Abstract
Objective
This study aimed to analyze the correlation between urinary crystals and urinary calculi.
Methods
Clinical data, including urinary crystal types, were collected from 237 patients with urinary calculi. The detection rate of urine crystals and their correlation with stone composition were analyzed. The receiver operating characteristic curve analysis was used to determine the best cut-off value for predicting stone formation risk based on calcium oxalate crystals in urine.
Results
Calcium oxalate was the most common component in 237 patients. Among them, 201 (84.81%) patients had stones containing calcium oxalate. In these patients, calcium oxalate crystals were detected in 45.77% (92/201) of cases. In different groups of calcium oxalate stones, calcium oxalate crystals accounted for more than 90% of the total number of crystals detected in each group. The detection rate of calcium oxalate crystals was higher in first-time stone formers than in recurrent patients. The receiver operating characteristic curve analysis suggested a cut-off value of 110 crystals/μL for predicting stone formation, validated with 65 patients and 100 normal people.
Conclusion
Calcium oxalate crystals in urine can predict the composition of calcium oxalate stones and indicate a higher risk of stone formation when the number exceeds 110 crystals/μL. This non-invasive method may guide clinical treatment and prevention strategies.
Keywords: Calculus, Urine crystal, Calcium oxalate crystal, Predict model
1. Introduction
Urinary calculi are a common disease in urology, ranking the first in hospitalized patients. Epidemiological data from European and American countries indicate that the incidence of urinary calculi is from 2% to 20% [1]. The overall incidence of urinary calculi in China ranges from 1% to 5%, and that in the south is as high as 5% to 10%. The annual new incidence rate is 150–200 cases per 100 000 people, and 25% of the patients required hospitalization [2]. The latest survey revealed that about 1/17 of Chinese adults have kidney stones [3]. In recent years, the incidence of urinary calculi in China is on the rise and has become one of the three high-risk areas in the world. Urinary calculi are a highly recurrent disease that may lead to inflammation, oxidative damage, and renal dysfunction in the kidneys when large stones form or recur multiple times [4]. Therefore, the development of a simple and effective method to predict stone composition may be helpful for early treatment and prevention of recurrence.
As we all know, stones mainly include five types, including calcium oxalate stones, calcium phosphate stones, struvite stones, uric acid stones, and cystine stones. Among them, calcium oxalate stones were the most common and exist as either the monohydrate or dihydrate [5]. Although various underlying factors and diseases have been identified to induce calcium oxalate stones, most cases are idiopathic and relate with the Randall's plaque [6,7]. Randall's plaques are a mixture of calcium phosphate crystals, collagen fibers, and molecules involved in the inflammatory response that disrupts the papillary urothelium of the kidney, leading to calcium oxalate crystal aggregation and stone formation [8]. However, the process from urine supersaturation to crystal nucleation, growth, and aggregation is essential for the formation and progression of all stone types [9]. In each type of urolithiasis, the formation of crystals in the urine is a necessary initial step in stone formation in each type of urolithiasis [10]. Therefore, crystalluria can provide the evidence of the propensity of urine to form stones.
The formation of calcium oxalate crystals is mainly determined by the excessive concentration of calcium ions and oxalate ions leading to excessive urine saturation. Although calcium oxalate crystals can also be found in healthy people, Robert et al. [11] compared the urine of healthy people and patients with stones and found that the diameter of calcium oxalate crystals in patients was 2–3 times than that of normal people, and they were easy to aggregate into clusters. Therefore, calcium oxalate crystals in urine provide a natural indicator of calcium oxalate stone formation propensity and are of great value in predicting stone composition, assessing stone recurrence and whether the treatment is effective.
One of the characteristics of stones is easy recurrence, which also highlights the importance of prevention. Despite great progress in diagnosis, surgery, and various treatments, there were still major deficiencies in the prevention of stones. While there are currently no drugs that dissolve existing calcium stones, it is possible to prevent new stone formation through behavioral and nutritional interventions, depending on stone composition. To know the specific composition of the stone, it can only be obtained by surgical removal of the stone. At present, there is still a lack of corresponding indicators to accurately predict composition of the stone by non-surgical methods. Therefore, 237 patients with stones were retrospectively studied in this study. The correlation between calcium oxalate crystals and calcium oxalate stones was emphatically studied in patients with calcium oxalate stones. At the same time, we further evaluated the accuracy of calcium oxalate crystals in predicting calcium oxalate stones, providing a basis for formulating stone prevention and treatment policies for this special population.
2. Patients and methods
2.1. Patients' information
Clinical data such as urinary crystal types and other clinical data of 237 patients with urinary calculi admitted to the Department of Urology, the First Affiliated Hospital of Nanjing Medical University, China, from March 2022 to August 2022 were collected with permission of usage in the present study. All cases were diagnosed as urolithiasis by one or more imaging examinations such as urinary system color Doppler ultrasound, urinary system plain film, intravenous urography, CT, or various endoscopy. Stones were taken from procedures such as percutaneous nephrolithotomy, ureteroscopic lithotripsy, and open or laparoscopic lithotripsy. All the patients provided written informed consents, and the protocol was approved by the Ethical Committee of the First Affiliated Hospital of Nanjing Medical University (approval number 2024-SR-307).
2.2. Analysis of stone composition
Totally 237 patients with urinary calculi were detected by the infrared spectrum automatic analysis system of the lithotripsy center in our hospital. Before testing, we washed the stains and residues on the stone surface with clean water and distilled water, and then dried it in an oven at 100 °C for about 5 min. If the diameter of the stone was ≤3 mm, the stone was grounded directly in an agate bowl. If the diameter of the stone was >3 mm, the stone was cut open and the central part was taken. We utilized a balance to take 2 mg of stone samples and 100–200 mg of pure potassium bromide, which had been completely dried beforehand, and then put them into an agate bowl and ground them in the same direction. Then, the mixture was pressurized to 20 MPa with a tablet press and maintained for 30 s to form a translucent tablet with a thickness of 0.3–0.5 mm, which was quickly put into the infrared spectrometer for scanning.
2.3. Analysis of urine crystallization
First, 10 mL of fresh first-voided morning urine sample was collected from patients between 7 a.m. and 7:30 a.m. Urine samples were kept in containers without preservatives and stored at room temperature. Urine samples brought to the laboratory within 1 h of voiding were kept at room temperature and were rapidly processed. The iChem VELOCITY Urine Chemistry Analyzer (Beckman Coulter, CA, USA) and iQ®200 Automatic Urine Formation Analyzer (Beckman Coulter, CA, USA) were used to detect urine composition and the type of crystallization, and quantify the number of crystals. The urine was then centrifuged for 5 min at a relative centrifugal force of 400 m/s2. After centrifugation, the supernatant urine was poured or aspirated at once, and 1 mL of liquid was retained at the bottom of the centrifuge tube. Then the urine sediment was thoroughly mixed; an appropriate amount of urine was dropped on the FAST READ 102 counting plate (Biosigma Spa, Venice, Italy); the crystal morphology was observed by a phase contrast microscope and the number of crystals in 10 large squares was counted. Finally, combined with the results of the urine sediment analyzer and microscope, the type of crystallization was determined. According to the guidance of the relevant literature [12], we controlled the entire test process within 2 h.
2.4. The identification and verification of the cut-off value for calcium oxalate crystals
Based on the number of calcium oxalate crystals in 193 patients with calcium oxalate stones and 63 normal people, we used SPSS 21.0 statistical software (IBM, Armonk, NY, USA) to analyze the receiver operating characteristic curve of calcium oxalate crystals to screen the best cut-off. The maximum value of sensitivity plus specificity minus 1 is the cutoff value. In addition, we collected calcium oxalate crystals from 65 patients with calcium oxalate stones and 100 normal people to verify the accuracy of the cut-off value. In the validation group, we re-examined the samples that did not detect crystallization at the first time on the second day.
2.5. Statistical analysis
SPSS 21.0 statistical software was used to analyze and process the research data. The measurement data conforming to the normal distribution were expressed as means with standard deviations; the counting data were expressed as percentages; and the χ2 test was used for comparisons between groups. The Wilcox test was used to analyze differences in data distribution between groups. The p<0.05 was considered to be statistically significant.
3. Results
3.1. Overall distribution of stone components
In 237 patients with urinary stones, the most common component was pure calcium oxalate stones accounting for 40.93% (97/237), followed by calcium oxalate mixed carbonate apatite stones accounting for 35.02% (83/237). In addition, among the 237 patients with calculi, males were more common, accounting for 65.82% (156/237); the most common site of calculi was the kidney, accounting for 56.54% (134/237), and the patients with first occurrence were the main ones, accounting for 65.82% (156/237). Considering the limited number of samples, 200 patients with calcium oxalate stones were selected as the main participants in this study, including 48.50% (97/200) of pure calcium oxalate stones, 41.50% (83/200) of calcium oxalate mixed carbonate apatite stones, and 10.00% (20/200) of calcium oxalate mixed uric acid stones (Supplementary Table 1).
3.2. Correlation analysis of urinary crystals and urinary calculi
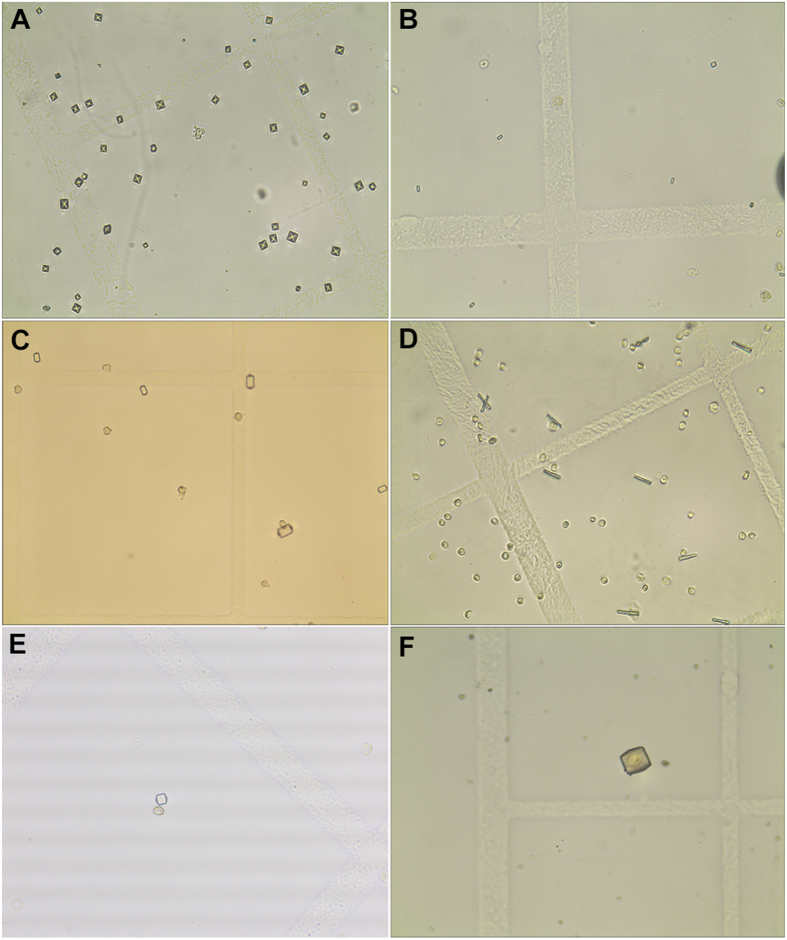
Overall, the detection rate of crystals in morning urine of 237 patients with urinary calculi was about 48.10% (114/237), and the matching degree between crystal types and stone components was about 91.23% (104/114). In the stone composition group with a sample size of 15 or more, the detection rates of crystals were all above 40%, and the matching rates between crystals and the stone composition were all greater than 66.67% (Table 1). Moreover, the most common crystal component was calcium oxalate crystals accounting for about 83.93% (94/112). Fig. 1 presented the morphology of urine crystals under the microscope. Therefore, we focused on the correlation between calcium oxalate crystals and calcium oxalate stones. Among the 201 patients with calcium oxalate stones, i.e., pure calcium oxalate stones, calcium oxalate mixed carbonate apatite, calcium oxalate mixed uric acid stones, and calcium oxalate mixed l-cystine stones, the detection rate of calcium oxalate crystals was about 45.77% (92/201). Among calcium oxalate mixed carbonate apatite stones, the detection rate of calcium oxalate crystals was the highest, about 51.81% (43/83), followed by pure calcium oxalate stones, with a detection rate of 43.30% (42/97). In addition, the detection rate of calcium oxalate crystals was significantly lower in patients with other types of stones, such as 6.25% in struvite mixed carbonate apatite stones and 6.67% in uric acid stones (Table 1).
Table 1.
The crystal detection, match, mismatch, and COC detection rate in various urinary calculi.
| Stone composition | Total | Urinary crystal |
None urinary crystal | Crystallization detection rate, % | Match, % | Mismatch,% | COC detection rate, % | |||
|---|---|---|---|---|---|---|---|---|---|---|
| COC | PC | UC | CC | |||||||
| Calcium oxalate calculus | 97 | 42 | 3 | 1 | 0 | 51 | 47.42 | 91.30 | 8.70 | 43.30 |
| Calcium oxalate mixed carbonate apatite | 83 | 43 | 1 | 0 | 0 | 39 | 53.01 | 97.73 | 2.27 | 51.81 |
| Calcium oxalate mixed uric acid calculus | 20 | 7 | 0 | 2 | 0 | 11 | 45.00 | 100 | 0 | 35.00 |
| Struvite mixed carbonate apatite | 16 | 1 | 5 | 1 | 0 | 9 | 43.75 | 71.43 | 28.57 | 6.25 |
| Uric acid calculus | 15 | 1 | 1 | 4 | 0 | 9 | 40.00 | 66.67 | 33.33 | 6.67 |
| Struvite calculus | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Carbonate apatite mixed hydroxyapatite | 1 | 1 | 0 | 0 | 0 | 0 | 100 | 0 | 100 | 100 |
| Calcium oxalate mixed l-cystine calculus | 1 | 0 | 0 | 0 | 1 | 0 | 100 | 100 | 0 | 0 |
| Carbonate apatite mixed l-cystine calculus | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| l-cystine calculus | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Total | 237 | 95 | 10 | 8 | 1 | 123 | 48.10 | 91.23 | 8.77 | 40.08 |
COC, calcium oxalate crystal; CC, cystine crystal; PC, phosphate crystal; UC, urate crystal.
Figure 1.
The morphology of urine crystals under the microscope (10×40). (A) The morphology of dihydrated calcium oxalate crystal; (B) The morphology of monohydrated calcium oxalate crystal; (C) Type I morphology of phosphate crystals; (D) Type II morphology of phosphate crystals; (E) Type I morphology of uric acid crystal; (F) Type II morphology of uric acid crystal.
3.3. Difference of the calcium oxalate crystal detection rate in different gender, age, stone location, and disease history
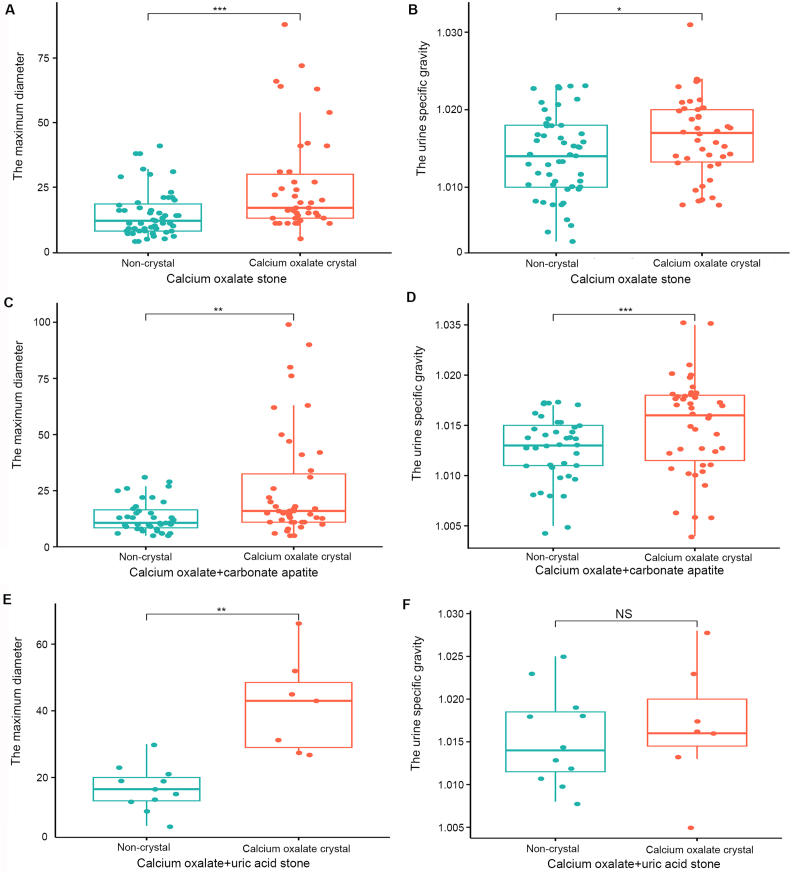
Due to the limited sample size, we selected calcium oxalate crystals in patients with calcium oxalate stones as the main research object. Our study found that the detection rate of calcium oxalate crystals in patients with calcium oxalate stones was not significantly different between males and females (p=0.659) and varied significantly with age (p=0.040). Besides, we observed a statistically significant difference in the detection rates of calcium oxalate crystals based on the stone location (p=0.023). This suggested that the location of the stones within the urinary tract influences the likelihood of detecting calcium oxalate crystals. Additionally, the presence of calcium oxalate crystals were more readily detected in first diagnosed patients than in recurrent patients (p=0.040) (Table 2). Moreover, we further analyzed the differences in the urine specific gravity and maximum stone diameter between the calcium oxalate crystal group and non-crystal group in patients with calcium oxalate stones. The results revealed that, except for the calcium oxalate mixed uric acid stone group where there was no difference in urine specific gravity, the urine specific gravity and the maximum stone diameter of the calcium oxalate crystal group were higher than those of the non-crystal group, and the difference was statistically significant (Fig. 2A–E). In the calcium oxalate mixed uric acid group, although the specific gravity of urine in the calcium oxalate crystal group was higher than that in the non-crystal group, the difference was not statistically significant (Fig. 2F).
Table 2.
Differences in the detection of calcium oxalate crystals in various clinical phenotypes (n=92).
| Characteristics | Calcium oxalate mixed carbonate apatite (n=43) | Calcium oxalate mixed uric acid calculus (n=7) | Calcium oxalate calculus (n=42) | p-Value |
|---|---|---|---|---|
| Gender, n (%) | 0.659 | |||
| Male | 30 (32.6) | 6 (6.5) | 29 (31.5) | |
| Female | 13 (14.1) | 1 (1.1) | 13 (14.1) | |
| Age, year, n (%) | 0.040 | |||
| ≤52 | 27 (29.3) | 2 (2.2) | 16 (17.4) | |
| >52 | 16 (17.4) | 5 (5.4) | 26 (28.3) | |
| Stone location, n (%) | 0.023 | |||
| Kidney and ureter | 12 (13.0) | 0 (0) | 18 (19.6) | |
| Ureter | 13 (14.1) | 2 (2.2) | 3 (3.3) | |
| Kidney | 18 (19.6) | 5 (5.4) | 21 (22.8) | |
| History, n (%) | 0.040 | |||
| First | 30 (32.6) | 2 (2.2) | 32 (34.8) | |
| Recrudescence | 13 (14.1) | 5 (5.4) | 10 (10.9) |
Figure 2.
Differences in the urine specific gravity and maximum stone diameter between calcium oxalate crystal and non-crystal groups. (A and B) Calcium oxalate stones; (C and D) Calcium oxalate mixed carbonate apatite stones; (E and F) Calcium oxalate mixed uric acid stones. ∗ p<0.05; ∗∗ p<0.01; ∗∗∗ p<0.001; NS, no significance.
3.4. Construction and verification of the calcium oxalate crystal prediction model
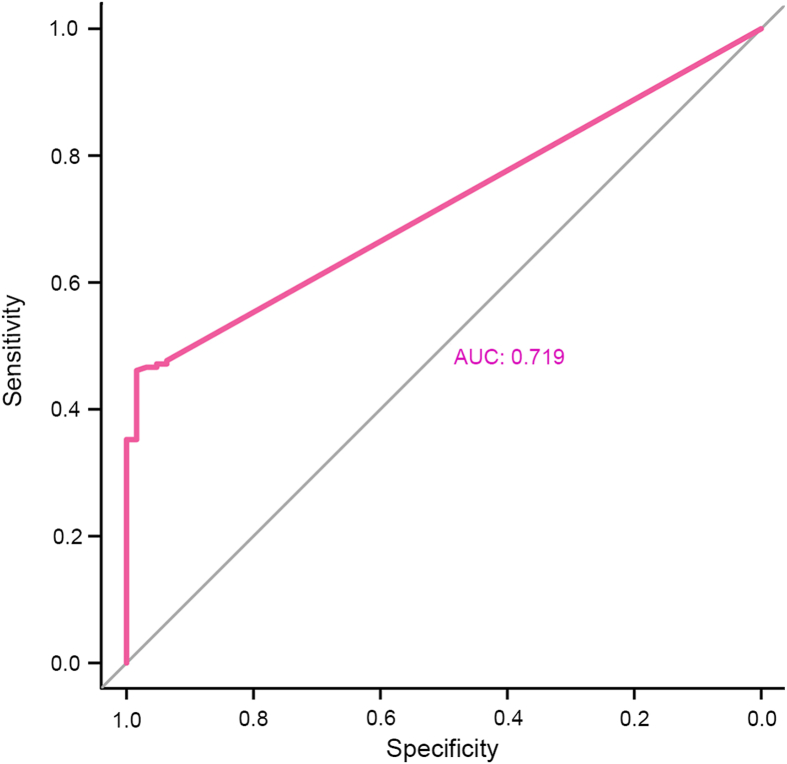
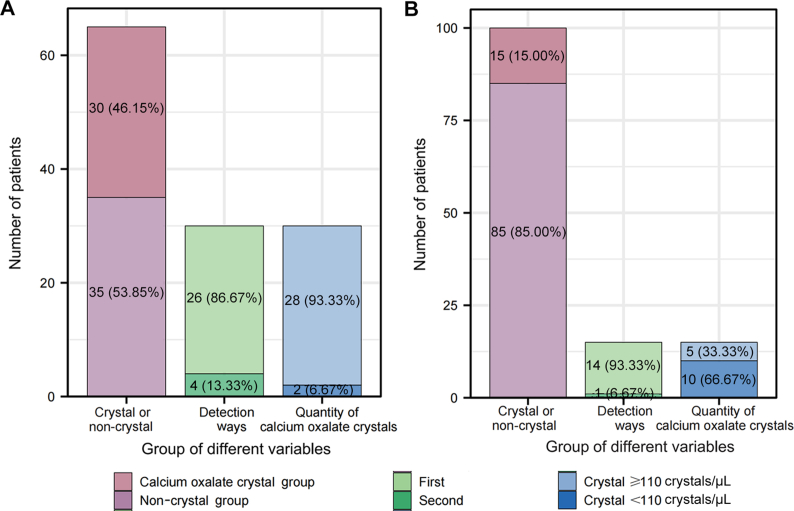
Through the receiver operating characteristic curve analysis of the number of calcium oxalate crystals in 193 patients with calcium oxalate stones and 63 normal people, we screened 110 crystals/μL as the cut-off value of calcium oxalate crystal considering both sensitivity and specificity (Fig. 3). Then, we further collected the number of calcium oxalate crystals from 65 patients with calcium oxalate stones and 100 normal people as the validation group to verify accuracy of the cut-off. The results showed that calcium oxalate crystals were detected in urine in 46.15% (30/65) patients with calcium oxalate stones. Among the 30 samples, 13.33% (4/30) were found to have calcium oxalate crystals for the second time. In addition, among 30 patients with calcium oxalate crystals, 28 had a calcium oxalate crystal count of ≥110 crystals/μL (Fig. 4A). In addition, the detection rate of calcium oxalate crystals was 15.00% (15/100) in normal people, and only one (6.67%) sample was found to have calcium oxalate crystals for the second time. Besides, 33.33% (5/15) of them had the number of calcium oxalate crystals ≥110 crystals/μL (Fig. 4B).
Figure 3.
Screening for the best cut-off for predicting the risk of stone formation through the receiver operating characteristic curve analysis. AUC, area under the curve.
Figure 4.
Validation of the best cut-off for predicting the risk of stone formation. (A) The distribution, retest results, and proportion of calcium oxalate crystal and non-crystal groups in the calcium oxalate stone validation group; (B) The distribution, retest results, and proportion of calcium oxalate crystals and non-crystal groups in the normal validation group.
4. Discussion
Urinary calculi are a common disease with a prevalence of 14.8% and a 5-year recurrence rate of 50% [12]. The prevalence may continue to increase due to factors such as lifestyle, dietary habits, and global warming [1,13]. In recent years, various minimally invasive procedures have become the main method for treating urinary calculi, but they have not reduced the recurrence rate of stones. Crystals are the important component of calculi; therefore, they may play a crucial role in the prevention and treatment of calculi.
The results of stone composition analyses indicated that urinary calculi were mainly calcium oxalate stones, with pure calcium oxalate stones and calcium oxalate mixed carbonated apatite being the most common, which is consistent with other studies [14,15]. In addition, in patients with calcium oxalate stones, calcium oxalate crystals are most frequently detected when the stone is located in the kidney. On the one hand, it is because the formation of stones started in the kidney [16]. Crystal nucleation occurs when the kidney produces supersaturated urine. Once the crystal nucleus is established in the kidney and exposures to urine, stones may grow through encrustation [17,18]. Besides, glycosaminoglycans are potent inhibitors of calcium oxalate growth and aggregation in urine. The normal urothelium is covered by the glycosaminoglycan layer that not only resists bacterial adhesion but also inhibits crystal nucleation and acts as a barrier to crystal adhesion [19,20]. On the other hand, when the stone is located in the ureter, it tends to block the ureter and cause hydronephrosis, leading to the deposition of crystals in the kidney [21]. The urine specific gravity is higher in the crystal group in patients with calcium oxalate stones. The reason may be that the formation of stones is caused by long-term exposure to supersaturated urine. When a large number of crystals are present in urine, urine specific gravity in urine may increase.
The formation of crystals was a necessary initial step in the development of every urinary stone disease [22]. Therefore, crystal formation has been widely employed in experimental studies aimed at evaluating conditions ranging from urine supersaturation to crystal nucleation, growth, and aggregation [23,24]. However, the correlations between urinary crystals and clinical phenotype, diagnosis and prognosis of patients with stones remain unclear. At the same time, it has become a trend to prevent the formation and recurrence of stones according to the composition of stones in patients [25]. However, the composition of stones may only be known after the operation, and there was still a lack of a simple and effective method to predict the composition of stones. Our study indicated that the detection rate of calcium oxalate crystals in patients with calcium oxalate stones was more than 40%, and more than 90% of the detected crystals were calcium oxalate crystals. Therefore, we hypothesized that urinary calcium oxalate crystals may serve as an effective biomarker for predicting stone composition, which may provide a simple and inexpensive tool for detecting and preventing stone formation. In addition, in the validation group, we found that retesting can increase the detection rate of calcium oxalate crystals by 13.33% in patients with calcium oxalate stones, while retesting can increase the detection rate of calcium oxalate crystals by 6.67% in the normal population. Therefore, we believe that the detection rate and accuracy of calcium oxalate crystal may be improved by re-examination. In addition, our study found that the risk of calcium oxalate stone formation was higher when the number of calcium oxalate crystals in urine was ≥110 crystals/μL. At the same time, some normal people with the number of calcium oxalate crystals ≥110 crystals/μL were also found to have renal crystals or small stones in the follow-up.
Our study still had certain limitations. This study was a single-center study and included only 237 patients with calculi. It is still necessary to carry out a multi-center study and expand the sample size to verify the results of our research. Moreover, our recognition of crystal types relies more on microscopy and has not yet been fully automated. In order to evaluate the correlation between urinary crystals and stones more generally, it is necessary to automate the detection of crystals.
5. Conclusion
Our study provided the evidence that calcium oxalate crystals had a high detection rate in calcium oxalate stones and could accurately predict the stone composition without surgery. The quantitative analysis of calcium oxalate crystals is helpful to guide the clinical treatment and prevention of calcium oxalate stone patients. Given that urine crystal testing is a reliable, simple, and inexpensive method, the determination of urine crystal may therefore be a useful clinical tool for predicting stone composition.
Author contributions
Study concept and design: Ninghong Song, Lujiang Yi, Chao Qin.
Data acquisition: Xi Zhang, Yang Zheng, Yichun Wang.
Data analysis: Xi Zhang, Xiyi Wei, Shuai Wang, Jie Zheng.
Drafting of manuscript: Jixiang Yao, Chen Xu, Zhijun Cao.
Critical revision of the manuscript: Xi Zhang, Ninghong Song.
Conflicts of interest
The authors declare no conflict of interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82071638 to Song N).
Footnotes
Peer review under responsibility of Tongji University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajur.2024.04.003.
Contributor Information
Chao Qin, Email: qinchao@njmu.edu.cn.
Lujiang Yi, Email: lj.yi@njmu.edu.cn.
Ninghong Song, Email: songninghong_urol@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Scales C.D., Smith A.C., Hanley J.M., Saigal C.S. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W., Fan J., Huang G., Li J., Zhu X., Tian Y., et al. Prevalence of kidney stones in mainland China: a systematic review. Sci Rep. 2017;7 doi: 10.1038/srep41630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng G., Mai Z., Xia S., Wang Z., Zhang K., Wang L., et al. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. 2017;120:109–116. doi: 10.1111/bju.13828. [DOI] [PubMed] [Google Scholar]
- 4.Alexander R.T., Hemmelgarn B.R., Wiebe N., Bello A., Morgan C., Samuel S., et al. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345 doi: 10.1136/bmj.e5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan S.R., Pearle M.S., Robertson W.G., Gambaro G., Canales B.K., Doizi S., et al. Kidney stones. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh P., Enders F.T., Vaughan L.E., Bergstralh E.J., Knoedler J.J., Krambeck A.E., et al. Stone composition among first-time symptomatic kidney stone formers in the community. Mayo Clin Proc. 2015;90:1356–1365. doi: 10.1016/j.mayocp.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worcester E.M., Coe F.L. Clinical practice. Calcium kidney stones. N Engl J Med. 2010;363:954–963. doi: 10.1056/NEJMcp1001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kok D.J., Boellaard W., Ridwan Y., Levchenko V.A. Timelines of the “free-particle” and “fixed-particle” models of stone-formation: theoretical and experimental investigations. Urolithiasis. 2017;45:33–41. doi: 10.1007/s00240-016-0946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlayson B. Physicochemical aspects of urolithiasis. Kidney Int. 1978;13:344–360. doi: 10.1038/ki.1978.53. [DOI] [PubMed] [Google Scholar]
- 10.Frochot V., Daudon M. Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. Int J Surg. 2016;36:624–632. doi: 10.1016/j.ijsu.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Robert M., Boularan A.M., Delbos O., Guiter J., Descomps B. Study of calcium oxalate crystalluria on renal and vesical urines in stone formers and normal subjects. Urol Int. 1998;60:41–46. doi: 10.1159/000030201. [DOI] [PubMed] [Google Scholar]
- 12.Fink H.A., Wilt T.J., Eidman K.E., Garimella P.S., MacDonald R., Rutks I.R., et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians clinical guideline. Ann Intern Med. 2013;158:535–543. doi: 10.7326/0003-4819-158-7-201304020-00005. [DOI] [PubMed] [Google Scholar]
- 13.Brikowski T.H., Lotan Y., Pearle M.S. Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci U S A. 2008;105:9841–9846. doi: 10.1073/pnas.0709652105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Z., Zeng G., Yang H., Li J., Tang K., Wang G., et al. The status and characteristics of urinary stone composition in China. BJU Int. 2020;125:801–809. doi: 10.1111/bju.14765. [DOI] [PubMed] [Google Scholar]
- 15.Burns J.R., Finlayson B., Gauthier J. Calcium oxalate retention in subjects with crystalluria. Urol Int. 1984;39:36–39. doi: 10.1159/000280941. [DOI] [PubMed] [Google Scholar]
- 16.Robertson W.G. Potential role of fluctuations in the composition of renal tubular fluid through the nephron in the initiation of Randall's plugs and calcium oxalate crystalluria in a computer model of renal function. Urolithiasis. 2015;43(Suppl 1):93–107. doi: 10.1007/s00240-014-0737-1. [DOI] [PubMed] [Google Scholar]
- 17.Khan S.R., Hackett R.L. Urolithogenesis of mixed foreign body stones. J Urol. 1987;138:1321–1328. doi: 10.1016/s0022-5347(17)43592-0. [DOI] [PubMed] [Google Scholar]
- 18.Worcester E.M. Urinary calcium oxalate crystal growth inhibitors. J Am Soc Nephrol. 1994;5:S46–S53. doi: 10.1681/ASN.V55s46. [DOI] [PubMed] [Google Scholar]
- 19.Lieske J.C., Leonard R., Toback F.G. Adhesion of calcium oxalate monohydrate crystals to renal epithelial cells is inhibited by specific anions. Am J Physiol. 1995;268:F604–F612. doi: 10.1152/ajprenal.1995.268.4.F604. [DOI] [PubMed] [Google Scholar]
- 20.Gill W.B., Jones K.W., Ruggiero K.J. Protective effects of heparin and other sulfated glycosaminoglycans on crystal adhesion to injured urothelium. J Urol. 1982;127:152–154. doi: 10.1016/s0022-5347(17)53650-2. [DOI] [PubMed] [Google Scholar]
- 21.Cavanaugh C., Perazella M.A. Urine sediment examination in the diagnosis and management of kidney disease: core curriculum 2019. Am J Kidney Dis. 2019;73:258–272. doi: 10.1053/j.ajkd.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Berg W., Schnapp J.D., Schneider H.J., Hesse A., Hienzsch E. Crystaloptical and spectroscopical findings with calcium oxalate crystals in the urine sediment: a contribution to the genesis of oxalate stones. Eur Urol. 1976;2:92–97. doi: 10.1159/000471970. [DOI] [PubMed] [Google Scholar]
- 23.Azoury R., Garside J., Robertson W.G. Calcium oxalate precipitation in a flow system: an attempt to simulate the early stages of stone formation in the renal tubules. J Urol. 1986;136:150–153. doi: 10.1016/s0022-5347(17)44761-6. [DOI] [PubMed] [Google Scholar]
- 24.Robertson W.G., Peacock M., Nordin B.E. Calcium crystalluria in recurrent renal-stone formers. Lancet. 1969;2:21–24. doi: 10.1016/s0140-6736(69)92598-7. [DOI] [PubMed] [Google Scholar]
- 25.Daudon M., Hennequin C., Boujelben G., Lacour B., Jungers P. Serial crystalluria determination and the risk of recurrence in calcium stone formers. Kidney Int. 2005;67:1934–1943. doi: 10.1111/j.1523-1755.2005.00292.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.