The growth of geographic atrophy (GA) over 4 years in the Age-Related Eye Disease Study (AREDS) was shown to be dependent on the baseline area of GA.1 However, 3 articles have demonstrated that the square root transformation of lesion area measurements eliminates the dependence on baseline lesion size for both the test-retest variability and the growth rates.2–4 This square root transformation strategy was applied to the AREDS data.
Methods.
Area measurements of GA collected from 181 eyes of 181 AREDS study patients and followed up longitudinally for up to 4 years were analyzed using the square root transformation strategy.2 The correlations between growth of the lesion area over 4 years and baseline lesion size were performed on the original area scale and on the square root area scale with both Pearson and Spearman correlation analyses. In a second analysis, lesions were divided into the published categories of baseline lesion size expressed as disc areas (<0.75, 0.75 to <4, and ≥4 disc areas), and lesion growth was calculated and compared using the standard area measurements (millimeters squared) and the square root of these same measurements (millimeters).1 The influences of the assigned treatments (zinc and antioxidants) were also analyzed using the square root transformation strategy. These analyses were performed using generalized estimating equations with correlation structure modeled as first-order autoregressive.5
Results.
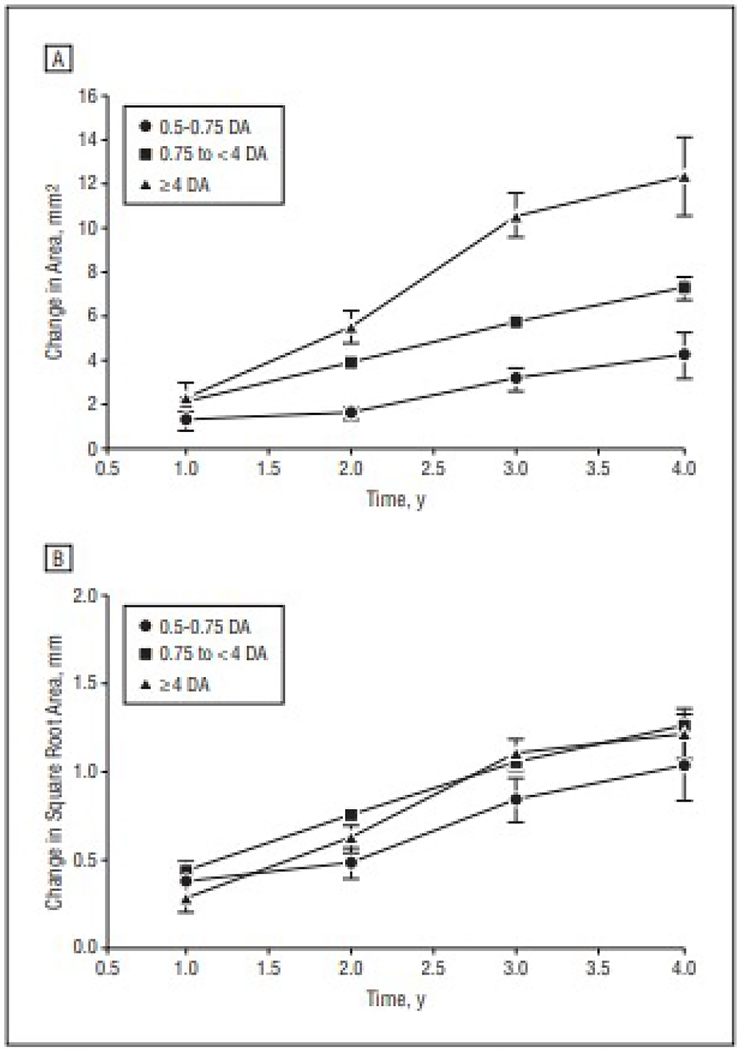
Application of the square root transformation to the area measurements eliminated the statistical significance of the correlations between growth and baseline area. Four-year growth rates were significantly correlated with baseline lesion size using the original area measurements (Pearson r = 0.53, P<.001; Spearman r = 0.49, P<.001), but there was no statistically significant correlation once the area measurements underwent the square root transformation strategy (Pearson r=0.10, P=.30; Spearman r=0.17, P=.08). When the baseline lesions were divided into different size categories, there was a statistically significant difference in the growth of GA using the original area measurements (P<.001) (Figure, A) but no statistically significant difference after applying the square root transformation strategy (P=.34) (Figure, B). Zinc and antioxidant treatment and their interaction were not associated with decreased lesion growth using either the original area or square root area measurements (all P>.28).
Figure.
Change in area of geographic atrophy using the standard area measurements and the square root transformation of these area measurements. A, Original analysis showing the change in area over 4 years for each of the baseline size categories. B, Revised analysis showing the change in the square root of the area measurements for each of the baseline size categories. DA indicates disc areas.
Comment.
The square root transformation strategy reduced the association between growth rates and baseline area measurements in AREDS, which confirmed our recent reports.2–4 This square root strategy simplifies the design and enrollment of clinical trials by eliminating the need to either specify a range of lesion sizes or adjust for lesion size in the analysis. When this square root transformation strategy is used in studies designed to test treatments that may slow the progression of GA in dry age-related macular degeneration, fewer patients with a wider range of lesion sizes can be enrolled compared with the number of patients that would be needed in studies using the standard area measurements.2 While this reanalysis of the AREDS data did not alter the conclusion about the lack of efficacy when using zinc and antioxidants for the treatment of GA, the square root strategy did eliminate the artifactual dependence of growth on baseline size within the range of lesion sizes enrolled in AREDS. However, very small lesions when GA first appears and very large lesions nearing the end of their growth cycle are expected to grow more slowly based on the natural history of GA in age-related macular degeneration. This is not evident from the subgroup data because there were few very small and few very large lesions in the analysis.
Funding/Support:
This work was supported by an unrestricted grant from Research to Prevent Blindness, core center grant P30 EY014801 to the University of Miami from the National Eye Institute, and grant W81XWH-09–1-0675 from the US Department of Defense.
Footnotes
Conflict of Interest Disclosures: Dr Ferris is one of the inventors on US patent 6 660 297, “Nutritional Supplement to Treat Macular Degeneration,” issued on December 9, 2003, and owned by Bausch & Lomb; Dr Ferris has assigned his interest in the patent to the US government and receives government compensation. Dr Rosenfeld is a consultant to Acucela, Boehringer Ingelheim, Chengdu Kanghong Biotech, and Oraya; receives research support from Advanced Cell Technology, Alexion Pharmaceuticals, and GlaxoSmithKline; receives research support and lecture fees from Carl Zeiss Meditec; and is an advisor to Sucampo Pharmaceuticals and ThromboGenics.
Contributor Information
Mr William J. Feuer, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida.
Dr Zohar Yehoshua, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida.
Dr Giovanni Gregori, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida.
Dr Fernando M. Penha, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida.
Dr Emily Y. Chew, National Eye Institute, Bethesda, Maryland.
Dr Frederick L. Ferris, National Eye Institute, Bethesda, Maryland.
Dr Traci E. Clemons, EMMES Corporation, Rockville, Maryland.
Dr Anne S. Lindblad, EMMES Corporation, Rockville, Maryland.
Dr Philip J. Rosenfeld, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida.
References
- 1.Lindblad AS, Lloyd PC, Clemons TE, et al. ; Age-Related Eye Disease Study Research Group. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127 (9):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yehoshua Z, Rosenfeld PJ, Gregori G, et al. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology. 2011;118(4):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregori G, Wang F, Rosenfeld PJ, et al. Spectral domain optical coherence tomography imaging of drusen in nonexudative age-related macular degeneration. Ophthalmology. 2011;118(7):1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118(12): 2434–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. [DOI] [PubMed] [Google Scholar]