Significance
The field exploring the diagnostic and therapeutic potential of white matter lesions by targeting abnormal ErbB signaling has been silenced for many years given the fact that neither knockout of neuregulin 1 nor ErbB3/ErbB4 alters central nervous system (CNS) myelin. We use a pan-ErbB strategy that definitely blocks endogenously activated ErbB receptors including ErbB1-ErbB4, and provide firm evidence that ErbB signaling is required for oligodendrocyte myelination. Beyond this, by using in vivo and in vitro oligodendrocyte-stage specific manipulation, we are able to demonstrate that ErbB signaling can alter axonal conduction and cognition additionally by regulating aerobic glycolysis in mature oligodendrocytes. These findings reveal the mental health implications of varied oligodendrocyte vulnerabilities induced by ErbB dysregulation.
Keywords: ErbB signaling, myelin, oligodendrocyte differentiation stage, working memory, lactate
Abstract
White matter (WM) abnormalities are an emerging feature of schizophrenia, yet the underlying pathophysiological mechanisms are largely unknown. Disruption of ErbB signaling, which is essential for peripheral myelination, has been genetically associated with schizophrenia and WM lesions in schizophrenic patients. However, the roles of ErbB signaling in oligodendrocytes remain elusive. Here, we used an in vivo pan-ErbB inhibition strategy and demonstrated the functions of endogenous ErbB receptors in oligodendrocytes. Through analyses of the cellular, histological, biochemical, behavioral, and electrophysiological differences in mice with manipulated ErbB activities in oligodendrocytes at different differentiation stages, we found that ErbB signaling regulates myelination and aerobic glycolysis in oligodendrocytes, and both functions are required for working memory. ErbB inhibition in oligodendrocytes at early differentiation stages induces hypomyelination by suppressing the myelinating capacity of newly formed oligodendrocytes. In contrast, ErbB inhibition in mature oligodendrocytes alters neither myelination nor oligodendrocyte numbers, but accelerates axonal conduction decline under energy stress. Mechanistically, ErbB inhibition attenuates K-Ras activities, leading to the reduced expression of lactate dehydrogenase A that promotes aerobic glycolysis in mature oligodendrocytes. Supplementation of L-lactate restores axonal conduction and working memory capacity that are suppressed by ErbB inhibition in mature oligodendrocytes. These findings emphasize the indispensable roles of ErbB signaling in WM integrity and function and provide insights into the multifaceted contributions of WM abnormalities to cognitive impairment.
Adolescence is the critical period for the central nervous system (CNS) to completely develop and mature. In particular, CNS myelin generated by oligodendrocytes (OLs) is one of the most developmentally active components in the adolescent brain. This makes CNS myelin a highly susceptible target in psychiatric disorders such as schizophrenia that typically develops during adolescence. A growing body of literature points to abnormalities in the structure, component proteins, or regulating molecules of CNS myelin in schizophrenic patients (1, 2). Particularly, white-matter microstructural changes that can be examined by structural brain imaging techniques are sensitive to the patient age, symptom characteristics, and genetic loading (3). Given that it is one of the most promising features that can be periodically examined in patients, understanding the biological mechanisms underlying schizophrenia-related white matter (WM) abnormalities is crucial for developing diagnostic criteria and therapeutic targets.
A few myelin protein–encoding genes, including PLP1, MOG, MAG, and MPZL1, exhibit genetic associations with schizophrenia. However, most of these variants have only been identified in the Han Chinese population (4). In contrast, tyrosine kinase receptor ERBB4 and its ligand neuregulin 1 (NRG1) show aberrant expression and genetic polymorphisms associated with schizophrenia in many populations (4, 5). NRG1/ErbB signaling has been proven essential for peripheral nervous system (PNS) myelination (5, 6). Studies combining genetic linkage analysis and brain imaging techniques have linked NRG1 and ERBB4 variability to reduced WM density and integrity in human subjects (7, 8).
In addition to the extensively studied NRG1 and ERBB4, many other ligands and receptors in the ErbB signaling pathway are implicated in schizophrenia (4). However, the role of ErbB signaling in CNS myelination remains elusive due to the contradictory reports from different research groups (9–12). Heterozygous NRG1 type III mutant mice are reported to exhibit hypomyelination in the brain (12), whereas genetic ablation of all types of NRG1 or ErbB4 induces neither developmental alteration nor pathogenesis in the WM of mutant mice (9). ERBB3 is another ErbB receptor that specifically binds to the NRG family ligands and shows a genetic association with schizophrenia (13). One research group reported that ErbB3 depletion in OLs from postnatal day 19 (P19) results in adult hypomyelination (10), whereas several other groups reported that ErbB3 knockout from the embryonic stage does not influence OLs and CNS myelin (9, 11).
Among the four ErbB receptors, EGFR (i.e., ErbB1) binds specifically to the epidermal growth factor (EGF) family ligands (5). Notably, EGFR and its ligand EGF also exhibit genetic polymorphisms and aberrant expression that are associated with schizophrenia (14–17). Nevertheless, hypomorphic EGFR mutant mice display only delayed myelination in the brain, which is attributed to impaired oligodendrogenesis during early development (18). Schizophrenia is a complex polygenic disorder. It is particularly noteworthy that gene–gene interactions among variants in the NRG-ERBB and EGF-ERBB pathways increase susceptibility to schizophrenia (16, 19). However, whether NRG-ErbB and EGF-ErbB signaling synergistically regulate myelin development and function has not been investigated.
In the CNS, OL precursor cells (OPCs) undergo terminal mitosis and differentiate into newly formed OLs (NFOs). NFOs progress from premyelinating OLs to newly myelinating OLs. Myelinating OLs effectively generate myelin sheaths in a short time window before further progressing into mature OLs (MOs) that maintain the myelin sheath (20, 21). We demonstrated that, regardless of which ErbB receptor is primarily bound by the ligand, both NRG-ErbB and EGF-ErbB receptors can be activated in OPCs and differentiated OLs. We adopted an inducible pan-ErbB strategy that blocked the activities of any activated ErbB receptors in OL-lineage cells in vivo. This strategy allowed us to focus on the functions of ErbB signaling in OLs without the need to characterize the complex ErbB ligands in the niche. By using two valuable research mouse tools that help distinguish the outcomes induced by defects of OLs at different differentiation stages, Sox10+/rtTA (Tet-on) targeting OPC-NFOs and Plp-tTA (Tet-off) targeting MOs (22), we were able to demonstrate that disruption of ErbB signaling causes myelination-dependent and -independent WM abnormalities, and prove that both pathophysiological changes lead to cognitive deficits.
Results
ErbB Inhibition in Oligodendrocytes during Early Differentiation Stages Induces Hypomyelination.
We characterized the expression of ErbB receptor members in subcortical WM regions at different postnatal days. The results revealed that EGFR was expressed with relatively stable levels during P20-P40, while ErbB4 gradually decreased after P20. ErbB2 was barely detectable in mouse CNS myelin after P15 by western blotting, despite comparable mRNA levels of ErbB2 to EGFR or ErbB4 detected by real-time RT-PCR. Note that subcortical WM regions isolated from mice before P5 contained few myelin components. ErbB3 expression increased dramatically from P5 and plateaued during P25-P30 (SI Appendix, Fig. S1 A–C). To analyze which receptors are activated by NRG or EGF family ligands in OLs, we stimulated primarily cultured OPCs and postmitotic OLs, which were purified and in relatively synchronized differentiation stages in vitro, with NRG1 (binding ErbB3/4 only), EGF (binding EGFR only), or HB-EGF (binding both EGFR and ErbB4) for 10 min. Unexpectedly, all three ErbB ligands exhibited the potential to increase tyrosine phosphorylation of ErbB1-4 receptors, despite NRG1 demonstrating stronger agonistic effects on ErbB4 than on ErbB1-3 receptors (Fig. 1 A and B). These results indicate that, regardless of the stimulating ligands, NRG-ErbB and EGF-ErbB receptors collaborate in the same biological processes. Therefore, the functions of ErbB signaling in OLs may not be accurately evaluated by investigating mice with knockout of only EGFR or ErbB3/4.
Fig. 1.
ErbB inhibition induces hypomyelination in Sox10-dnEGFR mice, but not in Plp-dnEGFR mice. (A and B) Activation of ErbB receptors (pErbB) by HB-EGF, EGF, or NRG1 treatments for 10 min in OPCs (A) and differentiated OLs (B). The immunofluorescence results indicate the synchronized OL stages in vitro. (C and D) Representative (C) and statistical western blotting results (D) of the white matter from Sox10-dnEGFR (SE) and littermate controls (Ctrl). (E) Phosphorylation of ErbB receptors examined in NFOs purified from indicated mice at indicated ages by western blotting. (F) Electron microscope images of the corpus callosum (CC), optic nerve (ON), and prefrontal cortex (PFC) from Sox10-dnEGFR and littermate controls at 44 dwd. g-ratio was calculated for myelinated axons and averaged g-ratio were analyzed by unpaired t test. (G-J) Western blotting results of the white matter (G, H), purified NFOs (I), as well as electron microscope (J) for Plp-dnEGFR (PE) and littermate controls (Ctrl) at indicated ages. In (F), Ctrl n = 4, SE n = 3 for CC; Ctrl n = 4, SE n = 5 for ON; Ctrl n = 3, SE n = 3 for PFC. In (J), n = 3 for each group. For (D, E, H, I), shown are data of indicated mice that have been normalized by those of littermate controls. n = 3 pairs for each group.
To investigate the functions of endogenous ErbB signaling in vivo, we first generated Sox10+/rtTA;TRE-dnEGFR (Sox10-dnEGFR) mice (SI Appendix, Fig. S2 A and B), an inducible mouse model expressing a dominant negative mutant of EGFR (dnEGFR) in OPC-NFOs upon doxycycline (Dox) treatment. When overexpressed, dnEGFR efficiently blocks the activation of any endogenous ErbB receptors under either NRG or EGF stimulation (23). In line with this concept, phosphorylation of ErbB3 and ErbB4 was reduced in the WM of Sox10-dnEGFR mice at P35 after 14 days with Dox feeding (dwd). Phosphorylation of EGFR was unchanged in Sox10-dnEGFR mice at P35 (Fig. 1 C and D), consistent with our previous findings in Sox10-ErbB2V664E mice, where expressing a constitutively activated ErbB2 mutant in OPC-NFOs activated endogenous ErbB3 and ErbB4, but not EGFR (22). However, both pEGFR and pErbB4 were downregulated in NFOs purified from Sox10-dnEGFR mice at P35 (Fig. 1E and SI Appendix, Fig. S3 A–C), suggesting the pan-ErbB inhibition by dnEGFR. Since Sox10-rtTA targets OPCs and NFOs comprising 20% or less of total cells in WM after adolescence (22), the varying phosphorylation levels of ErbB receptors in Sox10-dnWGFR WM may be due to dilution by non-dnEGFR-expressing cells.
Myelin thickness and ultrastructures in the WM of Sox10-dnEGFR and littermate control mice at P35 with 14 dwd did not show significant differences (SI Appendix, Fig. S2C). Therefore, we raised these mice to adulthood with continuous Dox feeding. Phosphorylation of EGFR, instead of ErbB3 or ErbB4, was noticeably reduced in the WM of Sox10-dnEGFR mice at P65 (Fig. 1 C and D), and reduction in both pEGFR and pErbB4 were detected in purified NFOs from Sox10-dnEGFR mice at P65 (Fig. 1E). For Sox10-dnEGFR mice at P65 with 44 dwd, axons in the corpus callosum (CC) and optic nerve (ON) were hypomyelinated with myelinated axon densities unchanged (Fig. 1F and SI Appendix, Fig. S2D). Consistently, myelin basic protein (MBP) was reduced in the WM of Sox10-dnEGFR mice at P65 (Fig. 1 C and D).
We further crossed Plp-tTA and TRE-dnEGFR to generate Plp-tTA;TRE-dnEGFR (Plp-dnEGFR) mice (SI Appendix, Fig. S2 E and F). At P35 in these mice, with dnEGFR expression in MOs for 14 days post Dox-withdrawal (dpd) at P21, western blotting revealed a significant suppression of phosphorylation in EGFR, ErbB3, and ErbB4 (Fig. 1 G and H), consistent with their overactivation in Plp-ErbB2V664E mice (22). No differences in CNS myelin were observed in Plp-dnEGFR and littermate control mice at P35 with 14 dpd (SI Appendix, Fig. S2 G and H).
We extended our investigation to P65, where dnEGFR continued to functionally suppress ErbB receptor activities in the WM of Plp-dnEGFR mice (Fig. 1 G and H). Since Plp-tTA does not target OL stages before MOs, no dnEGFR overexpression or pErbB reduction was observed in purified NFOs from Plp-dnEGFR mouse brains (Fig. 1I and SI Appendix, Fig. S3D), indicating no possible off-target effects of dnEGFR on non-dnEGFR expressing cells. At P65 with 44 dpd, brains of Plp-dnEGFR and littermate mice exhibited no differences in MBP levels (Fig. 1 G and H) or myelin ultrastructures (Fig. 1J and SI Appendix, Fig. S2H). Extending Dox treatments beyond P90 neither relieved hypomyelination in Sox10-dnEGFR mice nor induced myelin alterations in Plp-dnEGFR mice (SI Appendix, Fig. S2 I and J). These results consolidated that blocking endogenous ErbB signaling in MOs does not affect CNS myelin development, whereas in OPC-NFOs it induces hypomyelination.
ErbB Inhibition in Oligodendrocytes during Early Differentiation Stages Increases Oligodendrocyte Numbers.
To investigate whether hypomyelination is induced by a decrease in myelinating cell numbers, we examined the OL-lineage cells in Sox10-dnEGFR and Plp-dnEGFR mice. Unexpectedly, the densities of Olig2+ (all OL-lineage cells), NG2+ (OPCs), and CC1+Olig2+ cells (postmitotic OLs) significantly increased in the CC of Sox10-dnEGFR mice at P65 with 44 dwd (Fig. 2 A and C), although this alteration was not detected at P35 with 14 dwd (SI Appendix, Fig. S4 A and B). In contrast, no differences in Olig2+, CC1+Olig2+, or NG2+ cell densities were observed in WM of Plp-dnEGFR mice and littermate controls at either P35 or P65 (Fig. 2 B and D and SI Appendix, Fig. S4 C and D).
Fig. 2.
ErbB inhibition induces OL number increases in Sox10-dnEGFR mice, but not in Plp-dnEGFR mice. (A and B) Olig2+, CC1+, and NG2+ cells in the CC of indicated mice at indicated ages were examined by immunostaining. (C and D) Statistical results of Olig2+, CC1+Olig2+, and NG2+ cell densities in the CC of Sox10-dnEGFR mice (SE) and littermate controls (Ctrl) at P65 with 44 dwd, or Plp-dnEGFR mice (PE) and littermate controls at P65 with 44 dpd. Data were from repeated immunostaining of three mice for each group. (E–G) Representative images (E and F) and the ratio of densities of proliferating OL-lineage cells (Olig2+Ki67+) to those of total Olig2+ OLs (G, n = 3 for each group) examined in indicated mice at indicated ages. (H) Temporal expression levels of dnEGFR in primarily cultured OPCs examined by real-time RT-PCR. Note that dnEGFR increased in Sox10-dnEGFR (SE) OPCs with Dox treatments for over 2 d, whereas it was not expressed in Plp-dnEGFR (PE) OPCs, n = 3. (I) Time course variation of ErbB phosphorylation levels in response to EGF or NRG1 in cultured OPCs from indicated mice. Statistical data were pErbB/β-tubulin ratio after normalization to vehicle treatment (Veh) in each batch of experiments and analyzed by the paired t test. *P < 0.05, **P <0.01, Ctrl-EGF vs. SE-EGF; #P < 0.05, ##P <0.01, Ctrl-NRG1 vs. SE-NRG1, n = 3. (J) Proliferating OPCs (EDU+PDGFRα+) in response to EGF (treatments for 1 d) examined in OPCs purified from indicated mice with or without Dox treatments for 4 d. For SE OPCs in comparison with control OPCs: Ctrl with vehicle treatments (veh), n = 10; SE with veh, n = 9; Ctrl with EGF treatments (EGF), n = 10; SE with EGF, n = 7. For PE OPCs in comparison with control OPCs: Ctrl with veh, n = 10; PE with veh, n = 10; Ctrl with EGF, n = 9; PE with EGF n = 9.
The increase in OL numbers was attributed to the proliferation of OPCs (Ki67+Olig2+) detected in the WM of Sox10-dnEGFR mice at P65 (Fig. 2 E and G and SI Appendix, Fig. S5A), where apoptotic cells (TUNEL+) were as minimal as in control mice (SI Appendix, Fig. S5C). In contrast, WM in Plp-dnEGFR mice, which did not show changes in OL numbers, exhibited no differences in Ki67+Olig2+ or TUNEL+ status (Fig. 2 F and G and SI Appendix, Fig. S5 B and D). However, ErbB signaling does not negatively regulate OPC proliferation, as evidenced by EGF increasing the numbers of EdU+ OPCs in vitro (Fig. 2J). Moreover, Sox10-dnEGFR OPCs, which overexpressed dnEGFR and paninhibited ErbB activities in response to either EGF or NRG1 (Fig. 2 H and I), lost the EdU+-number-increase responses to EGF (Fig. 2J and SI Appendix, Fig. S6 A and B), indicating a requirement for ErbB activation in OPC proliferation. Therefore, the enhanced OPC proliferation and increased OL numbers in adult Sox10-dnEGFR mice do not seem to be directly induced by ErbB disruption in OPCs, but rather an indirect consequence through cell communication during brain development.
ErbB Inhibition Suppresses NFO Myelinating Capacity to Lead to Hypomyelination.
ErbB inhibition occurs in our mouse models after P21, when OPC proliferation and differentiation have passed their synchronous peak period and become more sporadic, contributing to de nova myelinogenesis and myelin remodeling in the adult brain (21, 24). With the increase in OL number, there must be other events impairing myelination capacity in the brains of Sox10-dnEGFR mice during adolescence to adulthood. Given that both Sox10-dnEGFR and Sox10-ErbB2V664E mice exhibit hypomyelination (22), they likely share a molecular or cellular deficit in myelination. We performed RNA-seq analyses of subcortical WM tissues and identified 68 genes with similar expression tendencies in Sox10-ErbB2V664E and Sox10-dnEGFR mice during adolescence (Fig. 3A, SI Appendix, Fig. S7 A–D, and Datasets S1 and S2). Notably, in addition to Gsn and Itgb4, characteristic genes for myelinating OLs (25), Enpp6, Itpr2, and Slc12a2, characteristic genes for NFOs, also exhibited significantly reduced expression in both mouse lines. Transcription of these genes was persistently reduced in Sox10-dnEGFR WM at P65 (Fig. 3B). Although NFO numbers became very few and differences became indistinguishable by P65, in situ hybridization of Enpp6 (26) and immunostaining of TCF4 (27), which specifically label NFOs, confirmed their deficiency in Sox10-dnEGFR mice at P35 (Fig. 3 C and D and SI Appendix, Fig. S8A). In vivo time-lapse imaging has shown that NFOs are in the most active stage for accomplishing myelination of axons (20). In an in vitro assay covering NFO-to-MO progression processes, Sox10-dnEGFR NFOs, expressing dnEGFR that paninhibited ErbB receptors (Fig. 3 E and F), failed to respond to EGF or NRG1 in generating MBP+ myelinating OLs (Fig. 3G). The progression of myelinating NFOs into MOs was unaffected by Sox10-dnEGFR, as indicated by the ratio of MOs to total OLs (ASPA+/Olig2+) in Sox10-dnEGFR mice being similar to littermate control mice at P65, despite the increase in MO (ASPA+) numbers (Fig. 3H). Nevertheless, the increased MO numbers did not overcome the reduction in the transcription of myelin protein genes Mbp, Mag, and Mog in Sox10-dnEGFR mice at P65 (Fig. 3B), consistent with electron microscope observations. Therefore, NFOs with ErbB inhibition generated in Sox10-dnEGFR mice after Dox treatments from P21 exhibited impaired myelinating capabilities, resulting in deficits in myelinogenesis and remodeling since adolescence, ultimately culminating in adult hypomyelination. In contrast, in vivo transcription of NFO characteristic genes and myelin protein genes, the ASPA+ MO numbers, and in vitro induction of MBP+ myelinating NFOs by ErbB ligands were unaffected in Plp-dnEGFR mice (Fig. 3 B and G and SI Appendix, Fig. S8 B–D).
Fig. 3.
ErbB inhibition impairs the myelinating capacity of NFOs. (A) Heat maps of Z value are presented for 68 genes with similar expression tendencies in the WM of Sox10-ErbB2V664E (soxEb vs. treEb) and Sox10-dnEGFR (soxEG vs. treEG) mice as identified by RNA-seq analyses of WM tissues isolated from three pairs of Sox10-ErbB2V664E and littermate control mice at P30 with 9 dwd, or Sox10-dnEGFR and littermate control mice at P35 with 14 dwd. The detailed RNA-seq data have been deposited in the GEO and SRA database and can be found at GEO: GSE123491. (B) Transcription levels of characteristic genes of OLs at different differentiation stages in the WM of indicated mice. Shown are real-time RT-PCR results of indicated mice that have been normalized by those of littermate controls. Mouse pairs for each group n = 3. (C) In situ hybridization results of Enpp6 and immunostaining results of TCF4 in the CC of indicated mice at indicated ages. (D) Statistical analyses of Enpp6+ or TCF+ cell densities, as well as the percentages of NFOs in total OLs (TCF4+/Olig2+) in Sox10-dnEGFR (SE) mice and littermate controls (Ctrl) at P35 or P65. Data were from repeated immunostaining of three mice for each group. (E) Temporal expression levels of dnEGFR in cultured Sox10-dnEGFR (SE) and control (Ctrl) OLs at different days after triiodothyronine (T3) induction as examined by real-time RT-PCR, n = 3. (F) Time course variation of ErbB phosphorylation levels in response to EGF or NRG1 in cultured NFOs from indicated mice after 2-d T3 induction. Statistical data were pErbB/β-tubulin ratio after normalization to vehicle treatment (Veh) in each batch of experiments and analyzed by the paired t test. *P < 0.05, Ctrl-EGF vs. SE-EGF; #P < 0.05, Ctrl-NRG1 vs. SE-NRG1 n = 3. (G) Newly myelinating OLs (MBP+) induced by EGF or NRG1 from OPCs after 1-d T3 induction. For analysis of Sox10-dnEGFR (SE) NFOs: Control (Ctrl) with vehicle treatment (veh) n = 9, SE with veh n = 10; Ctrl with EGF treatment (EGF) n = 10, SE with EGF n = 9; Ctrl with NRG1 treatment (NRG1) n = 9, SE with NRG1 n = 10. For analysis of Plp-dnEGFR (PE) NFOs: Ctrl with veh n = 10, PE with veh n = 10; Ctrl with EGF n = 10, PE with EGF n = 10; Ctrl with NRG1 n = 10, PE with NRG1 n = 10. (H) Immunostaining results of MOs (ASPA+Olig2+) in the CC of Sox10-dnEGFR (SE) and littermate controls (Ctrl) at indicated ages. Statistical data of ASPA+ cell densities and the ratio of ASPA+ to Olig2+ cell numbers were from repeated immunostaining of three mice for each group.
ErbB Inhibition in MOs Disrupts Cognitive Function in the Absence of Myelin Alteration.
It is noteworthy that while Plp-dnEGFR WM did not exhibit alterations in the expression of myelin protein genes and NFO genes, there was a reduction in the transcription of Itpr2, Gsn, and Itgb4 (Fig. 3B). These results suggest that OLs in Plp-dnEGFR mice were not as normal as we assumed. Before delving into mechanistic investigations, we first evaluated whether ErbB signaling in MOs is crucial for brain functions by comparing the behavioral performance of Sox10-dnEGFR and Plp-dnEGFR mice.
While showing minimal differences in the PNS (SI Appendix, Fig. S9 A–F), Sox10-dnEGFR mice performed worse than control mice in the rotarod test (Fig. 4A), and were slightly hypoactive in the open field test (Fig. 4B). Nevertheless, they performed normally in analyses of anxiety (central/peripheral zone analysis), stereotyped behavior, social interaction (potential autistic-like phenotype), sensory gating (prepulse inhibition analysis), and tests for depression (forced swim and tail suspension tests) (Fig. 4 C–E and SI Appendix, Fig. S10 A and B). Interestingly, Plp-dnEGFR mice performed normally, similar to the controls in most tests, except showing subtle hyperactivity in the open field test (Fig. 4 G–K and SI Appendix, Fig. S10 C and D). These differing results from the two mouse models imply that the impaired motor coordination is attributed to hypomyelination in the CNS.
Fig. 4.
ErbB inhibition in MOs impairs working memory in the absence of myelin alteration. Behavioral performance of adult Sox10-dnEGFR mice with littermate controls, or Plp-dnEGFR mice with littermate controls. (A-F) Behavioral performance of adult Sox10-dnEGFR mice and littermate controls. (G-L) Behavioral performance of adult Plp-dnEGFR mice and littermate controls. Shown are motor coordination assessed by the rotarod test (A and G), locomotive activity assessed by open field tests (B and H), zone analysis of open field tests (C and I), social interests assessed by social interaction tests (D and J), sensory gating assessed by PPI tests (E and K), and working memory assessed by the eight-arm radial water maze test (F and L). In (A), controls n = 12, Sox10-dnEGFR n = 12. In (B and C), control n = 11, Sox10-dnEGFR n = 13. In (D), control n = 12, Sox10-dnEGFR n = 13. In (E), control n = 10, Sox10-dnEGFR n = 12. In (F), control n = 7, Sox10-dnEGFR n = 12. In (G), control n = 13, Plp-dnEGFR n = 12. In (H and I), control n = 19, Plp-dnEGFR n = 14. In (J), control n = 14, Plp-dnEGFR n = 13. In (K), control n = 14, Plp-dnEGFR n = 12. In (L), control n = 10, Plp-dnEGFR n = 14. Data were analyzed by two-way ANOVA test, except for those from the visible platform test in eight-arm radial water maze that were analyzed by the unpaired t test. Illustrative examples of the travel pathways of control or mutant mice were included in (D, F, J, and L). In illustrative examples in (F and L), green circles indicate the last arms with a hidden platform, while red crosses indicate the arms with used platforms in the past three trials.
We further tested these mice in the eight-arm radial water maze, a paradigm analyzing working memory capacity. Not only Sox10-dnEGFR mice, which had CNS hypomyelination, but also Plp-dnEGFR mice, which did not have myelin alteration, made significantly more working memory errors than control mice (Fig. 4 F and L and Movie S1). Note that they had normal eyesight as assessed in the visible platform test, as well as similar reference memory errors that indicated unaltered spatial recognition and memory (Fig. 4 F and L). This phenotype in Plp-dnEGFR mice reveals that working memory deficiency can be directly caused by ErbB inhibition in MOs through a myelination-independent mechanism.
ErbB Inhibition in MOs Suppresses Axonal Conduction under Energy Stress.
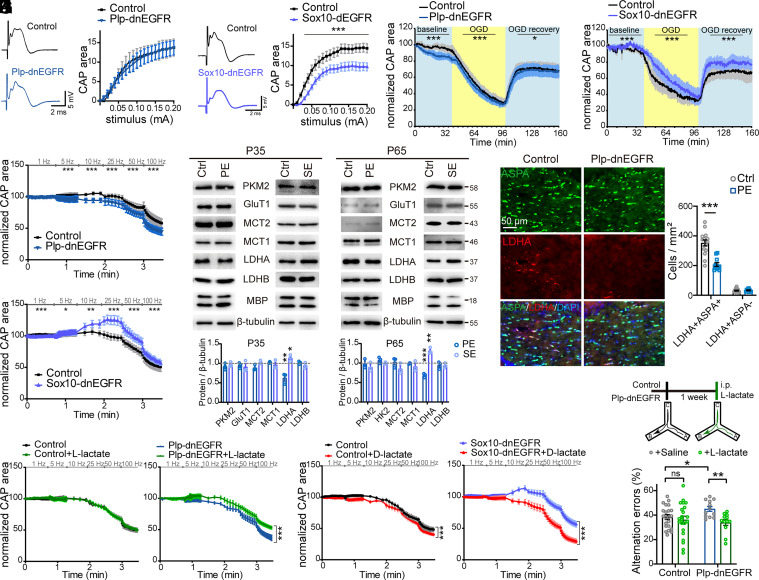
To determine which functions were impaired in the WM tracts of Plp-dnEGFR mice, we acutely isolated the ONs from adult mice and recorded electrical stimulus-evoked compound action potentials (CAPs). Maximal CAPs, which represent excitation of all axons in the nerves, were similar in Plp-dnEGFR ONs and control nerves (Fig. 5A). In contrast, they were reduced in Sox10-dnEGFR ONs (Fig. 5B). These results indicate that basic axonal conduction was not affected in Plp-dnEGFR WM tracts, whereas it was impaired in Sox10-dnEGFR WM tracts that exhibited hypomyelination.
Fig. 5.
ErbB inhibition in MOs suppresses LDHA expression and axonal conduction under energy stress. (A and B) Axonal excitability is similar in Plp-dnEGFR ONs and control nerves (A), but decreased in Sox10-dnEGFR ONs in comparison with controls (B). CAPs of ONs generated by electrical stimuli with intensities at stepped increase (0 to 0.2 mA) were recorded ex vivo. Data were from 3 to 7 ONs of 3 to 5 mice for each group. (C and D) Normalized CAPs generated with stimuli at maximal intensities were declined by OGD for Plp-dnEGFR (C) and Sox10-dnEGFR ONs (D). OGD was started for the recorded nerves after 1-h baseline stimulation, and stopped after another hour by restoring the bathing media to oxygenated ACSF. Initial CAPs were recorded after 30-min baseline stimulation. The areas under CAPs were measured and normalized to the initial levels. Data were from 4 to 8 ONs of 3 to 5 mice for each group. (E and F) Normalized CAPs generated in ONs from indicated mice with stimuli at frequency changes from 5 to 100 Hz. Data were from 4 to 8 ONs of 3 to 5 mice for each group. (G) Indicated proteins examined by western blotting in the WM of Sox10-dnEGFR (SE) mice or Plp-dnEGFR (PE) mice, in comparison with that of littermate controls (Ctrl). (H) Statistical results of experiments in (G). Data of the protein levels in WM of indicated mice have been normalized by those of littermate controls. n = 3 for each group. (I) Immunostaining results of LDHA and ASPA in the CC of Plp-dnEGFR and littermate control mice at P65. Statistical data were from repeated immunostaining of three mice for each group. (J) L-lactate restored the declined CAPs in Plp-dnEGFR ONs. Data were from 6 to 10 ONs of 3 to 5 mice for each group. (K) D-lactate suppressed the expanded CAPs in Sox10-dnEGFR ONs. Data were from 5 to 12 ONs of 3 to 6 mice for each group. (L) Working memory deficits in Plp-dnEGFR mice were rescued by systemic L-lactate as assessed by the Y maze test. Control with saline or L-lactate n = 22; Plp-dnEGFR with saline or L-lactate n = 12.
Next, we challenged the ONs by incubating them in the oxygen-glucose deprivation (OGD) conditions for 60 min. CAPs gradually declined in control ONs, ultimately reaching 30% of initial levels (Fig. 5 C and D). However, in Plp-dnEGFR ONs under OGD conditions, CAP failure was slightly accelerated and aggravated (Fig. 5C). In contrast, for Sox10-dnEGFR ONs under the same conditions, CAP drops from baseline levels was delayed and attenuated (Fig. 5D). Upon restoration of glucose and oxygen levels in the bathing medium, CAPs in control and Plp-dnEGFR ONs recovered to 60% of baseline initial levels (Fig. 5 C and D). However, in Sox10-dnEGFR ONs, CAPs recovered to 80% of initial levels (Fig. 5D).
It is notable that continuous electrical stimulation caused a baseline CAP decline in Plp-dnEGFR ONs, whereas it caused a baseline CAP enhancement in Sox10-dnEGFR ONs compared to their initial levels before the OGD (Fig. 5 C and D). Therefore, we further examined axonal conduction under physiological conditions with increasing energy demands generated by neuronal activities (28, 29). By stimulating ONs with several trains of short bursts with frequencies increasing from 1 to 100 Hz, electrical stimuli at 5 to 25 Hz amplified CAPs, while stimuli at 50 to 100 Hz induced smaller CAP decay, from initial levels in Sox10-dnEGFR ONs (Fig. 5F). In contrast, for Plp-dnEGFR ONs, both groups of stimuli significantly exacerbated CAP decay from initial levels (Fig. 5E).
ErbB Inhibition in MOs Suppresses the Energy Substrate Supply to Electrically Active Axons.
Note that MO numbers were not altered in Plp-dnEGFR mice, where ErbB inhibition occurred in MOs, whereas ErbB receptors were not inhibited in MOs of Sox10-dnEGFR mice, which exhibited increased MO numbers (SI Appendix, Fig. S10F). Therefore, the contrasting capacities of Sox10-dnEGFR and Plp-dnEGFR ONs to maintain CAPs under energy-challenging conditions suggest that ErbB signaling in MOs determines glia-axon energy coupling efficiency. Supporting this idea, we observed a significant decrease in lactate dehydrogenase A (LDHA), the LDH subunit promoting glycolytic products including lactate (30, 31), in Plp-dnEGFR WM (Fig. 5 G and H). The reduction in the bulk levels of LDHA in WM tissues was attributable to the decreased number of ASPA+ MOs that have detectable LDHA levels (Fig. 5I). Other enzymes and transporters in glycolysis pathways linking OLs and axons, including PKM2, GluT1, LDHB, MCT1, and MCT2, were unchanged (Fig. 5 G and H). Interestingly, Sox10-dnEGFR WM exhibited increased LDHA levels in MOs (Fig. 5 G and H), consistent with its relative resistance to energy stress. Lactate is known as one of the major energy substrates that MOs provide to axons (28, 32, 33). Consistent with this notion, supplementation with L-lactate rescued accelerated CAP decline in Plp-dnEGFR ONs during high-frequency stimulation (Fig. 5J). Conversely, D-lactate, a metabolically inert isoform competing for endogenous lactate uptake and inhibiting LDH activity (29, 34), suppressed the relatively expanded CAPs in Sox10-dnEGFR ONs (Fig. 5K). Furthermore, systemic L-lactate supplementation rescued working memory deficits in Plp-dnEGFR mice (Fig. 5L). Of note, L-lactate administration in control mice showed no statistical significance in improving axonal conduction or cognition (Fig. 5 J and L). These results underscore the saturation of endogenous energy substrates produced by aerobic glycolysis in normal WM tracts, highlighting the crucial role of oligodendroglial ErbB signaling in glia-axon metabolic coupling.
K-Ras Mediates the Effect of ErbB Receptors on LDHA Expression in MOs.
To explore the downstream signaling of ErbB receptors in OLs, we analyzed genes showing opposite expression tendencies in the RNA-seq results of Sox10-ErbB2V664E and Sox10-dnEGFR WM tissues (Fig. 6A). We identified 50 genes enriched in GO terms related to signaling pathways, cell division, or cell death (SI Appendix, Fig. S7C). It is noteworthy that the expression of btc and kras was increased in Sox10-ErbB2V664E and decreased in Sox10-dnEGFR WM (Fig. 6A). Btc encodes betacellulin, an EGF family member that binds to both EGFR and ErbB4, suggesting a positive feedback loop of ErbB signaling in WM, potentially contributing to pan-ErbB activation in OL-lineage cells.
Fig. 6.
K-Ras mediates the effect of ErbB receptors on LDHA expression in MOs. (A) Heat maps of Z value are presented for 50 genes with opposite expression tendencies in the WM of Sox10-ErbB2V664E (soxEb vs. treEb) and Sox10-dnEGFR (soxEG vs. treEG) mice as identified by RNA-seq analyses. (B) Active K-Ras (K-Ras-GTP) levels in WM from Plp-dnEGFR (PE) or Sox10-dnEGFR (SE) mice and their littermate controls (Ctrl) at P65. n =3 for each group in statistical results. (C) ErbB ligands in WM tissues of Plp-dnEGFR (PE) or Sox10-dnEGFR (SE), and their littermate controls mice (Ctrl) were examined by western blotting. n = 3 for each group in statistical results. (D) Temporal expression levels of dnEGFR in cultured Plp-dnEGFR OLs after different days of T3 induction as examined by real-time RT-PCR. OL maturation levels were represented by transcriptional levels of myelin protein MOBP. n = 3. (E) Time course variation of ErbB phosphorylation levels in response to EGF or NRG1 in cultured MOs from indicated mice after 6-d T3 induction. Statistical data were pErbB/β-tubulin ratio after normalization to vehicle treatment (Veh) in each batch of experiments and analyzed by the paired t test. *P < 0.05, Ctrl-EGF vs. SE-EGF; #P < 0.05, Ctrl-NRG1 vs. SE-NRG1. n = 3. (F and G) Representative western blotting (F) and immunostaining (G) results of LDHA alteration induced by EGF treatments or EGF+BI-2852 cotreatments for 1 d in control MOs (Ctrl) or EGF treatments in Plp-dnEGFR MOs (PE), respectively. MOs (MBP+) were maintained in vitro for 6 or more days after differentiation induced by T3. (H) Statistical results of the ratio of LDHA levels induced by EGF treatments to those of vehicle treatments (Veh) in control MOs (Ctrl), Plp-dnEGFR MOs (PE), or control MOs with BI-2852 treatments (Ctrl+BI), as examined by western blotting, immunostaining, and real-time RT-PCR. For WB and real-time RT-PCR, n = 3 for each group. For IF, Ctrl, n = 7; PE, n = 7; Ctrl+BI, n = 6. (I) Western blotting results of LDHA alteration induced by EGF treatments for 1 d in control MOs (Ctrl) or KRA-533 (1.0 μM) treatments for 1 d in Plp-dnEGFR MOs (PE). (J) Statistical results of the ratio of LDHA levels induced by EGF treatments to those of vehicle treatments (Veh) in control MOs (Ctrl-EGF), or KRA-533 treatments to those of vehicle treatments in Plp-dnEGFR MOs (PE-KRA). n = 3 for each group. (K) Real-time RT-PCR results of LDHA alteration induced by 0.5 μM (PE-KRA-0.5) or 1.0 μM KRA-533 (PE-KRA-1.0) for 1 d in Plp-dnEGFR MOs in comparison with vehicle treatments in Plp-dnEGFR MOs (PE-Veh). E3E4 stands for primers detecting ldha CDS sequence across exon 3 and exon 4. E5E6 stands for primers detecting ldha CDS sequences across exon 5 and exon 6. n =3 for each group. (L) Schematic illustration of the effects of ErbB signaling that promote the myelinating capacity in NFOs and aerobic glycolysis in MOs.
The GTPase K-Ras is upstream of both Akt and Erk pathways that are known to be crucial for CNS myelin development (35–37). Consistent with the changes in LDHA levels (Fig. 5 G and H), K-Ras activity (K-Ras-GTP) was downregulated in WM tissues from Plp-dnEGFR mice, whereas it was upregulated in tissues from Sox10-dnEGFR mice (Fig. 6B). In Sox10-dnEGFR WM, where MO number increased without dnEGFR expression (Fig. 3 E and H), the elevated K-Ras activity may result from environmental factors altered by dnEGFR-expressing cells or their interactions. Indeed, Amphiregulin (AR), an ErbB ligand in the EGF subfamily, increased in WM tissues from Sox10-dnEGFR mice (Fig. 6C). No ErbB ligand decrease was observed in WM tissues from Plp-dnEGFR mice; instead, NRG1 type III increased there (Fig. 6C). These results suggest that endogenous ErbB inhibition downregulates K-Ras activities in Plp-dnEGFR MOs.
To investigate ErbB signaling in regulating LDHA levels in MOs, we established an in vitro MO model that was maintained for more than 6 d after differentiation induction, and confirmed dnEGFR overexpression along with ErbB inhibition in Plp-dnEGFR MOs (Fig. 6 D and E). EGF increased LDHA levels in control MOs, as assessed by immunostaining, western blotting, and real-time RT-PCR. Interestingly, NRG1, which prefers to activate ErbB4 (Figs. 1 A and B, 2I, and 3F) and showed weaker agonistic effects than EGF on MOs (Fig. 6E), did not increase LDHA expression in MOs (SI Appendix, Fig. S11 A and B). In contrast, AR, as an EGF-like ligand, increased LDHA expression in control and Sox10-dnEGFR MOs. Intriguingly, overexpression of dnEGFR inhibited EGF- or AR-stimulated LDHA expression in Plp-dnEGFR MOs (Fig. 6 F–H and SI Appendix, Fig. S11 C and D).
Rather than using K-Ras-depleted cells or tissues that could influence intracellular molecules through developmental effects, we employed the selective K-Ras inhibitor BI-2852 (38) in combination with EGF treatments in MOs. Importantly, BI-2852 cotreatments, which inhibited Akt and Erk activation without blocking ErbB activation induced by EGF (SI Appendix, Fig. S12A), suppressed EGF-stimulated LDHA expression in control MOs at both mRNA and protein levels (Fig. 6 F–H). Conversely, treatments with KRA-533, a selective K-Ras agonist (39), increased LDHA expression at both mRNA and protein levels in Plp-dnEGFR MOs, concomitant with Erk and Akt activation (Fig. 6 I–K and SI Appendix, Fig. S12B). Therefore, K-Ras can mediate the effects of ErbBs on LDHA expression in MOs.
Discussion
Genetic variants in schizophrenic patients are associated with reduced expression or loss of function in ErbB receptors or ligands (5, 40, 41). The present study reveals that the loss of ErbB functions in OLs has two types of adverse impacts on WM tracts: hypomyelination and axonal energy deficiency. By using the mouse models Sox10-dnEGFR and Plp-dnEGFR, which help distinguish the consequences induced by ErbB disruption in OPC-NFOs from those in MOs, we demonstrated that ErbB inhibition in OPC-NFOs impairs the myelinating capacity of NFOs, resulting in hypomyelination in adulthood. Simultaneously, ErbB inhibition in MOs blocks the supply of energy substrates to axons, disrupting axonal conduction within electrically active neural circuits. Importantly, both defects contribute to working memory deficits, firmly supporting that both myelination-dependent and -independent oligodendropathy can be primary causes of the cognitive symptoms of schizophrenia.
The behavioral phenotypes exhibited in Sox10-dnEGFR mice provide direct evidence supporting the known concept that myelin integrity is fundamental to the cognitive performance of patients (42). Given that energy stress resistance was enhanced in Sox10-dnEGFR WM tracts (Fig. 5 D and F), but Sox10-dnEGFR mice still exhibited working memory deficits (Fig. 4F), the structural integrity of myelin outweighs the efficiency of glia-axon metabolic coupling in determining cognitive performance. Targeting NFO deficiency may be more important in treatments aimed at combating WM abnormalities induced by ErbB dysregulation.
In contrast, Plp-dnEGFR mice serve as a good model to affirm the myelination-independent contributions of OLs to higher brain function, given the fact that dual inhibition of NRG-ErbB and EGF-ErbB signaling in MOs does not affect myelin or OL numbers in adolescent and adult brains (Figs. 1 and 2). Studies on mice with OL-specific depletion of Cox10, Kir4.1, or NR1 demonstrate that the metabolic products of glycolysis in MOs are important for axonal conductivity, with L-lactate being a major form for axonal energy consumption (28, 32, 33). ErbB signaling has been revealed to regulate aerobic glycolysis in cancer cells, where the expression of PKM2, LDHA, and GluT1 is increased to optimize cells for the Warburg effect (43, 44). It was unexpected that ErbB signaling in MOs is indispensable only for LDHA expression (Fig. 5 G and H), highlighting a cell-specific event regulated by ErbB signaling. Importantly, the conversion of pyruvate to lactate by LDHA regenerates NAD+, which fuel the conversion of glyceraldehyde-3-phosphate to 1,3- bisphosphoglycerate and ensures the rapid flux of aerobic glycolysis (31). Through regulation of LDHA levels, ErbB signaling can regulate energy substrate production in MOs to maintain axonal conduction under energy stress, and accelerate the glycolytic flux that provides multiple intermediates for biosyntheses (Fig. 6L). Whether LDHA activities in MOs are critical for myelin or axons deserves further investigation. However, a recent report indicates that investigating the roles of LDHA in the OL lineage may be challenging because LDHA expression in adult MOs is difficult to be detected (45). Indeed, in Plp-dnEGFR mice and their littermate controls, LDHA immunoreactivities were found in sparsely distributed MOs (Fig. 5I), suggesting that only MOs in a certain state have detectable LDHA. We were unable to detect LDHA immunoreactivities in individual MOs of Sox10-dnEGFR mice and their littermate controls. It is uncertain whether the mouse line background influences the immunostaining sensitivities of LDHA. More careful investigations are necessary to elaborate the effects of LDHA in OLs.
For the field that debates the roles of ErbB receptors in the CNS myelin, our results demonstrated the inhibition of both EGFR binding to the EGF family ligands and ErbB3/ErbB4 binding to the NRG family ligands in the WM of Sox10-dnEGFR and Plp-dnEGFR mice (Fig. 1 C–I). This indicates that EGFR and ErbB3/ErbB4 are all functionally activated in OLs in vivo. The finding that either EGF- or NRG-family ligand can activate all endogenous ErbB receptors in OPCs or differentiated OLs (Fig. 1 A and B) also suggests that endogenous ErbB signaling is not solely conveyed by EGF-ErbB or NRG-ErbB receptors. The inhibition of in vitro proliferation by dnEGFR in Sox10-dnEGFR OPCs, which mimics early development in vivo, is reminiscent of the reduced oligodendrogenesis phenotype in hypomorphic EGFR mutant mice (wa2) before P15 (18). However, wa2 mice do not exhibit the same OL and myelin deficits at P60 as Sox10-dnEGFR mice, indicating the redundant effects from other ErbB receptors after early development. The opposite OPC proliferation phenomena in Sox10-dnEGFR mice at 2 mo old further suggest that the requirement for ErbB receptors in OL-lineage cells has age-dependent heterogeneity, which deserves further investigation.
The present study using OL stage-specific mouse models makes an advance by revealing that the inhibition of ErbB signaling induces adolescent NFO deficiency, leading to hypomyelination. The phenomenon that ErbB deficiency in OLs causes adult hypomyelination, despite an increase in OL numbers, in Sox10-dnEGFR mice is reminiscent of observations in CNP-DN-ErbB4 mice, which have only NRG-ErbB receptor activities inhibited in OL-lineage cells (46). The hypomyelination phenotype observed in Sox10-dnEGFR mice with Dox induction from P21 aligns with those seen in Plp-creERT;ErbB3flox/flox mice (10), emphasizing a critical period between P21 and P35 for NRG-ErbB regulation of myelin development. The phenotype discrepancies between CNP-DN-ErbB4/Plp-creERT;ErbB3flox/flox and conditional knockout mice of ErbB3, ErbB4, ErbB3/ErbB4, or NRG1 may be explained by the critical role of ErbB3 in myelin formation during P21-P35 (10), while it is dispensable for OL differentiation before P11 (9, 11). Supporting this explanation, we found that ErbB3 mRNA levels are the highest among ErbB receptors in postnatal WM after P10, and reach a plateau within P25-P30 (SI Appendix, Fig. S1 A–C). There are ErbB receptor preferences for each ErbB ligand and agonistic effect specificities for OLs at different differentiation stages, despite the potential for full activation of ErbB receptors in OLs (Figs. 1 A and B, 2I, 3F, and 6E). It warrants further investigation whether the distributions of ligands and receptors determine the vulnerability of ErbB signaling in OLs in different brain regions and at different ages.
By overcoming the challenges of multiple ligands and complicated ErbB receptor compositions, our mouse models exhibited consistent in vivo and in vitro phenotypes, elucidating the effects of endogenous ErbB signaling in the OL lineage. Nevertheless, although we detected pErbB reduction in NFOs acutely isolated from Sox10-dnEGFR mice (Fig. 1E), we cannot exclude the possibility that the pErbB alterations in NFOs from Plp-dnEGFR mice (Fig. 1I) have been lost due to the lengthy dissociation procedures. The development of in situ examination approaches for pErbB will be beneficial to definitively determine the specificities and efficiencies of endogenous ErbB manipulations in each cell in the future.
Materials and Methods
Animals.
Plp-tTA transgenic mice (47) were from the RIKEN BioResource Center (Stock No. RBRC05446). Sox10+/rtTA mice were from Dr. Michael Wegner (University Erlangen-Nurnberg, Germany). Transgenic mice TRE-ErbB2V664E (Stock No. 010577) and TRE-dnEGFR (Stock No. 010575) were from the Jackson Laboratory (23).
Statistical Analyses.
Statistical analyses other than for RNA-seq data were performed using Prism (GraphPad) and presented as mean ± SEM. Unless otherwise indicated, two-tailed unpaired Student’s t test was used for analysis between two groups with one variable, one-way ANOVA test was used for analysis among three or more groups with one variable, and two-way ANOVA test was used to determine difference among groups with two variables. Statistical significance was set at *P < 0.05, **P < 0.01, ***P < 0.001.
A detailed description of Materials and Methods is provided in SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Performances recorded for Sox10-dnEGFR mice and Plp-dnEGFR mice, as well as their controls, in the 4th trial of eight arm radial water maze at the test day.
Acknowledgments
We thank Zhengdong Wei, Xiaoyan Lu, Kaiwei Zhang, Youguang Yang, and Shasha Zhang in Hangzhou Normal University for the assistance in electron microscope image analyses, and Wanhua Shen for the assistance in electrophysiological experiments. This work was supported by the Ministry of Science and Technology of China (2021ZD0201700 to Y.T.), the national natural science foundation of China (32170956, 31371075, and 31871030 to Y.T.), the Start-up Research Fund of Southeast University (RF1028623294 to Y.T.), the key project of Zhejiang provincial natural science foundation of China (2022XHSHH004 to J.-M.J.), and the young scientists project of Zhejiang provincial natural science foundation of China (LQ19H090009 to J.-M.J.).
Author contributions
Y.T. designed research; X.H., Q.Z., T.L., Q.H., H.L., Y.X., X.N., L.H., and H.H. performed research; M.Q., Y.S., and J.-M.J. contributed new reagents/analytic tools; X.H., Q.Z., T.L., Q.H., H.L., Y.X., and Y.T. analyzed data; and Y.T. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission J.R.C. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
RNA-seq data have been deposited in GEO and SRA database (GSE123491) (48). All other data are included in the manuscript and/or supporting information.
Supporting Information
References
- 1.Peters B. D., Karlsgodt K. H., White matter development in the early stages of psychosis. Schizophr. Res. 161, 61–69 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uranova N. A., Vikhreva O. V., Rachmanova V. I., Orlovskaya D. D., Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: A postmortem morphometric study. Schizophr. Res. Treat. 2011, 325789 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly S., et al. , Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry 23, 1261–1269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen N. C., et al. , Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat. Genet. 40, 827–834 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Mei L., Nave K. A., Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 83, 27–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nave K. A., Salzer J. L., Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 16, 492–500 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Winterer G., et al. , Association of 5’ end neuregulin-1 (NRG1) gene variation with subcortical medial frontal microstructure in humans. Neuroimage 40, 712–718 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Zuliani R., et al. , Genetic variants in the ErbB4 gene are associated with white matter integrity. Psychiatry Res. 191, 133–137 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkmann B. G., et al. , Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 59, 581–595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makinodan M., Rosen K. M., Ito S., Corfas G., A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmucker J., et al. , erbB3 is dispensable for oligodendrocyte development in vitro and in vivo. Glia 44, 67–75 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Taveggia C., et al. , Type III neuregulin-1 promotes oligodendrocyte myelination. Glia 56, 284–293 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Li D., Feng G., He L., Case-control study of association between the functional candidate gene ERBB3 and schizophrenia in Caucasian population. World J. Biol. Psychiatry 10, 595–598 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Hanninen K., et al. , Epidermal growth factor a61g polymorphism is associated with the age of onset of schizophrenia in male patients. J. Psychiatr. Res. 41, 8–14 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Anttila S., et al. , Association of EGF polymorphism with schizophrenia in Finnish men. Neuroreport 15, 1215–1218 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Benzel I., et al. , Interactions among genes in the ErbB-Neuregulin signalling network are associated with increased susceptibility to schizophrenia. Behav. Brain Funct. 3, 31 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futamura T., et al. , Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol. Psychiatry 7, 673–682 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Aguirre A., Dupree J. L., Mangin J. M., Gallo V., A functional role for EGFR signaling in myelination and remyelination. Nat. Neurosci. 10, 990–1002 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Norton N., et al. , Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 141B, 96–101 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Czopka T., Ffrench-Constant C., Lyons D. A., Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes E. G., Orthmann-Murphy J. L., Langseth A. J., Bergles D. E., Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 21, 696–706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X., et al. , Sustained ErbB activation causes demyelination and hypomyelination by driving necroptosis of mature oligodendrocytes and apoptosis of oligodendrocyte precursor cells. J. Neurosci. 41, 9872–9890 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., et al. , A role for ErbB signaling in the induction of reactive astrogliosis. Cell Discov. 3, 17044 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young K. M., et al. , Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron 77, 873–885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., et al. , An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao L., et al. , Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 19, 1210–1217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye F., et al. , HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci. 12, 829–838 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saab A. S., et al. , Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trevisiol A., et al. , Monitoring ATP dynamics in electrically active white matter tracts. Elife 6, e24241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheppard S., et al. , Lactate dehydrogenase A-dependent aerobic glycolysis promotes natural killer cell anti-viral and anti-tumor function. Cell Rep. 35, 109210 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunt S. Y., Vander Heiden M. G., Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Funfschilling U., et al. , Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Looser Z. J., et al. , Oligodendrocyte-axon metabolic coupling is mediated by extracellular K(+) and maintains axonal health. Nat. Neurosci. 27, 433–448 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tekkok S. B., Brown A. M., Westenbroek R., Pellerin L., Ransom B. R., Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J. Neurosci. Res. 81, 644–652 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Yang H. W., et al. , Cooperative activation of PI3K by Ras and Rho family small GTPases. Mol. Cell 47, 281–290 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flores A. I., et al. , Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 28, 7174–7183 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii A., Fyffe-Maricich S. L., Furusho M., Miller R. H., Bansal R., ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci. 32, 8855–8864 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kessler D., et al. , Drugging an undruggable pocket on KRAS. Proc. Natl. Acad. Sci. U.S.A. 116, 15823–15829 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu K., et al. , Small molecule KRAS agonist for mutant KRAS cancer therapy. Mol. Cancer 18, 85 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison P. J., Law A. J., Neuregulin 1 and schizophrenia: Genetics, gene expression, and neurobiology. Biol. Psychiatry 60, 132–140 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Pedrosa E., et al. , Analysis of a promoter polymorphism in the SMDF neuregulin 1 isoform in Schizophrenia. Neuropsychobiology 59, 205–212 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kujala P., Portin R., Ruutiainen J., The progress of cognitive decline in multiple sclerosis. A controlled 3-year follow-up. Brain 120, 289–297 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Makinoshima H., et al. , Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. J. Biol. Chem. 289, 20813–20823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang W., et al. , ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 14, 1295–1304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spate E., et al. , Downregulated expression of lactate dehydrogenase in adult oligodendrocytes and its implication for the transfer of glycolysis products to axons. Glia 72, 1374–1391 (2024), 10.1002/glia.24533. [DOI] [PubMed] [Google Scholar]
- 46.Roy K., et al. , Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl. Acad. Sci. U.S.A. 104, 8131–8136 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inamura N., et al. , Gene induction in mature oligodendrocytes with a PLP-tTA mouse line. Genesis 50, 424–428 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Tao Y., Hu X., Data from “Gene expression profiling in the white matter with oligodendroglial stage-specific activation or inhibition of ErbB receptors”. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse123491. Deposited 08 December 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Performances recorded for Sox10-dnEGFR mice and Plp-dnEGFR mice, as well as their controls, in the 4th trial of eight arm radial water maze at the test day.
Data Availability Statement
RNA-seq data have been deposited in GEO and SRA database (GSE123491) (48). All other data are included in the manuscript and/or supporting information.