Abstract
Objective
Biologic valves dominate tricuspid valve replacement, yet data on different valve types are lacking. We compare the survival and durability of porcine and pericardial tricuspid prostheses.
Methods
A retrospective review of consecutive patients undergoing tricuspid valve replacement with porcine (N = 542) or pericardial (N = 144) prostheses between 1975 and 2022 was performed using a prospectively maintained institutional database. Concurrent procedures were included. Cox proportional hazards and logistic regression were performed.
Results
Patients who received the porcine prosthesis, compared with pericardial, were younger (56 ± 17 years vs 63 ± 15 years) and more likely to present urgently (55% porcine, 44% pericardial); however, there were no differences in redo status or concomitant operations. Ten-year survival was not significantly different between the porcine and pericardial groups (35% ± 3% vs 28% ± 4%, respectively, P = .2). The 10-year cumulative incidence of structural valve deterioration (porcine 9% ± 2%, pericardial 11% ± 3%, P = .8), reoperation for structural valve deterioration (porcine 5% ± 1%, pericardial 4% ± 2%, P = .06), and severe regurgitation (porcine 4% ± 1%, pericardial 5% ± 2%, P = .7) were not significantly different between groups. The failure mode was similar, with no difference in severe stenosis (porcine 32/47 [68%], pericardial 11/16 [69%], P = .9) or severe regurgitation (porcine 18/47 [38%], pericardial 7/16 [44%], P = .7). On regression analysis, valve type was not associated with survival (P = .6). Valve type was not associated with structural valve deterioration (P = .1) or reoperation for structural valve deterioration (P = .9).
Conclusions
In our series, there were no differences in survival or durability between porcine and pericardial valves. In most patients undergoing tricuspid valve replacement, the choice of porcine versus pericardial prosthesis is unlikely to affect clinical outcomes.
Key Words: pericardial bioprosthetic, porcine bioprosthetic, reoperation, structural valve deterioration, tricuspid regurgitation, tricuspid stenosis, tricuspid valve
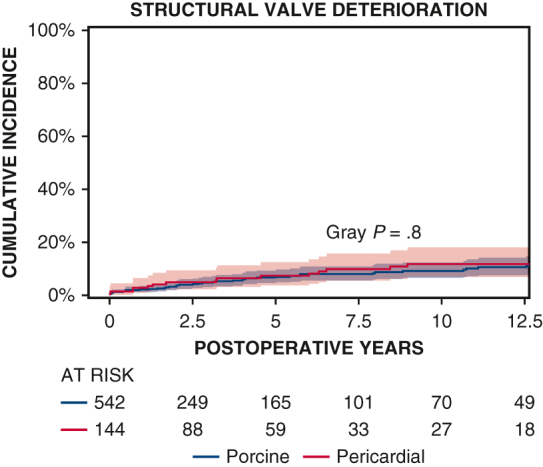
No difference in structural deterioration after TVR over time.
Central Message.
Porcine and pericardial prostheses in TVR demonstrate similar durability, with no difference in structural valve deterioration, reoperation, regurgitation, or stenosis.
Perspective.
In this large single-institution study, we present clinical and echocardiography data of porcine and pericardial tricuspid valves. We found no significant differences in survival or durability (structural valve deterioration, failure mode, mean gradient, and reoperation) between porcine and pericardial valves. Our study can inform surgeons on valve choice, particularly as newer devices become available.
See Discussion on page 88.
Biologic valves dominate tricuspid valve replacement (TVR).1 In a large study of isolated tricuspid valve operations using the Society of Thoracic Surgeons Adult Cardiac Surgery Database, 92.4% of patients undergoing valve replacement received a biologic valve compared with a mechanical valve.1 Unfortunately, guidelines surrounding tricuspid valve surgery are limited due to a paucity of literature, including robust studies on late outcomes based on valve type.2,3 Furthermore, with a 1-year all-cause mortality after TVR of 25%, further studies on this challenging patient population are essential.4
Commercially available biologic prostheses for the mitral or tricuspid position first became available in 1974 with the Edwards porcine model 6625. In 2000, the pericardial bioprosthetic tricuspid valve was introduced in the United States with the Edwards 6900 model, followed by the 7200 and 7300 series. To date, only 1 large series has directly compared porcine (n = 199) and pericardial (n = 342) prostheses in the tricuspid position.5 At 5 years, there was no difference in all-cause mortality, but the cumulative incidence (CI) of reoperation and prosthetic valve stenosis were higher in the pericardial valves compared with porcine valves.5 Most studies on biologic TVR are small and heterogeneous, and comparative series are challenged as new models and brands are introduced.6, 7, 8 Recent studies that compare biologic and mechanical valves in the tricuspid position do not report the biologic valve type and models included, thereby assuming identical durability and characteristics in the biologic models.6, 7, 8
This study was undertaken to directly compare porcine and pericardial tricuspid prostheses, with extended follow-up at a single institution. Given that both porcine and pericardial prostheses are available on the market today, the goal of the study was to provide insight into valve choice for surgeons.
Patients and Methods
Patient Population
A retrospective analysis was performed using a prospectively maintained institutional database on consecutive patients who underwent TVR with biologic valves between 1975 and 2022. Patients were divided into 2 groups: porcine and pericardial prostheses. Concurrent procedures were included. The choice of tricuspid prosthesis was driven by market availability and surgeon preference. Waiver of informed consent was approved by the Institutional Review Board (Pro00105933, approved June 22, 2020).
Study Outcomes
The primary end point was the CI of structural valve deterioration, which excluded deterioration due to endocarditis. Secondary end points included patient survival and CI of reoperation for structural valve deterioration, severe tricuspid regurgitation, and severe tricuspid stenosis. Tricuspid valve findings on postoperative echocardiography were reported according to the American Society of Echocardiography guidelines.9 Structural valve deterioration was defined as onset of severe tricuspid stenosis, severe tricuspid regurgitation, or tricuspid reoperation for prosthetic stenosis/regurgitation not due to endocarditis.10 Severe tricuspid stenosis was defined as a mean mitral gradient of 10 mm Hg or more. Calculated valve orifice area was not reliably reported in our cohort.2 Follow-up data were obtained from the electronic medical record with linkage to multiple national hospital databases and the National Death Index.
Statistical Methods
Binary outcomes were compared with Pearson's chi-square or Fisher exact test as appropriate and presented as frequency counts and percentages (n [%]). Continuous outcomes were compared with Wilcoxon rank-sum or t test as appropriate, and summarized with mean and SD or median and interquartile range. CI curves were compared using Gray's test.
A competing-risk analysis was performed to compute CI of end points other than survival occurring over time using the variables in Table E1. Death, cardiac transplantation, and insertion of a left ventricular assist device were censored at the time of last echocardiography follow-up. Outcomes based on echocardiography findings were censored for the last known echocardiogram. Missing data were handled with complete case analysis after checking the assumption of missing completely at random.
To correct for differences between the porcine and pericardial groups, Cox proportional hazard analysis was used for patient survival after checking for hazard proportionality. Variables were chosen based on clinical judgement, prior literature, and univariate covariables associated with porcine and pericardial grouping (Table E1). Survival estimates were presented as ± SE. Survival curves were compared using the log-rank test.
Statistical software was SAS 9.4 (SAS Institute Inc). Waiver of informed consent was approved by the Institutional Review Board (Pro00105933, approved June 22, 2020).
Results
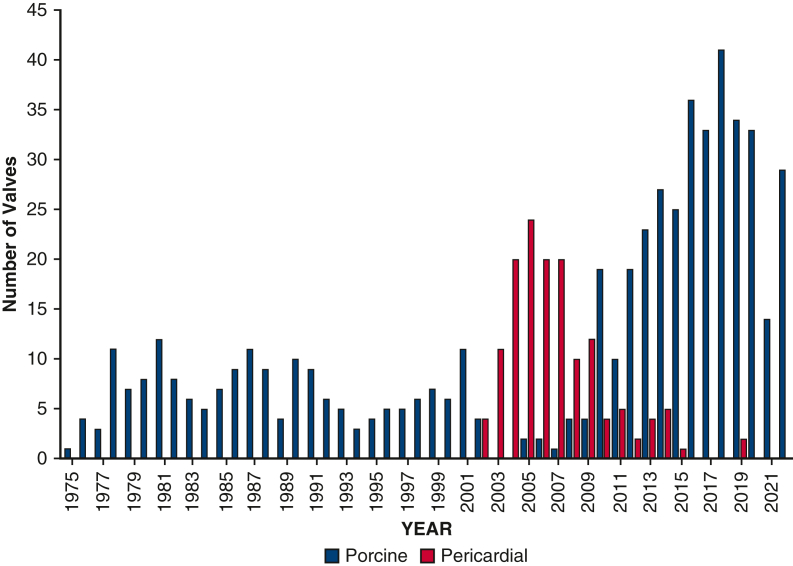
From 1975 to 2022, 686 consecutive patients underwent TVR with biological prostheses (Figure E1). The porcine prostheses (N = 542) implanted included Mosaic (N = 292) (Medtronic, Inc), Edwards Model 6625 (N = 129) (Edwards Lifesciences), Hancock (N = 65) (Medtronic, Inc), Biocor (N = 42) (Abbott Laboratories), and Epic (N = 32) (Abbott Laboratories). The pericardial prostheses implanted included the Edwards Models 6900 or 7300 TFX (N = 144) (Edwards Lifesciences) beginning in 2002. The median patient survival in this series was 5.1 (0.7-11.3) years. One-year mortality was 24% ± 2%. Median clinical follow-up of patients was 2.3 (0.5-6.3) (range, 0-41) years after porcine replacement and 4.0 (1.7-7.5) (range, 0-19) years after pericardial replacement. Median echocardiography follow-up of patients was 1.1 (0.1-4.4) (range, 0-30) years after porcine replacement and 1.6 (0.2-5.2) (range, 0-17) years after pericardial replacement. Completeness of echocardiography follow-up of living patients was 122 of 236 (52%) at 5 years and 54 of 104 (52%) at 10 years. Data missingness was less than 5% for patient demographics. For patients living more than 30 days, postoperative echocardiography data missingness was 17% (106/619). A total of 33 surgeons were included; however, 10 surgeons performed 80% (550/686) of cases. Patients who received the porcine prosthesis, compared with pericardial, were younger (56 ± 17 years vs 63 ± 15 years) and more likely to present urgently (porcine 55%, pericardial 44%) with endocarditis (porcine 21%, pericardial 13%) and history of intravenous drug use (porcine 13%, pericardial 5%) (Table 1). However, pericardial patients were more likely to have class 3 to 4 heart failure (pericardial 76%, porcine 67%). Groups demonstrated no difference in redo status, concomitant operations, or severity of tricuspid regurgitation.
Figure E1.
Institutional volume of patients undergoing TVR with porcine and pericardial prostheses by operative year.
Table 1.
Preoperative and intraoperative data
| Variable | Porcine | Pericardial | P value |
|---|---|---|---|
| N | 542 | 144 | |
| Age (y) | 56 ± 17 | 63 ± 15 | <.001 |
| Male | 223 (41%) | 54 (38%) | .05 |
| White | 398 (74%) | 108 (75%) | .80 |
| Hypertension | 276 (51%) | 97 (67%) | .001 |
| Class III-IV heart failure | 363 (67%) | 109 (76%) | .05 |
| Coronary artery disease | 87 (16%) | 33 (23%) | .05 |
| Prior cardiac surgery | 296 (55%) | 85 (59%) | .80 |
| Urgent | 298 (55%) | 63 (44%) | .02 |
| Severe tricuspid regurgitation | 499 (92%) | 133 (92%) | .90 |
| Intravenous drug abuse | 70 (13%) | 7 (5%) | .007 |
| Tricuspid disease etiology | |||
| Endocarditis | 119 (22%) | 18 (13%) | .01 |
| Rheumatic disease | 76 (14%) | 22 (15%) | .70 |
| Tricuspid device failure | 36 (7%) | 7 (5%) | .40 |
| Functional tricuspid disease | 252 (46%) | 83 (58%) | .02 |
| Other tricuspid etiology | 59 (11%) | 14 (10%) | .70 |
| Tricuspid size | 30 ± 3 | 28 ± 2 | <.001 |
| Sternotomy | 389 (72%) | 287 (52%) | <.001 |
| Cardiopulmonary bypass time (min) | 197 ± 91 | 205 ± 81 | .15 |
| Crossclamp time (min) | 83 (58, 118) | 108 (70, 155) | .005 |
| No crossclamp | 215/542 (40%) | 65/144 (49%) | .04 |
| Isolated tricuspid replacement | 281/542 (52%) | 65/144 (45%) | .15 |
| Coronary artery bypass grafting | 33 (6%) | 12 (8%) | .30 |
| Aortic valve replacement | 79 (15%) | 23 (16%) | .70 |
| Mitral valve repair | 36 (7%) | 23 (16%) | .001 |
| Mitral valve replacement | 164 (30%) | 40 (28%) | .60 |
| Left ventricular assist device | 14 (2.6%) | 2 (1.4%) | .40 |
| Maze procedure | 14 (3%) | 5 (3%) | .60 |
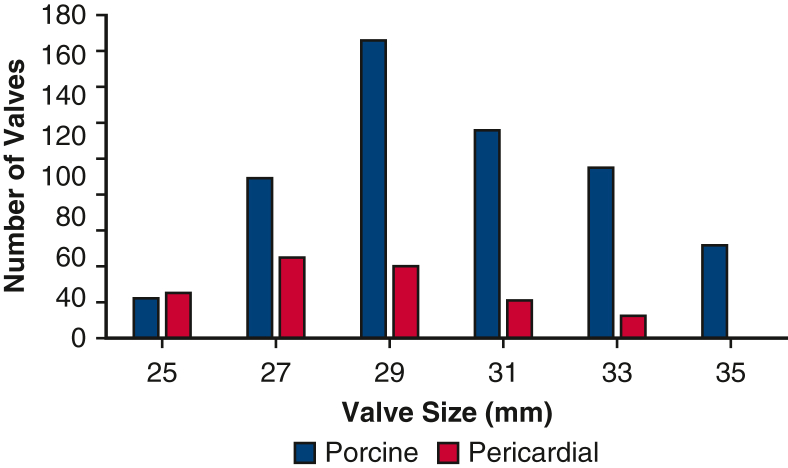
Intraoperatively, patients experienced similar cardiopulmonary bypass times, but the pericardial group had longer crossclamp times (Table 1). After adjustment with regression analysis, valve type was not associated with clamp time (P = .2). The porcine group was more likely to receive sternotomy (porcine 389/542 [72%], pericardial 67/144 [47%]). Mean valve size was 30 ± 3 for porcine and 28 ± 2 for pericardial valves (Figure E2). Approximately half of the patients in each group underwent isolated TVR (porcine 281/542 [52%], pericardial 65/144 [45%], P = .15). Concurrent procedures (coronary artery bypass grafting, aortic valve replacement, mitral valve replacement, left ventricular assist devices, and Maze procedure) were similar between groups. Postoperatively, patients with porcine and pericardial valves experienced similar 30-day mortality, stroke, reoperation for bleeding, and implantation of permanent pacemaker (Table 2). The porcine group was more likely to have renal injury (porcine 95/542 [18%], pericardial 12/144 [8%]), prolonged ventilation (porcine 84/542 [16%], pericardial 13/144 [9%]), and longer length of stay (17 days vs 13 days, respectively).
Figure E2.
Distribution of tricuspid valve sizes for porcine and pericardial prostheses.
Table 2.
Patient outcomes, unmatched
| Outcome | Porcine | Pericardial | P value |
|---|---|---|---|
| N | 542 | 144 | |
| Postoperative death | 67 (12%) | 13 (9%) | .30 |
| Postoperative stroke | 19 (4%) | 5 (3%) | 1.0 |
| Postoperative reoperation for bleeding | 45 (8%) | 42 (8%) | .90 |
| Postoperative permanent pacemaker | 98 (18%) | 23 (16%) | .60 |
| Postoperative new atrial fibrillation | 46 (8%) | 4 (3%) | .02 |
| Postoperative renal injury | 95 (18%) | 12 (8%) | .007 |
| Prolonged ventilation (>48 h) | 84 (16%) | 13 (9%) | .05 |
| Length of stay (median days) | 17 (10, 31) | 13 (9, 21) | .0003 |
| 10-y survival | 35% ± 3% | 28% ± 4% | .20 |
| 10-y CI structural valve deterioration | 9% ± 2% | 11% ± 3% | .80 |
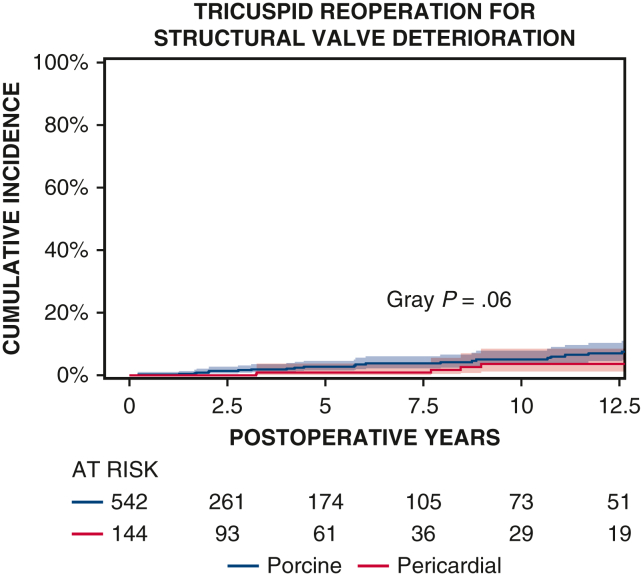
| 10-y CI tricuspid reoperation for structural valve deterioration | 5% ± 1% | 4% ± 2% | .06 |
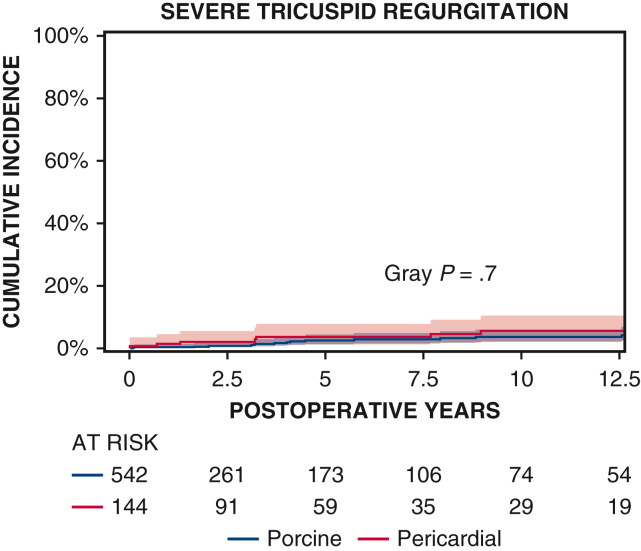
| 10-y CI severe tricuspid regurgitation | 4% ± 1% | 5% ± 2% | .70 |
| 10-y CI severe tricuspid stenosis | 6% ± 1% | 7% ± 2% | .60 |
Postoperative, 30-day or index hospitalization; CI, cumulative incidence.
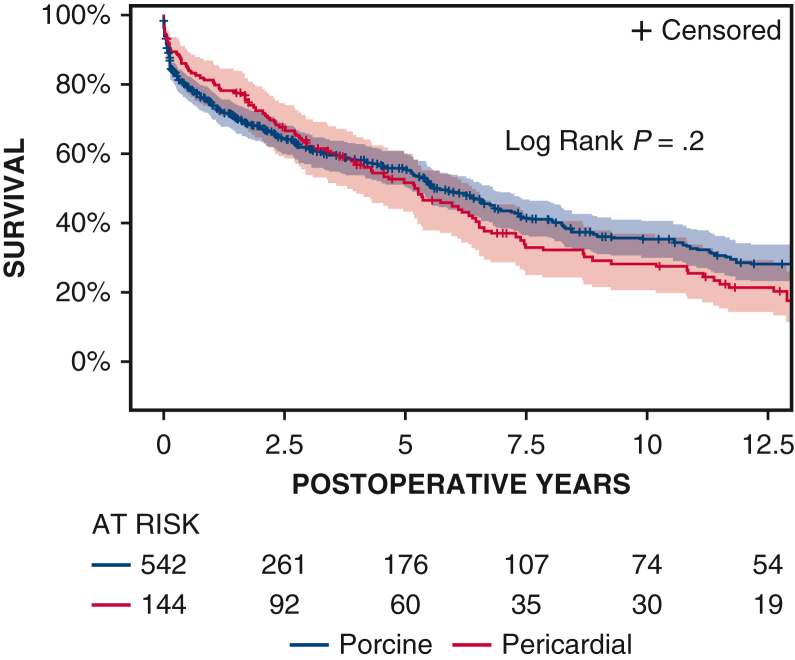
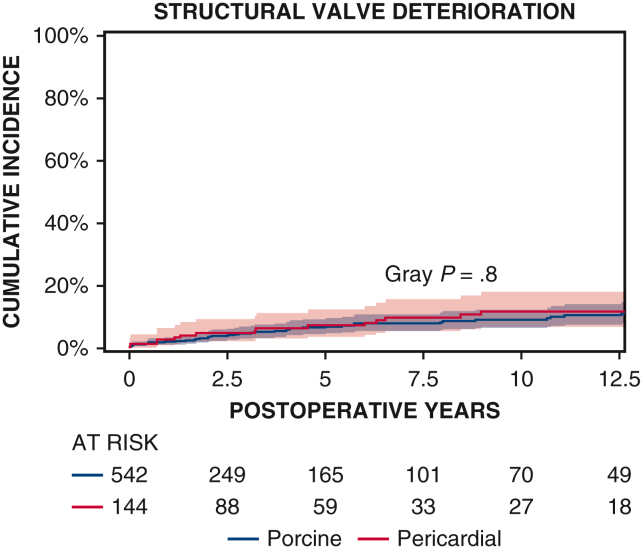
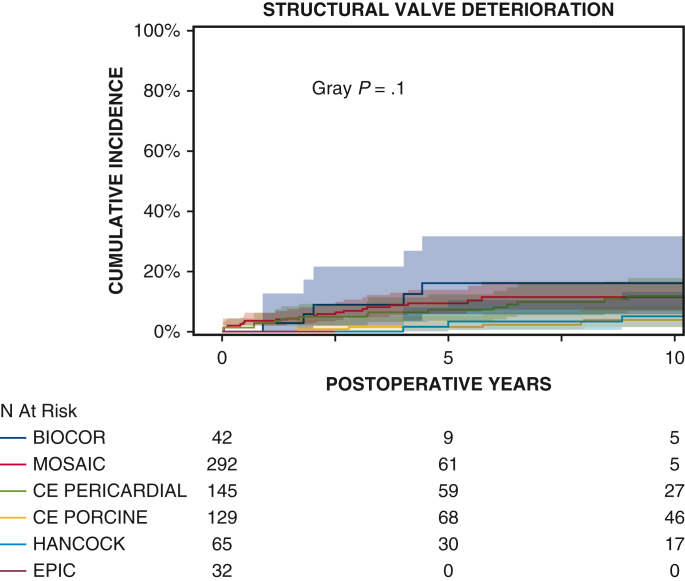
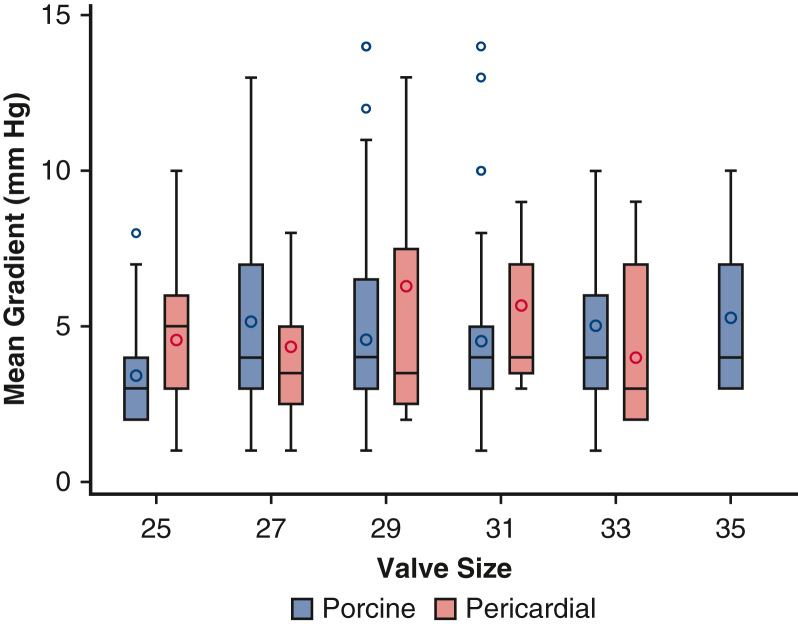
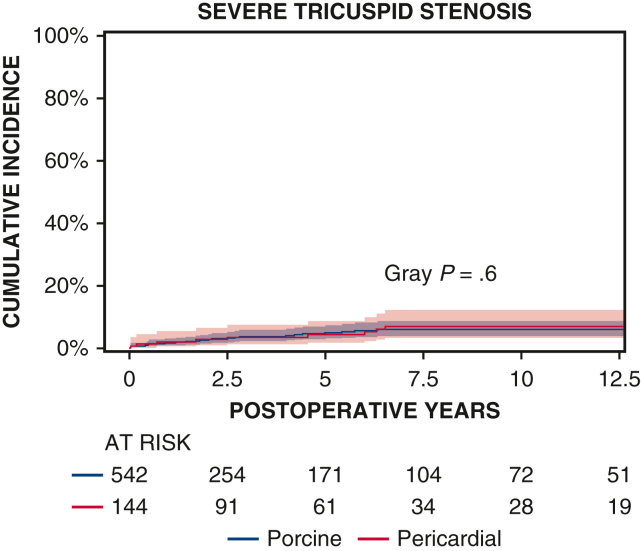
Ten-year survival was not significantly different between patients with porcine and pericardial valves (35% ± 3% vs 28% ± 4%, P = .2) (Figure 1 and Table 2). The 10-year CI of structural valve deterioration (porcine 9% ± 2%, pericardial 11% ± 3%, P = .8), reoperation for structural valve deterioration (porcine valve 5% ± 1%, pericardial valve 4% ± 2%, P = .06), and severe regurgitation (porcine 4% ± 1%, pericardial 5% ± 2%, P = .7) were not significantly different between the porcine and pericardial groups (Figures 2, 3, and E3; Table 2). Likewise, there was no difference in structural valve deterioration between valve models (P = .1) (Figure 4). The failure mode was similar, with a similar incidence of severe stenosis (porcine 32/47 [68%], pericardial 11/16 [69%], P = .9), and severe regurgitation (porcine 18/47 [38%], pericardial 7/16 [44%], P = .7). Porcine and pericardial valves demonstrated identical median tricuspid gradients at the last echocardiography (4 [3-6] mm Hg in each group, (P = .7) (Figure 5). Likewise, the 10-year CI of severe stenosis was similar (6% ± 1%, 7% ± 2%, P = .6) (Figure E4 and Table 2).
Figure 1.
Kaplan–Meier estimates of overall survival in patients undergoing tricuspid valve surgery with porcine and pericardial prostheses. Red and blue color bars indicate 95% CIs.
Figure 2.
CI curves of structural valve deterioration after TVR with porcine and pericardial prostheses. Red and blue color bars indicate 95% CIs.
Figure 3.
CI curves of tricuspid reoperation for structural valve deterioration after TVR with porcine and pericardial prostheses. Red and blue color bars indicate 95% CIs.
Figure E3.
CI curves of severe tricuspid regurgitation after TVR with porcine and pericardial prostheses. Red and blue color bars indicate 95% CIs.
Figure 4.
CI curves of tricuspid structural valve deterioration after TVR with the 6 valve prosthesis models studied. Red and blue color bars indicate 95% CIs.
Figure 5.
Mean tricuspid valve gradient at last echocardiography based on valve size for porcine and pericardial prostheses. Red boxes represent pericardial valves, and blue color boxes indicate porcine valves. Lower and upper borders of the box mark the 25th and 75th percentiles. Horizontal bar within the box marks the median, with circles showing the mean. Lower and upper whiskers show the minimum and maximum values of nonoutliers.
Figure E4.
CI curves of severe tricuspid stenosis after TVR with porcine and pericardial prostheses. Red and blue color bars indicate 95% CIs.
Multivariable Cox model analysis demonstrated that the multivariable correlates of death were older age (hazard ratio [HR], 1.02, P < .0001), class 3 to 4 heart failure (HR, 1.16, P = .001), hemodialysis (HR, 1.3, P = .01), lung disease (HR, 1.4, P = .01), and radiation therapy (HR, 1.2, P = .01). Bioprosthetic valve type (porcine vs pericardial) was not significantly associated with survival (P = .6). On multivariate logistic regression, the valve type (P = .1) and size (P = .8) were not associated with structural valve deterioration. Reoperation for structural valve deterioration was associated with younger age (<50 years) (HR, 1.05, P < .0001), but not valve type (P = .9).
To account for operative year, the analysis was repeated with patients who underwent TVR from 2002 to 2015 only (porcine = 140, pericardial = 142). Similar to results from the larger cohort (1975-2022), there were no differences between valve types for 10-year survival (porcine 31% ± 5%, pericardial 28% ± 4%, P = .5), 10-year structural valve deterioration (porcine 14% ± 3%, pericardial 12% ± 3%, P = .4), or reoperation for structural valve deterioration (porcine 6% ± 2%, pericardial 4% ± 2%, P = .2).
Discussion
In this study, we compared durability of porcine and pericardial prosthetic valves used in TVR. With an all-cause 1-year mortality of 25% in patients who undergo TVR, studies on improving outcomes in this patient population by informing surgeon decision-making are imperative.4 Porcine and pericardial tricuspid valves demonstrated similar survival, structural valve deterioration, reoperation for structural valve deterioration, severe tricuspid regurgitation, and severe tricuspid stenosis at 10 years. After adjustment, valve type (porcine and pericardial) was not associated with survival, structural valve deterioration, or reoperation for structural valve deterioration. Even after the analysis was limited to operations performed after 2002 when porcine and pericardial were available simultaneously, there were no differences in survival, structural valve deterioration, or reoperation for structural valve deterioration.
To our knowledge, this is one of the largest comparative analyses of porcine and pericardial tricuspid valve prosthetics.5 Other published series comparing biologic prostheses are small and report variable late durability of TVR.11,12 Studies comparing mechanical and biologic tricuspid replacement offer larger cohorts and late follow-up, but unfortunately combine all tissue valve models into a single group for the analyses, which eliminates access to the outcomes based on valve types.6,7,13,14
Similar to our findings, Sohn and colleagues5 reported no difference in all-cause mortality. Sohn and colleagues5 and Kang and colleagues11 reported more tricuspid stenosis and tricuspid reoperation in pericardial valves, even after multivariable correction for differences in underlying patient characteristics. Our study found no difference in tricuspid stenosis or tricuspid reoperation between pericardial versus porcine valves; however, we specifically investigated reoperation for structural valve deterioration with exclusion of patients with prosthetic valve endocarditis. Unlike other studies, we included echocardiography data in our definition of durability, which enhanced granularity of outcome. We reported no difference between valve type in structural valve deterioration and failure mode. The incidence of severe regurgitation and stenosis was similar between porcine and pericardial valves.
The Mosaic porcine prosthesis accounted for 292 of the porcine valves in our tricuspid series. In the mitral position, studies report conflicting results on the durability of porcine and pericardial prostheses. Eric Jamieson and colleagues15 and Uchino and colleagues16 reported a higher incidence of late regurgitation for porcine mitral prosthetics. Buete and colleagues17 reported a significantly higher incidence of late structural valve deterioration as well as reoperation for structural valve deterioration in patients with pericardial valves. Conversely, in a meta-analysis comparing porcine and pericardial mitral valves, porcine valves demonstrated higher freedom from SVD.18 Different valve models may have contributed to disparate results in the studies, with third-generation porcine Mosaic valves in Buete and colleagues'17 and Malvindi and colleagues'18 series and first-generation porcine valves in Jamieson and colleagues'15 series. However, in our current series of tricuspid replacements, there was no significant difference in durability between any biological valve models, including first-generation porcine valves, pericardial valves, and later-generation porcine prostheses, which includes the Mosaic porcine prosthesis (Figure 4). The applicability of data from biologic valves in the mitral position may not be generalizable to the same valve models in the tricuspid position due to different hemodynamic profiles and poor survival of patients who underwent TVR (Figures 1 and 2).4
This study demonstrates that both porcine and pericardial prostheses provide similar clinical and echocardiographic outcomes. Still, despite similar durability, most patients undergoing TVR did not outlive the prosthesis, regardless of valve type and operative year.
Study Limitations
Our study is a retrospective analysis from a single center over 47 years. Not all models of porcine or pericardial biological valves were examined. The practically accessible definition of severe tricuspid stenosis as a mean gradient of 10 mm Hg instead of a valve area of less than 1 cm2 may have inaccuracy due to variation in cardiac index. The data document drift in patient selection and operative technique over time between the 2 series, and measured variables may not fully account for differences in patient selection or operative technique. The use of multivariable analysis cannot entirely adjust for selection bias of choice of prosthesis, particularly in the era of pericardial valves when porcine was available. Valve manufacturing and anticalcification techniques are proprietary and difficult to differentiate from the valve lines themselves. Finally, this study includes a patient population with a known median life expectancy of less than 10 years; likewise, the study design is limited in discerning differences between bioprostheses that commonly have durability over 20 years (Figure 2).
Conclusions
We present our large institutional experience of TVR and compare the durability of porcine and pericardial prostheses. Remarkably, we found no significant differences in durability between porcine and pericardial valves in the tricuspid position, despite comparison of many valve models in a wide variety of patients over many decades. We assessed durability through multiple metrics, including clinic outcomes (survival and reoperation) and echocardiography findings (prosthetic valve regurgitation, stenosis, and mean gradient). Therefore, this study can inform surgeon decision-making on valve type, particularly as newer devices become available. In most patients undergoing TVR, the choice of porcine versus pericardial prosthesis is unlikely to affect clinical outcomes.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/long-term-outcomes-of-porcine-7137.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Waiver of informed consent was approved by the Institutional Review Board (Pro00105933, approved June 22, 2020).
Appendix E1
Table E1.
Variables used in multivariable analysis of outcomes
| Pericardial vs porcine prosthesis |
| Age of patient |
| Gender |
| Rheumatic mitral disease etiology |
| Functional mitral disease etiology |
| Endocarditis |
| Coronary artery disease |
| Hypertension |
| Tricuspid prosthesis size |
| Endocarditis |
| Urgency |
| White race |
| Severe tricuspid regurgitation |
| Smoking |
| Class III-IV heart failure |
| Weight |
| Intravenous drug abuse |
| Prior operation |
| Atrial fibrillation |
References
- 1.Chen Q., Bowdish M.E., Malas J., et al. Isolated tricuspid operations: the Society of Thoracic Surgeons Adult Cardiac Surgery database analysis. Ann Thorac Surg. 2023;115(5):1162–1170. doi: 10.1016/j.athoracsur.2022.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2021;162(2):e183–e353. doi: 10.1016/j.jtcvs.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 4.Kundi H., Popma J.J., Cohen D.J., et al. Prevalence and outcomes of isolated tricuspid valve surgery among Medicare beneficiaries. Am J Cardiol. 2019;123(1):132–138. doi: 10.1016/j.amjcard.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Sohn S.H., Kang Y., Kim J.S., Hwang H.Y., Kim K.H., Choi J.W. Long-term clinical outcomes of tricuspid valve replacement using bovine versus porcine valves: a nationwide population-based study. Eur J Cardiothorac Surg. 2023;64(1) doi: 10.1093/ejcts/ezad151. [DOI] [PubMed] [Google Scholar]
- 6.Liu P., Xia D.S., Qiao W.H., et al. Which is the best prosthesis in an isolated or combined tricuspid valve replacement? Eur J Cardiothorac Surg. 2021;59(1):170–179. doi: 10.1093/ejcts/ezaa273. [DOI] [PubMed] [Google Scholar]
- 7.Patlolla S.H., Saran N., Schaff H.V., et al. Prosthesis choice for tricuspid valve replacement: comparison of clinical and echocardiographic outcomes. J Thorac Cardiovasc Surg. 2024;167(2):668–679.e2. doi: 10.1016/j.jtcvs.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Y., Sun Y., Li N., et al. Long-term outcomes of bioprosthetic and mechanical tricuspid valve replacement after left-sided valves surgery. Ann Thorac Cardiovasc Surg. 2023;29(6):307–314. doi: 10.5761/atcs.oa.23-00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoghbi W.A., Adams D., Bonow R.O., et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Dvir D., Bourguignon T., Otto C.M., et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137(4):388–399. doi: 10.1161/CIRCULATIONAHA.117.030729. [DOI] [PubMed] [Google Scholar]
- 11.Kang Y., Hwang H.Y., Sohn S.H., Choi J.W., Kim K.H., Kim K.B. Comparative analysis of structural valve deterioration after bioprosthetic tricuspid valve replacement: bovine pericardial versus porcine valves. Artif Organs. 2021;45(8):911–918. doi: 10.1111/aor.13909. [DOI] [PubMed] [Google Scholar]
- 12.Wiedemann D., Rupprechter V., Mascherbauer J., et al. Tricuspid valve replacement: results of an orphan procedure - which is the best prosthesis? J Cardiovasc Surg (Torino) 2018;59(4):626–632. doi: 10.23736/S0021-9509.18.10392-2. [DOI] [PubMed] [Google Scholar]
- 13.Sohn S.H., Kang Y., Kim J.S., Hwang H.Y., Kim K.H., Choi J.W. Early and long-term outcomes of bioprosthetic versus mechanical tricuspid valve replacement: a nationwide population-based study. J Thorac Cardiovasc Surg. 2024;167(6):2117–2128.e11. doi: 10.1016/j.jtcvs.2023.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Kang Y., Hwang H.Y., Sohn S.H., Choi J.W., Kim K.H., Kim K.B. Fifteen-year outcomes after bioprosthetic and mechanical tricuspid valve replacement. Ann Thorac Surg. 2020;110(5):1564–1571. doi: 10.1016/j.athoracsur.2020.02.040. [DOI] [PubMed] [Google Scholar]
- 15.Eric Jamieson W.R., Marchand M.A., Pelletier C.L., et al. Structural valve deterioration in mitral replacement surgery: comparison of Carpentier-Edwards supra-annular porcine and perimount pericardial bioprostheses. J Thorac Cardiovasc Surg. 1999;118(2):297–304. doi: 10.1016/S0022-5223(99)70220-5. [DOI] [PubMed] [Google Scholar]
- 16.Uchino G., Murakami H., Mukohara N., et al. Modes of the bioprosthetic valve failure of the porcine and pericardial valves in the mitral position. Eur J Cardiothorac Surg. 2022;62(1) doi: 10.1093/ejcts/ezab506. [DOI] [PubMed] [Google Scholar]
- 17.Beute T.J., Goehler M., Parker J., et al. Long-term outcomes of mosaic versus perimount mitral replacements: 17-year follow-up of 940 implants. Ann Thorac Surg. 2020;110(2):508–515. doi: 10.1016/j.athoracsur.2019.10.075. [DOI] [PubMed] [Google Scholar]
- 18.Malvindi P.G., Mastro F., Kowalewski M., et al. Durability of mitral valve bioprostheses: a meta-analysis of long-term follow-up studies. Ann Thorac Surg. 2020;109(2):603–611. doi: 10.1016/j.athoracsur.2019.07.024. [DOI] [PubMed] [Google Scholar]