Abstract
Inherited antithrombin deficiency is an autosomal dominant thrombophilia, resulting from genetic variations in the serpin family C member 1 (SERPINC1) gene. Antithrombin deficiency increases the risk of venous thromboembolism (VTE) compared to the general population. In this report, a novel missense variant of SERPINC1, c.40A>G, p.Arg14Gly that predicts to cause secretion defect of antithrombin, was identified in two related patients: a 65-year-old man with chronic thromboembolic pulmonary hypertension (CTEPH) after acute pulmonary embolism, and his son with early onset of VTE. Treatment with direct oral anticoagulants and catheter interventions led to successful outcomes for both patients. These cases highlight the importance of screening testing for inherited antithrombin deficiency and intrafamilial survey in patients with VTE and CTEPH.
Learning objectives
Inherited antithrombin deficiency is associated with a strong risk of venous thromboembolism (VTE). However, the relationship between inherited antithrombin deficiency and chronic thromboembolic pulmonary hypertension (CTEPH) remains unclear. The influence of antithrombin deficiency on developing CTEPH following acute pulmonary embolism requires further investigation. This report emphasizes the importance of screening for thrombophilic factors in cases of VTE and CTEPH.
Keywords: Balloon pulmonary angioplasty, Catheter-directed thrombolysis, Chronic thromboembolic pulmonary hypertension, Genetic variant, Inherited antithrombin deficiency, Venous thromboembolism
Introduction
Inherited antithrombin deficiency is a genetic disorder caused by variants in the antithrombin gene, SERPINC1. This deficiency can be classified into two subtypes based on plasma antithrombin levels: type I, a quantitative defect with reduced antithrombin activity and antigen levels, and type II, a qualitative defect with normal antigen levels but impaired inhibitory activity [1]. SERPINC1 consists of seven exons and is located at chromosome bands 1q25.1. In Japan, large-scale genetic analyses in patients with antithrombin deficiency have previously been reported [2,3]. Antithrombin deficiency increases the risk of venous thromboembolism (VTE) compared to the general population, type I deficiency having a significantly higher risk of VTE than type II deficiency [4]. In contrast, the relationship between antithrombin deficiency and chronic thromboembolic pulmonary hypertension (CTEPH) is not yet fully understood, including the potential impact on the risk of developing CTEPH following acute pulmonary embolism (PE). This report presents two related cases, a father exhibiting CTEPH after acute PE and a son with early-onset of VTE, both carrying the same novel SERPINC1 missense variant.
Case reports
Case 1 (the proband)
A 65-year-old man presented to a local hospital with persistent dyspnea on exertion for 3 years. The patient was diagnosed with acute PE and proximal deep vein thrombosis (DVT) and had been treated with apixaban (10 mg daily) for 6 months. However, his symptoms had no improvement. Subsequently, he was referred to our hospital. No family history of VTE was reported when he visited our hospital. Laboratory tests revealed normal D-dimer levels (<0.5 μg/mL; normal range: 0–1.0 μg/mL), decreased levels of both antithrombin activity (62 %; normal range: 80–120 %) and antigen (14.7 mg/dL; normal range: 23.6–33.5 mg/dL), consistent with type I antithrombin deficiency. Contrast-enhanced computed tomography (CT) revealed webs and bands (Fig. 1A). Pulmonary perfusion scintigraphy detected multiple perfusion defects (Fig. 1B). The results of right heart catheterization (RHC) were as follows: mean pulmonary artery pressure, 34 mmHg; pulmonary artery wedge pressure, 10 mmHg; pulmonary vascular resistance, 5.2 units. The results of RHC and pulmonary angiography confirmed the diagnosis of CTEPH. The patient underwent five sessions of balloon pulmonary angioplasty. Three months after the final session, the patient's dyspnea improved, and RHC showed improvement of PH (mean pulmonary artery pressure, 19 mmHg; pulmonary vascular resistance, 2.1 units). The patient had no recurrence of thrombosis with continuation of apixaban for one year. Clinical characteristics of case 1 are summarized in Table 1.
Fig. 1.
The findings of contrast-enhanced computed tomography (CT), perfusion scintigraphy, and venography. (A) Contrast-enhanced CT showing webs and bands in the right pulmonary artery (yellow arrows). (B) Pulmonary perfusion scintigraphy identified multiple perfusion defects. (C) Contrast-enhanced CT findings: the upper CT image identifies thrombi in both pulmonary arteries (yellow arrows). The middle CT image reveals thrombi in the left-sided inferior vena cava (red arrow). The lower CT image shows thrombi in the bilateral iliac veins. (D) Venography before intervention revealed the bilateral vein occlusion. (E) Venography after catheter intervention illustrates recanalization of the bilateral veins.
Table 1.
Clinical characteristics of case 1 and case 2.
| Case 1 | Case 2 | |
|---|---|---|
| Age of admission (years) | 65 | 18 |
| Sex | Male | Male |
| Diagnosis | PE, DVT, CTEPH | PE, DVT |
| Provoked VTE risk | No | No |
| Thrombophilia testing | ||
| Antithrombin activity (%) | 62.0 | 57.0 |
| Antithrombin antigen (mg/dL) | 14.7 | 16.2 |
| Protein S activity (%) | 123 | 84 |
| Free protein S antigen (%) | 125 | 72 |
| Protein C activity (%) | 148 | 96 |
| Protein C antigen (%) | 128 | 114 |
| LAC dRVVT | 1.77 | 2.42 |
| aCL-IgG (units/mL) | 7 | <4 |
| Anti-β2GPI (units/mL) | <1.3 | <1.3 |
| Anticoagulant therapy | ||
| Parenteral | No | No |
| Oral | Apixaban | Rivaroxaban |
| Continuation | Yes | Yes |
| Additional therapy | BPA | IVCF/CDT |
| Recurrence | No | No |
| Genetic variant | SERPINC1, c.40A>G | SERPINC1, c.40A>G |
| Amino acid substitution | p.Arg14Gly | p.Arg14Gly |
Normal range of antithrombin activity: 80–120 %. Normal range of antithrombin antigen: 23.6–33.5 mg/dL.
PE, pulmonary embolism; DVT, deep vein thrombosis; CTEPH, chronic thromboembolic pulmonary hypertension; VTE, venous thromboembolism; LAC dRVVT, lupus anticoagulant with dilute Russell's viper venom time; aCL, anti-cardiolipin; β2GPI, β2-glycoprotein I; BPA, balloon pulmonary angioplasty; IVCF, inferior vena cava filter; CDT, catheter-directed thrombolysis.
Case 2 (the proband's son)
An 18-year-old man, the proband's son, presented with leg pain and swelling and was diagnosed with VTE at a nearby hospital. Despite rivaroxaban treatment, his symptoms did not improve, and he was transferred to our hospital for further management. The patient had no significant medical history, but his father had been treated for CTEPH following acute PE and DVT (case 1). Laboratory data revealed elevated levels of D-dimer (18.9 μg/mL) and decreased levels of both antithrombin activity (57 %) and antigen (16.2 mg/dL), indicating type I antithrombin deficiency. Contrast-enhanced CT demonstrated fresh thrombi in the left and right pulmonary arteries and from the medial portion of the left-sided inferior vena cava (IVC) to the right distal vein and left femoral vein (Fig. 1-C). Thus, the patient was diagnosed with VTE caused by type I antithrombin deficiency. Upon admission, the patient was orally administered with rivaroxaban and underwent IVC filter placement and catheter-directed thrombolysis (CDT). Rivaroxaban was also continued during CDT. Venography before intervention revealed the bilateral vein occlusion (Fig. 1D). CDT, catheter aspiration, and balloon angioplasty of both lower veins were also performed. Venography confirmed bilateral vein recanalization (Fig. 1E), and the IVC filter was removed. The patient's symptoms improved, and he had not experienced a recurrence of VTE with continuation of rivaroxaban for 1 year. Clinical characteristics of case 2 are summarized in Table 1.
Genetic testing and family pedigree
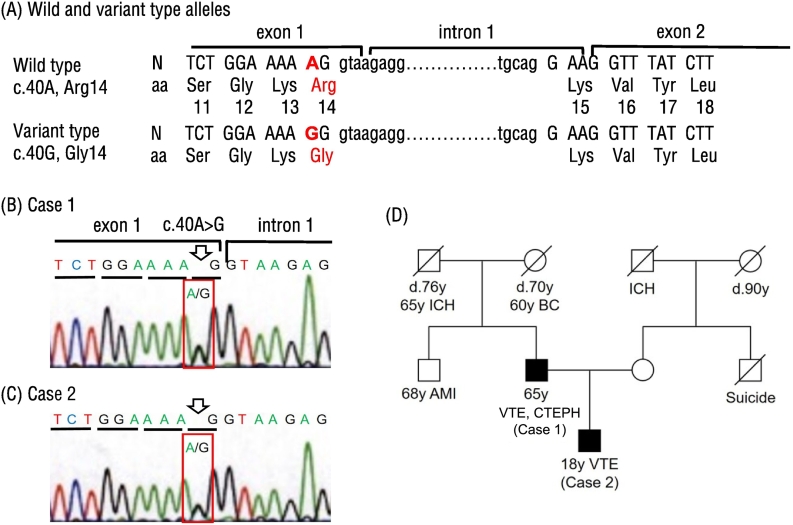
Informed consent was obtained from both patients. We only sequenced SERPINC1 and did not perform genetic analysis of other genes. Genetic analysis was conducted using Sanger sequencing, which identified a novel c.40A>G variant with a replacement of AGG (arginine) with GGG (glycine) at the amino acid position 14, p.Arg14Gly, in the signal sequence of pre-antithrombin in both patients (Fig. 2A-C). We did not identify any other genetic variants in SERPINC1. No other family member had a history of VTE and CTEPH (Fig. 2D). The mother in case 2 demonstrated a normal antithrombin activity level of 108 %.
Fig. 2.
Identification of c.40A>G variant causing p.Arg14Gly replacement in two cases. (A) Nucleotide sequences of wild and variant alleles of SERPINC1. Deduced amino acid sequences are also given. Nucleotide variant and amino acid replacement identified in this study are shown in red. (B, C) Nucleotide sequence of the variant allele in cases 1 and 2. A heterozygous c.40A>G variant leading to a p.Arg14Gly replacement in the pre-antithrombin signal sequence was identified in each case. Open arrows indicate the c.40A>G sites in red brackets. (D) In the family pedigree, two affected cases are denoted by filled symbols.
N, nucleotide sequence; aa, deduced amino acid sequence; Arg, arginine; Gly, glycine; Leu, leucine; Lys, lysine; Ser, serine; Tyr, tyrosine; Val, valine; AMI, acute myocardial infarction; BC, breast cancer; CTEPH, chronic thromboembolic pulmonary hypertension; ICH, intracerebral hemorrhage; VTE, venous thromboembolism.
Pre-antithrombin has positively charged amino acids Lys-Arg-Lys at positions 13–15 in the signal sequence consisting of 32 amino acids (Fig. 2A). The Arg14 residue in the signal sequence is highly conserved across various mammalian species, indicating its crucial function in antithrombin secretion in mammals. Both patients carried heterozygous p.Arg14Gly variant (Fig. 2B, C) and exhibited decreased activity and antigen levels of plasma antithrombin (Table 1). Therefore, pre-antithrombin variant with Gly14 in the signal sequence is presumed to cause severe secretion defects.
Another aspect for antithrombin deficiency in these patients is that the c.40A>G heterozygous variant is located at the adjacent consensus splice donor site, a G/guaa sequence (/; splice junction) [5], of SERPINC1 (Fig. 2A-C). We predicted the splicing events in the pre-mRNA sequence of pre-antithrombin using Splice AI, a computational tool for predicting splicing [5], and found that c.40A>G is predicted to show a donor loss of 0.13. This suggests that c.40A>G may not seriously affect the correct splicing event of pre-mRNA sequence of pre-antithrombin.
Discussion
Inherited antithrombin deficiency, a rare autosomal dominant genetic disorder, significantly increases the risk of VTE [4]. In case 2, the patient developed VTE at a relatively young age of 18 years. However, the patient's father (Case 1) had been diagnosed with VTE and CTEPH at the older age of 65 years. Therefore, it remains unclear whether this novel SERPINC1 variant influenced the onset age.
CTEPH is commonly considered a complication of acute PE, given the high proportion of CTEPH patients having a documented history of acute PE (74.8 %) in the large European database [6]. On the other hand, in Japanese patients, only 37.2 % of CTEPH patients have a previous history of acute PE, and there are other notable differences, including a higher proportion of females in CTEPH patients (Japanese registry, 72.9 % vs. international registry, 49.9 %) and fewer coagulopathies (Japanese registry, 11.7 % vs. international registry, 31.9 %) among Japanese patients compared with international patients [7]. These differences suggest the possibility of a distinct phenotype and genotype of CTEPH in Japanese and Europeans. The relationship between inherited antithrombin deficiency and CTEPH remains to be fully understood. Antithrombin deficiency was identified in 1.1 % of CTEPH patients reported from China [8], and 0.7 % from Europe [6]. A European database study confirmed previous VTE and recurrent VTE as risk factors for developing CTEPH [9]. Additionally, the higher incidence of first and recurrent VTE is associated with inherited antithrombin deficiency. Therefore, inherited antithrombin deficiency might be a risk factor for developing CTEPH, but there is currently insufficient evidence to establish a direct association between inherited antithrombin deficiency and CTEPH, due to the low prevalence.
There are two possible effects of the c.40A>G, p.Arg14Gly variant on antithrombin, one is the secretion defect and other is the splicing abnormality. The missense variant p.Arg14Gly is located in the signal sequence (32 amino acids) of pre-antithrombin in both patients. The signal sequences are short peptides located generally at the N-terminal region of secretory proteins and target the precursor protein to translocation sites on the appropriate membrane such as endoplasmic reticulum [10]. The canonical signal sequence is characterized by a short positively charged stretch of amino acids close to the N-terminus and a central hydrophobic α-helical region, followed by a cleavage site by a signal peptidase at the C-terminal end. The positively charged amino acids may help membrane translocation of the polypeptide [10]. The missense variant p.Arg14Gly causes a replacement of a positively charged amino acid Arg with a neutral amino acid Gly. Arg14 in the signal sequence of pre-antithrombin is highly conserved in mammals. Therefore, it is assumed that the p.Arg14Gly variant affects the normal secretion of antithrombin.
As for c.40A>G, it is located at the consensus splice donor site. However, the splice prediction showed a minor effect (only 13% loss) of c.40A>G, suggesting that c.40A>G may not seriously affect the correct splicing event of pre-mRNA sequence of pre-antithrombin. The pre-antithrombin p.Arg14Gly variant causes possible severe antithrombin deficiency and c.40A>G makes a small contribution to the likely splicing defect. The reduction in plasma antithrombin activity and antigen levels due to dual effects may contribute to the increased risk of VTE in both patients.
This gene variant may explain the development of CTEPH after acute PE in case 1 and the early onset of VTE in case 2. Of course, it remains unclear whether the novel SERPINC1 variant influenced the phenotype of these two patients.
In conclusion, we reported a familial onset of inherited antithrombin deficiency caused by the novel genetic variant. These cases highlight the importance of screening testing for inherited antithrombin deficiency and intrafamilial survey in patients with VTE and CTEPH.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
Acknowledgment
None.
Consent statement
Written informed consents were obtained from both patients for the publication of this case report.
References
- 1.Pabinger I., Thaler J. How I treat patients with hereditary antithrombin deficiency. Blood. 2019;134:2346–2353. doi: 10.1182/blood.2019002927. [DOI] [PubMed] [Google Scholar]
- 2.Miyata T., Sato Y., Ishikawa J., Okada H., Takeshita S., Sakata T., Kokame K., Kimura R., Honda S., Kawasaki T., Suehisa E., Tsuji H., Madoiwa S., Sakata Y., Kojima T., et al. Prevalence of genetic mutations in protein S, protein C and antithrombin genes in Japanese patients with deep vein thrombosis. Thromb Res. 2009;124:14–18. doi: 10.1016/j.thromres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Sekiya A., Taniguchi F., Yamaguchi D., Kamijima S., Kaneko S., Katsu S., Hanamura M., Takata M., Nakano H., Asakura H., Ohtake S., Morishita E. Causative genetic mutations for antithrombin deficiency and their clinical background among Japanese patients. Int J Hematol. 2017;105:287–294. doi: 10.1007/s12185-016-2142-8. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuguro M., Sakata T., Okamoto A., Kameda S., Kokubo Y., Tsutsumi Y., Sano M., Miyata T. Usefulness of antithrombin deficiency phenotypes for risk assessment of venous thromboembolism: type I deficiency as a strong risk factor for venous thromboembolism. Int J Hematol. 2010;92:468–473. doi: 10.1007/s12185-010-0687-5. [DOI] [PubMed] [Google Scholar]
- 5.Jaganathan K., Kyriazopoulou Panagiotopoulou S., McRae J.F., Darbandi S.F., Knowles D., Li Y.I., Kosmicki J.A., Arbelaez J., Cui W., Schwartz G.B., Chow E.D., Kanterakis E., Gao H., Kia A., Batzoglou S., et al. Predicting splicing from primary sequence with deep learning. Cell. 2019;176(535–48) doi: 10.1016/j.cell.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Pepke-Zaba J., Delcroix M., Lang I., Mayer E., Jansa P., Ambroz D., Treacy C., D’Armini A.M., Morsolini M., Snijder R., Bresser P., Torbicki A., Kristensen B., Lewczuk J., Simkova I., et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe N., Sugiura T., Tatsumi K. Recent progress in the diagnosis and management of chronic thromboembolic pulmonary hypertension. Respir Investig. 2013;51:134–146. doi: 10.1016/j.resinv.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Lian T.Y., Liu J.Z., Guo F., Zhou Y.P., Wu T., Wang H., Li J.Y., Yan X.X., Peng F.H., Sun K., Xu X.Q., Han Z.Y., Jiang X., Wang D.L., Miao Q., et al. Prevalence, genetic background, and clinical phenotype of congenital thrombophilia in chronic thromboembolic pulmonary hypertension. JACC Asia. 2022;2:247–255. doi: 10.1016/j.jacasi.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonderman D., Wilkens H., Wakounig S., Schafers H.J., Jansa P., Lindner J., Simkova I., Martischnig A.M., Dudczak J., Sadushi R., Skoro-Sajer N., Klepetko W., Lang I.M. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33:325–331. doi: 10.1183/09031936.00087608. [DOI] [PubMed] [Google Scholar]
- 10.Owji H., Nezafat N., Negahdaripour M., Hajiebrahimi A., Ghasemi Y. A comprehensive review of signal peptides: structure, roles, and applications. Eur J Cell Biol. 2018;97:422–441. doi: 10.1016/j.ejcb.2018.06.003. [DOI] [PubMed] [Google Scholar]