Abstract
Purpose
To biomechanically compare primary medial patellofemoral ligament (MPFL) repair (MPFLr) augmented with a reinforced bioinductive implant (RBI) to the native MPFL ligament and a semitendinosus (semi-T) MPFL reconstruction (MPFLR) at time zero.
Methods
Four fresh-frozen matched pair cadavers (8 knees) were used to biomechanically compare the native MPFL to augmented MPFLr (n = 4) and semi-T MPFLR (n = 4). The native MPFL (n = 8) was isolated, preserving the femoral and patellar attachments, and pulled to failure. The semi-T was harvested from 1 of the matched pairs and whipstitched, as was a 250-mm × 5-mm RBI. A standard double-bundle docking technique was utilized. The patella was potted and mechanically pulled parallel to the transverse axis until failure in both cohorts. Cyclic creep, load and displacement at failure, failure mode, and stiffness were recorded.
Results
Failure load was highest in the RBI with repair group (287 ± 130 N) compared to the native MPFL (219 ± 64 N) and the semi-T group (84 ± 29 N). No statistically significant difference in failure load between the RBI augmentation with repair group and the native ligament (P = .19) were found. The semi-T reconstruction group failed at the least amount of displacement (7.93 ± 3.4 mm) compared to the native MPFL (20.9 ± 9 mm) (P < .01) and the RBI with repair group (33.2 ± 17.7 mm) (P < .02). At 10 mm of displacement, the RBI group (8.3 ± 1.2 N/mm) demonstrated stiffness in the midrange compared to the native MPFL (14.1 ± 7.1 N/mm). Early anchor/tendon pullout failure on the patella side was noted in the semi-T group compared to the RBI group. One reconstruction was excluded from analysis due to poor bone quality.
Conclusions
No statistically significant difference was seen between the augmented MPFL repair and the native MPFL in load-to-failure testing. The augmented MPFL repair was observed to have biomechanical properties similar to the native MPFL. MPFLr with RBI augmentation provided consistent stiffness at clinically relevant displacement.
Clinical Relevance
Primary MPFL repair and reconstruction using the semi-T graft, while effective, are nevertheless imperfect procedures. MPFL repair has been shown to have higher instability recurrence rates, while the stiffness profile of MPFLR with semi-T is higher than the native MFPL and may lead to knee stiffness, loss of motion, or cartilage damage. The results of this time-zero biomechanical study indicate that the use of an RBI for augmentation of a primary MPFL repair may be a viable alternative to traditional MPFL repair or reconstruction using a semi-T graft.
Patella dislocations typically result in disruption of the medial patella-femoral ligament (MPFL). The MPFL provides stability against lateral translation of the patella with the knee flexed at 30°.1,2 It has a native ligament strength of approximately 200 ± 50 N based upon a cadaver study.3 Injuries to the MPFL may occur on either the patellar or femoral side of the ligament, with varying recurrence rates accompanying the site of disruption in first-time dislocators.2 Recurrent patella dislocations are commonly treated surgically based upon the patient’s pathology. Despite improved understanding of each treatment option, inherent risks and benefits remain.4
Due to high recurrent instability rates associated with primary MPFL repair (MPFLr), MPFL reconstruction (MPFLR) is becoming more common.5,6 Multiple studies have concluded that MPFLR can provide good outcomes with acceptable rates of re-dislocation.7, 8, 9, 10, 11 However, despite improved surgical techniques, the combined complications associated with MPFLR have been reported to be approximately 25% in the highest risk population of children and adolescents.12 Complications such as recurrent instability, anterior knee pain, acceleration of patella-femoral cartilage damage, and loss of motion have been associated with MPFLR. Furthermore, technical issues, including graft pullout from the patella, patella fracture, and overtensioning of the graft, have also been identified.11,13, 14, 15, 16, 17, 18 Graft choice is also a concern, with debate surrounding the use of auto- versus allograft as well as tendon choice.3,19, 20, 21, 22 Multiple studies have been performed using semitendinosus (semi-T) grafts for MPFLR, and concern has been documented over the increased stiffness of the graft compared to the native ligament, leading to persistent pain, loss of motion, and increased risk of patella-femoral arthritis.19,21,23
A reinforced bioinductive scaffold may be indicated for the reinforcement of soft tissue where weakness exists. The scaffold, commonly referred to as a reinforced bioinductive implant (RBI), is composed of highly porous type I collagen and bioresorbable poly (L-lactide) microfilaments. The 3-dimensional open, porous, scaffold allows for strength at time zero of implantation (141 N) and encourages tissue induction and cellular infiltration.24,25 It comes prepackaged as either a 5-mm × 250-mm implant or as a 23-mm × 30-mm implant. It has been used in a variety of applications for soft tissue augmentation and has been shown to promote new tissue growth and maturation.25
The purpose of this study was to biomechanically compare a primary MPFLr augmented with an RBI to the native MPFL ligament and to a semi-T double-bundle MPFLR at time zero. The hypothesis is that the biomechanics of the RBI-augmented MPFL repair mimics the native MPFL.
Methods
Four matched pairs of cadaveric limbs (8 knees) were obtained for this study. Cadaveric parameters included no history of knee surgery, trauma, or osteoporosis, and a body mass index above 20. All 8 native MPFL tendons were isolated and biomechanically tested first. The specimens were then randomized into 2 groups of 4 specimens each to receive either semi-T MPFLR or the augmented RBI MPFLr (BioBrace; ConMed). The knees were randomized so that when 1 cadaveric knee was assigned to a group, the contralateral knee from the same cadaveric specimen was assigned to the other cohort. Cadaveric semi-T autografts were harvested from each cadaver specimen.
Preparing the Native MPFL
All dissections were performed by the senior surgeon (S.M.). The MPFL was isolated, preserving the femoral and patellar attachments. The patellar and quadriceps tendons were resected off the patella, isolating the patella from all soft tissue except for the MPFL attachment. The MPFL footprint was measured (distance from patellar to femoral insertions and widths of both insertion points). The femur was secured to a cylindrical fixture with at least 3 pins through the bone to secure it. The femur was fixed at 37° ± 2°, internally rotated with 0° set at where the posterior femoral condyle line is horizontal to allow the line of force to be parallel to the horizontal axis of the patella. This experimental design is similar to the study by Mountney et al.4,23 The patella was then potted and pulled to failure in all 8 specimens.
MPFL Repair/Reconstruction Technique
Once the native MPFL was pulled to failure, 1 leg underwent a semi-T MPFLR while the contralateral leg underwent augmented MPFLr.
Graft Prep
The RBI (5 × 250 mm) and semi-T autografts were trimmed to 220 mm in length. Neither the RBI nor the semi-T were tapered at their respective ends after length trimming. The respective widths of each semi-T ranged between 6 and 7 mm, with the diameter measured through a sizing block of 4 mm after being stitched (Table 1). A No. 2 suture was used to whipstitch the ends of each graft at least 25 mm from each graft end.
Table 1.
Graft Width and Diameters of the 4 Individual Semi-T Autografts Used for the Reconstruction Group
| Specimen | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Graft width at free end (single strand), mm | 6 | 7 | 6 | 6.5 |
| Graft diameter after stitching, mm | 4 | 4 | 4 | 4 |
NOTE. Specimen and graft 3 (highlighted in bold) were ultimately discarded due to poor patella bone quality resulting in fracture of the graft socket.
Patellar Fixation
Patellar fixation for both the MPFL repair and reconstruction was accomplished utilizing a dual-anchor/socket docking technique as has previously been described in the literature.26 Two parallel 4.5-mm sockets were drilled on the superior two-thirds of the patella to a depth of 25 mm. The graft was looped through the button fixation, and both ends of the graft were marked at 25 mm. The whipstitched ends were passed through the anchor eyelets (Fig 1). Two 4.75-mm PEEK anchors (Argo Knotless Suture Anchor; ConMed) were inserted into the superior two-thirds of the patella 15 mm apart. To ensure the graft was fully seated into the sockets alongside the anchors, the whipstitch had to slightly protrude from the socket and the black line at 25 mm had to be flush with the bone, as shown in Figure 2.
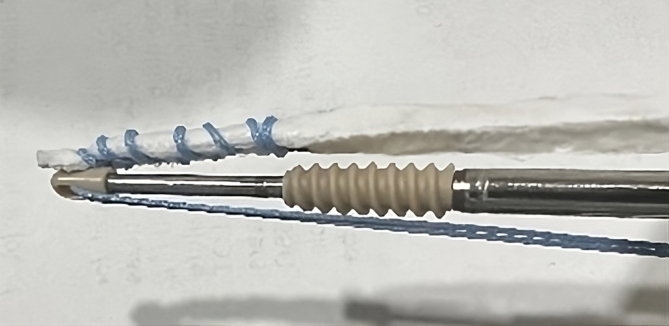
Fig 1.
The reinforced bioinductive implant alongside the PEEK anchor. The end of the 5-mm whipstitched implant is at the apex of the anchor inserter to allow for complete seating into the patella socket.
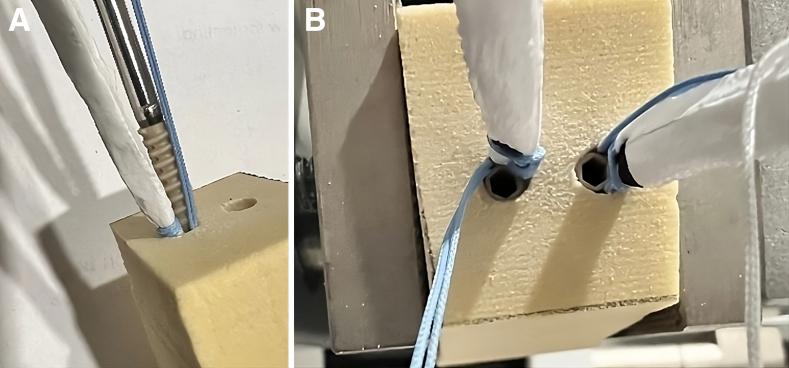
Fig 2.
(A) The reinforced bioinductive implant and 4.75-mm PEEK anchors being docked into a 4.5-mm socket on a Sawbones model. (B) The completed patellar fixation in Sawbones example with the implant seated in the sockets.
Femoral Fixation
Femoral fixation for both the MPFL repair and reconstruction was the same. A 3.5-mm beath pin was inserted just anterior and distal to the adductor tubercle for femoral fixation overlying the native attachment of the MPFL. A unicortical bone tunnel was drilled for femoral button fixation and then over-reamed with a 5-mm drill bit to a depth of 40 mm to dock the graft on the femoral end. The button was passed through the tunnel using the beath pin tip, pulling the pin through the femur to deliver the suture. An adjustable button fixation device (Infinity Femoral Adjustable Loop Button; ConMed) was secured and tensioned to ensure reapproximation of the native MPFL length measured previously.
For the augmented MPFLr group, after the double-bundle construct was secured, the native MPFL was then repaired using 2 interrupted No. 2 sutures in a side-to-side manner incorporating the RBI into the ligament repair. The sutures were placed in a box configuration at the location of native ligament failure (Fig 3 A and B). The construct was then retensioned to ensure the graft was load sharing alongside the repaired native tendon.
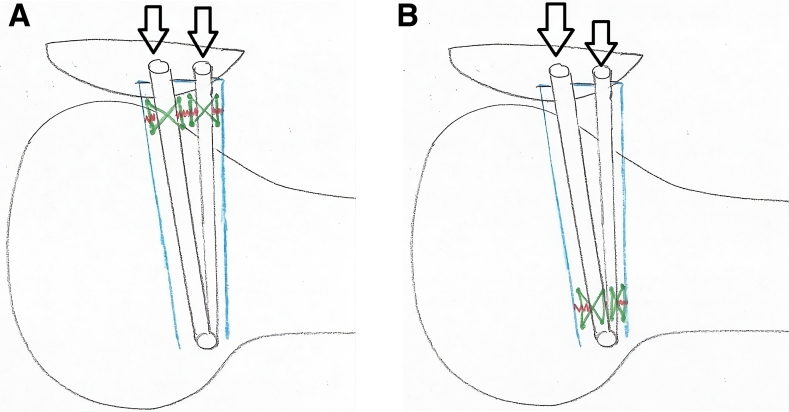
Fig 3.
The images demonstrate a lateral view of the medial patellofemoral ligament repair. The blue outline denotes the native medial patellofemoral ligament. The red lines denote the location of tear, either approximate to the patella (A) or the femoral (B) attachment sight. The green lines denote the “box” configuration of suture placement to complete the primary repair of the native ligament tear with incorporation of the reinforced bioinductive implant (black arrows).
Mechanical Testing
Each native MPFL (n = 8), augmented MPFLr (n = 4), and semi-T MPFLR (n = 4) were cyclically preconditioned and pulled to failure. Load and displacement, failure mode, and stiffness were recorded for all 3 groups. The gauge length was measured at a nominal force of 10 N. Each construct was preconditioned by a series of 10 cycles from 0 to 30 N at 1 Hz, and then the gauge length was measured again at 10 N nominal force to record creep in the construct (Fig 4). The construct was then brought down to 0 N and then pulled at 25 mm/min until failure.26 Load (N) versus displacement (mm) was recorded at 100 Hz. The load at failure was the value at which the load was 20% of its maximum, and stiffness was calculated in multiple regions of clinically relevant displacements.
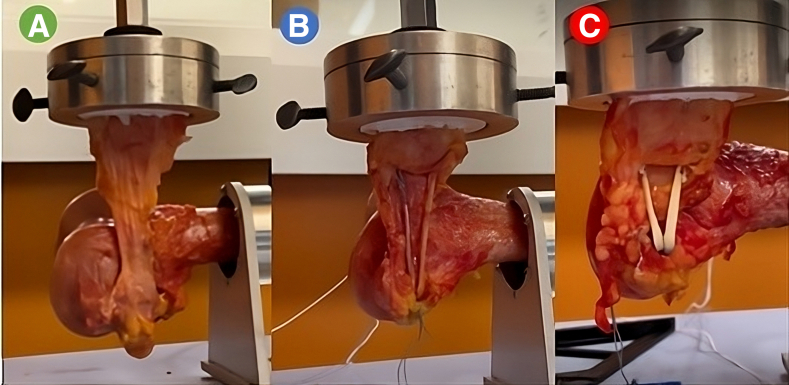
Fig 4.
The cadaveric biomechanical test setups for the native medial patellofemoral ligament (A), semitendinosis medial patellofemoral ligament reconstruction (B), and augmented medial patellofemoral ligament repair (C).
Statistical Methods
A Ryan-Joiner test was used to demonstrate normality. A paired t test was performed using MINITAB (Minitab) to compare all 3 groups in cyclic creep, load at 5 mm, failure load, and displacement at failure. For all comparisons, P < .05 was considered to indicate a statistically significant difference.
Results
The load versus displacement curves of the native MPFL, reconstruction, and augmented repair can be seen in Figure 5. The first 10 mm of displacement is shown as displacement past 10 mm is not clinically relevant. Results for all measured parameters are in Table 2, P values are located in Table 3, and stiffness values measured at various displacements can be seen in Table 4.
Fig 5.
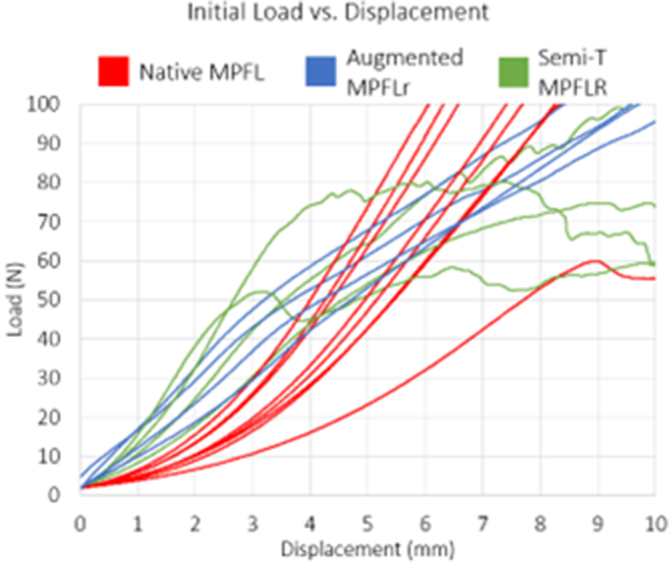
Load versus displacement curves of native MPFL, semi-T MPFLR, and augmented MPFLr. (MPFL, medial patellofemoral ligament; MPFLr, medial patellofemoral ligament repair; MPFLR, medial patellofemoral ligament reconstruction; semi-T, semitendinosus.)
Table 2.
Measured Values for All 3 Test Groups Evaluating Cyclic Creep, Load at 5 mm, Load to Failure (N), and Displacement at Failure (mm)
| Group | Cyclic Creep, mm | Load at 5 mm | Load at Failure, N | Displacement at Failure, mm |
|---|---|---|---|---|
| Native (n = 8) | 1.1 ± 0.9 | 51.4 ± 16 | 233 ± 59 | 20.7 ± 9.0 |
| Semi-T MPFLR (n = 3) | 1.6 ± 1.3 | 63.1 ± 12 | 84 ± 29 | 7.93 ± 3.4 |
| Augmented MPFLr (n = 4) | 1.7 ± 0.4 | 59.7 ± 6.4 | 287 ± 130 | 33.2 ± 17.7 |
MPFLr, medial patellofemoral ligament repair; MPFLR, medial patellofemoral ligament reconstruction; semi-T, semitendinosus.
Table 3.
Paired t Test Results Comparing All 3 Groups
| Paired t Test | Cyclic Creep, mm | Load at 5 mm | Load at Failure, N | Displacement at Max, mm | |
|---|---|---|---|---|---|
| Native MPFL vs | Augmented repair | .034∗ | .462 | .759 | .272 |
| Semi-T reconstruction | .475 | .295 | .049∗ | .077 | |
| Augmented repair vs reconstruction | .078 | .510 | .051 | .074 | |
MPFL, medial patellofemoral ligament; semi-T, semitendinosus.
Statistically significant difference, P < .05.
Table 4.
Stiffness Values Measured at Various Displacements for All 3 Test Groups
| Group | Stiffness, N/mm |
|||
|---|---|---|---|---|
| At 2 mm | At 5 mm | At 7 mm | At 10 mm | |
| Native (n = 8) | 7.5 ± 3.0 | 18.2 ± 5.3 | 19.5 ± 4.8 | 14.1 ± 7.1 |
| Semi-T MPFLR (n = 3) | 16.8 ± 4.7 | 7.1 ± 3.6 | 1.9 ± 3.8 | 1.9 ± 2.9 |
| Augmented MPFLr (n = 4) | 12.7 ± 2.3 | 9.3 ± 1.0 | 8.8 ± 1.2 | 8.3 ± 1.2 |
NOTE. The augmented MPFLr provided consistent stiffness at clinically relevant displacements.
MPFLr, medial patellofemoral ligament repair; MPFLR, medial patellofemoral ligament reconstruction; semi-T, semitendinosus.
Four of the native MPFLs failed at the femur, 2 failed at the patellar attachment, and 2 failed mid-substance. Average failure load was 233 ± 59 N, and displacement at failure was 20.7 ± 9.0 mm. At 5 mm of displacement (linear elastic region), stiffness was 18.2 ± 4.8 N/mm.
Three of the 4 semi-T MPFLR constructs failed at 7.93 ± 3.4 mm displacement at a load of 84 ± 29 N. The semi-T had an initial stiffness that was double that of the native MPFL at 2 mm of displacement (16.8 ± 4.7 N/mm vs 7.5 ± 3.0 N/mm, respectively). The semi-T reconstruction group failed prematurely compared to the native MPFL (7.93 vs 20.9 mm, respectively). The load at 5 mm for the semi-T MPFLR was similar to the native tissue, but after ∼8 mm, the construct had completely failed in contrast to the native MPFL, which at that point was in its most linear region. The semi-T reconstruction group had a statistically significantly lower failure load compared to native MPFL (P = .049). Three semi-T MPFLRs failed via anchor pullout at the patella. Examination of the anchor, socket, and graft after premature pullout indicated that the most likely cause of failure was due to incomplete seating of the graft into the socket. One of the reconstructions was excluded from analysis due to extremely poor bone quality of the patella. During attempted fixation of the graft to the patella, the bone bridge between the sockets failed prior to biomechanical testing. After consideration of alternative methods of fixating the graft to the patella, it was decided to exclude this specimen from testing to maintain consistency.
The augmented repair results demonstrate that the RBI provides supplemental strength to an MPFL repair. The augmented MPFLr group (n = 4) failed at 33.2 ± 17.7 mm of displacement, which was similar to the native MPFL displacement at failure (P = .272). Its average failure load was 287 ± 130 N, which was also similar to the native MPFL failure load (P = .759). The load at 5 mm of displacement was also similar to the native MPFL (P = .462). Two of the constructs failed due to the RBI tearing mid-substance while the other 2 failed via anchor pullout at the patella. The augmented MPFLr provided consistent stiffness at clinically relevant displacements.
Discussion
No statistically significant difference was seen between the augmented MPFL repair and the native MPFL in load-to-failure testing. The augmented MPFL repair was observed to have biomechanical properties similar to the native MPFL. The treatment of recurrent patella instability via soft tissue correction has gradually evolved from primary repair to MPFLR with hamstring tendon due to inferior outcomes of repair alone.27,28 Kruckeberg et al.5 reported 3-fold higher recurrent dislocation in patients who underwent MPFLr versus those with MPFLR. Puzzitiello et al.6 found similar findings, noting 36.9% recurrent dislocations in MPFLr compared to a 6.3% recurrence in a reconstruction cohort. Numerous systematic reviews have demonstrated this to be an acceptable treatment option with low re-dislocation rates.7, 8, 9, 10, 11,27,28 Both auto- and allograft tissues have been used for reconstruction, with allograft being favored in the adult populations.3,19, 20, 21, 22 A recent study has noted advantages of allograft include lack of donor site morbidity, predictable graft diameter, shorter operative time, decreased rate of recurrent instability, and avoidance of loss in terminal hamstring flexion.19 Despite these factors, the inherent risks of allograft tissue still exist, including the integrity of the graft structure after irradiation, graft elongation and the stigmata of its use in adolescent patients.
Within the highest risk population of children and adolescents, recurrent instability and complications have been reported between 0% and 25% with MPFLR using hamstring tendon.29 Parikh et al.29 retrospectively reviewed 179 patients who underwent hamstring MPFLR and reported a 16.2% complication rate. Of those, more than half were due to complaints of joint stiffness over time, loss of flexion, and patellofemoral arthrosis/pain. One of the largest drawbacks associated with hamstring MPFLR is excessive graft stiffness compared to the native MPFL.19,21,23 This is often associated with short- and long-term sequelae such as anterior knee pain, acceleration of patella-femoral cartilage damage, and loss of motion. If isometry of the reconstructed graft is not obtained, the high stiffness of the hamstring graft can exacerbate these symptoms.
Studies have demonstrated the maximum load and stiffness of the MPFL in human cadaveric testing has been variable, ranging from 145 ± 44 N at 18.9 mm of displacement to 208 ± 90 N at 26 mm of displacement.4,27,28 The latter was comparable to what was demonstrated in this study. Reasons for this variability in results may be due to cadaveric bone and tissue quality and MPFL dissection techniques.
The semi-T MPFLRs had an initial time-zero stiffness that was double that of the native MPFL at 2 mm of displacement. Unfortunately, the reconstruction group failed prematurely compared to the native MPFL, with 3 of the semi-T reconstructions failing via premature anchor pullout at the patella. This mechanism of failure was likely due to difficulty in docking the free ends of the tendon into patella sockets due to variability in tendon thickness. The ends of the semi-T grafts in this study were not tapered prior to suturing, only trimmed to a length of 220 mm. Examination of the tendon size revealed tendon diameters consistent with previous biomechanical studies.24 Based upon this and the tendon quality of the specimens, it was deemed by the authors to not risk compromise of the grafts by tapering them further. However, in retrospect, the diameter of the semi-T, combined with the suture and a 4.75-mm anchor placed into a 4.5-mm socket, most likely prevented full seating of the graft. This technical issue, combined with cadaveric bone quality, may explain the premature failures in this group. Conversely, premature failure was not seen in the RBI group. The authors believe this is due to the consistent 5-mm width of the scaffold, which allows for reproducible fixation. In the group that underwent RBI-augmented MPFLr, failure occurred at a load and displacement similar to the native MPFL. Two of the constructs failed due to the scaffold tearing mid-substance while the other 2 failed via anchor pullout at the patella. The augmented MPFLr provided consistent stiffness at clinically relevant displacements. While the semi-T MPFLR was initially stiffer, the construct failed prematurely compared to the native MPFL. The augmented MPFL repair was observed to have biomechanical properties similar to the native MPFL.
An alternative technique of addressing MPFL disruption by augmenting MPFLr with an RBI using a double-bundle docking technique has been presented in this biomechanical study. Augmented MPFLr, in terms of load to failure and stiffness, may be a viable option for treating MPFL injuries based upon its profile similarities to the native MPFL. While direct comparison of the semi-T MPFL group in the current study cannot be accomplished due to the premature failures, review of the literature demonstrates multiple studies on MPFLR load to failure and stiffness using the same docking technique at time zero. In separate studies, Russ et al.30 and Raoulis et al.31 reported load-to-failure and stiffness profiles (299.25 ± 99.87 N, 20.60 ± 6.78 N/mm; 253.5 ± 38.2 N, 37.8 ± 5.7 N/mm) that were greater than the native MPFL in our study (233 ± 59 N, 14.1 ± 7.1 N/mm). Extrapolating from the literature further, their loads to failure were in line with the RBI-augmented MPFLr (287 ± 130 N) in the present study, while the stiffness profile was higher in the semi-T groups versus the MPFLr (8.3 ± 1.2 N/mm). These findings are consistent with our hypothesis. Additionally, Wetzler et al.24 performed an anchor pullout biomechanical comparison in a cadaveric model of the RBI compared to the semi-T graft using a double-bundle docking technique. In their model, the semi-T graft ends were tapered to ensure adequate graft seating into the sockets. Their results demonstrated no statistical difference in pullout strength between the 2 groups (RBI = 249.3 ± 36.3 N; semi-T = 235.0 ± 113.6 N).
The findings of this time-zero study suggest that RBI-augmented MPFLr may hold promise as an alternative in treating MPFL injuries. Bioinductive implants have shown the ability to successfully yield new tissue, but to date, they have lacked structural strength at the time of implantation.7 The RBI utilized in this study combines both time-zero strength with the ability to create an environment for soft tissue ingrowth.25 The implant provides supplemental strength with a linear degradation of strength over 2 years before naturally resorbing. This combination of properties addresses previous drawbacks associated with soft tissue augmentation implants. Given the concerns of excessive stiffness with semi-T grafts and the associated complications, the augmented MPFLr may hold clinical relevance as a more native-like ligament construct. Future studies are warranted to further investigate this and collect long-term in vivo patient outcomes for those who undergo treatment of MPFL injuries with an RBI.
Limitations
This study has certain limitations. The age of the cadaveric bone (average = 54 years) is substantially greater than the treatment population for which these injuries typically occur. As such, factors such as pullout strength of the bone and tissue integrity of the harvested semi-T grafts may have been affected. It is unknown how much this impacted the results of the biomechanical testing. In the MPFLR group, premature failure of the fixation on the patella side was found in 3 of the specimens. It may be hypothesized that this was multifactorial based upon the bone quality and the graft size placed with anchor into the socket.
Another limitation of this study was the sample size, given the premature failures and poor bone quality. Furthermore, the authors acknowledge this is a time-zero biomechanical study, and clinical decision-making regarding the application of these data in vivo for human patients is limited.
Conclusions
No statistically significant difference was seen between the augmented MPFL repair and the native MPFL in load-to-failure testing. The augmented MPFL repair was observed to have biomechanical properties similar to the native MPFL. MPFLr with RBI augmentation provided consistent stiffness at clinically relevant displacement.
Disclosures
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: S.M. received financial support from the American Osteopathic Academy of Orthopedics and is a consultant or advisor for CONMED Corp. S.S. received financial support from the American Osteopathic Academy of Orthopedics and is a consultant or advisor for CONMED Corp. Z.R.B. received financial support from the American Osteopathic Academy of Orthopedics. E.B. received financial support from the American Osteopathic Academy of Orthopedics. E.F. received financial support from the American Osteopathic Academy of Orthopedics.
References
- 1.Conlan T., Garth W.P., Jr., Lemons J.E. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg. 1993;75:682–693. doi: 10.2106/00004623-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Hautamaa P.V., Fithian D.C., Kaufman K.R., Daniel D.M., Pohlmeyer A.M. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop. 1998;349:174–182. doi: 10.1097/00003086-199804000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Calvo Rodríguez R., Figueroa Poblete D., Anastasiadis Le Roy Z., et al. Reconstruction of the medial patellofemoral ligament: Evaluation of the clinical results of autografts versus allografts. Rev Esp Cir Ortop Traumatol. 2015;59:348–353. doi: 10.1016/j.recot.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Mountney J., Senavongse W., Amis A.A., Thomas N.P. Tensile strength of the medial patellofemoral ligament before and after repair or reconstruction. J Bone Joint Surg Br. 2005;87:36–40. [PubMed] [Google Scholar]
- 5.Kruckeberg B.M., Wilbur R.R., Song B.M., et al. Comparison of failure rates at long-term follow-up between MPFL repair and reconstruction for recurrent lateral patellar instability. Orthop J Sports Med. 2024;12 doi: 10.1177/23259671231221239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puzzitiello R.N., Waterman B., Agarwalla A., et al. Primary medial patellofemoral ligament repair versus reconstruction: Rates and risk factors for instability recurrence in a young, active patient population. Arthroscopy. 2019;35:2909–2915. doi: 10.1016/j.arthro.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Arendt E.A., Moeller A., Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19:1909–1914. doi: 10.1007/s00167-011-1516-y. [DOI] [PubMed] [Google Scholar]
- 8.Buckens C.F., Saris D.B. Reconstruction of the medial patellofemoral ligament for treatment of patellofemoral instability: A systematic review. Am J Sports Med. 2010;38:181–188. doi: 10.1177/0363546509353132. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B., Nyland J., Brand E., Curtin B. Medial patellofemoral ligament reconstruction for recurrent patellar dislocation: A systematic review including rehabilitation and return-to-sports efficacy. Arthroscopy. 2010;26:1384–1394. doi: 10.1016/j.arthro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Mikashima Y., Kimura M., Kobayashi Y., Miyawaki M., Tomatsu T. Clinical results of isolated reconstruction of the medial patellofemoral ligament for recurrent dislocation and subluxation of the patella. Acta Orthop Belg. 2006;72:65–71. [PubMed] [Google Scholar]
- 11.Smith T.O., Walker J., Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2007;15:1301–1314. doi: 10.1007/s00167-007-0390-0. [DOI] [PubMed] [Google Scholar]
- 12.Clark D., Metcalfe A., Wogan C., Mandalia V., Eldridge J. Adolescent patellar instability: Current concepts review. Bone Joint J Br. 2017;99:159–170. doi: 10.1302/0301-620X.99B2.BJJ-2016-0256.R1. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh K.M., Merican A.M., Iranpour F., Deehan D.J., Amis A.A. The effect of overstuffing the patellofemoral joint on the extensor retinaculum of the knee. Knee Surg Sports Traumatol Arthrosc. 2009;17:1211–1216. doi: 10.1007/s00167-009-0830-0. [DOI] [PubMed] [Google Scholar]
- 14.Lippacher S., Reichel H., Nelitz M. Patellar fracture after patellar stabilization. Orthopade. 2010;39:516–518. doi: 10.1007/s00132-010-1609-1. [DOI] [PubMed] [Google Scholar]
- 15.Matzkin E. Medial patellofemoral ligament reconstruction: Indications, technique, and outcomes. Arthroscopy. 2019;35:2970–2972. doi: 10.1016/j.arthro.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Reagan J., Kullar R., Burks R. MPFL reconstruction: Technique and results. Orthop Clin. 2015;46:159–169. doi: 10.1016/j.ocl.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Shah J.N., Howard J.S., Flanigan D.C., Brophy R.H., Carey J.L., Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40:1916–1923. doi: 10.1177/0363546512442330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaunat M., Erasmus P.J. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17:480–483. doi: 10.1007/s00167-008-0702-z. [DOI] [PubMed] [Google Scholar]
- 19.Aliberti G.M., Kraeutler M.J., Miskimin C., Scillia A.J., Belk J.W., Mulcahey M.K. Autograft versus allograft for medial patellofemoral ligament reconstruction: A systematic review. Orthop J Sports Med. 2021;9 doi: 10.1177/23259671211046639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flanigan D.C., Shemory S., Lundy N., Stitgen M., Long J.M., Magnussen R.A. Medial patellofemoral ligament reconstruction with allograft versus autograft tissue results in similar recurrent dislocation risk and patient-reported outcomes. Knee Surg Sports Traumatol Arthrosc. 2020;28:2099–2104. doi: 10.1007/s00167-020-05920-x. [DOI] [PubMed] [Google Scholar]
- 21.Hendawi T., Godshaw B., Flowers C., et al. Autograft vs allograft comparison in pediatric medial patellofemoral ligament reconstruction. Ochsner J. 2019;19:96–101. doi: 10.31486/toj.18.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliorini F., Trivellas A., Driessen A., Quack V., Tingart M., Eschweiler J. Graft choice for isolated MPFL reconstruction: Gracilis versus semitendinosus. Eur J Orthop Surg Traumatol. 2020;30:763–770. doi: 10.1007/s00590-020-02636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenschow S., Schliemann B., Gestring J., Herbort M., Schulze M., Kösters C. Medial patellofemoral ligament reconstruction: Fixation strength of 5 different techniques for graft fixation at the patella. Arthroscopy. 2013;29:766–773. doi: 10.1016/j.arthro.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Wetzler A., McMillan S., Brewer E., Patel A., Handy S., Wetzler M. No difference in pullout strength between a bio-inductive implant and a semitendinosus tendon graft in a biomechanical study of medial patellofemoral ligament repair augmentation. Arthrosc Sports Med Rehabil. 2024;6 doi: 10.1016/j.asmr.2023.100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMillan S., Arciero R., Ford E. The next frontier for rotator cuff augmentation? Strength + bio-induction. J Orthop Exp Innovat. 2021;2:1–7. [Google Scholar]
- 26.Rosinski A., Chakrabarti M., Gwosdz J., McGahan P.J., Chen J.L. Double-bundle medial patellofemoral ligament reconstruction with allograft. Arthrosc Tech. 2019;8:e513–e520. doi: 10.1016/j.eats.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arendt E.A. Anatomy and biomechanics of the patellar ligaments. Tecn Chir Orthop Traumatol. 2007;5:13–18. [Google Scholar]
- 28.Liu Z., Yi Q., He L., et al. Comparing nonoperative treatment, MPFL repair, and MPFL reconstruction for patients with patellar dislocation: A systematic review and network meta-analysis. Orthop J Sports Med. 2021;9 doi: 10.1177/23259671211026624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh S.N., Nathan S.T., Wall E.J., Eismann E.A. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41:1030–1038. doi: 10.1177/0363546513482085. [DOI] [PubMed] [Google Scholar]
- 30.Russ S.D., Tompkins M., Nuckley D., Macalena J. Biomechanical comparison of patellar fixation techniques in medial patellofemoral ligament reconstruction. Am J Sports Med. 2015;43:195–199. doi: 10.1177/0363546514550992. [DOI] [PubMed] [Google Scholar]
- 31.Raoulis V.A., Zibis A., Chiotelli M.D., et al. Biomechanical evaluation of three patellar fixation techniques for MPFL reconstruction: Load to failure did not differ but interference screw stabilization was stiffer than suture anchor and suture-knot fixation. Knee Surg Sports Traumatol Arthrosc. 2021;29:3697–3705. doi: 10.1007/s00167-020-06389-4. [DOI] [PubMed] [Google Scholar]