Highlights
-
•
53 patients with localized, recurrent, and metastatic RCC were treated with SABR.
-
•
2-year local control was 100%, and 3-year local control was 94.4%.
-
•
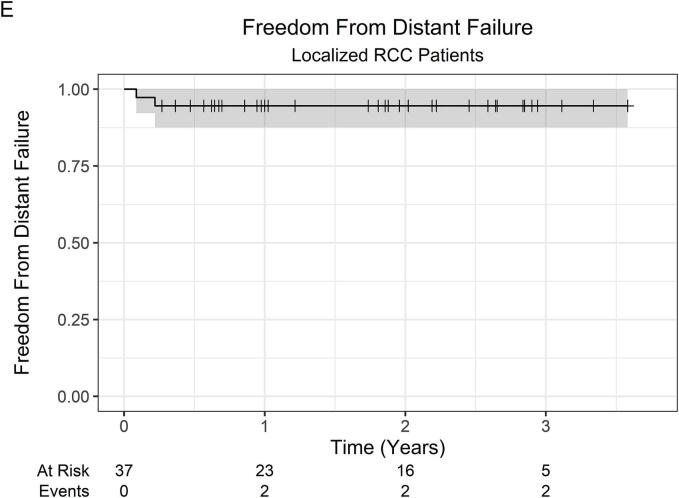
Among patients with localized RCC, 2-year freedom from distant failure was 94.6%.
-
•
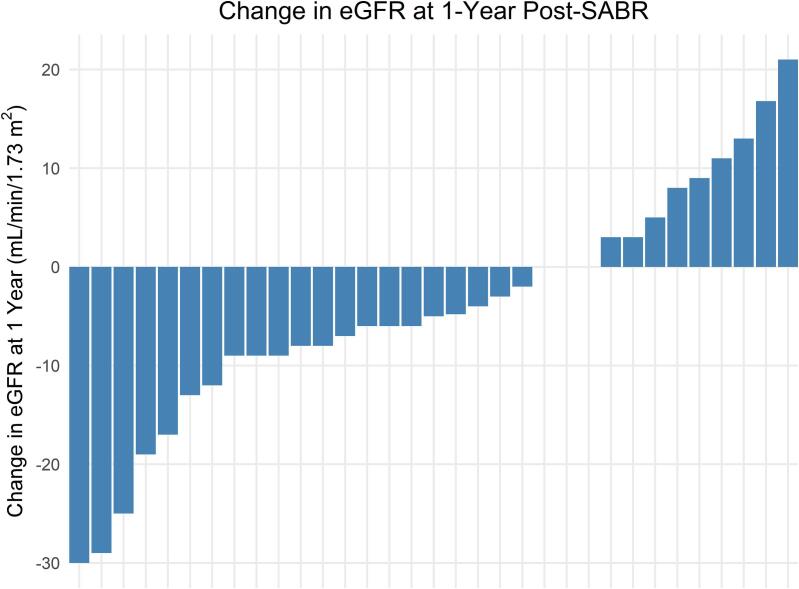
Median eGFR decline was 5 mL/min/1.73 m2 at 1-year post-SABR.
Keywords: Radiotherapy, Radiosurgery, Carcinoma, Renal Cell, Humans, Kidney
Abstract
Background and purpose
Stereotactic ablative body radiotherapy (SABR) is an effective treatment for localized renal cell carcinoma (RCC). However, the role of primary site SABR for locally recurrent or metastatic RCC is unclear. Here, we report outcomes of primary SABR across a diverse cohort of localized, recurrent, and metastatic RCC patients treated at our institution.
Materials and methods
RCC patients treated with SABR to lesions of the kidney or nephrectomy bed at our institution with at least 6 months of follow-up were included for analysis. Local control, overall survival, and freedom from distant failure were estimated using the Kaplan-Meier method. Estimated glomerular filtration rate (eGFR) was assessed at baseline and following SABR.
Results
Fifty-three patients received primary site SABR. Thirty-seven (70 %) patients had localized RCC, and 16 (30 %) had metastatic RCC. Seven (13 %) had locally recurrent RCC after prior surgery or ablation. The median tumor size was 4.5 cm (IQR 3.7–6.3). At a median follow-up of 23 months (IQR 12–35), 2-year local control was 100 %, and 3-year local control was 94.4 % (95 % CI 84.4 %–100 %). Among patients with initially localized disease, the 2-year freedom from distant failure was 94.6 % (95 % CI 87.6 %–100 %), and the 2-year overall survival was 66.5 % (95 % CI 51.9 %–85.2 %). Twelve (23 %) patients experienced acute grade 1–2 treatment-related toxicity (nausea, vomiting, or small bowel). There were no acute grade 3–4 toxicities. Two (3.8 %) patients developed late grade 3 gastrointestinal toxicity. The median baseline eGFR was 51 mL/min/1.73 m2 (IQR 38–77). At 1-year post-SABR, the median eGFR decline was 5 mL/min/1.73 m2 (IQR −3 to 9). One patient required dialysis following SABR.
Conclusion
This analysis demonstrates excellent local control rates across patients with localized, recurrent, and metastatic RCC treated with SABR. Treatment was associated with minimal eGFR decline.
1. Introduction
The incidence of renal cell carcinoma (RCC) in the United States has been steadily increasing, particularly among patients over the age of 65 [1]. Older patients are more likely to have reduced kidney function or comorbid conditions that preclude them from undergoing surgery, such as partial or radical nephrectomy. Nephrectomy is the gold standard for the treatment of localized RCC. However, for those who are medically unfit for surgery, alternative options include thermal ablation (TA) and stereotactic ablative body radiotherapy (SABR). The use of TA is generally restricted to T1a tumors, as local recurrence rates with TA for T1b tumors are suboptimal at ∼20–30 % [2]. Furthermore, tumor location near the renal pelvis or ureter correlates with a higher incidence of tumor progression and ureteral injury following TA [3], [4].
Given the limitations of TA, there has been increasing interest in SABR as a non-invasive treatment for inoperable RCC, particularly for larger tumors. While RCC was historically considered to be radioresistant based on early preclinical data [5], SABR has emerged as a highly effective treatment for localized RCC, with a 5-year local failure rate of only 5.5 % based on a multi-institutional meta-analysis from the International Radiosurgery Consortium of the Kidney (IROCK) [6]. Although the safety and efficacy of SABR have been established for T1 and select T2 tumors confined to the kidney [6], [7], [8], [9], [10], further work is needed to define the role of SABR for T3 tumors extending into renal vein or inferior vena cava (IVC).
For locally recurrent or metastatic RCC, treatment is multidisciplinary and may entail a combination of systemic therapy (e.g., immunotherapy or targeted agents), surgery (e.g., nephrectomy or metastasectomy), or radiotherapy (e.g., SABR to the primary tumor or metastatic sites). In the prior era of cytokine-based systemic therapy, cytoreductive nephrectomy was shown to confer a modest survival advantage across two prospective clinical trials [11], [12]. However, a more recent randomized controlled trial has challenged this notion, as sunitinib monotherapy was found to be non-inferior to nephrectomy followed by sunitinib [13]. The relevance of these data in the modern era is unclear, given the recent evolution of systemic therapy to immune checkpoint therapy combinations. In contrast to cytoreductive nephrectomy, very little is known regarding the efficacy of primary tumor SABR for metastatic RCC.
Further data are needed to elucidate the role of primary tumor SABR for patients with more advanced presentations of RCC (e.g., stage III-IV disease). Here, we report the outcomes of primary SABR to the kidney or to the nephrectomy bed across a diverse cohort of localized, recurrent, and metastatic RCC patients treated at our institution.
2. Materials and methods
2.1. Participants
All RCC patients treated with SABR to lesions of the kidney or the nephrectomy bed at our institution with a minimum post-treatment follow-up time of 6 months were included for analysis. Patients were deemed high risk of peri-operative complications and/or high risk for dialysis. All patients had radiographic evidence of tumor growth prior to referral for SABR. Pathologic confirmation via biopsy was routinely performed (94 % of patients).
Demographic characteristics, including age, gender, race, Eastern Cooperative Oncology Group (ECOG) performance status, and Karnofsky Performance Status (KPS) score, were retrospectively reviewed. The Charlson Comorbidity Index was calculated for each patient. Tumor characteristics were collected, including tumor stage, size, and location (unifocal, multifocal, or bilateral). RENAL nephrometry scores were calculated for all kidney tumors. Stage was determined according to the American Joint Committee on Cancer (AJCC) staging system, 8th edition. This study was approved by our institutional review board (IRB number 23-1674). Data were retrospectively collected and updated last in August 2024.
2.2. Procedures
The intervention in this study was SABR to the kidney tumor, or if locally recurrent, to the nephrectomy bed. SABR dosing regimens were selected per clinician discretion, ranging from 1 to 5 fractions. Appropriate radiotherapy regimens were selected based on tumor size and extent, proximity to organs-at-risk (OARs), and distance from the patient’s residence to the treatment facility. The radiotherapy dose for single-fraction SABR was 26 Gy. Three-fraction regimens ranged from 30-45 Gy cumulative dose (10–15 Gy/fraction), most commonly to 42 Gy or 45 Gy. Five-fraction regimens ranged from 40-50 Gy cumulative dose (8–10 Gy/fraction), most commonly to 50 Gy. Overall treatment time ranged from 1 day (for single-fraction SABR) to 2 weeks, delivered either once daily or once every other day. Biologically effective doses using an α/β ratio of 10 (BED10) were calculated using the linear-quadratic formula.
Before treatment, fiducial markers were placed by interventional radiology within the ipsilateral kidney at the time of biopsy. At simulation, patients were immobilized using a stereotactic body frame. A four-dimensional CT scan was used to account for respiratory motion. A pre-treatment MRI, if available, was fused to the planning CT scan for contouring. An internal target volume (ITV) was contoured for each tumor, including the total tumor excursion throughout the respiratory cycle. A 3–5 mm isotropic expansion was added to the ITV to form the planning target volume (PTV). If applicable, tumor thrombus extending into the renal vein or IVC was contoured and included in the ITV. A bowel planning organ-at-risk volume (PRV) margin of 2–3 mm was used during radiotherapy planning to account for possible intestinal motion. Institutional dose constraints, adapted from the Timmerman tables [14], were used to limit radiation dose to OARs. All patients were treated using a Varian TrueBeam linear accelerator, with daily kV imaging (triggered to fiducials) and cone-beam CT for setup verification. Concurrent or adjuvant systemic therapy was permitted during treatment; however, concurrent use of vascular endothelial growth factor receptor (VEGFR) inhibitors was generally avoided. Following treatment, CT or MRI scans were obtained at 3- to 6-month intervals for tumor assessment.
2.3. Outcome measures
Our primary outcome was local control (LC), measured from the date of SABR completion to the first evidence of radiographic local progression, as evaluated by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, censored at the last radiographic assessment. Secondary outcomes were overall survival (OS), defined as the time from the date of SABR completion to the date of death from any cause, and freedom from distant failure (FFDF), defined as the time from the date of SABR completion to the first documented distant progression, with death as a censoring event. FFDF was calculated only for patients with non-metastatic disease at initial diagnosis and treatment. Toxicities were reported according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Among patients with an intact ipsilateral kidney who were not on dialysis pre-SABR, renal function outcomes were assessed using estimated glomerular filtration rate (eGFR) data at baseline and 1-year, 2-years, and 3-years post-SABR.
2.4. Statistical analysis
Baseline characteristics were summarized using descriptive statistics. LC, OS, and FFDF were estimated using the Kaplan-Meier method. Linear modeling was used to test the association of 1-year post-SABR eGFR with the following clinical and treatment-related features: age, gender, race, Charlson Comorbidity Index, disease extent, baseline eGFR, ITV, PTV, ipsilateral kidney volume, BED10, and RENAL nephrometry score. All statistical tests were two-sided and performed at the 0.05 significance level using R version 4.3.1 [15].
3. Results
From March 2018 to April 2024, 65 patients with RCC were treated with primary site SABR at our institution. Of those, 53 patients had a post-treatment interval of at least 6 months and were included for analysis. Table 1 summarizes baseline patient, tumor, and treatment characteristics. Twenty-six (49 %) patients received single-fraction SABR (26 Gy), 15 (28 %) received 3 fractions (most commonly to 42–45 Gy), and 12 (23 %) received 5 fractions (most commonly to 50 Gy). Three (5.7 %) patients received concurrent immunotherapy with SABR, and 3 (5.7 %) patients received adjuvant immunotherapy with or without VEGFR inhibitor following SABR.
Table 1.
Baseline Characteristics.
| N = 531 | |
|---|---|
| Age (years) | 73 (65, 80) |
| Gender | |
| Male | 32 (60 %) |
| Female | 21 (40 %) |
| Race | |
| Black | 21 (40 %) |
| White | 27 (51 %) |
| Other | 5 (9.4 %) |
| Performance status | |
| Favorable (ECOG 0–1 or KPS ≥ 80) | 40 (75 %) |
| Unfavorable (ECOG 2 or KPS 70) | 13 (25 %) |
| Charlson comorbidity index | 8 (7, 9) |
| Solitary kidney | 8 (15 %) |
| Disease extent | |
| Localized | 35 (66 %) |
| Regional | 2 (3.8 %) |
| Metastatic | 16 (30 %) |
| Total number of metastases (if metastatic) | |
| 1–2 | 6 (38 %) |
| 3–5 | 4 (25 %) |
| >5 | 6 (38 %) |
| Locally recurrent after nephrectomy | 7 (13 %) |
| T stage (if not recurrent) | |
| 1 | 36 (78 %) |
| 2 | 3 (6.5 %) |
| 3 | 5 (11 %) |
| 4 | 2 (4.3 %) |
| N stage (if not recurrent) | |
| 0 | 44 (96 %) |
| 1 | 2 (4.3 %) |
| M stage (if not recurrent) | |
| 0 | 32 (70 %) |
| 1 | 14 (30 %) |
| Histology type (if biopsied) | |
| Clear cell | 32 (64 %) |
| Papillary | 14 (28 %) |
| Other RCC | 1 (2.0 %) |
| Non-diagnostic biopsy | 3 (6.0 %) |
| Tumor location | |
| Unifocal | 50 (94 %) |
| Multifocal | 2 (3.8 %) |
| Bilateral | 1 (1.9 %) |
| Maximum tumor dimension (cm) | 4.5 (3.7, 6.3) |
| RENAL nephrometry score (if not recurrent) | 8 (7, 9) |
| RENAL complexity group (if not recurrent) | |
| High | 7 (15 %) |
| Intermediate | 29 (63 %) |
| Low | 10 (22 %) |
| Dose and fractionation | |
| 26 Gy in 1 fraction | 26 (49 %) |
| 30 Gy in 3 fractions | 1 (1.9 %) |
| 42 Gy in 3 fractions | 3 (5.7 %) |
| 45 Gy in 3 fractions | 11 (21 %) |
| 40 Gy in 5 fractions | 3 (5.7 %) |
| 45 Gy in 5 fractions | 1 (1.9 %) |
| 50 Gy in 5 fractions | 8 (15 %) |
| BED10 (Gy) | 93.6 (93.6, 100.8) |
ECOG = Eastern Cooperative Oncology Group. KPS = Karnofsky Performance Status. RCC = renal cell carcinoma. BED10 = biologically effective dose using an α/β ratio of 10. IQR = interquartile range.
Median (IQR); n (%).
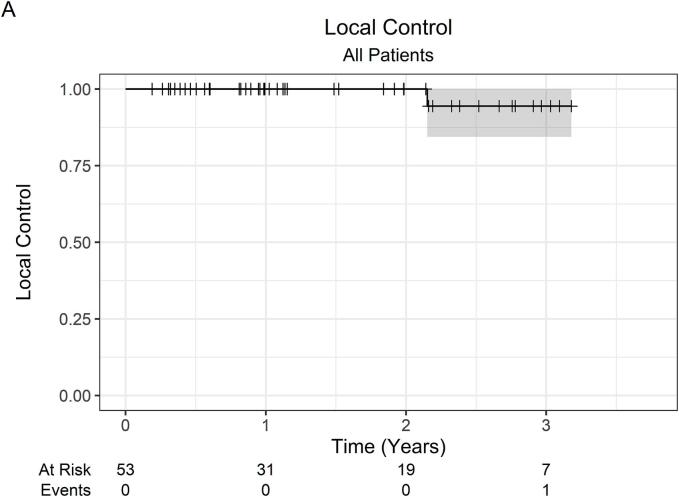
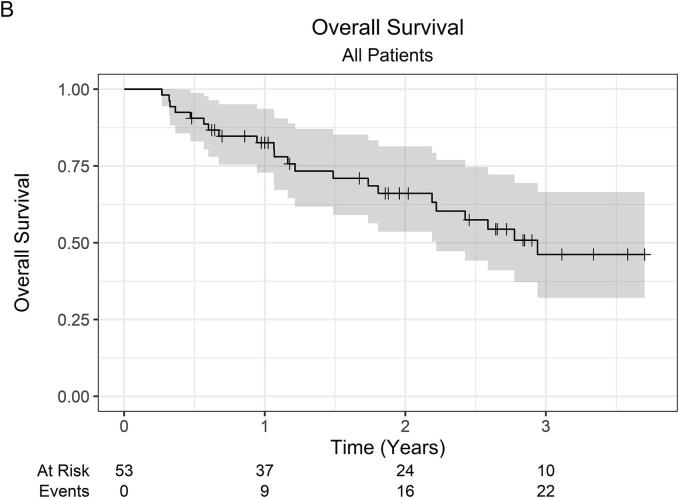
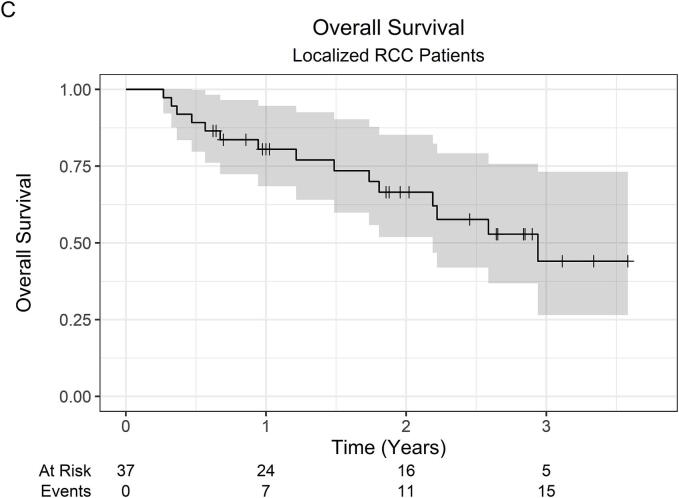
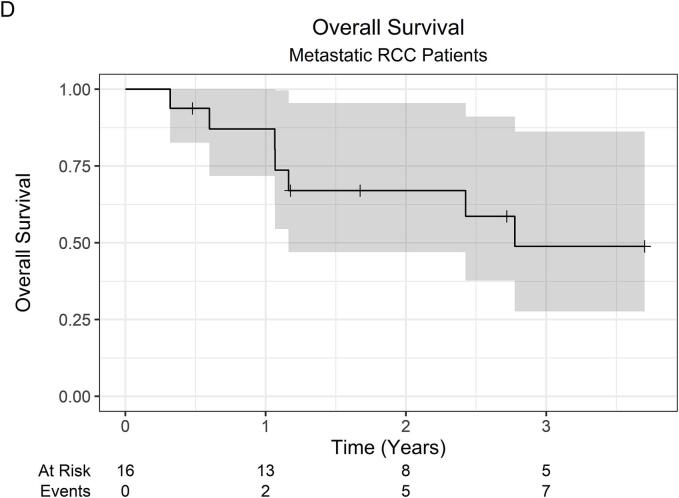
At a median follow-up of 23 months (IQR 12–35), the 2-year LC was 100 %, while the 3-year LC was 94.4 % (95 % CI 84.4 %–100 %). Across all 53 patients, there was 1 local failure. This patient was initially treated with primary tumor SABR (26 Gy in 1 fraction) along with SABR to oligometastatic sites, which were controlled without systemic therapy. Within 1-year post-SABR, the patient developed multiple new metastatic pulmonary nodules, for which pembrolizumab monotherapy was started, followed by the addition of axitinib for disease progression. Subsequently, at roughly 2-years post-SABR, the patient developed progressive renal vein and IVC tumor thrombus on surveillance imaging consistent with local failure. OS was 82.6 % (95 % CI 72.8 %–93.6 %) at 1 year, 66.1 % (95 % CI 53.7 %–81.4 %) at 2 years, and 46.2 % (95 % CI 32.1 %–66.5 %) at 3 years. The 2-year OS was 66.5 % (95 % CI 51.9 %–85.2 %) and 67.0 % (47.0 %–95.5 %) for localized RCC and metastatic RCC patients, respectively. Among 37 patients with initially localized disease, the 2-year FFDF was 94.6 % (95 % CI 87.6 %–100 %). Kaplan-Meier curves are shown in Fig. 1.
Fig. 1.
Kaplan-Meier curves for (A) local control (all patients), (B) overall survival (all patients), (C) overall survival (localized RCC patients), (D) overall survival (metastatic RCC patients), and (E) freedom from distant failure (localized RCC patients). Abbreviations: RCC = renal cell carcinoma.
Of the 53 patients, 20 were excluded from eGFR analysis due to baseline end-stage renal disease requiring dialysis (n = 5) or renal transplant (n = 1), history of ipsilateral nephrectomy (n = 7), or absence of eGFR data past 6 months, e.g., in the case of death within 6 months post-SABR (n = 7). Among the 33 remaining patients, the median baseline eGFR was 51 mL/min/1.73 m2 (IQR 38–77). At 1-year post-SABR, the median eGFR decline was 5 mL/min/1.73 m2 (IQR −3 to 9). Individual patient eGFR changes are shown in Fig. 2. The median drop in eGFR from baseline was 12 mL/min/1.73 m2 (IQR 8–17) at 2-years post-SABR (n = 18) and 11 mL/min/1.73 m2 (IQR 5–17) at 3-years post-SABR (n = 12). One (3.4 %) patient with a baseline eGFR of 27 mL/min/1.73 m2 required dialysis at 2 years post-SABR following an acute heart failure exacerbation. Only baseline eGFR was significantly associated with eGFR at 1 year with a drop of 8.4 mL/min/1.73 m2 (95 % CI 6.6–10) in 1-year eGFR for every 10 mL/min/1.73 m2 decrease in baseline eGFR (p < 0.001). The linear model results are summarized in Table 2.
Fig. 2.
Waterfall plot showing the distribution of individual patient eGFR changes at 1-year post-SABR among the 33 patients with an intact ipsilateral kidney not on dialysis at baseline. Abbreviations: eGFR = estimated glomerular filtration rate. SABR = stereotactic ablative body radiotherapy.
Table 2.
eGFR analysis.
| Characteristic | N | Beta | 95 % CI | p-value |
|---|---|---|---|---|
| eGFR, baseline | 33 | 8.4 | 6.6, 10 | <0.001 |
| Internal target volume, cc | 33 | 0.01 | −0.04, 0.07 | 0.6 |
| Planning target volume, cc | 33 | 0.02 | −0.02, 0.07 | 0.3 |
| Kidney volume, cc | 33 | 0.04 | −0.05, 0.13 | 0.3 |
| Charlson comorbidity index | 33 | −2.4 | −6.4, 1.5 | 0.2 |
| Race | 33 | |||
| Black | — | — | ||
| Other | 15 | −14, 44 | 0.3 | |
| White | 10 | −6.4, 27 | 0.2 | |
| Age | 33 | −0.22 | −1.0, 0.58 | 0.6 |
| BED10 | 33 | −0.04 | −0.91, 0.83 | >0.9 |
| Gender | 33 | |||
| Female | — | — | ||
| Male | 2.1 | −15, 19 | 0.8 | |
| Disease extent | 33 | |||
| Localized | — | — | ||
| Metastatic | 5.5 | −11, 22 | 0.5 | |
| Nephrometry score | 33 | −0.29 | −5.0, 4.4 | >0.9 |
Abbreviations: CI = confidence interval. eGFR = estimated glomerular filtration rate. BED10 = biologically effective dose using an α/β ratio of 10.
Twelve (23 %) patients experienced acute grade 1–2 treatment-related toxicity (nausea, vomiting, or small bowel). There were no acute grade 3–4 toxicities. However, 2 (3.8 %) patients experienced late grade 3 treatment-related toxicity. One patient developed a renal-cecal fistula (attributed to SABR with adjuvant pembrolizumab/axitinib) and subsequently underwent elective nephrectomy with hemicolectomy. Another patient with a history of gastritis developed a radiation-induced gastric ulcer with recurrent bleeding, requiring subtotal gastrectomy. Toxicity outcomes are summarized in Table 3.
Table 3.
Treatment-Related Toxicities Among All 53 Patients.
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Any toxicity (acute or late)* | 4 (8 %) | 8 (15 %) | 2 (4 %) |
| Nausea (acute) | 4 (8 %) | 8 (15 %) | 0 |
| Vomiting (acute) | 3 (6 %) | 2 (4 %) | 0 |
| Small bowel (acute) | 1 (2 %) | 0 | 0 |
| Colorenal fistula (late) | 0 | 0 | 1 (2 %) |
| Gastric ulcer (late) | 0 | 0 | 1 (2 %) |
Worst toxicity grade (1, 2, 3, or 4).
4. Discussion
To our knowledge, this is the first study reporting outcomes of primary site SABR across a broad cohort of localized, recurrent, and metastatic RCC patients. We included patients with more advanced stages of RCC, such as patients with T2b-T4 tumors, metastatic disease, or local recurrence following prior nephrectomy or ablation. Despite the heterogeneous patient population in this analysis, primary SABR demonstrated excellent LC rates of 94.4 % at 3 years with an overall favorable toxicity profile.
SABR is an established and guideline-recommended treatment for medically inoperable RCC [16]. The most compelling data to support its use for localized RCC are from large, multi-institutional efforts, such as the IROCK meta-analysis [6], [7], [8] and FASTRACK II trial [9]. The IROCK meta-analysis demonstrated a 5-year local failure rate of 5.5 % among RCC patients with a median tumor diameter of 4.0 cm [6]. The FASTRACK II trial enrolled 70 patients with T1-2aN0-1 RCC treated with SABR, either 26 Gy in 1 fraction for smaller tumors or 42 Gy in 3 fractions for larger tumors, and demonstrated a 1-year LC rate of 100 % [9]. Thus far, no prospective trials have directly compared SABR to other local therapies, such as nephrectomy or TA. For medically inoperable T1a RCC, TA is effective at achieving durable tumor control, with 5-year local recurrence-free survival rates of roughly 90 % [17], [18]. However, the outcomes of TA for T1b tumors are less impressive, with local recurrence rates of ∼20–30 % [2]. Therefore, SABR is the preferred treatment modality for larger tumors (i.e., ≥T1b) in medically inoperable patients.
The role of SABR for metastatic RCC has been evolving in the contemporary era of immunotherapy and targeted agents. This is partly due to emerging evidence suggesting that SABR has immunomodulatory effects that might act synergistically with immune checkpoint inhibitors [19], [20]. While numerous studies have investigated cytoreductive nephrectomy for metastatic RCC [11], [12], [13], the role of cytoreductive SABR is less clear [20], [21]. A pilot study by Singh et al. showed that upfront SABR followed by cytoreductive nephrectomy was safe and feasible [20]. A prospective phase I trial by Correa et al. demonstrated the safety and feasibility of SABR as an alternative to cytoreductive nephrectomy for inoperable patients with metastatic RCC [21]. Two ongoing trials are investigating the role of primary tumor SABR for metastatic RCC in the era of modern systemic therapy agents: NRG-GU012 SAMURAI (NCT05327686) and CYTOSHRINK (NCT04090710). Aside from local therapy to the primary tumor, metastasis-directed SABR could potentially be used to delay the initiation of systemic therapy for patients with oligometastatic RCC [22], [23].
Data to support SABR for locally recurrent RCC are limited to case reports and retrospective studies [24], [25], [26]. A retrospective analysis by Liu et al. demonstrated that patients with recurrent localized RCC who received local therapy (e.g., surgery or SABR) had significantly longer progression-free survival compared to those who received systemic therapy alone [25]. Among the 106 patients analyzed, 39 (36.8 %) were treated with primary SABR to their recurrent disease, with a 1-year LC rate of 96.6 %. Our study included 7 (13 %) patients with locally recurrent tumors, none of whom experienced a local failure over the follow-up period of this study.
Of the 46 patients without locally recurrent tumors, 5 (11 %) had T3 disease with renal vein or IVC tumor thrombus. After SABR monotherapy to the primary tumor (including tumor thrombus), none developed local tumor progression. Patients with renal vein or IVC tumor thrombus are at increased risk of complications such as varicocele, Budd-Chiari syndrome, or pulmonary embolism [27], thus underscoring the importance of prompt treatment. Unfortunately, the standard definitive treatment in this setting is both complex and morbid, consisting of radical nephrectomy with thrombectomy. Therefore, there has been interest in utilizing neoadjuvant SABR to downstage tumors and reduce surgical complexity, particularly for those with IVC wall invasion [28], [29]. Alternatively, there is evidence for responsiveness of IVC thrombi to neoadjuvant immune checkpoint therapy prior to surgery [30]. For patients who are unfit or unwilling to undergo surgery, SABR monotherapy could be considered. Freifeld et al. demonstrated that SABR to the IVC thrombus with or without SABR to the primary tumor or metastatic sites was safe and feasible, with thrombus regression in 7/12 (58 %) patients and symptom palliation in 5/5 (100 %) patients [31]. Similarly, our results demonstrate that SABR monotherapy for T3 tumors may be considered in lieu of surgery for patients with medically inoperable RCC.
Our analysis demonstrated clinically acceptable eGFR decline following primary SABR, with a median eGFR decline of 5 mL/min/1.73 m2 at 1 year, 12 mL/min/1.73 m2 at 2 years, and 11 mL/min/1.73 m2 at 3 years. This is comparable to the median eGFR decline reported from IROCK of 5.5 mL/min/1.73 m2 at 1 year, up to 14.2 mL/min/1.73 m2 at 5 years [6]. In the FASTRACK II trial, baseline mean eGFR decreased by 10.8 mL/min/1.73 m2 at 1 year and by 14.6 mL/min/1.73 m2 at 2 years, while plateauing thereafter [9]. Similar to the findings from our cohort, they showed that only baseline eGFR was associated with 12-month post-treatment eGFR, with an average reduction of 8.4 mL/min/1.73 m2 at 12 months per 10 mL/min/1.73 m2 lower baseline eGFR. A retrospective analysis by Ali et al. showed a median eGFR decline of 14 mL/min/1.73 m2 following SABR, with worse baseline chronic kidney disease being the strongest predictor for eGFR decline [32]. Overall, our renal function outcomes confirm that SABR is safe for medically inoperable patients with localized, recurrent, and metastatic RCC.
Several limitations should be acknowledged. First, the retrospective nature of the analysis introduces the potential for selection bias and premature loss of follow-up. Second, the relatively small sample size and single-institution nature of this study limit the generalizability of its findings. Third, the limited follow-up period of this study may not capture relevant long-term outcomes, such as durable treatment response rates or long-term eGFR decline. Finally, variations in SABR dosing or SABR targets (in the case of metastasis-directed radiotherapy) and the use of concurrent or adjuvant systemic therapies could influence overall treatment outcomes and toxicity profiles.
In conclusion, our findings demonstrate that primary site SABR is an effective and well-tolerated treatment option for patients with localized, recurrent, and metastatic RCC. SABR offers excellent short-term LC rates with favorable toxicity outcomes and minimal eGFR decline. Though further prospective trials are needed, primary site SABR shows promise for treating more advanced stages of RCC in the contemporary era of immunotherapy combinations.
CRediT authorship contribution statement
Daniel Huang: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Project administration. Connor Lynch: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Writing – review & editing, Visualization. Lucas M. Serra: Software, Formal analysis, Writing – review & editing. Randy F. Sweis: Writing – review & editing. Paul J. Chang: Investigation, Writing – review & editing. Walter M. Stadler: Writing – review & editing. Russell Z. Szmulewitz: Writing – review & editing. Peter H. O’Donnell: Writing – review & editing. Abhinav Sidana: Writing – review & editing. Scott E. Eggener: Writing – review & editing. Arieh L. Shalhav: Writing – review & editing. Stanley L. Liauw: Writing – review & editing. Sean P. Pitroda: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Walter M. Stadler has acted as a member of the Data and Safety Monitoring Board for AstraZeneca, Merck, Pfizer, and Treadwell Therapeutics, and a consultant for AstraZeneca, Aveo, Caremark/CVS, EMA Wellness, Fortress Biotech, and XenCor. Sean P. Pitroda is co-founder and Chief Medical Officer of PersonaDx (including equity) and reports intellectual property unrelated to the current manuscript.
Contributor Information
Daniel Huang, Email: Daniel.Huang@uchicagomedicine.org.
Connor Lynch, Email: Connor.Lynch@uchicagomedicine.org.
Lucas M. Serra, Email: Lucas.Serra@uchicagomedicine.org.
Randy F. Sweis, Email: rsweis@bsd.uchicago.edu.
Paul J. Chang, Email: pchang1@bsd.uchicago.edu.
Walter M. Stadler, Email: Walter.Stadler@uchicagomedicine.org.
Russell Z. Szmulewitz, Email: rszmulew@bsd.uchicago.edu.
Peter H. O’Donnell, Email: podonnel@bsd.uchicago.edu.
Abhinav Sidana, Email: Abhinav.Sidana@bsd.uchicago.edu.
Scott E. Eggener, Email: seggener@bsd.uchicago.edu.
Arieh L. Shalhav, Email: ashalhav@bsd.uchicago.edu.
Stanley L. Liauw, Email: sliauw@bsd.uchicago.edu.
Sean P. Pitroda, Email: spitroda@uchicago.edu.
References
- 1.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Rembeyo G., Correas J.-M., Jantzen R., Audenet F., Dariane C., Delavaud C., et al. Percutaneous ablation versus robotic partial nephrectomy in the treatment of cT1b renal tumors: oncologic and functional outcomes of a propensity score-weighted analysis. Clin Genitourin Cancer. 2020;18:138–147. doi: 10.1016/j.clgc.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Hao G., Hao Y., Cheng Z., Zhang X., Cao F., Yu X., et al. Local tumor progression after ultrasound-guided percutaneous microwave ablation of stage T1a renal cell carcinoma: risk factors analysis of 171 tumors. Int J Hyperthermia. 2018;35:62–70. doi: 10.1080/02656736.2018.1475684. [DOI] [PubMed] [Google Scholar]
- 4.Wah T.M., Irving H.C., Gregory W., Cartledge J., Joyce A.D., Selby P.J. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int. 2014;113:416–428. doi: 10.1111/bju.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deschavanne P.J., Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol. 1996;34:251–266. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 6.Siva S., Ali M., Correa R.J.M., Muacevic A., Ponsky L., Ellis R.J., et al. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: an individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney) Lancet Oncol. 2022;23:1508–1516. doi: 10.1016/S1470-2045(22)00656-8. [DOI] [PubMed] [Google Scholar]
- 7.Siva S., Correa R.J.M., Warner A., Staehler M., Ellis R.J., Ponsky L., et al. Stereotactic ablative radiotherapy for ≥T1b primary renal cell carcinoma: a report from the international radiosurgery oncology consortium for kidney (IROCK) Int J Radiat Oncol. 2020;108:941–949. doi: 10.1016/j.ijrobp.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Correa R.J.M., Louie A.V., Staehler M., Warner A., Gandhidasan S., Ponsky L., et al. Stereotactic radiotherapy as a treatment option for renal tumors in the solitary kidney: a multicenter analysis from the IROCK. J Urol. 2019;201:1097–1104. doi: 10.1097/JU.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 9.Siva S., Bressel M., Sidhom M., Sridharan S., Vanneste B.G.L., Davey R., et al. Stereotactic ablative body radiotherapy for primary kidney cancer (TROG 15.03 FASTRACK II): a non-randomised phase 2 trial. Lancet Oncol. 2024;25:308–316. doi: 10.1016/S1470-2045(24)00020-2. [DOI] [PubMed] [Google Scholar]
- 10.Hannan R., McLaughlin M.F., Pop L.M., Pedrosa I., Kapur P., Garant A., et al. Phase 2 trial of stereotactic ablative radiotherapy for patients with primary renal cancer. Eur Urol. 2023;84:275–286. doi: 10.1016/j.eururo.2023.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanigan R.C., Roy V., Crawford E.D. Nephrectomy followed by interferon Alfa-2b compared with interferon Alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001 doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 12.Mickisch G., Garin A., Van Poppel H., De Prijck L., Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/S0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 13.Méjean A., Ravaud A., Thezenas S., Colas S., Beauval J.-B., Bensalah K., et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379:417–427. doi: 10.1056/NEJMoa1803675. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman R. A story of hypofractionation and the table on the wall. Int J Radiat Oncol. 2022;112:4–21. doi: 10.1016/j.ijrobp.2021.09.027. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team (2024). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; n.d.
- 16.Siva S., Louie A.V., Kotecha R., Barber M.N., Ali M., Zhang Z., et al. Stereotactic body radiotherapy for primary renal cell carcinoma: a systematic review and practice guideline from the International Society of Stereotactic Radiosurgery (ISRS) Lancet Oncol. 2024;25:e18–e28. doi: 10.1016/S1470-2045(23)00513-2. [DOI] [PubMed] [Google Scholar]
- 17.Olweny E.O., Park S.K., Tan Y.K., Best S.L., Trimmer C., Cadeddu J.A. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61:1156–1161. doi: 10.1016/j.eururo.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Zagoria R.J., Pettus J.A., Rogers M., Werle D.M., Childs D., Leyendecker J.R. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77:1393–1397. doi: 10.1016/j.urology.2010.12.077. [DOI] [PubMed] [Google Scholar]
- 19.Chow J., Hoffend N.C., Abrams S.I., Schwaab T., Singh A.K., Muhitch J.B. Radiation induces dynamic changes to the T cell repertoire in renal cell carcinoma patients. Proc Natl Acad Sci. 2020;117:23721–23729. doi: 10.1073/pnas.2001933117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A.K., Winslow T.B., Kermany M.H., Goritz V., Heit L., Miller A., et al. A pilot study of stereotactic body radiation therapy combined with cytoreductive nephrectomy for metastatic renal cell carcinoma. Clin Cancer Res. 2017;23:5055–5065. doi: 10.1158/1078-0432.CCR-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa R.J.M., Ahmad B., Warner A., Johnson C., MacKenzie M.J., Pautler S.E., et al. A prospective phase I dose-escalation trial of stereotactic ablative radiotherapy (SABR) as an alternative to cytoreductive nephrectomy for inoperable patients with metastatic renal cell carcinoma. Radiat Oncol. 2018;13:47. doi: 10.1186/s13014-018-0992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang C., Msaouel P., Hara K., Choi H., Le V., Shah A.Y., et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-centre, feasibility, phase 2 trial. Lancet Oncol. 2021;22:1732–1739. doi: 10.1016/S1470-2045(21)00528-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Schoenhals J., Christie A., Mohamad O., Wang C., Bowman I., et al. Stereotactic ablative radiation therapy (SAbR) used to defer systemic therapy in oligometastatic renal cell cancer. Int J Radiat Oncol. 2019;105:367–375. doi: 10.1016/j.ijrobp.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kataki K.J., Gupte A., Madhavan R., Beena K., Dutta D., Holla R., et al. Case report on stereotactic body radiation therapy for locally recurrent renal cell carcinoma after partial nephrectomy in a patient with single kidney. South Asian J Cancer. 2019;08:135–136. doi: 10.4103/sajc.sajc_7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Zhang X., Ma H., Tian L., Mai L., Long W., et al. Locoregional recurrence after nephrectomy for localized renal cell carcinoma: feasibility and outcomes of different treatment modalities. Cancer Med. 2022;11:4430–4439. doi: 10.1002/cam4.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maclean J, Breau RH, Scheida N, Malone S. Durable control of locally recurrent renal cell carcinoma using stereotactic body radiotherapy. BMJ Case Rep 2014:bcr2014206015. doi: 10.1136/bcr-2014-206015. [DOI] [PMC free article] [PubMed]
- 27.Sunela K.L., Kataja M.J., Kellokumpu-Lehtinen P.I. Changes in symptoms of renal cell carcinoma over four decades. BJU Int. 2010;106:649–653. doi: 10.1111/j.1464-410X.2010.09241.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Liu Z., Peng R., Liu Y., Zhang H., Wang G., et al. Neoadjuvant stereotactic ablative body radiotherapy combined with surgical treatment for renal cell carcinoma and inferior vena cava tumor thrombus: a prospective pilot study. BMC Urol. 2024;24:31. doi: 10.1186/s12894-024-01405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margulis V., Freifeld Y., Pop L.M., Manna S., Kapur P., Pedrosa I., et al. Neoadjuvant SABR for renal cell carcinoma inferior vena cava tumor thrombus—safety lead-in results of a phase 2 trial. Int J Radiat Oncol. 2021;110:1135–1142. doi: 10.1016/j.ijrobp.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labbate C., Hatogai K., Werntz R., Stadler W.M., Steinberg G.D., Eggener S., et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer. 2019;7:66. doi: 10.1186/s40425-019-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freifeld Y., Pedrosa I., Mclaughlin M., Correa R.M., Louie A.V., Maldonado J.A., et al. Stereotactic ablative radiation therapy for renal cell carcinoma with inferior vena cava tumor thrombus. Urol Oncol Semin Orig Investig. 2022;40:166.e9–166.e13. doi: 10.1016/j.urolonc.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali M., Koo K., Chang D., Chan P., Oon S.F., Moon D., et al. Low rate of severe-end-stage kidney disease after SABR for localised primary kidney cancer. Radiat Oncol. 2024;19:23. doi: 10.1186/s13014-024-02413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]