Abstract
Background
Rigid age limits in the current allocation system for post-mortem donor kidneys in Germany may have problematic effects. The new German national transplantion registry enables data analysis with respect to this question.
Methods
Using anonymized data from the German national transplantion registry, we extracted and evaluated information on the recipients and postmortem donors of kidneys that were allocated in Germany through Eurotransplant over the period 2006–2020.
Results
Data on 19 664 kidney transplantations in Germany from 2006 to 2020 were analyzed. The median waiting time for kidney transplantation was 5.8 years. Persons under age 18 waited a median of 1.7 years; persons aged 18 to 64, 7.0 years; and persons aged 65 and older, 3.8 years. Over the period of observation, post-mortem kidneys were transplanted into 401 people of age 64 (2.0% of all organ recipients) and 1,393 people of age 65 (7.1% of all organ recipients). The difference in waiting times between allocation programs for persons under age 65 (ETKAS, „Eurotransplant Kidney Allocation System“) and those aged 65 and older (ESP, „Eurotransplant Senior Program“) increased over the period of observation, from 2.6 years in 2006–2010 to 4.1 years in 2017–2020.
Conclusion
The rigid age limits in the current allocation rules for post-mortem kidney donations in Germany are prolonging the waiting times for transplants among patients aged 18 to 64. We think these rules need to be fundamentally reassessed.
Kidney transplantation is the best treatment for kidney failure both in terms of life expectancy and quality of life (1–3). However, not all patients with kidney failure have access to this therapy in Germany due to a long-standing shortage of kidney donors.
Given this scarcity, the rules for the allocation of organs are extremely important to these patients. In Germany, Eurotransplant International Foundation (ET) is responsible for allocating kidneys of deceased donors according to the guidelines of the German Medical Association (Bundesärztekammer) (5). Since 1999, kidney allocation programs have remained virtually unchanged in their core (Box) (6).
Box. Kidney allocation via Eurotransplant.
The majority of transplanted kidneys from deceased donors are allocated via Eurotransplant (ET) in two standard programs, the Eurotransplant Kidney Allocation System (ETKAS, introduced in 1996) on the one hand and the European Senior Program (ESP, introduced in 1999) on the other. In addition, a small number of special cases with very high immunological risk are allocated via the Acceptable Mismatch Program (AM, introduced in 1989); furthermore, there are other special programs for transplantations of more than one organ and very urgent cases.
ETKAS allocates kidneys from donors aged <65 to recipients aged <65. Organ allocation is based on a points system, with the person on the waiting list with the highest number of points receiving the organ offer. Points are awarded for waiting time, regional/national allocation, prior organ transplantations, and immunological factors. In all programs, the first day of ongoing dialysis is counted as the start of the waiting time. Children receive extra points up to their 18th birthday. Germany is the only country where the pediatric bonus cease completely upon reaching the age of majority.
ESP allocates kidneys of donors aged ≥ 65 to recipients aged ≥ 65. In this program, kidneys are initially allocated within the region (= close to the place of organ harvesting); the waiting time is the main factor that determines to which patient the organ is allocated. Furthermore, Germany is the only ET member state where there is a mandatory choice for patients aged ≥ 65 years between remaining in ETKAS or participating in ESP; in all other ET member states, participation in ESP is mandatory for persons aged ≥ 65 years.
If it is impossible to allocate an organ via the regular allocation programs—because, for example, no matching recipients are available—, then it is permitted to deviate from the usual allocation algorithms in order to prevent the loss of the organ (so-called rescue allocation). This is why, in rare cases, persons listed in ETKAS do receive an offer for transplantation of a kidney from a donor older than 65 years.
From an ethical point of view, when allocating an organ, potential societal benefits of organ transplantation must be weighed against an individual‘s right to equal opportunities (7). In addition, section 12 (3) of the German Transplantation Act (Transplantationsgesetz, TPG) stipulates that the rules for organ allocation must be based on the current state of medical science, while also taking in particular the urgency and the likelihood of a successful outcome into account when allocating organs.
Despite the current lack of publicly available data on the age distribution of kidney donors and the corresponding kidney recipients in Germany, it is assumed that the allocation rules result in age discrimination due to two rigid age limits:
The first age limit is the 18th birthday. As the so-called child bonus is lost at this age, the probability of an organ offer being made to young adults decreases significantly from this age (8, 9).
The second critical age limit is the 65th birthday. From this age, it is possible to transition from the allocation algorithm for persons under age 65 (Eurotransplant Kidney Allocation System, ETKAS) to the Eurotransplant Senior Program (ESP).
Initially, ESP was introduced in an effort to use overall more organs from older donors for transplants. In the past, kidneys from older donors were often not accepted for transplantation over fears that these organs would not survive long enough. (8). So far published isolated data from organ recipients suggest that currently the waiting times differ significantly between ETKAS and ESP (8, 10).
Thanks to the transplant registry that was established in 2016 as required by law, it is now possible for the first time to bring together nationwide data records of organ donors and corresponding organ recipients (11). Based on an analysis of these data sets, we present in this article previously unknown facts on the significance of age for the allocation of donor kidneys at the federal level. In addition, we describe the implications of the current allocation algorithms.
Methods
Our analyses are based on the retrospective data set of the German National Transplantation Registry (data from 1 January 2006 to 31 December 2016) as well as the currently available set of new data (prospective data from 1 January 2017 to 31 December 2020).The data sets contain information from transplantation centers, Eurotransplant (ET) and the German Organ Procurement Organization (Deutsche Stiftung Organtransplantation, DSO) about kidney donors (post-mortem and living kidney donation) as well as recipients of solid organs, the procured organs, transplantation procedures, and the allocation procedures. Furthermore, the data sets include follow-up data of living donors and kidney recipients (12).
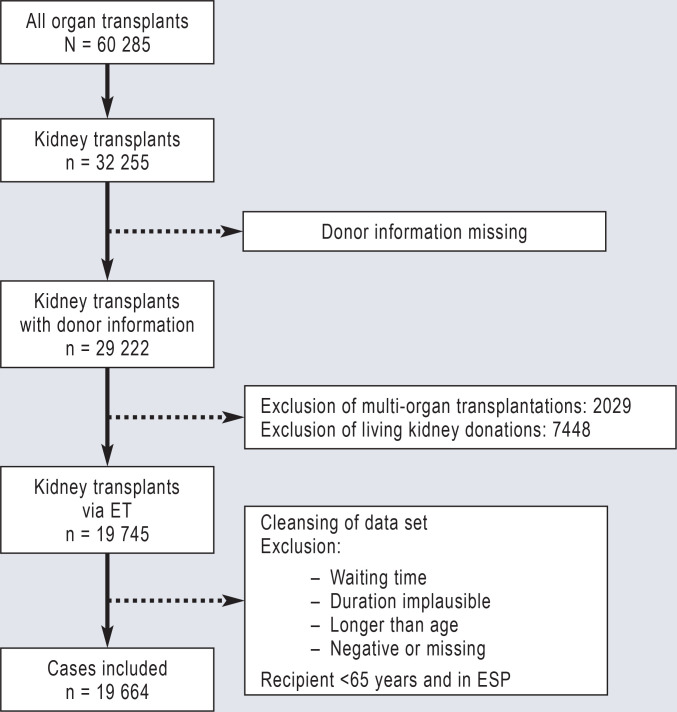
From these data, all kidney transplantations after post-mortem organ donation were identified where the recipients were listed in ETKAS, ESP or the Acceptable Mismatch Program (AM program) for a kidney transplantation. Data sets on transplantations with implausible or missing entries were excluded from the analysis, as were living donations and transplantations with allocation of kidneys simultaneously with other organs (eFigure 1).
eFigure 1.
Flow diagram of data set cleansing. The first step was to filter the kidney transplantations from all organ transplantations included in the data set and exclude entries with missing donor information. The second step was to exclude all living organ and multi-organ transplantations, because these recipients do not receive organs via the standard allocation programs for kidneys at Eurotransplant (ET). The last step was to clean the data set for implausible entries. The resulting entries on post-mortem kidney donations were then evaluated in the further analyses.
ESP, Eurotransplant Senior Program
The age at the time of organ transplantation was analyzed to present the age distributions of organ donors and organ recipients. The start of the waiting time for an organ was considered to be the date stored for the start of ongoing dialysis; in the case of a pre-emptive listing (n = 180), the listing date was taken as the start of the waiting time.
The development environment and programming language R (version 4.3.0) with the packages “tidyverse” (13), “readxl“ (14), “gtsummary“ (15), “grid“ (16), “Gmisc“ (17), and “cowplot“ (18) were used for data processing and preparation.
Results
The analyzed data sets from the German National Transplantation Registry comprised entries from a total of 60 285 organ transplantations, of which 32 255 were kidney transplantations. After excluding living donations, multi-organ transplantations and adjusting for cases with implausible entries, 19 664 transplantations with kidneys from 12 356 deceased donors remained, which were then evaluated (eFigure 1).
The recipients of the organs were listed in 71.0% of cases in the ETKAS, in 26.9% of cases in the ESP and in the remaining cases (2.1%) in the AM program. While men were the organ recipients in about two thirds of cases, they accounted for only about half of organ donations (eTable).
eTable. Characteristics of post-mortem donor kidneys and kidney recipients in the various allocation systems at Eurotransplant 2006–2020.
| Characteristics |
Total
N = 19 664* |
AM
n = 422* |
ESP
n = 5 288* |
ETKAS
n = 13 954* |
| Sex of recipient | ||||
| Male | 12 436 (63%) | 185 (44%) | 3 638 (69%) | 8 613 (62%) |
| Female | 7 228 (37%) | 237 (56%) | 1 650 (31%) | 5 341 (38%) |
| Sex of donor | ||||
| Male | 10 325 (53%) | 228 (54%) | 2 481 (47%) | 7 616 (55%) |
| Female | 9 339 (47%) | 194 (46%) | 2 807 (53%) | 6 338 (45%) |
| Recipient age (years) | 57 (46–66) | 49 (41–56) | 68 (66–71) | 51 (42–59) |
| Donor age (years) | 57 (46–67) | 49 (40–57) | 72 (68–76) | 51 (42–58) |
| Waiting time (yrs) | 5.8 (3.2–8.0) | 4.7 (3.2–7.1) | 3.7 (2.3–5.2) | 6.9 (4.2–8.6) |
* n (%); Median (IQR)
AM, Acceptable Mismatch Program; ESP, European Senior Program; ETKAS, Eurotransplant Kidney Allocation System; IQR, interquartile range
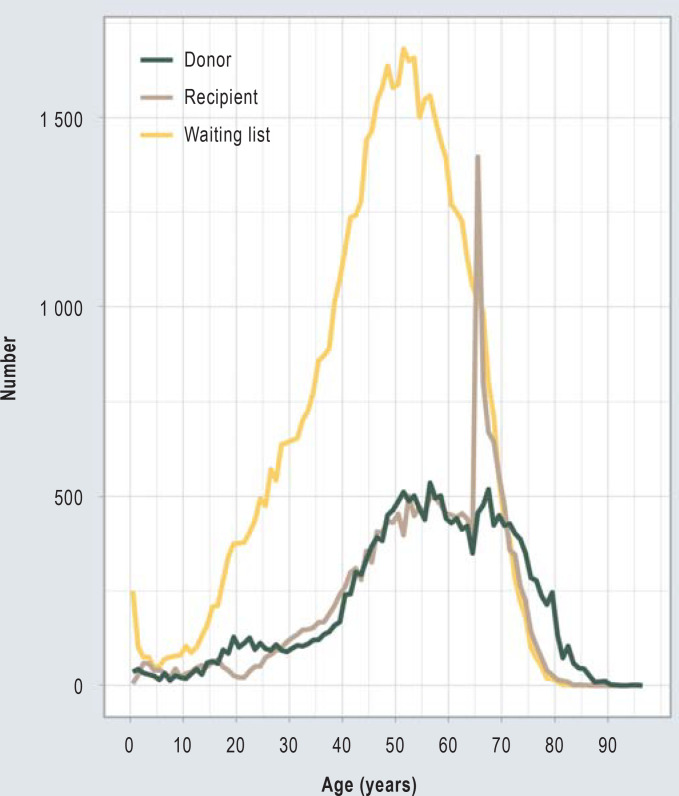
The median age of organ recipients was 49.1 years at the beginning of the waiting time. The age distribution shows an increase in the number of organ donors with increasing age, with a maximum at around the age of 55, after which it falls again, with the median age of all organ donors being 57. In addition, there is a noticeable peak around the age of 20.
Likewise, the age distribution of organ recipients shows a maximum around age 55 (median 57 years) and then initially drops again. For the 66th and 67th year of life, however, there is a distinctly disproportionate increase in kidney recipients, which is not reflected in the age distribution of organ donors or patients on the waiting list. While only 401 64-year-old persons (2.1% of all organ recipients) received a transplant kidney, 1393 65-year-old persons received a transplant kidney (7.1% of all organ recipients) during the same period of time (Figure 1).
Figure 1.
Age distribution of patients on the waiting list and age distribution of kidney donors and recipient in Germany 2006–2020. Only post-mortem kidney transplantations to organ recipients listed at Eurotransplant in the ETKAS, ESP or AM program were evaluated. With patients on the waiting list (n = 49 323), only initial listings were included. For waiting list patients, the age at first start of dialysis was evaluated, for organ donors and recipients their age at the time of organ transplantation.
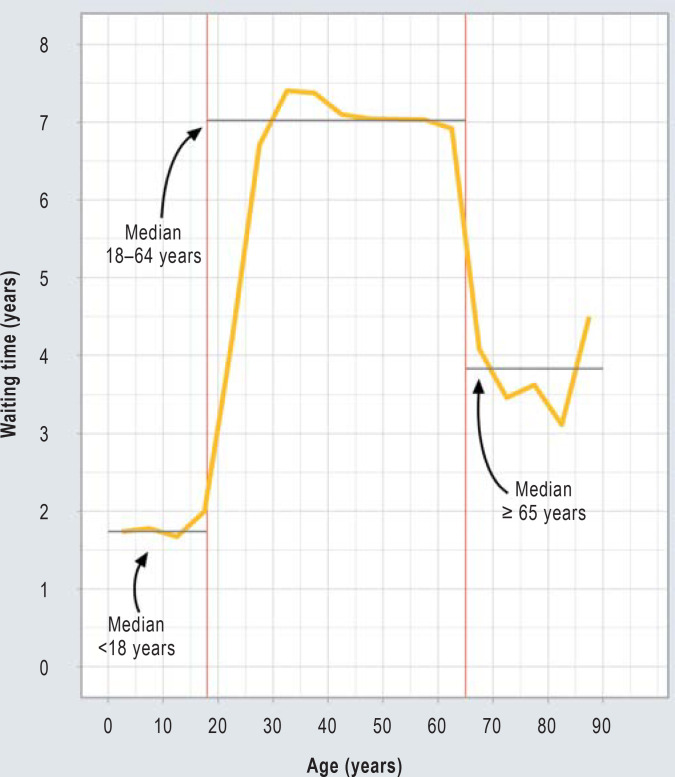
The median waiting time of organ recipients from the start of dialysis to kidney transplantation was 5.8 years. The median waiting time for a transplant kidney was strongly associated with age. Children younger than 18 years waited 1.7 years. After the 18th birthday, there is a sharp increase in waiting time. The median waiting time for persons aged between 18 and 64 was 7.0 years. Upon reaching the age of 65, a second sudden change in waiting time is noticeable. Persons over the age of 65 waited a median of 3.8 years for a kidney transplantation (Figure 2).
Figure 2.
Age-dependent waiting times for kidney transplantation in Germany 2006–2020. For the graphical representation, 5-year age groups were formed, the median waiting time was determined and the individual data points were linked. The median waiting times are shown for the following groups: <18 years (1.7 years [IQR 0.8–2.6]), 18–64 years (7.0 years [IQR 4.9–8.7]) and ≥ 65 years (3.8 years [IQR 2.3–5.5]). The red markers indicate ages 18 and 65.
Waiting times also differed significantly between the various allocation programs: In ETKAS, the median waiting time was 6.9 years, in the AM program 4.7 years and in ESP only 3.7 years (eTable). The waiting time within ETKAS also varied dependent on age. While persons aged 18 to 64 years waited 7.1 years for a kidney transplantation in ETKAS, the waiting time for organ recipients over 65 years of age was only 5.4 years in ETKAS (Table 1).
Table 1. Characteristics and waiting times of kidney recipients in the various allocation systems at Eurotransplant 2006–2020, depending on age.
| Age 18–64 years | Age <18 years | Age ≥65 years | |||||||
|
Total
N = 12 756* |
AM
n = 387* |
ETKAS
n = 12 369* |
Total
N = 746* |
AM
n = 6* |
ETKAS
n = 740* |
Total
N = 6 162* |
AM
n = 29* |
ESP
n = 5 288* |
ETKAS
n = 845* |
| Waiting time (years) | |||||||||
| 7.0 (4.9–8.7) |
4.8 (3.2–7.3) |
7.1 (5.0–8.8) |
1.7 (0.8–2.6) |
2.3 (1.3–3.4) |
1.7 (0.8–2.6) |
3.8 (2.3–5.5) |
3.7 (3.2–4.9) |
3.7 (2.3–5.2) |
5.4 (2.6–8.7) |
* Median (IQR); AM, Acceptable Mismatch Program; ESP, European Senior Program; ETKAS, Eurotransplant Kidney Allocation System; IQR, interquartile range
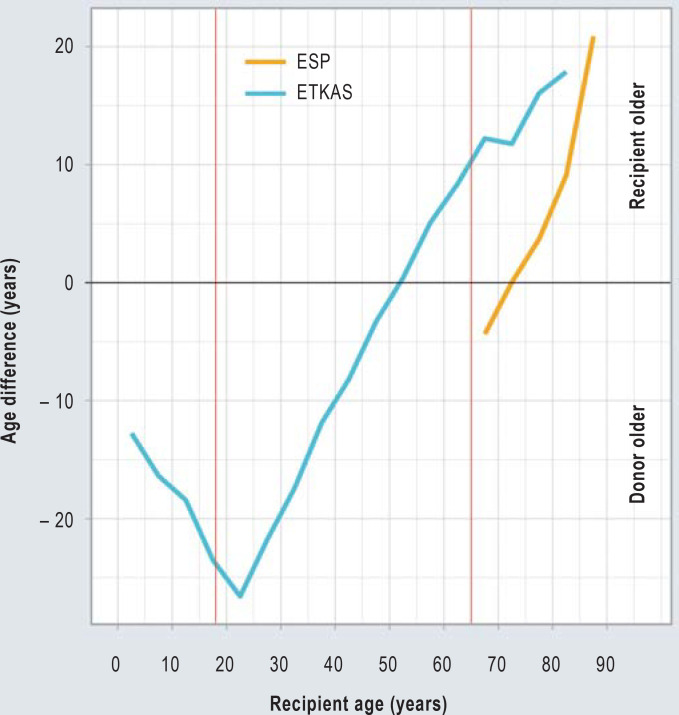
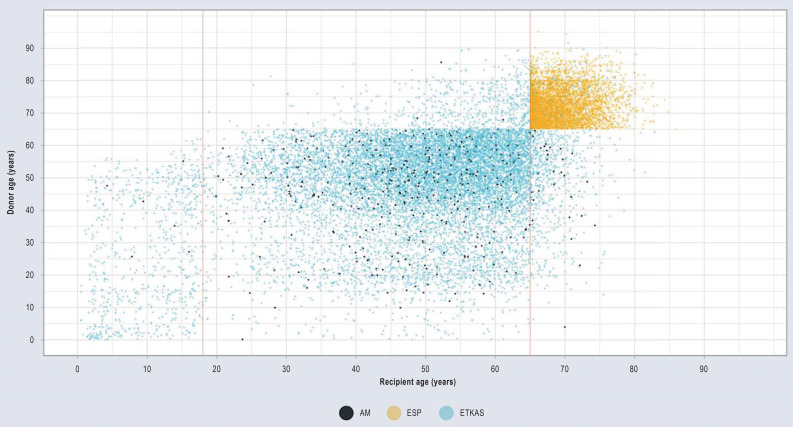
Although the median ages of donors and recipients were close to each other (eTable), there were significant variations between age strata and allocation programs. Organ recipients aged 18 to 30 in particular regularly received considerably older organs (Figure 3). In individual cases, these age differences amounted to several decades (eFigure 2). In ESP, organ recipients also received older organs (Figure 3); on average, the kidneys transplanted in ESP originated from donors who were 4 years older than the recipients (eTable).
Figure 3.
Age differences between post-mortem kidney donors and kidney recipients in Germany 2006–2020. Organ allocations by the Eurotransplant Kidney Allocation System (ETKAS, blue) and the European Senior Program (ESP, yellow) are depicted separately. The red markers indicate an age of 18 and 65 years, respectively. A negative age difference means that the donor is older, a positive age difference means that the recipient is older. For the graphical representation, 5-year age groups were formed for donors and recipients; for each of these, the median age difference was calculated.
eFigure 2.
Comparison of donor and recipient age by allocation program in Germany 2006–2020. Each plotted point corresponds to a single evaluated kidney transplantation (black dots: Acceptable Mismatch Program (AM); yellow dots: Eurotransplant Senior Program (ESP), blue dots: Eurotransplant Kidney Allocation System (ETKAS). The red markers indicate an age of 18 and 65 years, respectively. The wide variation indicates that there is a large discrepancy between the age of recipients and donors.
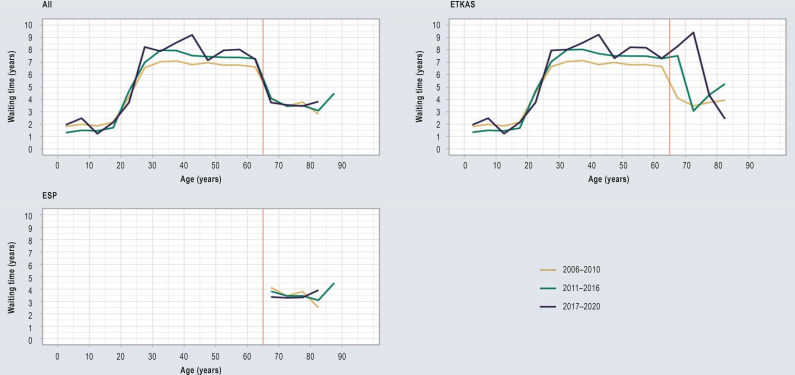
The difference in waiting times between ETKAS and ESP increased continuously between 2006 and 2020. Compared to the years 2006–2010, the median waiting time in ETKAS was 0.9 years longer, while it decreased in ESP by 0.6 years. Furthermore, an increasingly large number of organs were allocated to ETKAS-listed recipients via the rescue allocation procedure (Table 2, eFigure 3).
Table 2. Characteristics and waiting times of post-mortem donor kidneys and kidney recipients in the various allocation systems at Eurotransplant over the years 2006–2020.
| Period 2006–2010 | Period 2011–2016 | Period 2017–2020 | |||||||||
|
Total
N = 9768*1 (1 954/yr)*2 |
AM
n = 134*1 |
ESP
n = 2478*1 |
ETKAS
n = 7156*1 |
Total
N = 8249*1 (1650/yr)*2 |
AM
n = 234*1 |
ESP
n = 2289*1 |
ETKAS
N = 5726*1 |
Total
N = 1 647*1 (330/yr)*2 |
AM
n = 54*1 |
ESP
n = 521*1 |
ETKAS
n = 1072*1 |
| Recipient age (years) | |||||||||||
| 56 (45–65) |
48 (40–55) |
68 (66–70) |
50 (41–58) |
58 (47–66) |
49 (42–57) |
69 (67–72) |
52 (43–60) |
60 (48–67) |
52 (45–57) |
69 (67–72) |
53 (40–61) |
| Donor age (years) | |||||||||||
| 55 (45–67) |
47 (41–56) |
71 (68–75) |
50 (42–58) |
57 (47–68) |
50 (41–57) |
72 (69–76) |
52 (42–59) |
60 (48–69) |
45 (32–57) |
72 (68–77) |
53 (41–60) |
| Waiting time (years) | |||||||||||
| 5.9 (3.3–7.6) |
4.7 (3.4–6.8) |
4.0 (2.4–5.3) |
6.6 (4.0–8.0) |
5.9 (3.2–8.6) |
4.7 (3.2–7.3) |
3.6 (2.3–5.1) |
7.2 (4.5–9.4) |
5.3 (2.8–8.9) |
4.5 (3.1–7.7) |
3.4 (2.2–4.7) |
7.5 (3.7–9.9) |
| Rescue allocation | |||||||||||
| 609 (6.2%) |
0 (0%) |
38 (1.5%) |
571 (8.0%) |
835 (10%) |
0 (0%) |
78 (3.4%) |
757 (13%) |
215 (13%) |
0 (0%) |
13 (2.5%) |
202 (19%) |
*1 n (%); median (IQR); *2 mean; yr, year; AM, Acceptable Mismatch Program; ESP, European Senior Program; ETKAS, Eurotransplant Kidney Allocation System;
IQR, interquartile range
eFigure 3.
Development of the waiting time among transplant kidney recipients in Germany over the years 2006–2020. To present this information in a graphic, the periods 2006–2010, 2011–2016 and 2017–2020 were plotted separately. Five-year age groups were formed, the median waiting time was determined and the individual data points were linked. The plotting was carried out for all kidney transplant recipients together as well as separately for the Eurotransplant Kidney Allocation System (ETKAS) and the European Senior Program (ESP).
Discussion
In our study, we analyzed for the first time data of the German National Transplantation Registry on the allocation of kidneys of deceased organ donors on a federal level. When considering the distribution of age and waiting time, two prominent findings emerge:
Two circumstances bring about a significant change in the waiting time: reaching adulthood and the 65th birthday.
There are a disproportionate number of 65– and 66-year-old organ recipients
The reason behind this is the rigid age limits set in the current allocation system. The first of these limits is the age at which children are declared adults for the purposes of the allocation system. Up to 2010, this age was 16 years in all Eurotransplant countries; it was then increased to 18 years (19). Currently, children in Germany who are on the waiting list for a kidney transplantation in ETKAS receive a pediatric bonus until their 18th birthday and preferentially younger kidneys from pediatric donors. This bonus is waived entirely in Germany upon reaching the age of 18 (20). Germany is not the only country in the world to take this approach. A rigid age limit for the prioritization of children and young adults when allocating transplant kidneys was and remains a common feature of many allocation programs; this is how it is handled in many European countries and in the United States (21, 22). However, the transition from pediatric to adult health care is a particularly critical phase in the life of children living with chronic disease; it is generally accepted that this should not be rigidly linked to the 18th birthday and, especially in transplantation medicine, this rigid limit poses a major challenge for everyone involved (23–25). Other ET member states have taken the highly variable adolescence process in children with kidney failure into account and introduced in 2023 that the pediatric bonus is granted beyond the 18th birthday and gradually decreases until the age of 30; Germany is the only exception in that it has not adopted this new rule (20).
The second rigid age limit in the current allocation system for donor kidneys in Germany is the 65th birthday from which it is possible to transition from ETKAS to ESP (20). The majority of people over 65 receive donor kidneys within ESP. In this allocation system, organ of donors aged over 65 are allocated to recipients over 65 years of age. Thanks to the comparatively higher supply of organs from older donors, the waiting time is significantly shorter in ESP compared to ETKAS. When ESP was introduced, this effect was purposely factored in to promote equal opportunities for individuals in need of a kidney transplant. The advantage of a shorter waiting time in ESP comes with the disadvantage that the organs are older and therefore have a shorter transplant lifespan than organs from younger donors (26, 27).
But why does this lead to the disproportionately high number of 65– and 66-year-old recipients? On turning 65, people on the waiting list for kidney transplantation meet the entry requirements for ESP and can transfer their previously accumulated waiting time to it (20). If these persons have already waited several years in ETKAS and then have themselves transferred to ESP when they turn 65, they immediately are very likely to qualify for a transplantation due to the waiting time they have already completed.
There is a need to discuss this rigid age limit for a variety of ethical reasons: First of all, this age limit collides with the principle of individual equality of opportunity, as it cannot be justified objectively why, for example, a 65-year-old individual should have the right to receive a donor kidney significantly faster than a 64-year-old person. In addition, the rigid age limit is also not helpful when viewed against the principle of individual benefit. In principle, younger persons can also benefit from older donor kidneys with regard to their life expectancy, even if the transplant kidneys should fail at some point before their death (28). This applies in particular when waiting times for a donor organ are as long as they currently are in ETKAS (29). Furthermore, it can be argued that allocating organs to younger persons is of greater benefit to society as a whole, since younger persons naturally have a better life expectancy after kidney transplantation than older persons and thus fewer younger persons die with a functioning kidney transplant (30).
In order to ensure maximum benefit from the allocation of a donor kidney, the life expectancy of the recipient should be in line with the expected functional life of the transplant. The age of the recipient and the age of the donor are the best individual predictors of life expectancy and the functional life of a transplant kidney (26, 27, 31). From this perspective, it is therefore useful to perform age-matching, i.e. to allocate young organs to young persons and older organs to older persons (7). During the last revision of the US allocation system, the concept of life years from transplantation (LYFT) was discussed at great length. This concept is based on the idea that an analysis is carried out for each donated organ to determine which potential recipient would gain the most years of life from the transplantation and allocates this organ to the respective person. At that time, it was calculated that the concept had the potential to save thousands of years of life in the United States every year (32, 33). Ultimately, however, it was not implemented because parts of society considered it to be too unequal and discriminatory against older and ill persons (7).
In 2014, a complex system was introduced as a compromise. In this system, a kidney donor profile index (KDPI) is calculated to estimate transplant survival and the “estimated posttransplant survival score“ (EPTS) is determined in order to rank recipient survival. Based on this system, 20% of transplant kidneys with the most favorable prognosis were allocated to the 20% of recipients with the best statistical life expectancy. The remaining transplant kidneys are allocated irrespective of the expected benefit so that all candidates on the waiting list have a chance to receive a kidney transplant (34).
The UK also implemented a similar procedure. There, the expected recipient life expectancy and transplant lifespan is estimated by calculating a recipient risk index and a donor risk index; in the case of a good match, allocation bonus points are awarded. This approach is intended to ensure that fewer older organ recipients die while their transplant is still functioning and that fewer young organ recipients require a second transplant because they survive their first transplant (35).
Apart from these considerations, the wording of the German Transplantation Act (TPG) also gives no reason to accept the age limit of 65 years for ESP. The limit is set arbitrarily and thus not based on the current state of scientific evidence. Furthermore, it does not improve the chances of success of the kidney transplantation and does not account for urgency. What is more: The waiting time differences between the two allocation algorithms ETKAS and ESP are getting increasingly divergent. On the one hand, this is due to a decreasing waiting time in ESP, on the other hand to an increasing waiting time in ETKAS. Given that the demographic development suggests that this trend will continue, this aspect is an additional reason to question the current allocation rules.
Limitations
With this data from the Transplantation Registry, information on donors and recipients of transplant organs can be brought together for the first time in Germany. Our analyses are based on two separate data sets. The data for the years 2006–2016 is obtained from the anonymized retrospective data set. Due to the fact that it is mandatory to communicate the variables that we have evaluated (age, gender, date of start of dialysis and date of transplantation) to ET, the quality of these data is good and we were able to almost completely analyze the transplantations recorded for this period.
The second data set evaluated contains information for the years 2017–2020 that was communicated prospectively. The total number of evaluable transplantations in this data set was considerably lower compared to the retrospective data set. That the documentation is incomplete is most likely due to the fact that the communication of personal data for the prospective data set is only permitted with express consent, while this was not required for the old retrospective data set (12). For this reason, no personal data are recorded in many cases. However, given that the allocation rules have not changed significantly during this period and that the increase in waiting time observed by us is consistent with the results of a recently published study which is based on data provided directly by ET, we assume that it is possible to achieve robust results even with a smaller number of evaluable transplantations.
Conclusion
Organ allocation is a very complex process, in which many other aspects, such as immunological (36) and logistical factors (10), must be taken into account, apart from those addressed in this article. Our findings, however, reveal problems that are attributable to the more or less arbitrary rigid age limits set in the allocation algorithms. These problems make a fundamental debate on the rules for the allocation of kidneys of deceased donors urgently necessary.
Acknowledgments
Translated from the original German by Ralf Thoene, M.D.
Footnotes
Conflict of interest statement
BK received research funding from Sanofi.
HUZ received third-party funding from the German Federal Ministry of Education and Research (BMBF) (funding code: 01ZX1912A).
AR is a Member of the Standing Commission on Organ Transplantation of the German Medical Association (BÄK, Bundesärztekammer) and a member of the Specialist Advisory Board of the German National Transplantation Registry.
SS is a member of the Ethics and Steering Committee of the EU-funded project Torque Teno Virus Monitoring.
FB is secretary to the board of the German Transplantation Society (DTG, Deutsche Transplantationsgesellschaft). He received reimbursement of travel expenses from DTG and ET. In addition, he is a member of the Eurotransplant Registry Advisory Committee and the Eurotransplant Liver and Intestine Advisory Committee.
The remaining authors declare that no conflict of interest exists.
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surgery. 2015;150:252–259. doi: 10.1001/jamasurg.2014.2038. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transpl. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 4.Deutsche Stiftung Organtransplantation. Organspende und Transplantation in Deutschland. Jahresbericht 2022. https://dso.de/organspende/statistiken-berichte/jahresbericht (last accessed on 20 May 2023) [Google Scholar]
- 5.Bundesärztekammer. Richtlinie gem. § 16 Abs. 1 S. 1 Nrn. 2 und 5 TPG für die Wartelistenführung und Organvermittlung zur Nierentransplantation vom 14.10.2022. DOI: 10.3238/arztebl.2023.RiliOrgaWlOv_neubek- (last accessed on 4 July 2024) [Google Scholar]
- 6.Langer RM, Cohen B, Rahmel A. History of Eurotransplant. Transplant Proc. 2012;44:2130–2131. doi: 10.1016/j.transproceed.2012.07.125. [DOI] [PubMed] [Google Scholar]
- 7.Stegall MD, Stock PG, Andreoni K, Friedewald JJ, Leichtman AB. Why do we have the kidney allocation system we have today? A history of the 2014 kidney allocation system. Hum Immunol. 2017;78:4–8. doi: 10.1016/j.humimm.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Echterdiek F, Heemann U. Allokation von Nieren im Zeitalter des Organmangels in Deutschland. Die Nephrologie. 2023;18:72–77. [Google Scholar]
- 9.Schicktanz S, Simon A, Raphael S, Ahlert M. The ethical debate over child priority in post-mortem organ allocation: a scoping review and practical-ethical outlook. Transplant Rev. 2020;34 doi: 10.1016/j.trre.2020.100543. 100543. [DOI] [PubMed] [Google Scholar]
- 10.Zecher D, Tieken I, Wadewitz J, Zeman F, Rahmel A, Banas B. Regional differences in waiting times for kidney transplantation in Germany. Dtsch Arztebl Int. 2023;120:393–399. doi: 10.3238/arztebl.m2023.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deutsches Transplantatregister. https://transplantations-register.de/ (last accessed on 20 May 2023) [Google Scholar]
- 12.Deutsches Transplantatregister. https://transplantations-register.de/register-datenschutz (last accessed on 14 May 2024) [Google Scholar]
- 13.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4:1686. [Google Scholar]
- 14.Wickham H, Bryan J, Kalicinski M, et al. Package ‘readxl’. Version, 13. 2019:1. [Google Scholar]
- 15.Daniel DS, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible summary tables with the gtsummary package. RJ. 2021;13:570–580. [Google Scholar]
- 16.Murrell P. The grid graphics package. R News. 2002;2:14–19. [Google Scholar]
- 17.Gordon M. Gmisc: descriptive statistics, transition plots, and more. R Package, Version 2016. :1. [Google Scholar]
- 18.Wilke CO, Wickham H, Wilke MCO. Package ‘cowplot’. Streamlined plot theme and plot annotations for ‘ggplot2. 2019:1. [Google Scholar]
- 19.Tönshoff B, Boer JD, Rahmel A, Heemann U. Änderungen der Allokation in der pädiatrischen Nierentransplantation bei Eurotransplant. Transplantationsmedizin. 2011:47–57. [Google Scholar]
- 20.Eurotransplant. Eurotransplant Manual. Chapter 4: Kidney (ETKAS and ESP). Version 2023 [Google Scholar]
- 21.Harambat J, van Stralen KJ, Schaefer F, et al. Disparities in policies, practices and rates of pediatric kidney transplantation in Europe. Am J Transpl. 2013;13:2066–2074. doi: 10.1111/ajt.12288. [DOI] [PubMed] [Google Scholar]
- 22.Engen RM, Smith JM, Bartosh SM. The kidney allocation system and pediatric transplantation at 5 years. Pediatr Transplant. 2022;26 doi: 10.1111/petr.14369. e14369. [DOI] [PubMed] [Google Scholar]
- 23.Gesellschaft für Transitionsmedizin. S3-Leitlinie: Transition von der Pädiatrie in die Erwachsenenmedizin. Version 1.1 vom 22.04.2021. https://register.awmf.org/de/leitlinien/detail/186-001 (last accessed on 4 July 2024) [Google Scholar]
- 24.Sawyer SM, Drew S, Yeo MS, Britto MT. Adolescents with a chronic condition: challenges living, challenges treating. Lancet. 2007;369:1481–1489. doi: 10.1016/S0140-6736(07)60370-5. [DOI] [PubMed] [Google Scholar]
- 25.Akchurin OM, Melamed ML, Hashim BL, Kaskel FJ, Del Rio M. Medication adherence in the transition of adolescent kidney transplant recipients to the adult care. Pediatr Transplant. 2014;18:538–548. doi: 10.1111/petr.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 27.Schulte K, Klasen V, Vollmer C, Borzikowsky C, Kunzendorf U, Feldkamp T. Analysis of the eurotransplant kidney allocation algorithm: how should we balance utility and equity? Transplant Proc. 2018;50:3010–3016. doi: 10.1016/j.transproceed.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Sáez MJ, Arcos E, Comas J, Crespo M, Lloveras J, Pascual J. Survival benefit from kidney transplantation using kidneys from deceased donors aged ≥75 years: a time-dependent analysis. Am J Transpl. 2016;16:2724–2733. doi: 10.1111/ajt.13800. [DOI] [PubMed] [Google Scholar]
- 29.Massie AB, Luo X, Chow EKH, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transpl. 2014;14:2310–2316. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 30.United States Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2023 USRDS Annual Data Report: epidemiology of kidney disease in the United States. [Google Scholar]
- 31.Clayton PA, McDonald SP, Snyder JJ, Salkowski N, Chadban SJ. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am J Transpl. 2014;14:1922–1926. doi: 10.1111/ajt.12761. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe RA, McCullough KP, Schaubel DE, et al. Calculating life years from transplant (LYFT): methods for kidney and kidney-pancreas candidates. Am J Transpl. 2008;8:997–1011. doi: 10.1111/j.1600-6143.2008.02177.x. [DOI] [PubMed] [Google Scholar]
- 33.Stegall MD. Developing a new kidney allocation policy: the rationale for including life years from transplant. Am J Transpl. 2009;9:1528–1532. doi: 10.1111/j.1600-6143.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- 34.OPTN Policy 8. Allocation of kidneys, effective date 5/2/2024. https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf (last accessed on 24 May 2024) [Google Scholar]
- 35.NHS Blood and Transplant. POL186/18—Kidney Transplantation: deceased donor organ allocation october 2023. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/33034/pol186-080423.pdf (last accessed on 5 July 2024) [Google Scholar]
- 36.Williams RC, Opelz G, McGarvey CJ, Weil EJ, Chakkera HA. The risk of transplant failure with HLA mismatch in first adult kidney allografts from deceased donors. Transplantation. 2016;100:1094–1102. doi: 10.1097/TP.0000000000001115. [DOI] [PMC free article] [PubMed] [Google Scholar]