Abstract
Objective
To highlight the role of hyper accuracy three-dimensional (3D) reconstruction in facilitating surgical planning and guiding selective clamping during robot-assisted partial nephrectomy (RAPN).
Methods
A transperitoneal RAPN was performed in a 62-year-old male patient presenting with a 4 cm right anterior interpolar renal mass (R.E.N.A.L nephrometry score 7A). An abnormal vasculature was observed, with a single renal vein and two right renal arteries originating superiorly to the vein and anterior, when dividing in their segmental branches. According to the hyper accuracy 3D (HA3D®) rainbow model (MEDICS Srl, Turin, Italy), one branch belonging to one of the segmental arteries was feeding the tumor. This allowed for an accurate prediction of the area vascularized by each arterial branch. The 3D model was included in the intraoperative console view during the whole procedure, using the TilePro feature. A step-by-step explanation of the procedure is provided in the video attached to the present article.
Results
The operative time was 90 min with a warm ischemia time on selective clamping of 13 min. Estimated blood loss was 180 mL. No intraoperative complication was encountered and no drain was placed at the end of the procedure. The patient was discharged on postoperative Day 2, without any early postoperative complications. The final pathology report showed a pathological tumor stage 1 clear cell renal cell carcinoma with negative surgical margins.
Conclusion
The present study and the attached video illustrate the value of 3D rainbow model during the planning and execution of a RAPN with selective clamping. It shows how the surgeon can rely on this model to be more efficient by avoiding unnecessary surgical steps, and to safely adopt a “selective” clamping strategy that can translate in minimal functional impact.
Keywords: Hyper accuracy three-dimensional rainbow model, Augmented reality, Clear cell renal cell carcinoma, Robot-assisted partial nephrectomy, Selective clamping
1. Introduction
Approaching a renal mass with a detailed and precise understanding of key anatomical landmarks is essential for the success of a robot-assisted partial nephrectomy (RAPN) procedure. Several three-dimensional (3D) models were developed [1], to foresee intraoperative anatomy and adequately plan key surgical steps, such as artery clamping. Moreover, the selective clamping, achieved with the accurate reproduction ensured by the 3D model [2], could reduce the impact of the procedure on surgical-related outcomes, such as warm ischemia time, which may affect postoperative renal function impairment [3,4].
Among the different technologies under investigation [5,6], hyper-accuracy 3D virtual models (3DVMs) proved their usefulness in avoiding the ischemia of healthy tissue, and to be predictive of a successful partial nephrectomy (PN) [7].
In the present study, we applied hyper accuracy 3D (HA3D®) rainbow model (MEDICS Srl, Turin, Italy) to a PN procedure, in order to highlight its role in facilitating surgical planning and guiding selective clamping during RAPN.
2. Materials and methods
2.1. The HA3D® rainbow model
The technique for the construction of the 3D model was already described in detail elsewhere [8]. Briefly, high-resolution CT scan images are processed using a dedicated software produced by M3DICS (MEDICS Srl, Turin, Italy) to create a HA3D® virtual model of the kidney. This model allows analyzing the renal vasculature, collecting system, and tumor location and characteristics. The “dynamic region growing” method is used to obtain a detailed virtual reconstruction of the renal pedicle and tumor feeding arteries. This method, originally described by Yoshida et al. [9], relies on the Voronoi's algorithm, a mathematical algorithm able to divide 3D space among pre-determined points or lines calculating the minimal distance between them. Renal arteries are reconstructed up to the segmental arteries. The precise information about renal vascularization represents a significant benefit of the HA3D® model, allowing the surgeon to safely plan selective and superselective clamping of the renal vessels. Segmentation of the renal parenchyma is performed by selectively thresholding the images, separating different voxels and grouping them based on their gray scale values. The resulting 3DVMs are then reviewed by bioengineers and urologists together, to assess their accuracy compared to the original images. Subsequently, a mathematical HA3D® model is created, along with an interactive 3D-PDF file that allows for navigation during preoperative surgical planning and for surgical navigation intraoperatively.
2.2. Surgical technique
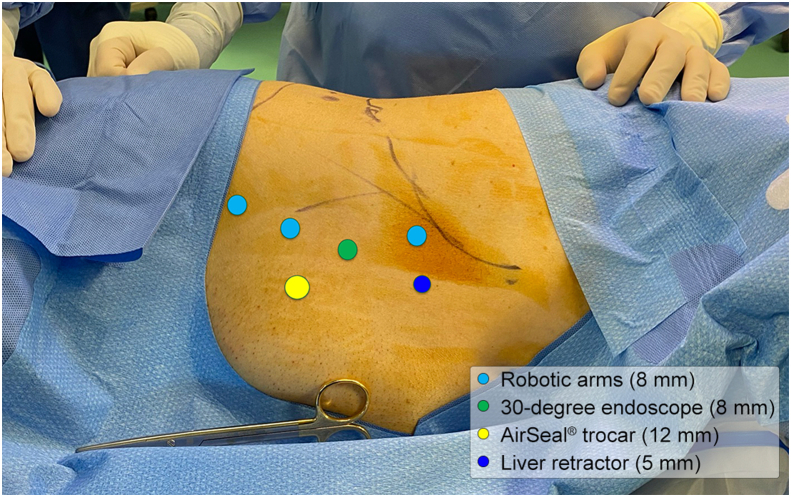
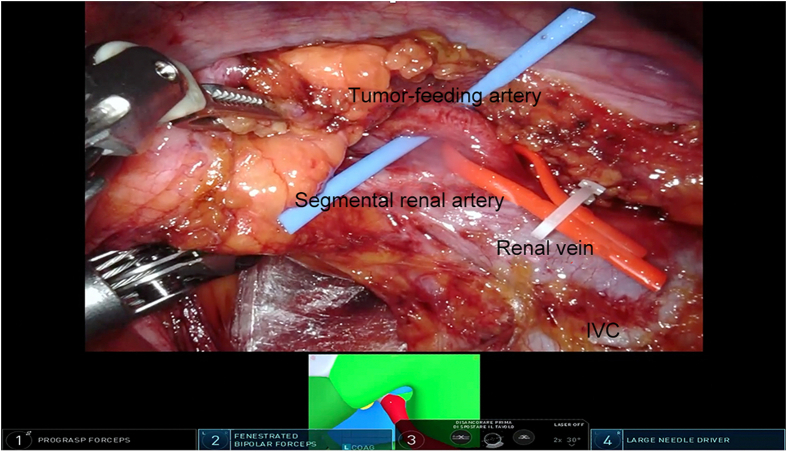
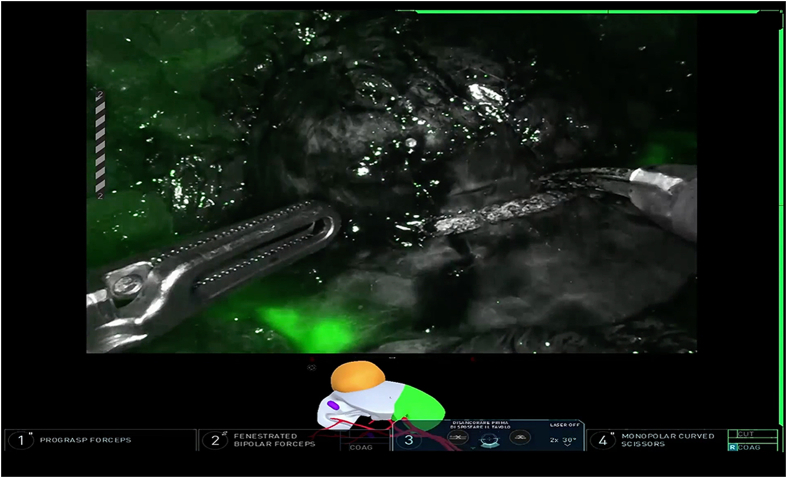
Transperitoneal RAPN was performed in a 62-year-old male patient presenting with a 4 cm right anterior interpolar renal mass (R.E.N.A.L nephrometry score 7A) [10] and a normal preoperative renal function. The procedure was performed after the patient's informed consent was obtained. The patient provided written informed consent for the reproduction of images and video of the procedure for scientific purposes. An abnormal vasculature was observed, with a single renal vein and two right renal arteries originating superiorly to the vein and anteriorly when dividing into their segmental branches. According to the HA3D® rainbow model, one branch belonging to one of the segmental arteries was feeding the tumor (Fig. 1). This allowed for an accurate prediction of the area vascularized by each arterial branch. The 3D model was included in the intraoperative console view during the whole procedure, using the TilePro feature. The Supplementary Video 1 illustrates the value of HA3D® rainbow model during the planning and execution of the procedure. The procedure was carried out with the DaVinci Xi robotic surgical system (Intuitive, Sunnyvale, CA, USA), docked from the back of the patient. The patient was placed in a modified flank position; four 8-mm trocars were inserted in a transperitoneal fashion on the pararectal line and two ancillary trocars were placed for the assistant, one 12-mm trocar for AirSeal® (CONMED, Utica, NY, USA) and another 5-mm trocar for liver retraction (Fig. 2). Adequate exposure of the kidney was achieved by incising the line of Toldt and medializing the right colon. Next, the duodenum was dissected from the anterior surface of Gerota fascia to expose the anterior surface of the inferior vena cava. After visualization of inferior vena cava, isolation of the renal vessels was performed. The anterior renal artery was not dissected at its origin; its branches were instead directly isolated with a lateral approach to identify the branch directly feeding the renal mass. Once renal vasculature was identified and fully isolated (Fig. 3), defatting of the kidney was performed in order to clearly recognize the renal mass. Selective clamping of the branch supplying the tumor was achieved with a bulldog clamp, guided by the HA3D® rainbow model which allowed for a prompt and easy identification of the vessel. After the bulldog positioning, the adequate devascularization of the kidney was verified with injection of indocyanine green and subsequent utilization of the Firefly™ fluorescence imaging vision system (Intuitive, Sunnyvale, CA, USA) (Fig. 4). Enucleoresection was conducted by combining wedge resection and enucleation. At first, the parenchyma surrounding the renal mass was scored to point out the borders of the resection. Thus, enucleoresection was carried out by blunt and sharp dissection, dividing the adhesions between the tumor and normal renal parenchyma. Once the renal mass was completely excised, the renorrhaphy was performed. The medullary layer was carried out with a 3/0 V-Lock barbed suture in a continuous fashion. The bulldog clamp was then removed, according to an early unclamping technique. After bulldog removal, persistent arterial bleeding from the tumor resection bed was observed. Therefore, the medullary layer was completed with a second suture, using the same stitch. The cortical layer was obtained with interrupted 0 monofilament sutures, and the resection bed's edges were approximated using the sliding clip technique. Accurate hemostasis was achieved, with the aid of FloSeal (Baxter International, Deerfield, IL, USA) and SURGICEL SNoW™ (Ethicon Inc, Raritan, NJ, USA), and the absence of any residual bleeding was verified. The specimen was bagged; robotic instruments were removed; and the robot was undocked. After specimen extraction, fascia and skin were closed.
Figure 1.
Accurate reproduction of the kidney by the hyper accuracy three-dimensional (HA3D®) rainbow model (MEDICS Srl, Turin, Italy). (A) Renal anatomy; (B) Tumor vascularization.
Figure 2.
Trocar placement for right robot-assisted partial nephrectomy. Light blue: 8 mm trocars for robotic arms; green: 8 mm trocar for 30-degree endoscope; yellow: 12 mm trocar for the assistant with the AirSeal® system (CONMED, Utica, NY, USA); dark blue: 5 mm trocar for liver retraction.
Figure 3.
Isolation of the elements of the renal pedicle. IVC, inferior vena cava.
Figure 4.
Devascularization of renal mass after selective clamping confirmed with injection of indocyanine green.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajur.2023.08.006
The following is/are the supplementary data related to this article:
Step-by-step robot-assisted partial nephrectomy performed with the hyper accuracy three-dimensional (HA3D®) rainbow model (MEDICS Srl, Turin, Italy).
3. Results
The operative time was 90 min with a warm ischemia time on selective clamping of 13 min. Estimated blood loss was 180 mL. No intraoperative complication was encountered, and no drain was placed at the end of the procedure. The catheter was removed on postoperative Day 1 and the patient discharged on postoperative Day 2, without any early postoperative complications. The final pathology report showed a pathological tumor stage 1 clear cell renal cell carcinoma with negative surgical margins. An assessment of the postoperative renal function performed 3 months after the procedure showed a normal estimated glomerular filtration rate value (>90 mL/min/1.73 m2).
4. Discussion
In this study, we present a case of a patient undergoing RAPN with selective clamping performed with the support of the HA3D® rainbow model. Even though different experiences of renal surgery with the aid of 3D models have already been presented [11], our experience with HA3D® rainbow model raises some points of great interest when considering the implications of its potential routine use when planning RAPN.
The model allows for increased efficiency by avoiding unnecessary surgical steps, and the safe adoption of a selective clamping strategy that can reduce the functional impact on postoperative outcomes. PN proved non-inferiority in terms of oncological outcomes (cancer-specific survival and overall survival) with respect to radical nephrectomy [12,13]. Furthermore, it led to better preservation of kidney function, potentially lowering the risk of cardiovascular disease [14]. Nevertheless, the role of ischemia time on postoperative kidney function is still a controversial matter. Many studies address it, together with other variables such as blood loss and preoperative renal function, as a pivotal factor in reducing renal function decrease after PN. According to a recent review, a shorter ischemia time showed no or less impact on postoperative renal function in patients undergoing RAPN [15]. This is further supported by results obtained by Antonelli et al. [16], which demonstrated that a warm ischemia time over 10 min could lead to a higher functional impairment according to postoperative radionuclide renal scan. This underlines the importance of minimizing the ischemia time to preserve kidney function. Nevertheless, this debated matter could be overcome with the adoption of a selective clamp approach. As a matter of fact, a comparison between selective or superselective clamp and main artery clamp showed superior renal function in the former group [17]. Nevertheless, the main artery clamp remains the technique of choice for the majority of surgical approaches to the kidney, even when it could be avoided [18]. Robot-assisted approach can help in reversing this trend, due to the possibility of accurate isolation of the components of the renal hilum. The efficacy of HA3D® rainbow model in aiding the surgeon to perform selective clamping has already been pointed out [8]. In particular, the use of HA3D® rainbow model led to an increase in selective clamping and a lower impairment of postoperative renal function, compared to procedures carried out without the 3D model, thus supporting the use of this tool in RAPN [3]. Further evidence comes from a recently published case series by Amparore et al. [4], which investigated the added value of integrating the assessment of perfusion regions into the HA3D® model. Perfusion regions were identified by dividing the parenchyma in different areas according to its vascularization, potentially avoiding ischemia to healthy tissue. The HA3D® model led to a high rate of selective clamp (76.7%), with a great concordance between preoperative and intraoperative plans for renal pedicle management (96.1%). Selective clamp allowed for better preservation of postoperative renal function, evaluated by 99mTc-mercaptoacetyltriglycine renal scintigraphy, compared to global clamping [4].
The detailed understanding of perfusion areas, colored in different ways, permits meticulous planning of which arteries to clamp in order to obtain a bloodless field and spare ischemia to healthy parenchyma. Indeed, in the present case, the surgeon was aware of the anatomical variant preoperatively and he consequently planned the whole procedure. The 3D model made it possible to directly approach the tumor-feeding arterial branch during hilar dissection and to safely obtain selective clamping, avoiding global ischemia. Thus, the preoperative knowledge of the structures constituting renal vasculature helped the surgeon in performing selective clamp with no further risk of bleeding.
An additional value of this tool is the possibility to juxtapose the intraoperative view with the 3D reconstruction model, which may overcome the intrinsic limit of two-dimensional images (i.e., computed tomography, magnetic resonance imaging) allowing for direct visualization of critical structures before and during surgery [19]. The contemporary visualization of the surgical field and the 3D model helps to decode renal anatomy during surgery. This could be of great interest for what concerns complex cases and surgeons at the beginning of their learning curve. Even if very limited evidence is available for what concerns RAPN in bigger renal masses, this approach is increasingly adopted also for clinical tumor stage 2 (cT2) tumors, in order to spare as much normal parenchyma as possible [20]. As a matter of fact, guidelines from the main urological society agree that PN should be proposed to patients with cT2 renal masses, whenever technically feasible [21]. This is particularly true for patients in which a nephron-sparing approach is unavoidable, such as patients with solitary kidneys, bilateral renal masses, and severely impaired pre-operative renal functions. The use of a 3D model in more complex cases could maximize the possibility of a successful PN, especially when the indication is mandatory [22]. This is particularly true in the case of multiple ipsilateral renal masses, which pose a higher risk of post-operative renal function impairment. In this setting, a minimally invasive approach is able to guarantee a high rate of success and preserve postoperative renal function. The higher rate of selective clamping and enucleation technique, and the reduction of collecting system violation achieved with the aid of 3DVMs are crucial in this subset of patients, since a more efficient surgery is required to ensure improved postoperative outcomes [23]. Furthermore, the use of a HA3D® rainbow model could prompt the choice of a selective clamp approach, even in challenging cases, enhancing the functional safety of the procedure in this subgroup of patients [4]. This technology is able not only to facilitate procedure success in very complex cases, but also to change surgical strategy according to preoperative planning, guiding the surgical indication towards more conservative approaches [24].
Augmented reality could facilitate the acquisition of operative skills necessary to perform surgical procedures, with lower risks for the patients and more accessibility for the trainees. The role of 3D models in this field has already been explored by Ahmadi and Liu [25] that concluded that models and simulators seem to represent an efficient strategy, especially for the more demanding steps of the procedures in terms of technical skills. The implementation and standardization of this learning mechanism could provide a useful field of application of these models and could lower the learning curve for RAPN.
Even if the debate is still open about some controversies in renal surgery, such as factors influencing postoperative outcomes and the effective role and optimal duration of clamping during PN, the use of 3D models could enable the surgeon to minimize the impact of the surgical procedure on intraoperative and postoperative outcomes. Some aspects that limit their spread need to be mentioned, such as their cost and the development process. Nevertheless, the HA3D® rainbow model seems a valuable tool to facilitate surgical planning and guide selective clamping during RAPN.
5. Conclusion
The present study and the attached video illustrate the value of 3D rainbow model during the planning and execution of a RAPN with selective clamping. It shows how the surgeon can rely on this model to be more efficient by avoiding unnecessary surgical steps, and to safely adopt a “selective” clamping strategy that can translate in minimal functional impact.
Author contributions
Study concept and design: Riccardo Autorino, Daniele Amparore, Francesco Porpiglia, Cristian Fiori.
Data acquisition: Antonio Franco, Enrico Checcucci.
Data analysis: Francesco Ditonno, Antonio Franco.
Drafting of manuscript: Francesco Ditonno, Celeste Manfredi.
Critical revision of the manuscript: Riccardo Autorino, Francesco Porpiglia, Daniele Amparore, Alessandro Antonelli, Marco De Sio, Cosimo De Nunzio.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Tongji University.
References
- 1.Piramide F., Kowalewski K.F., Cacciamani G., Rivero Belenchon I., Taratkin M., Carbonara U., et al. Three-dimensional model-assisted minimally invasive partial nephrectomy: a systematic review with meta-analysis of comparative studies. Eur Urol Oncol. 2022;5:640–650. doi: 10.1016/j.euo.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Amparore D., Piramide F., Verri P., Checcucci E., De Cillis S., Piana A., et al. New generation of 3D virtual models with perfusional zones: perioperative assistance for the best pedicle management during robotic partial nephrectomy. Curr Oncol. 2023;30:4021–4032. doi: 10.3390/curroncol30040304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amparore D., Pecoraro A., Checcucci E., Piramide F., Verri P., De Cillis S., et al. Three-dimensional virtual models' assistance during minimally invasive partial nephrectomy minimizes the impairment of kidney function. Eur Urol Oncol. 2022;5:104–108. doi: 10.1016/j.euo.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Amparore D., Piramide F., Checcucci E., Verri P., De Cillis S., Piana A., et al. Three-dimensional virtual models of the kidney with colored perfusion regions: a new algorithm-based tool for optimizing the clamping strategy during robot-assisted partial nephrectomy. Eur Urol. 2023;84:418–425. doi: 10.1016/j.eururo.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Hyde E.R., Berger L.U., Ramachandran N., Hughes-Hallett A., Pavithran N.P., Tran M.G.B., et al. Interactive virtual 3D models of renal cancer patient anatomies alter partial nephrectomy surgical planning decisions and increase surgeon confidence compared to volume-rendered images. Int J Comput Assist Radiol Surg. 2019;14:723–732. doi: 10.1007/s11548-019-01913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacciamani G.E., Shakir A., Tafuri A., Gill K., Han J., Ahmadi N., et al. Best practices in near-infrared fluorescence imaging with indocyanine green (NIRF/ICG)-guided robotic urologic surgery: a systematic review-based expert consensus. World J Urol. 2020;38:883–896. doi: 10.1007/s00345-019-02870-z. [DOI] [PubMed] [Google Scholar]
- 7.Pecoraro A., Amparore D., Checcucci E., Piramide F., Carbonaro B., De Cillis S., et al. Three-dimensional virtual models assistance predicts higher rates of “successful” minimally invasive partial nephrectomy: an Institutional analysis across the available trifecta definitions. World J Urol. 2023;41:1093–1100. doi: 10.1007/s00345-023-04310-5. [DOI] [PubMed] [Google Scholar]
- 8.Porpiglia F., Fiori C., Checcucci E., Amparore D., Bertolo R. Hyperaccuracy three-dimensional reconstruction is able to maximize the efficacy of selective clamping during robot-assisted partial nephrectomy for complex renal masses. Eur Urol. 2018;74:651–660. doi: 10.1016/j.eururo.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K., Takamatsu A., Nohara T., Yoneda N., Inoue D., Koda W., et al. Renal artery-based kidney segmentation on CT for patients with renal cell carcinoma: feasibility of segmental artery clamping simulation. Eur J Radiol Open. 2023;10 doi: 10.1016/j.ejro.2022.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutikov A., Uzzo R.G. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Porpiglia F., Amparore D., Checcucci E., Autorino R., Manfredi M., Iannizzi G., et al. Current use of three-dimensional model technology in urology: a road map for personalised surgical planning. Eur Urol Focus. 2018;4:652–656. doi: 10.1016/j.euf.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 12.MacLennan S., Imamura M., Lapitan M.C., Omar M.I., Lam T.B.L., Hilvano-Cabungcal A.M., et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol. 2012;62:1097–1117. doi: 10.1016/j.eururo.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Huang W.C., Elkin E.B., Levey A.S., Jang T.L., Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–62. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kates M., Badalato G.M., Pitman M., McKiernan J.M. Increased risk of overall and cardiovascular mortality after radical nephrectomy for renal cell carcinoma 2 cm or less. J Urol. 2011;186:1247–1253. doi: 10.1016/j.juro.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 15.Di Lascio G., Sciarra A., Del Giudice F., Salciccia S., Busetto G.M., De Berardinis E., et al. Which factors can influence post-operative renal function preservation after nephron-sparing surgery for kidney cancer: a critical review. Cent European J Urol. 2022;75:14–27. doi: 10.5173/ceju.2021.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonelli A.D., Cindolo L., Sandri M., Veccia A., Annino F., Bertagna F., et al. The role of warm ischemia time on functional outcomes after robotic partial nephrectomy: a radionuclide renal scan study from the clock randomized trial. World J Urol. 2023;41:1337–1344. doi: 10.1007/s00345-023-04366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cacciamani G.E., Medina L.G., Gill T., Abreu A., Sotelo R., Artibani W., et al. Impact of surgical factors on robotic partial nephrectomy outcomes: comprehensive systematic review and meta-analysis. J Urol. 2018;200:258–274. doi: 10.1016/j.juro.2017.12.086. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman L., Barod R., Dalela D., Diaz-Insua M., Abaza R., Adshead J., et al. Use of main renal artery clamping predominates over minimal clamping techniques during robotic partial nephrectomy for complex tumors. J Endourol. 2017;31:149–152. doi: 10.1089/end.2016.0678. [DOI] [PubMed] [Google Scholar]
- 19.Porpiglia F., Amparore D., Checcucci E., Manfredi M., Stura I., Migliaretti G., et al. Three-dimensional virtual imaging of renal tumours: a new tool to improve the accuracy of nephrometry scores. BJU Int. 2019;124:945–954. doi: 10.1111/bju.14894. [DOI] [PubMed] [Google Scholar]
- 20.Suek T., Davaro F., Raza S.J., Hamilton Z. Robotic surgery for cT2 kidney cancer: analysis of the National Cancer Database. J Robot Surg. 2022;16:723–729. doi: 10.1007/s11701-021-01300-w. [DOI] [PubMed] [Google Scholar]
- 21.Ljungberg B., Albiges L., Abu-Ghanem Y., Bedke J., Capitanio U., Dabestani S., et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399–410. doi: 10.1016/j.eururo.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Porpiglia F., Checcucci E., Amparore D., Piramide F., Volpi G., Granato S., et al. Three-dimensional augmented reality robot-assisted partial nephrectomy in case of complex tumours (PADUA ≥10): a new intraoperative tool overcoming the ultrasound guidance. Eur Urol. 2020;78:229–238. doi: 10.1016/j.eururo.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Turri F., Piramide F., Dell'oglio P., de Groote R., Lambert E., di Maida F., et al. Comment on: “Techniques and outcomes of robot-assisted partial nephrectomy for the treatment of multiple ipsilateral renal masses”. Minerva Urol Nephrol. 2023;75:398–400. doi: 10.23736/S2724-6051.23.05353-3. [DOI] [PubMed] [Google Scholar]
- 24.Amparore D., Piramide F., Pecoraro A., Verri P., Checcucci E., De Cillis S., et al. Identification of recurrent anatomical clusters using three-dimensional virtual models for complex renal tumors with an imperative indication for nephron-sparing surgery: new technological tools for driving decision-making. Eur Urol Open Sci. 2022;38:60–66. doi: 10.1016/j.euros.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmadi H., Liu J.J. 3-D imaging and simulation for nephron sparing surgical training. Curr Urol Rep. 2016;17:58. doi: 10.1007/s11934-016-0614-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Step-by-step robot-assisted partial nephrectomy performed with the hyper accuracy three-dimensional (HA3D®) rainbow model (MEDICS Srl, Turin, Italy).