Abstract
Objective:
To develop a clinical classification system for age-related macular degeneration (AMD).
Design:
Evidence-based investigation, using a modified Delphi process.
Participants:
Twenty-six AMD experts, 1 neuro-ophthalmologist, 2 committee chairmen, and 1 methodologist.
Methods:
Each committee member completed an online assessment of statements summarizing current AMD classification criteria, indicating agreement or disagreement with each statement on a 9-step scale. The group met, reviewed the survey results, discussed the important components of a clinical classification system, and defined new data analyses needed to refine a classification system. After the meeting, additional data analyses from large studies were provided to the committee to provide risk estimates related to the presence of various AMD lesions.
Main Outcome Measures:
Delphi review of the 9-item set of statements resulting from the meeting.
Results:
Consensus was achieved in generating a basic clinical classification system based on fundus lesions assessed within 2 disc diameters of the fovea in persons older than 55 years. The committee agreed that a single term, age-related macular degeneration, should be used for the disease. Persons with no visible drusen or pigmentary abnormalities should be considered to have no signs of AMD. Persons with small drusen (<63 μm), also termed drupelets, should be considered to have normal aging changes with no clinically relevant increased risk of late AMD developing. Persons with medium drusen (≥63–<125 μm), but without pigmentary abnormalities thought to be related to AMD, should be considered to have early AMD. Persons with large drusen or with pigmentary abnormalities associated with at least medium drusen should be considered to have intermediate AMD. Persons with lesions associated with neovascular AMD or geographic atrophy should be considered to have late AMD. Five-year risks of progressing to late AMD are estimated to increase approximately 100 fold, ranging from a 0.5% 5-year risk for normal aging changes to a 50% risk for the highest intermediate AMD risk group.
Conclusions:
The proposed basic clinical classification scale seems to be of value in predicting the risk of late AMD. Incorporating consistent nomenclature into the practice patterns of all eye care providers may improve communication and patient care.
Age-related macular degeneration (AMD) is the leading cause of irreversible central vision loss in developed countries. Prevalence data suggest that AMD will affect more than 3 million people in the United States by 2020.1 With the aging of populations, not only in the United States but also globally, AMD will become an increasingly prevalent and important condition worldwide. There have been rapid developments of therapies that today can halt or even reverse aspects of vision loss resulting from AMD among those most severely affected, and there is promise of future therapies that may prevent vision loss. Hence, having a common framework for describing the clinical phenotype of AMD is essential to facilitate efficient evaluation of therapeutic approaches and to improve communication among eye care providers and their patients. Indeed, with the high costs of such therapies and increasing calls for comparison of cost effectiveness, using a standard classification system for AMD will facilitate research in this field.
Currently, several AMD classification schemes, grading systems, and severity scales have been developed in an effort to provide standards to assist clinicians and researchers in the diagnosis and management of this important disorder.2–10 Most of these have been based on standardized grading of color fundus photographs and some have been considered to be potentially useful for clinical work. There is at present no universally accepted precise definition, including both initial diagnosis and staging, of the AMD phenotype for either clinical or research purposes. There is not even consensus on basic terminology, with some groups using AMD and others using age-related maculopathy or ARM or ARMD. Furthermore, terms such as early and intermediate have different meanings in various classification systems. Finally, there may be a variety of entities worldwide that are termed AMD, but that have differing progression characteristics associated with dissimilar causes and risk factors (genetic and phenotypic). As such, the AMD classification system proposed in this document focuses on the clinical phenotype associated with the development of large drusen and pigmentary abnormalities leading to neovascular AMD, geographic atrophy (GA), or both. It is hoped that the consensus recommendations from this committee will result in a simple, unified classification scheme that can be used worldwide.
Current Classification System Issues
The purpose of this report is to describe the modified Delphi process used to arrive at common terminology for a clinical classification system for AMD.11,12 This process is intended as a consensus-establishing technique that combines the scientific literature with expert opinion.13,14
Early stages of AMD usually are asymptomatic and have been characterized, across various classification systems, by the presence of drusen and pigmentary alterations within 2 disc diameters of the fovea. Previous classifications2–5 of AMD have included descriptions of some or all of the following: (1) drusen size (e.g., large vs. small), character (e.g., soft vs. hard), location, number, and area; (2) hyperpigmentation size, location, and area; and (3) hypopigmentation size, location, and area.
Multiple studies of white populations, mostly of European origin,4–9 have identified large soft or indistinct drusen, or both, and pigmentary abnormalities as being strongly associated with the subsequent development of late AMD (generally characterized as either neovascular AMD, GA, or both). Although it generally has been recognized that large drusen and pigmentary abnormalities provide evidence of various early stages of AMD, and although several schemes have been validated as useful in classifying AMD and its evolution, there remains a lack of consensus on the topic. Furthermore, most ophthalmologists do not currently seem to use a specific standardized clinical disease severity scale in their daily clinical practice.
Progression of AMD to GA, neovascular AMD, or detachments of the retinal pigment epithelium (RPE) may be associated with loss of visual acuity. These have been categorized in several ways, including (1) serous, drusenoid, fibrovascular, and hemorrhagic RPE detachments; (2) sub-RPE, subretinal, and intraretinal neovascularization; and (3) foveal-sparing and foveal-involving GA. The classifications of the phenotypes of these late changes in AMD are agreed on more widely than those regarding earlier stages of AMD. However, identifying patients at increased risk of these late AMD changes developing remains a most important goal, and it is therefore important to develop an evidence-based classification system for earlier forms of AMD. Establishment of consensus, particularly regarding the features that constitute early AMD, would provide a common nomenclature for comprehensive ophthalmologists, retinal experts, and the scientific community. Having a classification system that segregates patients based on probabilities of actual or potential loss of vision will become particularly important as additional forms of therapy for early stages of the disease are discovered. Specific risk groups can be chosen for studies and appropriate sample sizes can be developed. However, it is acknowledged that classification based on color photographs or biomicroscopy alone ignores changes relevant to the disorder, such as accumulation of autofluorescent material (lipofuscin) in the RPE, the development of reticular pseudodrusen, or the loss of rod photo-receptor cells. However, the classification system presented in this report provides guidance for broad clinical phenotypes.
As currently used, the term AMD is likely to have different causes leading to a final common pathway. More detailed phenotype information will be necessary for most research purposes. Because of this, as noted above, this discussion is limited to the phenotype of AMD that is widely recognized as evolving from small to large drusen with subsequent pigmentary abnormalities and eventual development of late AMD. As a first step, phenotype characteristics also are limited to those that can be identified by common ophthalmology office equipment, including an ophthalmoscope and a slit lamp with accessory lenses, to enhance its widespread applicability around the world. Although this proposed classification system is intended for clinical phenotyping, more detailed classification schemes based on new imaging technologies, genetic testing, and visual function evaluation are important to refine and expand the phenotypes of both early and late stages of AMD, and there are efforts underway to validate their usage in a more sophisticated classification system.10
Materials and Methods
The first steps in this process were to assemble a working group made up of 7 experts who were attending the January 2011 Arnold and Mabel Beckman Initiative for Macular Research conference to discuss how the process might move forward and to make specific recommendations regarding the next appropriate steps. These included a review of prior AMD classifications, a selection of additional experts in the field to establish a formal AMD classification committee, an agreement to use a modified Delphi process to learn if a consensus could be established, and finally, a plan to consider 2 separate classification schemes: a simplified (or basic) clinical system, in which only clinical examination equipment is necessary, and a scientific system that uses a variety of potential imaging and other approaches to specify unique phenotypes.
The principles of a basic clinical classification system, which is the focus of this report, include the following characteristics: (1) clinically usable in the vast majority of settings, (2) consistent with skill sets of most eye care providers, (3) requires only routine office examination room equipment (ophthalmoscope and slit lamp), (4) allows segregation of patient prognoses with and without therapies, (5) based on the best available current scientific evidence, (6) has the minimum number of stages required to segregate clinically important risk groups, (7) can be linked easily to more detailed scientific schemes that include a variety of imaging and function methods as well as genotypes, and (8) able to be upgradable and remain a work in progress. The relative importance of these characteristics is a matter of opinion, and the use of a Delphi type process of an expert group to develop consensus seemed appropriate. The details of this Delphi process are included in Appendix 2 (available at http://aaojournal.org).
After the 2011 Beckman conference, an AMD classification committee was established. Selection of the committee members was based on demonstrated expertise in the field, known diverse opinions, clinical and research interests, and geographic variation. The committee ultimately consisted of 26 international experts in AMD, 1 neuro-ophthalmologist, 2 nonvoting chairmen, and 1 nonvoting methodologist. In addition, a science writer was included to document the proceedings and also a project manager to assure efficient functioning of the group. At the initial meeting of the entire committee, in August 2011, selected literature, images, and current classification schemes were reviewed. After the August meeting, the co-chairmen reviewed the impressions and comments voiced by committee. This led to additional analysis of the Age-Related Eye Disease Study (AREDS) data in an effort to provide more clarity regarding the role of drusen and pigmentary abnormalities as risk factors for progression to late AMD. These additional analyses from AREDS subsequently were distributed to all committee members, and a second survey instrument consisting of 9 statements was composed by the co-chairmen and methodologist (see Appendix 3, available at http://aaojournal.org) to focus on clinical staging based on consolidating results of the initial survey and comments and insights made by panel members at the meeting. The results of this Delphi process are presented herein and constituted the primary topic for the second committee meeting held in January 2012.
Results
In the original survey, a score of 7 through 9 indicating agreement for inclusion for a clinical staging system was achieved for 22 (28%) of 79 of statements, although consensus was noted in only 1 of these cases, with 21 being rated as equivocal and 0 rated as nonconsensus. Four statements were rated as inappropriate (median, 1–3) with consensus in 1 and equivocal in the 3 other statements. In the remaining 53 instances, agreement for inclusion was rated as uncertain (median, 4–6) with 9 disagreements, with the remaining 44 being equivocal. After a discussion of the committee member’s interpretations and ratings, along with a review of several additional analyses from the AREDS database, it was agreed that a basic clinical system should provide criteria to distinguish a normal macula from so-called normal aging changes and the latter from early AMD. This helped to organize and consolidate the original statements into the 9 rating statements for assessment in the second round of ratings (Appendix 3, available at http://aaojournal.org).
The public health impact of applying the term drusen to describe everything from minute to large drusen and lesions with very different characteristics and different relevance to risk of disease progression was discussed. There was agreement that this could be considered confusing for both patients and professionals, because the risk of progression to more advanced levels of AMD from small drusen is so low that they may be considered normal aging changes. This is in contrast to drusen that are larger (>63 μm or especially >125 μm), which can be demonstrated to have a definite, clinically important increase in the risk of progressing to late AMD.3–9 Using a different name for small drusen would solve this problem. One suggestion was to use the term drupelet (the small units of aggregate fruit found in raspberries or blackberries). This term is similar to drusen, but also would discriminate these very small deposits from the more easily identified medium or large drusen that are associated with clinical progression of disease (Fig 1). Although separating these small and larger drusen in name was appealing, some of the committee believed that it was unlikely that any group could institute such a widespread nomenclature change at this point. For other committee members, having a word other than drusen to identify these small lesions seemed appropriate because of the very different risk for progression to advanced AMD that has been demonstrated for larger drusen in the analyses performed for this article. The committee was in agreement that the terms wet and dry AMD were confusing. It is especially problematic that dry can have meanings extending from simple drusen to GA or even old disciform scars. Although the committee preferred to differentiate the disease states by using terms such as early or intermediate AMD (excluding small drusen), neovascular AMD, or GA, there was concern that the terms wet and dry may be so imbued in the vernacular that changing them at this time may be difficult. However, a step in the right direction would be to limit the term dry to GA and not refer to earlier stages of AMD as dry. This would provide lay terms for the 2 types of advanced AMD, dry for GA and wet for neovascular AMD and would eliminate confusion with dry being associated with earlier types of AMD. (Note: The historic measurements from film photographs using circle templates have been preserved in this manuscript to avoid confusion with earlier studies, although there has been increasing recognition that the size of the standard disc diameter, rather than the assumed 1500 μm,10 is actually closer to 1800 μm based on updated measurements.15–19 Using the current scaling standards, the size of the 63-μm circle traditionally used at reading centers is better estimated as 75 μm, whereas that of the 125-μm circle is 150 μm.) The committee preferred adoption of the most widely used term, AMD, as opposed to ARM or ARMD, and expressed a strong opinion that only 1 term (AMD) should be used.
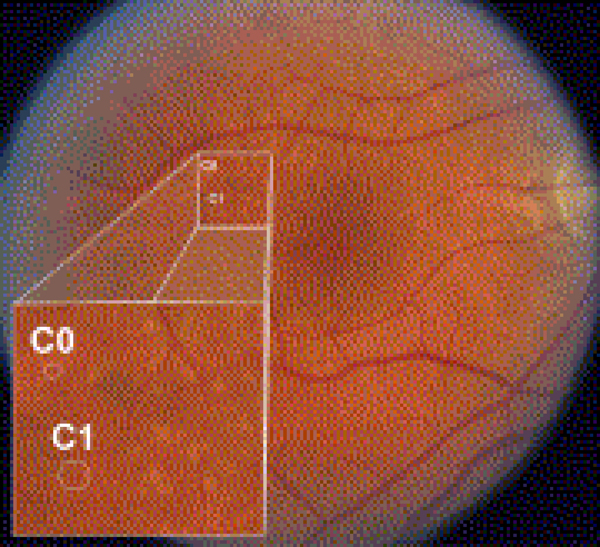
Figure 1.
In an eye with multiple drusen variants, the Age-Related Eye Disease Study drusen grading circles C0 (63-μm diameter) and C1 (125-μm diameter) are superimposed for size comparison. Small drusen are smaller than the C0 circle (drupelets). Lesions larger than C0 but less than C1 are considered medium drusen, and lesions larger than C1 are large drusen. Within the inset, drupelets and medium drusen are seen. Faint reticular drusen also may be seen in the superior macular region.
An analysis of the second ratings survey revealed that 8 of the 9 statements were rated with a median of 9, and the remaining item was rated 8.5, indicating agreement regarding appropriateness for use for clinical classification for all statements (see Appendix 3, available at http://aaojournal.org). Consensus was observed in 8 of 9 instances, with 1 statement being rated equivocal. There was no disagreement. Statement 4 (see Appendix 3, available at http://aaojournal.org) was the only instance of equivocal consensus associated with agreement on appropriateness. The issue posed by this statement was the relative importance of hyperpigmentary or hypopigmentary abnormalities in the absence of drusen as a significant risk factor for AMD. Careful review of Table 1, summarized from previously published AREDS data, reveals that if there were either hypopigmentary or hyperpigmentary abnormalities without any medium drusen (>63 μm but ≤125 μm), the risk of progressing to advanced AMD was very low, indicating that pigment changes unassociated with at least medium drusen are not an important risk factor for AMD progression (only 1 of 72 patients went on to be diagnosed with late AMD in 5 years, and that patient had pigmentary abnormalities in both eyes). In contrast, if medium drusen were present in one or both eyes, the additional presence of pigmentary abnormalities increased the 5-year risk of late AMD substantially (ranging from 5% to 20%, depending on the combinations of eyes with medium drusen and with pigmentary abnormalities). For this reason, we suggest the following definition: AMD pigmentary abnormalities are defined as hyperpigmentation or hypopigmentation present within 2 disc diameters of the center of the macula in eyes with drusen 63 μm or more in diameter and without known retinal disease entities or other reasons for such abnormalities. Requiring the presence of drusen 63 μm or more in diameter when attributing pigment abnormalities to AMD provided a solution that everyone could agree with when the committee met in January 2012.
Table 1.
Five-Year Rate of Developing Advanced AMD in AREDS Participants by Drusen Size and Degree of Pigmentary Abnormalities
| Drusen Size | Pigmentary Abnormalities None | Pigmentary Abnormalities One Eye | Pigmentary Abnormalities Both Eyes |
|---|---|---|---|
| None or small drusen | 0.4% (4/1017) | 0% (0/64) | 12.5% (1/8) |
| Intermediate drusen one eye no large drusen | 0.5% (2/449) | 5.0% (5/101) | 12.9% (4/31) |
| Intermediate drusen both eyes no large drusen | 2.1% (4/187) | 12% (6/50) | 20% (7/35) |
| Large drusen one eye | 3.9% (11/283) | 10.1% (17/168) | 25.6% (30/117) |
| Large drusen both eyes | 13% (27/208) | 27.3% (48/176) | 47.3% (150/317) |
AMD = age-related macular degeneration; AREDS = Age-Related Eye Disease Study.
The outcomes of the second survey were highly consistent with the AREDS simplified severity scale,5 in which risk categories for late AMD can be determined by clinical examination or evaluation of fundus photographs. The AREDS simplified system identifies 2 abnormalities found within 2 disc diameters from the fovea that determine a risk score for the patient (for clinical use, the committee believed this also could be considered the area within the arcades, excluding the peripapillary area): (1) 1 or more large drusen (≥125 μm in the smallest diameter), a distance approximating the width of a major branch retinal vein crossing the optic disc margin (Fig 2); and (2) any definite hyperpigmentary or hypopigmentary abnormalities associated with at least some drusen 63 μm or more in diameter, but not associated with known retinal disease entities or other reasons for such abnormalities.
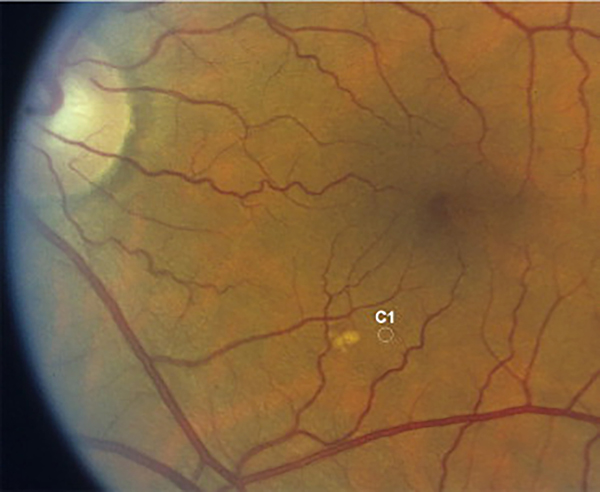
Figure 2.
The largest druse in this eye is slightly larger than the C1 (125 μm) circle and is classified as a large druse. Adjacent intermediate-sized drusen can be seen.
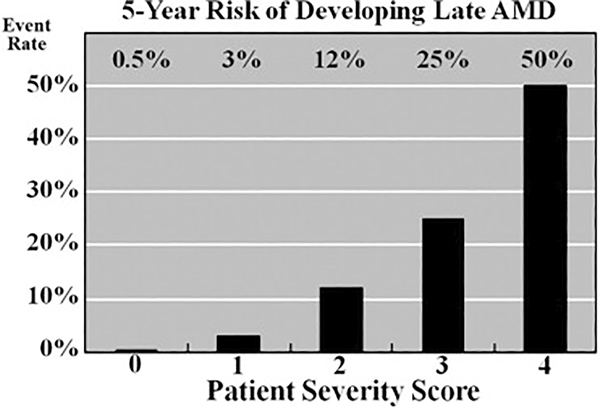
As seen in Table 1, patients with increasing combinations of these risk factors are at increasing risk of late AMD developing. By assigning a risk score of 1 for each risk factor in each eye, one can achieve a total maximum risk score of 2 per eye or 4 per patient. Thus, a 5-step severity scale of 0 (no risk factors) to 4 (both risk factors in both eyes) was established (Fig 3).4 The 5-year risk of late AMD developing increases by a factor of 100 between a score of 0 and a score of 4. There is low risk of 5-year progression to late AMD for scores of 0 and 1 and a doubling of risk between a score of 2 and 3 and a score of 3 and 4 (12%, 25%, and 50%, respectively). These risk estimates are averages for the entire population studied. The risk estimate would be modified up or down based on the presence or absence of other known demographic, environmental, or genetic risk factors.15,16 For example the estimated 5-year risk for a 75-year-old with a simple score of 4 would be lower than 50% for nonsmokers (38%) and higher for smokers (58%). The risk is especially sensitive to age. The effects of these factors on the simple score 5-year risk estimate can be seen using the recently published AMD risk calculator (available at: http://www.ohsucasey.com/amdcalculator; accessed June 18, 2012).20
Figure 3.
Graph showing age-related eye disease clinical severity scale for age-related macular degeneration (AMD), demonstrating the 5-year risk of developing advanced AMD for various risk groups. AREDS = Age-Related Eye Disease Study.
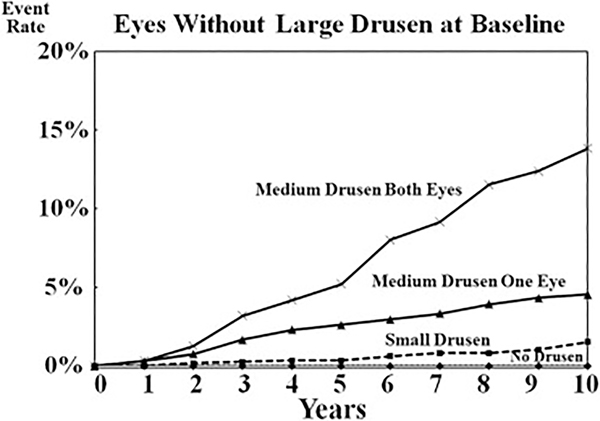
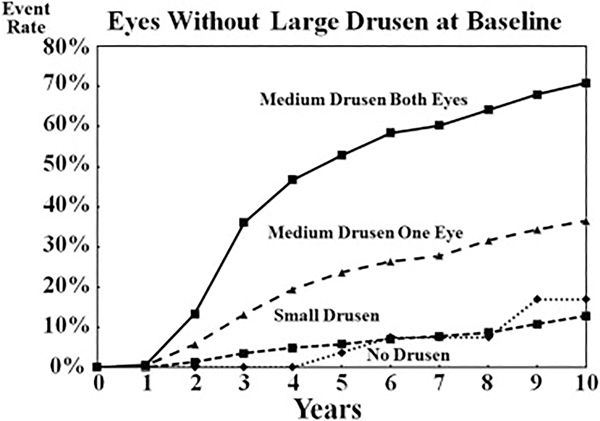
In addition to identifying large drusen and pigmentary abnormalities as risk factors, other special circumstances were considered and new analyses were performed before the second survey. Using the AREDS database, these analyses assessed the risk of progression to late AMD for persons without large drusen or pigmentary abnormalities. Results of these analyses are shown in Figures 4 and 5. For eyes without drusen and for eyes with only small drusen (drupelets), the risk for progression to late AMD, or even of large drusen developing, in 10 years is low. These data are consistent with the decision not to consider small drusen (drupelets) as part of the classification of AMD, but rather as a consequence of normal aging.
Figure 4.
Graph showing the 10-year risk of developing advanced age-related macular degeneration (AMD) in eyes without large drusen at baseline for various risk groups.
Figure 5.
Graph showing the 10-year risk of developing large drusen in eyes without large drusen at baseline for various risk groups.
Analyses of eyes with medium drusen (63–125 μm) provided evidence that as soon as drusen of this size were present, there was evidence of increasing risk of AMD progression. As can be seen in Figure 4, the presence of these medium drusen in one or both eyes was associated with an increased risk of progression to development of late AMD. When these medium drusen were present in both eyes, the risk of progression to late AMD was similar to the risk in persons with one simple-scale risk factor, so assigning a risk score of 0.5 to each eye with medium drusen seems appropriate.
Medium drusen also seem to be in the risk pathway for the development of large drusen, as seen in Figure 5. The 5-year risk of progression to large drusen is more than 50% for persons with medium drusen in both eyes and approximately 25% for those with medium drusen in one eye. By contrast, the 5-year risk for the development of large drusen in eyes with small or no drusen is less than 5%.
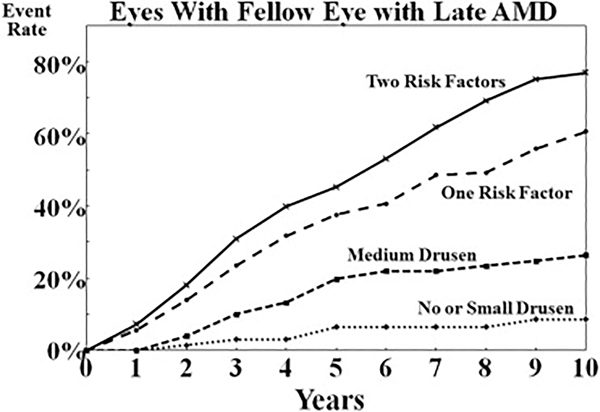
The risk of developing late AMD in the fellow eye of persons who already have late AMD in one eye can be estimated by giving the eye with late AMD a score of 2. Adding that to the risk score for the fellow eye provides a reasonable estimate for the risk of developing late AMD in that fellow eye (Fig 6). Persons with no drusen or only small drusen (drupelets) remain at relatively low risk. The risk increases with the presence of medium drusen, and the 5-year risk reaches roughly 50% for eyes with both large drusen and pigment abnormalities.
Figure 6.
Graph showing the 10-year risk of developing advanced age-related macular degeneration (AMD) in an eye with a fellow eye with late AMD for various risk groups.
Based on the review of the second survey and subsequent discussions, a proposed basic clinical classification 5-point scale was established by the committee (Table 2). The estimates of risk of progression for an individual eye was affected significantly by the status of the fellow eye, and the committee preferred this person-based risk assessment compared with a single eye-based assessment.
Table 2.
Proposed AMD Clinical Classification
| Classification of AMD | Definition (lesions assessed within 2 disc diameters of fovea in either eye) |
|---|---|
| No apparent aging changes | No drusen and No AMD pigmentary abnormalities* |
| Normal aging changes | Only drupelets (small drusen ≤63 μm) and No AMD pigmentary abnormalities* |
| Early AMD | Medium drusen >63 μm and ≤125 μm and No AMD pigmentary abnormalities* |
| Intermediate AMD | Large drusen > 125 μm and/or Any AMD pigmentary abnormalities* |
| Late AMD | Neovascular AMD and/or Any geographic atrophy |
AMD = age-related macular degeneration.
AMD pigmentary abnormalities = any definite hyper- or hypopigmentary abnormalities associated with medium or large drusen but not associated with known disease entities.
Discussion
Using a modified Delphi technique, the committee developed a 5-stage AMD classification scale (Table 2). This approach has been used in many fields to attempt to establish consensus regarding important questions, including classification systems.16–19 The process facilitates communication among a panel of experts regarding areas in which existing evidence-based information may be incomplete or unavailable. The focus of the technique is on the reliability of the expert group’s opinion rather than on individuals’ opinions.16 The committee agreed that development of this clinical classification was only a first step and that development of methods to collect detailed phenotype and genotype data systematically will be necessary to segregate AMD phenotypes for research purposes.
The results of this group effort to identify a basic clinical classification system for AMD indicate a strong level of consistency in the judgment of experts regarding the phenotype for a clinical staging system for AMD. Importantly, the committee agreed that normal aging changes, specifically small drusen (drupelets), should be differentiated from early AMD. The classification of early AMD should exist to separate persons with normal aging changes from those with intermediate AMD who have an increased risk of progressing to late AMD. Based on the review of these risks and discussion of their clinical implications, the committee agreed on a 5-stage classification scale for AMD (Table 2).
As noted earlier, several prior studies3–9 have attempted to correlate phenotypic features associated with the development of late-stage AMD. Although each of these has provided valid data, the differences between them create disparate classifications of AMD. The outcomes demonstrated in the discussions and surveys represented evidence of support for the AREDS simplified severity scale, in which risk categories for late AMD can be determined by clinical examination.4 A recent report20 concerning risk of AMD evaluated the phenotypic features in the AREDS simplified severity scale and additional variables, including: (1) demographic or environmental factors (age, gender, race, body mass index, hypertension and other cardiovascular diseases, diabetes, education level, sunlight exposure, history of skin malignancy, arthritis, and history of current and past medications and dietary supplements); and (2) genotype, in which patients were screened for genes previously reported to be associated with AMD.
The phenotypic variables had the largest hazard ratios for late AMD, ranging from 6.4 to 50.7. These were substantially different from the hazard ratios of 1.03 to 2.00 that were associated with all other variables in the model. Thus, these easily defined phenotypic features had the greatest predictive value for progression to late AMD. The proposed 5-stage basic classification system described by the committee is based on the data presented in the prior AREDS report,3 but they have been supplemented with additional information from the AREDS database.
A strength of using the clinical trial models for developing these risk factors is the careful long-term follow-up of a large cohort with various stages of disease, the large number of cases that progress to late AMD, and the power to assess risk factors. However, there are potential weaknesses in the clinical trial models. Although there is consistency across many studies that large drusen and pigmentary abnormalities are risk factors for progression to late AMD, and there is probably little question that increasing numbers of these risk factors are associated with increasing overall risk of late AMD developing, the magnitude of that risk may vary in different populations, and this needs further evaluation. The risks identified in Table 1 are for white populations of mostly European origin with consistent risks across epidemiologic studies and clinical trial populations. Blacks, Asians, Hispanics, and others may have differing risks of large drusen and pigmentary abnormalities developing and may well be at lower risk of progressing to late AMD for equivalent amounts of large drusen or pigmentary abnormalities. If the rates truly are lower, identifying the protective variables associated with this decreased risk, genetic or otherwise, will be important.21,22 In addition, it has been noted that the rates of progression to late AMD seem to be lower in published population-based studies compared with the AREDS trial population, but the risk ratios remain remarkably similar.22 This could be because of the selection of more severe cases in retina practices. However, data from the AREDS population suggest that this may not be the case. To address the question of whether AREDS enrolled a population with large drusen who were at particularly high risk of progression to late AMD, rates of progression to late AMD in the AREDS population enrolled with large drusen were compared with persons in AREDS who started without large drusen, but in whom large drusen developed during the course of the study. This should be the mildest possible group with large drusen because large drusen had to have developed within the previous year. The analysis of the rate of progression to late AMD in persons without large drusen or pigment abnormalities at the start of AREDS, but in whom those changes developed during the trial, demonstrated that the 5-year simple score rates of progression from the time large drusen were first noted to time late AMD developed were similar to the simple score rates for those who had these risk factors at study entry (data not shown). Although there may be unknown reasons why the clinical trial population may have higher progression rates than the population-based studies, there are factors that may bias the observed rates in both the clinical trials and the population-based studies. The trials may select for higher-risk patients because an intervention is involved, whereas population-based studies may have event rates biased in the downward direction. In most population studies, the participants are examined only at infrequent intervals, making it more likely that late AMD may develop in a study participant, but that the patient may die before being identified as having progressed. Second, after late AMD has developed in a person, he or she is more likely to undergo regular ophthalmic examinations. This may be a disincentive to return for the study examination and may result in an ascertainment bias, leading to an underestimate of the progression rate. Because of the small numbers of late AMD cases in the population-based studies, these potential ascertainment losses may have a considerable effect on risk estimates. Regardless of the exact risk, the relative risks of increasing amounts of large drusen and pigmentary abnormalities in both population-based studies and clinical trial populations seem to remain, making these lesions appropriate phenotypic criteria for identifying a person as having intermediate AMD.
The results of this study indicate that a committee of AMD specialists demonstrated a high degree of consensus in evaluating early signs of AMD. This classification system emphasizes several particularly important points:
Drusen size is important, and the presence of drupelets (small drusen <63 μm in diameter) was judged to pose very little risk of subsequent late AMD (Fig 4). This may indicate that the presence of drupelets only could be necessary, but not sufficient, for the development of large drusen and pigment abnormalities. Some other event may initiate the development of the risk phenotype.
The presence of pigmentary abnormalities within 2 disc diameters of the fovea, associated with at least some medium drusen (>63 but ≤125 μm) and not associated with known retinal entities or other reasons for such abnormalities, is associated with an increased risk of late AMD, and such eyes should be classified as having early AMD, even in the absence of drusen larger than 125 μm.
The proposed basic clinical classification scale seems to be of value in predicting AMD risk. Incorporating consistent nomenclature into the practice patterns of all eye care providers will improve communication and, we expect, patient care as well. This will become increasingly important as new preventive interventions for AMD become available. As noted earlier, this represents a work in progress, and future studies and community feedback should result in improvements. In addition, the committee currently is evaluating a variety of imaging methods and psychophysical tests in hopes of developing a scientific classification scheme in which important AMD phenotypes can be addressed.
Supplementary Material
Acknowledgment.
The authors thank Dr. Ronald Klein, Madison, Wisconsin, and Dr. Johanna Seddon, Boston, Massachusetts, for their helpful review and advice for this manuscript.
Supported by the Arnold and Mabel Beckman Initiative for Macular Research, Irvine, California.
Appendix: 1. Members of the Beckman Initiative for Macular Research Classification Committee
Committee Chairs: Frederick L. Ferris III, MD (National Eye Institute, National Institutes of Health) and C. P. Wilkinson, MD (Greater Baltimore Medical Center).
Committee: Alan Bird, MD (UCL Institute of Ophthalmology, Moorfields Eye Hospital), Dean Bok, PhD (Jules Stein Eye Institute), Neil M. Bressler, MD (The Wilmer Eye Institute), Usha Chakravarthy, MD (The Queen’s University of Belfast), Emily Chew, MD (National Eye Institute, National Institutes of Health), Karl Csaky, MD, PhD (Retina Foundation of the Southwest), Ronald Danis, MD (University of Wisconsin School of Medicine and Public Health), Matthew D. Davis, MD (University of Wisconsin School of Medicine and Public Health), Stuart L. Fine, MD (University of Colorado); Scott E. Fraser, PhD (California Institute of Technology), Robyn Guymer, MD, PhD (Melbourne University Centre for Eye Research), Gregory Hageman, PhD (John Moran Eye Center), Frank G. Holz, MD (University of Bonn), Tatsuro Ishibashi, MD (Kyushu University), Michael Klein, MD (Casey Eye Institute), Paul Lee, MD, JD (Kellogg Eye Center), Xiaoxin Li, MD (Peking University People’s Eye Center), Philip J. Luthert MBBS (UCL Institute of Ophthalmology), Paul Mitchell, MD, PhD (University of Sydney Centre for Vision Research), Kim Ramasamy, MD (Aravind Eye Hospital), Philip Rosenfeld, MD, PhD (Bascom Palmer Eye Institute), Stephen J. Ryan, MD (Doheny Eye Institute), Srinivas Sadda, MD (Doheny Eye Institute), Alfredo Sadun, MD, PhD (Doheny Eye Institute), Andrew P. Schachat, MD (Cole Eye Institute), Richard F. Spaide, MD (Vitreous Retina Macula Consultants of New York), Giovanni Staurenghi, MD (Luigi Sacco Eye Clinic University of Milan), Cynthia A. Toth, MD (Duke University Medical Center), and Johannes Vingerling, MD, PhD (Erasmus Medical Center).
Footnotes
Footnotes and Financial Disclosures
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
A list of all members of the Arnold and Mabel Beckman Initiative for Macular Research Classification Committee with affiliations can be found in Appendix 1.
References
- 1.Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–72. [DOI] [PubMed] [Google Scholar]
- 2.International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol 1995;39:367–74. [DOI] [PubMed] [Google Scholar]
- 3.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology 2006;113:260–6. [DOI] [PubMed] [Google Scholar]
- 4.Age-Related Eye Disease Study Research Group. Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol 2005;123:1484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research Group. A simplified severity scale for age-related macular degeneration: AREDS report no. 18. Arch Ophthalmol 2005;123:1570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2001;42:2237–41. [PubMed] [Google Scholar]
- 7.van Leeuwen R, Klaver CC, Vingerling JR, et al. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol 2003;121:519–26. [DOI] [PubMed] [Google Scholar]
- 8.Wang JJ, Rochtchina E, Lee AJ, et al. Ten-year incidence and progression of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology 2007;114:92–8. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Knudtson MD, et al. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology 2007;114:253–62. [DOI] [PubMed] [Google Scholar]
- 10.Zweifel AZ, Imamura Y, Spaide TC, et al. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology 2010;117:1775–81. [DOI] [PubMed] [Google Scholar]
- 11.Fitch K, Bernstein SJ, Aguilar MS, et al. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND; 2001. Available at: http://www.rand.org/content/dam/rand/pubs/monograph_reports/2011/MR1269.pdf. Accessed October 22, 2012. [Google Scholar]
- 12.Lee PP, Sultan MB, Grunden JW, et al. Assessing the importance of IOP variables in glaucoma using a modified Delphi process. J Glaucoma 2010;19:281–7. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MR, Lee PP, Weinreb RN, et al. , Glaucoma Modified RAND-Like Methodology Group. A panel assessment of glaucoma management: modification of existing RAND-like methodology for consensus in ophthalmology. Part I: methodology and design. Am J Ophthalmol 2008;145:570–4. [DOI] [PubMed] [Google Scholar]
- 14.Singh K, Lee BL, Wilson MR, et al. , Glaucoma Modified RAND-Like Methodology Group. A panel assessment of glaucoma management: modification of existing RAND-like methodology for consensus in ophthalmology. Part II: results and interpretations. Am J Ophthalmol 2008;145:575–81. [DOI] [PubMed] [Google Scholar]
- 15.Duke-Elder S, Wybar KC. System of Ophthalmology. vol. 2. The Anatomy of the Visual System. St. Louis, MO: Mosby; 1961:294–307. [Google Scholar]
- 16.Danis RP, Scott IU, Qin H, et al. , Diabetic Retinopathy Clinical Research Network. Association of fluorescein angiographic features with visual acuity and with optical coherence tomographic and stereoscopic color fundus photographic features of diabetic macular edema in a randomized clinical trial. Retina 2010;30:1627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis MD, Bressler SB, Aiello LP, et al. , Diabetic Retinopathy Clinical Research Network Study Group. Comparison of time-domain OCT and fundus photographic assessments of retinal thickening in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci 2008;49:1745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangaputra SJ, Won Pak JW, Peng Q, et al. , Studies of the Ocular Complications of AIDS Research Group. Transition from film to digital fundus photography in the Longitudinal Studies of the Ocular Complications of AIDS. Retina 2012;32:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lujan BJ, Wang F, Gregori G, et al. Calibration of fundus images using spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging 2008;39(suppl): S15–20. [DOI] [PubMed] [Google Scholar]
- 20.Klein ML, Francis PJ, Ferris FL III, et al. Risk assessment model for development of advanced age-related macular degeneration. Arch Ophthalmol 2011;29:1543–50. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Reynolds R, Rosner B, et al. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci 2012;53:1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein R, Klein BE, Myers CE. Risk assessment models for late age-related macular degeneration. Arch Ophthalmol 2011;129: 1605–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.