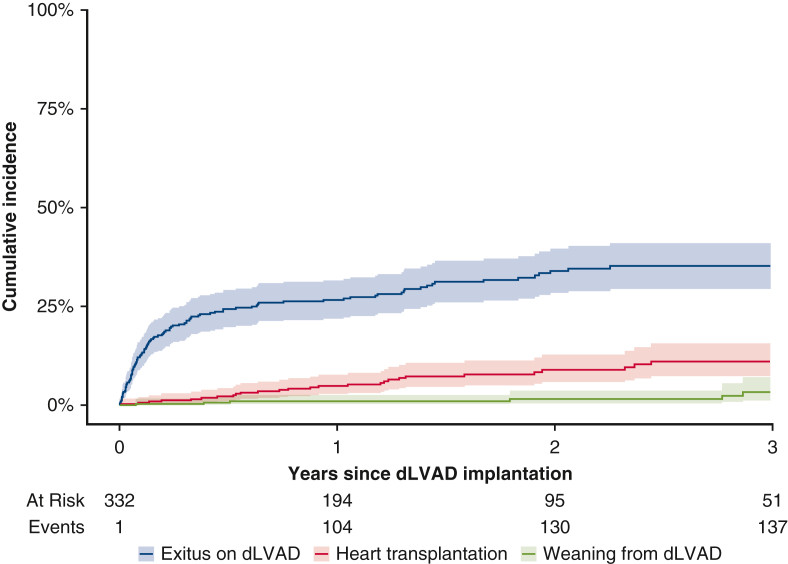
Cumulative incidence function of exitus on dLVAD with transplantation and weaning.
Central Message.
Durable LVAD implantation after tMCS with mAFP is a valid approach with good outcomes in this critical patient population. The predictive score may facilitate the selection process.
Perspective.
Advanced age, BMI >30 kg/m2, female sex, elevated total bilirubin, and low platelet counts were identified as risk factors for 1-year mortality. If platelet counts and bilirubin fail to normalize on mAFP, indicating continued venous congestion despite adequate perfusion, inadequate right ventricular recovery should be considered.
Temporary mechanical circulatory support (tMCS) plays a major role in the treatment of severe cardiogenic shock.1 In particular, venoarterial extracorporeal life support (VA-ECLS) offers the possibility of immediate resuscitation and hemodynamic stabilization facilitating end-organ recovery and restoration of cardiac compensation. In the absence of adequate myocardial recovery during tMCS, selected patients can be transitioned to durable mechanical circulatory support (dMCS), usually a durable left ventricular assist device (dLVAD), which is an established therapy for end-stage heart failure.1 Especially in European countries, where the availability of donor organs is limited, dMCS (eg, LVAD) implantation is frequently the only option in patients with inadequate ventricular function. However, recently reported results of a multicenter registry-based study focusing on VA-ECLS as a bridge to LVAD therapy showed a 1-year survival of only 53%.2
The Impella (Abiomed), a microaxial flow pump (mAFP) that provides blood flow of up to 5.5 L/minutes depending on the model (CP, 5.0, or 5.5), is being increasingly used for temporary ventricular support alone or in combination with ECLS3 as a bridge to recovery or bridge to decision and provides several advantages that may improve outcomes in this critical patient population. First, mAFPs actively unload the left ventricle, facilitating myocardial recovery, whereas VA-ECLS increases left ventricular afterload, jeopardizing myocardial recovery and potentially causing pulmonary congestion. Furthermore, owing to a longer possible duration of support, an mAFP can be used to wean patients from VA-ECLS, thereby reducing the duration of ECLS and consequently ECLS-related complications, which otherwise may adversely impact outcomes after dLVAD implantation.2,4
However, the currently published data on patients with dLVAD implantation following tMCS with a mAFP is limited to only a few small case series,5,6 resulting in a lack of reliable criteria for adequate patient selection and estimation of the optimal timing for transitioning patients to a dLVAD. Moreover, outcome data, especially information on perioperative and late complications, is missing. Thus, the main goal of the present study was to provide outcome data for this unique patient population and identify mortality predictors to facilitate appropriate decision making in dLVAD candidacy and the optimal timing for surgery.
Methods
Patient Population
This multicenter retrospective study presents the data of 332 consecutive patients who underwent dLVAD implantation following temporary support with an mAFP between January 2017 and October 2022 in 19 European centers (Online Data Supplement 1). All adult patients who were transitioned to dLVAD from tMCS with an mAFP were included. Patients who underwent implantation of a total artificial heart (TAH), biventricular assist device (BiVAD), or an LVAD other than the Heartmate 3 or HeartWare HVAD were excluded.
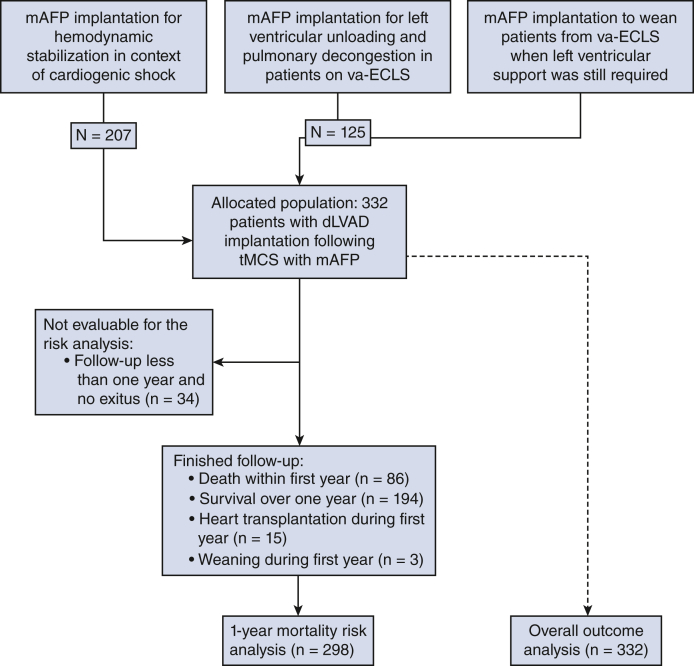
An mAFP was implanted for hemodynamic stabilization and optimization of end-organ perfusion in the context of cardiogenic shock, to facilitate left ventricular unloading and pulmonary decongestion in patients on concomitant VA-ECLS, or to wean patients from VA-ECLS when left ventricular support was still required (Figure 1). In all centers, the main goal was to wean the patients off MCS. Since all patients were on mAFP support, those who did not meet the weaning criteria and were not eligible for heart transplantation were considered for dLVAD therapy after adequate clinical neurologic evaluation, including computed tomography scans.
Figure 1.
CONSORT diagram. mAFP, Microaxial flow pump; VA-ECLS, venoarterial extracorporeal life support; dLVAD, durable left ventricular assist device; tMCS, temporary mechanical circulatory support.
Evaluation of eligibility for heart transplantation was performed in accordance with Eurotransplant protocol. Owing to the very limited availability of donor organs in European countries and consequent long waiting times, patients who although eligible for heart transplantation most likely would succumb to heart failure until transplantation instead were considered for bridge-to-transplantation therapy with a dLVAD. However, there was no protocol specifying when and how to proceed with dLVAD. The identification of patients for durable support was left at the center's discretion.
Preoperative, intraoperative, and postoperative data, including demographic information and laboratory and hemodynamic parameters, were collected. In particular, the last parameters before dLVAD implantation were collected, including, among others, renal and liver function tests, complete blood counts, and blood gas analysis. Both the MELD and MELD-XI scores were calculated for each individual patient and included in the analysis. Patients who survived at least 1 year, died, or underwent transplantation or weaning within the first year (n = 298) were included in the logistic regression analysis to identify risk factors for 1-year mortality (Figure 1). All patients (n = 332) were included in the outcome analysis.
Respiratory failure was considered if discontinuation of ventilatory support within the first postoperative week was not possible or if reintubation (excluding temporary intubation for operative, diagnostic or therapeutic procedures) or tracheostomy was required.7 Right heart failure (RHF) was considered only if mechanical support of the right ventricle was necessary. Furthermore, RHF was categorized as early, early postoperative, or late RHF according to the updated definitions of the Mechanical Circulatory Support Academic Research Consortium.7 Early stroke was defined as a perioperative event of ischemic or hemorrhagic stroke up to 30 days after dLVAD implantation.
The study protocol was approved by the individual Health Research Ethics Boards (EA2/196/21) on July 16, 2023. Patients provided informed written consent for the use of their pseudonymized data for research purposes. The need for informed written consent for the publication of their study data in this specific publication was waived by the Health Research Ethics Boards.
Statistical Analysis
Continuous variables are presented as mean and standard deviation (SD) or median and interquartile rage (IQR) in cases of non-normal data. For binary or ordinal data, absolute and relative frequencies are given. Differences between groups were tested using the t tests and Mann-Whitney U test (for normally and non-normally distributed continuous data), and the χ2 test with Yates continuity parameter or Fisher exact test were applied for categorical data.
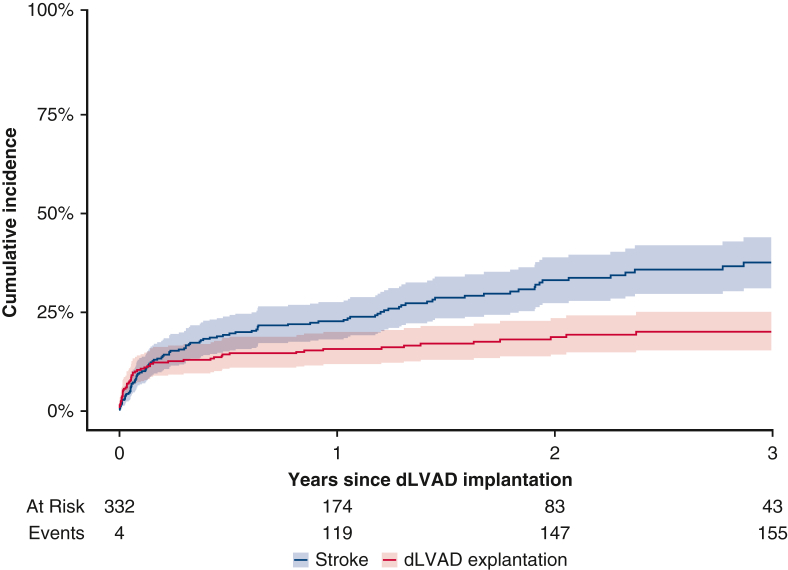
The primary endpoint was defined as 1-year mortality on dLVAD. Here 30-day mortality was not chosen as a primary endpoint because the number of patients who had died within 30 days was expected to be too small; given the study's retrospective nature, overall mortality was not chosen as a primary endpoint to avoid bias because of significant differences in follow-up time between patients and potentially missing follow-up data. Instead, the secondary endpoint was defined as overall outcome, including overall mortality as well as events of early postoperative and late complications, such as RHF, stroke or gastrointestinal bleeding (GIB). In Figure 2, the cumulative incidence of exitus during dLVAD support is displayed graphically in a cumulative incidence function (CIF) with transplantation and weaning as competing risks.8 Late complications, including stroke, driveline infection, GIB, and pump thrombosis, are presented as events per 100 patient-years. In Figure 3, the cumulative incidence of stroke is graphically displayed in a CIF with dLVAD explantation (exitus, weaning, or transplantation) as a competing risk. Multiple imputations with chained equations and 10 imputations were used to account for missing data.9 Covariates with >50% missing values were excluded from the analysis.
Figure 2.
Cumulative incidence function with 95% confidence intervals of exitus on durable left ventricular assist device (dLVAD) (blue) with transplantation (red) and weaning (green) as competing risks.
Figure 3.
Cumulative incidence function with 95% confidence intervals of stroke (blue) with durable left ventricular assist device (dLVAD) explantation (red) as a competing risk.
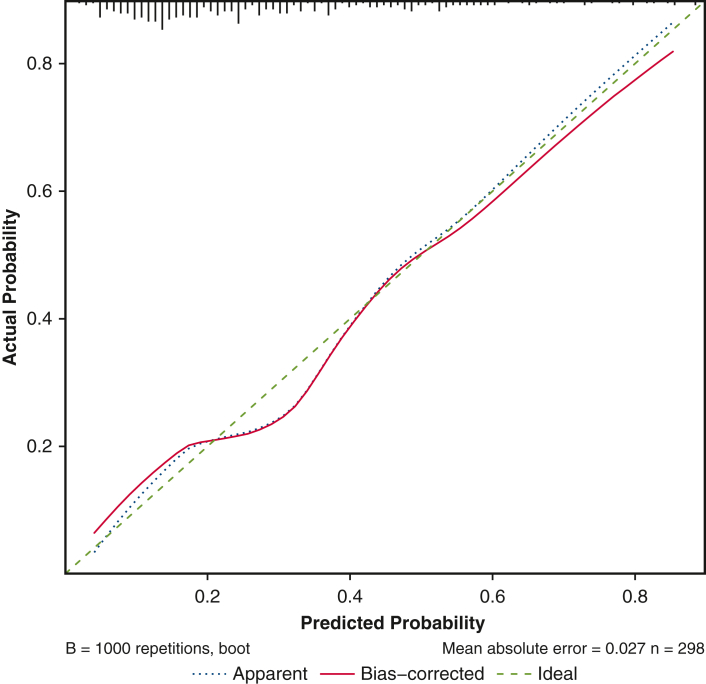
For all patients who either survived 1 year, underwent transplantation or weaning within the first year, or reached the primary endpoint (n = 298), a logistic regression analysis of explanatory variables was performed to predict the primary endpoint. Different parametric transformations and restricted cubic splines were considered to model the effect of continuous covariates. Prognostic factors for a multivariable logistic regression model were determined using the LASSO (least absolute shrinkage and selection operator).10 A multivariable logistic regression was evaluated with the resulting 5 prognostic factors, in which age was fitted with a constant term up to 55 years and a linear term for age >55 years. Finally, penalized regression coefficients were calculated to reduce overoptimism. We used the bootstrap resampling method to assess the stability of our final model and quantify its optimism. As a measure of predictive performance, the concordance index (C-index) was calculated and corrected from overoptimism by 1000 bootstrap samples. Calibration was verified using the Brier score,11 and the maximum absolute differences in predicted and calibrated probabilities (Emax) were calculated. A calibration plot is provided in Figure E1. Statistical analyses were performed using R version 4.03 (R Foundation for Statistical Computing).
Figure E1.
Calibration plot.
Results
Patient characteristics and outcomes are presented in Table 1. The mean patient age was 55 ± 12.49 years, and 280 of the patients (84.3%) were male. The mean body mass index (BMI) was 27.02 ± 4.89, and 99 patients (30.2%) had diabetes mellitus. The leading cause of severe cardiogenic shock necessitating tMCS was acute myocardial infarction (32.2%), followed by acute decompensation of ischemic cardiomyopathy (28.6%) or dilated cardiomyopathy (27.1%). Ninety-nine patients (29.8%) required cardiopulmonary resuscitation (CPR) before mAFP support. At the time of dLVAD implantation, 127 patients (38.3%) had a history of atrial fibrillation, 55 (17.7%) had a history of previous cardiac surgery, and 36 (11.7%) had a history of stroke.
Table 1.
Overall patient characteristics and outcomes (N = 332)
| Characteristic | Value |
|---|---|
| Demographics | |
| Age, y, mean ± SD | 55.4 (12.49) |
| Male sex, n (%) | 280 (84.3) |
| BMI, kg/m2, mean ± SD | 27.02 (4.89) |
| BSA, m2, mean ± SD | 2.01 (0.23) |
| Diabetes mellitus, n (%) | 99 (30.2) |
| Atrial fibrillation, n (%) | 127 (38.3) |
| History of stroke, n (%) | 36 (11.7) |
| History of cardiac surgery, n (%) | 55 (17.7) |
| CPR before Impella support, n (%) | 99 (30.2) |
| Diagnosis, n (%) | |
| Acute myocardial infarction | 107 (32.2) |
| Decompensated ischemic cardiomyopathy | 95 (28.6) |
| Decompensated dilated cardiomyopathy | 90 (27.1) |
| Fulminant myocarditis | 23 (6.9) |
| Other cardiomyopathy | 10 (3.0) |
| Other etiology | 7 (2.1) |
| Temporary mechanical circulatory support | |
| Impella 5.5 (%) | 92 (27.7) |
| Impella 5.0, n (%) | 153 (46.1) |
| Impella CP, n (%) | 87 (26.2) |
| Impella support duration, d, median (IQR) | 9.00 (5.00-14.00) |
| VA-ECLS before/during mAFP support, n (%) | 125 (39.2) |
| VA-ECLS duration, d, median (IQR) | 7.00 (4.00-10.00) |
| Outcomes | |
| 30-d survival rate, % | 87.8 |
| 1-y survival rate, % | 71.1 |
| Heart transplantation after dLVAD, n (%) | 32 (9.6) |
| Recovery and weaning from dLVAD, n (%) | 9 (2.6) |
| Right heart failure requiring RVAD implantation, n (%) | 70 (21.1) |
| Early intraoperative | 50 (15.1) |
| Early postoperative | 17 (5.1) |
| Late | 3 (0.9) |
| Reexploration of the surgical field after dLVAD implantation due to bleeding, n (%) | 77 (23.5) |
| Postoperative renal replacement therapy, n (%) | 109 (39.2) |
| Respiratory failure, n (%) | 103 (38.0) |
| Early postoperative stroke, n (%) | 30 (9.04) |
| Cause of death, n (%) | |
| Cardiogenic shock | 41 (12.3) |
| Septic shock | 26 (7.88) |
| Cerebral | 20 (6.0) |
| Respiratory failure | 6 (1.8) |
| dLVAD failure/thrombosis | 3 (0.9) |
| Hemorrhagic shock | 5 (1.5) |
| Palliative/malignancy | 6 (1.8) |
| Unknown | 8 (2.4) |
| Postoperative complications, EP100PY | |
| Ischemic stroke | 8.16 |
| Hemorrhagic stroke | 3.88 |
| Gastrointestinal bleeding | 9.59 |
| Driveline infection | 16.53 |
| Pump thrombosis | 4.29 |
| Total number of patient-years | 490 |
BMI, Body mass index; BSA, body surface area; CPR, cardiopulmonary resuscitation; IQR, interquartile range; VA-ECLS, venoarterial extracorporeal life support; mAFP, microaxial flow pump; dLVAD, durable left ventricular assist device; RVAD, right ventricular assist device; EP100PY, events per 100 patient-years.
The type of tMCS used in this population included the Impella 5.5 in 92 patients (27.7%), Impella 5.0 in 153 (46.1%), and Impella CP in 87 (26.2%), as well as VA-ECLS before or in addition to mAFP support3 in 125 patients (39.2%), with a median time on VA-ECLS of 7 days (IQR, 4-10 days). Moreover, 31 patients (9.3%) had an intra-aortic balloon pump before implantation of an Impella system, and 38 (14.9%) patients were switched from an Impella CP to an Impella 5.0 or 5.5 due to insufficient circulatory support. After a median time on Impella support of 9 days (IQR, 5-14 days), patients were transitioned to either a HeartMate 3 (n = 204) or a HeartWare HVAD (n = 128).
Complications and Outcome Data
Sixty-seven patients (19.0%) also had perioperative RHF necessitating temporary right ventricular assist device (RVAD) implantation. Postoperative complications included reexploration of the surgical field after dLVAD implantation due to bleeding in 77 patients (23.2%), postoperative renal failure necessitating renal replacement therapy in 109 (39.2%), and respiratory failure in 103 (38.0%), as well as early stroke in 30 (9.0%). In reference to the total of 490 patient-years, postoperative stroke occurred as 0.122 events per patient-year (EPPY), with ischemic stroke accounting for 0.086 EPPY and hemorrhagic stroke for 0.036 EPPY, and 0.035 EPPY being fatal. Furthermore, there were 0.096 EPPY of GIB, 0.165 EPPY of driveline infections, and 0.043 EPPY of pump thrombosis.
The leading cause of death on dLVAD support was multiple organ failure, followed by septicemia and stroke. Fifteen patients (4.5%) underwent heart transplantation, and 3 (0.9%) were weaned from dLVAD with subsequent device explantation during the first year; 32 (9.6%) patients underwent heart transplantation, and 9 (2.7%) were weaned from dLVAD with subsequent device explantation during the entire follow-up. Overall survival was 87.8% at 30 days and 71.1% at 1 year.
Survival Analysis
The differences in patient characteristics as well as in intraoperative and postoperative data on 1-year mortality are presented in Tables 2 and 3. Patients who died within the first year were older (mean age, 59.49 ± 11.86 years vs 53.96 ± 11.98 years) and had lower platelet counts (median, 105 [IQR, 82.25-149.50] × 10³/μL vs 142 [IQR, 92.50-213.25] × 10³/μL; P = .001), higher total bilirubin levels (median, 1.60 [IQR, 0.85-3.94] mg/dL vs 1.29 [IQR, 0.79-2.18] mg/dL; P = .020), and higher MELD (median, 18.5 [IQR, 12-26] vs 13 [IQR, 9-21.5]; P = .001) and MELD-XI scores (median, 17 [IQR, 11-23] vs 13 [IQR, 10-20]; P = .005) before dLVAD implantation. Moreover, specifically in patients with concomitant VA-ECLS, 75% of all patients with concomitant VA-ECLS who died within the first year were not weaned from VA-ECLS or were weaned within <1 day before transition to dLVAD, whereas 45.2% of 1-year survivors with concomitant VA-ECLS were weaned >1 day before dLVAD surgery.
Table 2.
Patient characteristics before durable mechanical circulatory support
| Parameter | 1-year survivors (N = 212) | 1-year nonsurvivors (N = 86) | P value | Missing values, % |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean ± SD | 53.96 ± 11.98 | 59.49 ± 11.86 | <.001 | 0.0 |
| Male sex, n (%) | 185 (87.3) | 68 (79.1) | .107 | 0.0 |
| BMI, kg/m2, mean ± SD | 26.73 ± 4.59 | 27.43 ± 5.36 | .259 | 1.5 |
| BMI >30 kg/m2, n (%) | 35 (16.7) | 23 (27.1) | .064 | 1.5 |
| BSA, m2, mean ± SD | 2.02 ± 0.23 | 2.00 ± 0.23 | .445 | 5.1 |
| Diabetes mellitus, n (%) | 61 (28.4) | 30 (36.1) | .250 | 1.2 |
| Atrial fibrillation, n (%) | 74 (34.9) | 41 (47.7) | .055 | 0.0 |
| Peripheral artery disease, n (%) | 16 (7.6) | 10 (12.0) | .330 | 1.5 |
| History of stroke, n (%) | 19 (9.6) | 12 (14.6) | .318 | 7.2 |
| History of cardiac surgery, n (%) | 32 (16.1) | 20 (25.6) | .097 | 6.6 |
| CPR before Impella support, n (%) | 62 (29.7) | 28 (32.0) | .680 | 1.2 |
| Diagnosis, n (%) | .605 | 0.0 | ||
| Acute myocardial infarction | 68 (32.1) | 26 (30.2) | ||
| Decompensated ischemic cardiomyopathy | 58 (27.4) | 26 (30.2) | ||
| Decompensated dilated cardiomyopathy | 58 (27.4) | 22 (25.6) | ||
| Fulminant myocarditis | 18 (8.5) | 5 (5.8) | ||
| Other cardiomyopathy | 7 (3.3) | 3 (3.5) | ||
| Other etiology | 3 (1.4) | 4 (4.7) | ||
| Data related to temporary mechanical circulatory support | ||||
| Impella type (%) | .163 | 0.0 | ||
| Impella 5.5 | 57 (26.9) | 25 (29.1) | ||
| Impella 5.0 | 105 (49.5) | 33 (38.4) | ||
| Impella CP | 50 (23.6) | 28 (32.6) | ||
| Impella access site (%) | .425 | 0.0 | ||
| Femoral artery | 53 (25.0) | 27 (31.4) | ||
| Axillary artery | 157 (74.1) | 58 (67.4) | ||
| Aorta | 2 (0.9) | 1 (1.2) | ||
| Impella support duration, d, median (IQR) | 9.00 (5.00-14.00) | 8.00 (5.50-14.00) | .426 | 3.3 |
| Upgrade from Impella CP to 5.0 or 5.5, n (%) | 25 (15.3) | 6 (10.3) | .471 | 25.8 |
| VA-ECLS before/during Impella support, n (%) | 77 (37.6) | 36 (44.4) | .348 | 3.9 |
| VA-ECLS duration, d, median (IQR) | 7.00 (4.00-10.00) | 8.00 (5.00-12.25) | .241 | 63.9 |
| Time between VA-ECLS weaning and dLVAD implantation, d, median (IQR) | 1.00 (0.00-9.00) | 0.00 (0.00-0.50) | .019 | 63.9 |
| VA-ECLS weaning ≤1 d before dLVAD implantation, n (%) | 40 (54.8) | 27 (75.0) | .054 | 63.9 |
| IABP before mAFP support, n (%) | 21 (9.9) | 8 (9.3) | 1.000 | 0.0 |
| Mobilization, n (%) | .350 | 9.0 | ||
| No mobilization | 65 (33.7) | 34 (43.0) | ||
| Mobilization in bed | 60 (31.1) | 22 (27.8) | ||
| Mobilization to the bedside | 26 (13.5) | 11 (13.9) | ||
| Mobilization out of bed | 30 (15.5) | 11 (13.9) | ||
| Mobilization out of the room | 12 (6.2) | 1 (1.3) | ||
| Blood loss during tMCS, mL, median (IQR) | 200.00 (5.00-1210.00) | 130.00 (7.50-1000.00) | .494 | 48.2 |
| Blood units during tMCS, median (IQR) | 4.00 (0.00-12.00) | 6.00 (0.00-22.50) | .221 | 19.0 |
| Other organ support | ||||
| Invasive ventilation, n (%) | 87 (42.0) | 36 (42.9) | 1.000 | 2.4 |
| Renal replacement therapy, n (%) | 55 (26.1) | 32 (37.2) | .076 | 0.3 |
| Inotropic score, median (IQR) | 3.00 (0.00-7.68) | 4.42 (0.25-7.49) | .123 | 12.6 |
| Vasoactive-inotropic score, median (IQR) | 3.10 (0.00-7.68) | 4.63 (0.25-7.73) | .095 | 12.6 |
| Last available hemodynamic parameters | ||||
| sPAP, mm Hg, median (IQR) | 37.50 (25.00-53.50) | 43.00 (29.25-52.75) | .300 | 47.6 |
| mPAP, mm Hg, median (IQR) | 26.00 (17.00-37.00) | 30.50 (24.25-40.75) | .105 | 48.5 |
| dPAP, mm Hg, median (IQR) | 20.50 (14.00-29.00) | 22.00 (18.25-27.00) | .284 | 48.8 |
| CVP, mm Hg, median (IQR) | 10.00 (7.00-15.00) | 11.00 (7.00-14.50) | .719 | 33.4 |
| Preoperative laboratory parameters, median (IQR) | ||||
| Platelets ×10³/μL | 142.00 (92.50-213.25) | 105.00 (82.00-149.50) | .001 | 1.8 |
| White blood cell count, ×10³/μL | 10.70 (8.70-14.22) | 11.70 (8.47-16.35) | .272 | 1.8 |
| Hemoglobin, mg/dL | 9.50 (8.50-10.50) | 9.40 (8.70-10.60) | .734 | 1.5 |
| Free hemoglobin, g/dL | 8.00 (5.23-12.70) | 8.20 (6.00-14.30) | .440 | 44.0 |
| Lactate dehydrogenase, mg/dL | 588.00 (423.50-902.50) | 610.00 (444.25-915.00) | .498 | 9.0 |
| Lactate, mmol/L | 1.10 (0.78-1.38) | 1.12 (0.80-1.56) | .219 | 0.9 |
| Base excess | 0.20 (−1.98 to 2.50) | 0.20 (−1.90 to 1.80) | .806 | 3.9 |
| pH | 7.43 (7.38-7.48) | 7.44 (7.40-7.47) | .536 | 1.8 |
| AST, U/L | 58.00 (35.00-123.30) | 68.50 (47.10-125.50) | .125 | 4.8 |
| ALT, U/L | 52.00 (30.00-96.50) | 60.50 (34.55-127.95) | .291 | 22.9 |
| GGT, U/L | 100.00 (59.00-189.00) | 102.50 (48.75-195.90) | .919 | 12.6 |
| Direct bilirubin, mg/dL | 0.94 (0.57-1.80) | 1.83 (1.01-3.42) | .004 | 61.7 |
| Total bilirubin, mg/dL | 1.29 (0.79-2.18) | 1.60 (0.85-3.94) | .020 | 2.4 |
| C-reactive protein, mg/dL | 8.12 (3.51-20.42) | 9.90 (4.80-20.60) | .232 | 2.7 |
| Haptoglobin, mg/dL | 10.00 (8.00-55.00) | 11.00 (8.00-35.65) | .984 | 66.3 |
| Albumin, mg/dL | 2.70 (2.10-3.10) | 2.59 (2.15-3.10) | .530 | 14.2 |
| INR | 1.20 (1.10-1.30) | 1.20 (1.10-1.40) | .198 | 2.4 |
| Urea, mg/dL | 47.00 (29.95-72.50) | 53.60 (33.65-98.17) | .054 | 2.1 |
| Creatinine mg/dL | 1.06 (0.80-1.71) | 1.17 (0.86-1.74) | .507 | 2.1 |
| MELD | 13.50 (9.00-21.50) | 18.50 (12.00-26.00) | .001 | 2.1 |
| MELD-XI | 13.00 (10.00-20.00) | 17.00 (11.00-23.00) | .005 | 2.1 |
BMI, Body mass index; BSA, body surface area; CPR, cardiopulmonary resuscitation; IQR, interquartile range; VA-ECLS, venoarterial extracorporeal life support; dLVAD, durable left ventricular assist device; IABP, intra-aortic balloon pump; mAFP, microaxial flow pump; tMCS, temporary mechanical circulatory support; sPAP, systolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; dPAP, diastolic pulmonary artery pressure; CVP, central venous pressure; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; INR, international normalized ratio; MELD, Model of End-Stage Liver Disease; MELD-XI, Model of End-Stage Liver Disease excluding international normalized ratio.
Table 3.
Intraoperative and postoperative data
| Parameter | 1-year survivors (N = 212) | 1-year nonsurvivors (N = 86) | P value |
|---|---|---|---|
| dLVAD type, n (%) | .508 | ||
| HeartWare HVAD | 88 (41.5) | 40 (46.5) | |
| HeartMate 3 | 124 (58.5) | 46 (53.5) | |
| Surgical data | |||
| Implantation on CPB, n (%) | 144 (69.6) | 59 (72.8) | .686 |
| Implantation on ECLS, n (%) | 39 (20.1) | 18 (23.7) | .629 |
| Minimally invasive surgery, n (%) | 15 (7.5) | 6 (8.0) | 1.000 |
| Concomitant valve surgery, n (%) | 13 (6.1) | 12 (14.0) | .048 |
| Surgery time, min, median (IQR) | 234.00 (170.00-290.00) | 250.00 (199.25-300.00) | .069 |
| Postoperative complications | |||
| Chest tube output 24 h after surgery, mL, median (IQR) | 600.00 (360.00-1200.00) | 800.00 (361.25-1310.00) | .210 |
| FFP during and within 24 h after surgery, units, median (IQR) | 6.00 (4.00-8.00) | 6.00 (4.00-9.00) | .420 |
| PRBC during and within 24 h after surgery, units, median (IQR) | 5.00 (3.00-8.00) | 7.00 (4.25-11.00) | <.001 |
| Platelet concentrates during and within 24 h after surgery, units, median (IQR) | 2.00 (0.00-4.00) | 3.00 (2.00-5.00) | .064 |
| Right heart failure requiring RVAD implantation, n (%) | 33 (15.6) | 34 (39.5) | <.001 |
| Time of right heart failure, n (%) | <.001 | ||
| Early intraoperative | 24 (10.8) | 24 (27.9) | |
| Early postoperative | 8 (3.8) | 9 (10.5) | |
| Late | 2 (0.9) | 1 (1.2) | |
| Weaning from RVAD, n (%) | 27 (75.0) | 12 (35.3) | <.001 |
| Duration of RVAD support, d, median (IQR) | 21.00 (13.00-25.00) | 16.00 (10.00-22.00) | .113 |
dLVAD, Durable left ventricular assist device; CPB, cardiopulmonary bypass; ECLS, extracorporeal life support; IQR, interquartile range; FFP, fresh frozen plasma; PRBC, packed red blood cells; RVAD, right ventricular assist device.
The results of the univariable and multivariable analyses are presented in Tables 4 and 5. The following parameters were identified as predictors of 1-year mortality: age (OR, 1.02; 95% CI, 0.98-1.06), specifically age >55 years (OR, 1.09; 95% CI, 1.02-1.16), BMI >30 kg/m2 (OR, 2.2; 95% CI, 1.14-4.25), female sex (OR for male sex, 0.43; 95% CI, 0.21-0.87), elevated total bilirubin (OR, 1.12; 95% CI 1.05-1.20), and low platelet count (OR, 0.996; 95% CI, 0.993-0.999). Based on the identified risk factors, the following score was calculated to estimate the probability of 1-year mortality:
Table 4.
Univariable logistic regression for 1-year mortality
| Variable | OR (95% CI) | P value |
|---|---|---|
| Age | 1.02 (1.02-1.07) | .0005 |
| Male sex | 0.55 (0.29-1.1) | .076 |
| BMI (kg/m2) | 1.00 (0.98-1.1) | .260 |
| BMI >30 kg/m2 | 1.80 (1.0-3.4) | .046 |
| BSA (m2) | 0.64 (0.21-2.0) | .444 |
| History of cardiac surgery | 1.80 (0.96-3.4) | .069 |
| Atrial fibrillation | 1.70 (1.2-8.0) | .041 |
| Diabetes mellitus | 1.40 (0.83-2.40) | .198 |
| Cardiopulmonary resuscitation | 1.20 (0.68-2.0) | .581 |
| Platelet count per 10/L | 0.95 (0.91-0.98) | .001 |
| Lactate (mmol/L) | 0.99 (0.80-1.20) | .905 |
| Total bilirubin per 10 mg/dL | 3.1 (1.6-6.2) | .001 |
| International normalized ratio | 1.9 (0.92-4.1) | .082 |
| Urea per 10 U/L | 1.1 (1-1.1) | .039 |
| Creatinine (mg/dL) | 1.1 (0.81-1.4) | .709 |
| Lactate dehydrogenase per 1000 mg/dL | 1.40 (0.97-1.90) | .071 |
| MELD per 10 | 1.8 (1.3-2.4) | <.001 |
| MELD-XI per 10 | 1.8 (1.2-2.6) | .003 |
| Blood units during mAFP support therapy per 10 units | 6.4 (1.0-40) | .047 |
| Renal replacement therapy | 1.7 (0.98-2.9) | .057 |
| Invasive ventilation | 1.0 (0.62-1.7) | .897 |
| VA-ECLS before/during mAFP support (%) | 1.3 (0.79-2.2) | .284 |
OR, Odds ratio; CI, confidence interval; BMI, body mass index; BSA, body surface area; MELD, Model of End-Stage Liver Disease; MELD-XI, Model of End-Stage Liver Disease excluding international normalized ratio; VA-ECLS, venoarterial extracorporeal life support; mAFP, microaxial flow pump.
Table 5.
Multivariable logistic regression for 1-year mortality
| Parameter | OR (95% CI) |
|---|---|
| Age (years up to 55) | 1.02 (0.98-1.06) |
| Age (years above 55) | 1.09 (1.02-1.16) |
| Male sex | 0.43 (0.21-0.87) |
| BMI >30 kg/m2 | 2.20 (1.14-4.25) |
| Total bilirubin per 10 mg/dL | 1.14 (1.05-1.20) |
| Platelet count per 10/L | 0.996 (0.993-0.999) |
OR, Odds ratio; CI, confidence interval; BMI, body mass index.
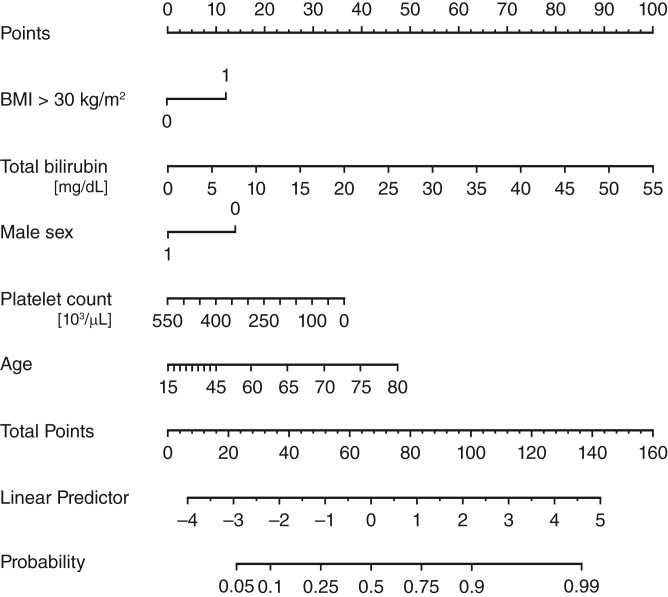
Probability of 1-year mortality = 1/(1 + exp(1.7008 – 0.0196 × age - 0.0828 (age −55 years)) – 0.1166 × total bilirubin value - 0.7896 × (BMI: 1 if > 30 kg/m2, 0 if < 30 kg/m2) + 0.8554 × (sex: 1 if male, 0 if female) + 0.0040 × platelet count value).
The model showed an adequate fit (likelihood ratio χ2, 61; 5 degrees of freedom; P < .001) and a good discriminative ability, with a C-index of 0.767 and Somers D of 0.53. With 200 bootstrap replicates, the estimated optimism is 0.033, resulting in an optimism-corrected C-index of 0.750 and Somers D of 0.49. A Brier score of 0.17 confirmed good model calibration, with Emax = 0.0223 as an index of unreliability. Figure 4 illustrates how the linear predictor is calculated from the values of the 5 risk parameters and in turn provides the estimate of mortality probability at 1 year.
Figure 4.
Nomogram illustrating the 1-year mortality estimate calculation. Depending on the values for the 5 risk factors—body mass index >30 kg/m2 (yes = 1, no = 0), total bilirubin in mg/dL, male sex (yes = 1, no = 0), platelet count × 10³/μL, age in years—the number of points for each of these can be read in the upper row. The total amount of points can then be converted into the linear predictor below, which in turn is used to estimate the probability of 1-year mortality in the row below. BMI, Body mass index.
Repeating the calculation of the predictive model derived from the HM3 patient subcohort excluding HVAD patients resulted in a C-index of 0.752. One-year survival was 72.9%. The differences in characteristics and preoperative data of the HM3 patients in terms of 1-year mortality are presented in Online Data Supplement 2.
Discussion
In this multicenter study, we found that bridging patients in cardiogenic shock with mAFP to implantation of a dLVAD is a valid concept, associated with good survival in these otherwise critically ill patients. We identified several risk factors for 1-year mortality and, based on these results, calculated a score for estimating survival after implantation of a dLVAD that may facilitate optimal patient selection for dLVAD surgery.
The previously performed analysis of a multicenter registry on patients bridged with VA-ECLS until durable MCS implantation reported inferior 30-day and 1-year survival rates of 77% and 53%, respectively.2 Given the poor outcomes,2,4 clinical practice was changed in most participating centers; patients are now rarely bridged with VA-ECLS alone and instead, if VA-ECLS was initially implanted, receive an mAFP as a left ventricular vent to optimize perfusion in the form of simultaneous circulatory support with mAFP and VA-ECLS,3 or to facilitate de-escalation from VA- ECLS.12 Patients in the present study were treated accordingly, and almost 40% of the patients had VA-ECLS implantation before or in addition to mAFP support. Thus, our results reflect the current clinical experience. However, direct comparison with previous studies focusing on patients with VA-ECLS before durable MCS therapy is precluded by the fact that our study population represents a highly selective cohort that does not include patients for whom mAFP therapy was deemed futile, whereas VA-ECLS implantation is frequently performed in patients during CPR, often with less available clinical data to properly evaluate the prognosis before initiation of tMCS. Interestingly though, VA-ECLS prior to and or during mAFP support was not associated with increased mortality in this study. Nevertheless, in patients with implantation of VA-ECLS for resuscitation and initial hemodynamic stabilization, subsequent mAFP implantation to facilitate early ECLS weaning while continuing support on mAFP until dLVAD implantation may reduce ECLS-associated complications, potentially improving outcomes.5,12 In fact, our results suggest that failure to wean from VA-ECLS before dLVAD surgery may be associated with 1-year mortality.
The main goal of bridge-to-bridge therapy is to stabilize hemodynamics and end-organ perfusion until long-term therapeutic options, particularly dLVAD, can be evaluated and implemented. Older age, elevated BMI, and female sex have been identified as predictors of adverse outcomes in dLVAD therapy as well,2,4 including in a recent analysis of the multicenter EUROMACS registry.13 In addition, similar to this study, parameters indicating liver failure were predictive of early mortality.2,4,13,14 In this study in particular, total bilirubin and platelet counts as well as urea levels and MELD and MELD-XI scores,15 which reflect renal and liver function, were predictive of 1-year mortality. Naturally, many clinicians would tend to wait for liver function to normalize, especially given the possibility of prolonged tMCS on mAFP in contrast to VA-ECLS. Surprisingly, however, time on mAFP support was not associated with survival. Moreover, prolonged support may increase the risk of bleeding complications and hemolysis necessitating administration of blood products, which was identified as a risk factor for 1-year mortality (OR per 10 units, 6.43; 95% CI, 1.02-41.50; P = .047).
On the other hand, the significantly higher incidence of perioperative RHF in the 1-year nonsurvivors suggests that signs of liver dysfunction as well as renal failure otherwise may be an indication of inadequate recovery of right heart function and congestion. This demonstrates an important advantage of tMCS with mAFP: while limited on VA-ECLS, mAFP support allows a better assessment of right ventricular function prior to dLVAD implantation. In fact, the overall incidence of perioperative RVAD implantation was twice as high in the MCS after ECLS cohort2 compared to our study population (42% vs 19%). This further highlights the importance of early VA-ECLS weaning before transitioning to dLVAD.
Finally, efforts toward normalizing platelet counts and total bilirubin should be undertaken to optimize the outcome of a transition from mAFP to dLVAD. In the context of cardiogenic shock, the most probable causes of thrombocytopenia and hyperbilirubinemia are liver dysfunction and MCS-related bleeding and hemolysis. Although immediate pump exchange or repositioning is an adequate treatment for the latter, rare causes of hemolysis—such as transfusion or drug-related side effects—also should be ruled out. Furthermore, platelet deprivation also may be caused by treatable causes, such as heparin-induced thrombocytopenia type II or sepsis. In cases of limited conjugation of bilirubin caused by liver dysfunction, occlusion of the common bile duct should be considered and if confirmed, treated accordingly. These conditions should be addressed on mAFP support before a transition to dLVAD to improve survival.
It remains challenging to assess the extent to which the patient's condition during tMCS on mAFP may affect survival following transition to dLVAD therapy. If platelet counts and bilirubin fail to normalize and MELD and MELD-XI scores do not improve, indicating continued liver failure despite adequate perfusion, inadequate right ventricular recovery should be considered. In this scenario, alternatives to dLVAD therapy may be more favorable, including implantation of a Bead or TAH as well as listing for heart transplantation, if eligible. Our proposed risk score may facilitate this decision by providing an individual estimate for death within the first year based on the patient's momentary clinical condition should dLVAD implantation be performed. A high probability of death may suggest consideration of alternatives for dLVAD or prolonging temporary support while waiting for improvement in the patient's condition.
Limitations
This study has several limitations, including its retrospective design. Nonetheless, it is the largest study on patients transitioned from mAFP to dLVAD published to date. Importantly, owing to limited experience, as well as the scarcity of literature and data on mAFP support and generally on tMCS prior to dLVAD implantation, there was no protocol specifying when and how to proceed with dLVAD. Identifying eligible patients for durable support was at the center's discretion, a major limitation of this study. Furthermore, the data presented herein are subject to heterogeneity of the tMCS provided, particularly patients with an Impella CP or an Impella 5.0/5.5 and patients with or without concomitant VA-ECLS. However, the aim of this study was to present outcome data of patients who received a dLVAD following tMCS with an mAFP regardless of the level of tMCS. Moreover, the Impella CP was replaced by the Impella 5.0/5.5 in patients in whom perfusion was insufficient during Impella CP support, suggesting that the circulatory support provided by the Impella CP was otherwise adequate. Also, given the fact that VA-ECLS was regularly implanted during CPR, followed by additional mAFP support after successful resuscitation, excluding these patients actually would result in increased bias due to a false representation of the patient population.
Conclusions
Our results show that using an mAFP for tMCS prior to a transition to a dLVAD is a valid concept with comparably good survival in this critical population. Although the selection of patients who may benefit from dLVAD and the optimal timing of the transition remain challenging, the predictive score may facilitate the selection process. Furthermore, when opting for a transition to a dLVAD, optimizing platelet counts as well as liver and renal function should be targeted. Specifically in patients with concomitant VA-ECLS, weaning from VA-ELCS before transitioning to dLVAD should be pursued.
Conflict of Interest Statement
M.O. is a member of the advisory board for A biomed. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Drs Bernhardt and Potapov share senior authorship.
Supplementary Data
Appendix E1
References
- 1.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Saeed D., Potapov E., Loforte A., et al. Transition from temporary to durable circulatory support systems. J Am Coll Cardiol. 2020;76:2956–2964. doi: 10.1016/j.jacc.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Eulert-Grehn J.J., Starck C., Kempfert J., Falk V., Potapov E. ECMELLA 2.0: single arterial access technique for a staged approach in cardiogenic shock. Ann Thorac Surg. 2021;111:e135–e137. doi: 10.1016/j.athoracsur.2020.06.084. [DOI] [PubMed] [Google Scholar]
- 4.Tsyganenko D., Gromann T.W., Schoenrath F., et al. Predictors of mid-term outcomes in patients undergoing implantation of a ventricular assist device directly after extracorporeal life support. Eur J Cardiothorac Surg. 2019;55:773–779. doi: 10.1093/ejcts/ezy351. [DOI] [PubMed] [Google Scholar]
- 5.Bertoldi L.F., Pappalardo F., Lubos E., et al. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J Crit Care. 2020;57:259–263. doi: 10.1016/j.jcrc.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 6.George T.J., Schaffer J.M., Harrington K.B., et al. Impact of preoperative Impella support on destination left ventricular assist device outcomes. J Card Surg. 2022;37:3576–3583. doi: 10.1111/jocs.16942. [DOI] [PubMed] [Google Scholar]
- 7.Kormos R.L., Antonides C.F.J., Goldstein D.J., et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: a consensus statement of the mechanical circulatory support academic research consortium. J Heart Lung Transplant. 2020;39:735–750. doi: 10.1016/j.healun.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 9.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 10.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B. 1996;58:267–288. [Google Scholar]
- 11.Gerds T.A., Cai T., Schumacher M. The performance of risk prediction models. Biom J. 2008;50:457–479. doi: 10.1002/bimj.200810443. [DOI] [PubMed] [Google Scholar]
- 12.Ott S., Lewin D., Nersesian G., et al. Improving survival in cardiogenic shock-a propensity score-matched analysis of the impact of an institutional allocation protocol to short-term mechanical circulatory support. Life (Basel) 2022;12:1931. doi: 10.3390/life12111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akin S., Soliman O., de By T.M.M., et al. Causes and predictors of early mortality in patients treated with left ventricular assist device implantation in the European Registry of Mechanical Circulatory Support (EUROMACS) Intensive Care Med. 2020;46:1349–1360. doi: 10.1007/s00134-020-05939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxhera B., Albert A., Ansari E., Godehardt E., Lichtenberg A., Saeed D. Survival predictors in ventricular assist device patients with prior extracorporeal life support: selecting appropriate candidates. Artif Organs. 2014;38:727–732. doi: 10.1111/aor.12386. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.A., Kato T.S., Shulman B.P., et al. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J Heart Lung Transplant. 2012;31:601–610. doi: 10.1016/j.healun.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.