Abstract
Aim
This Mendelian randomisation (MR) study endeavoured to delineate the causal relationship between periodontitis and adverse pregnancy outcomes (APOs), encompassing low birthweight (LBW), pre-term birth (PTB), stillbirth, miscarriage, and gestational hypertension (GH).
Methods
Utilising genetic instruments for periodontitis (acute and chronic periodontitis) from the Genome-Wide Association Study (GWAS) database among individuals of European descent, this study explored the causal relationship with adverse pregnancy outcomes, and vice versa. The Inverse Variance Weighted (IVW) method was employed as the primary analytical approach to assess causality, with MR-Egger serving as a sensitivity analysis method.
Results
The primary analytical method employed in this study, IVW, did not reveal any impact of periodontitis (acute and chronic periodontitis) on PTB, stillbirth, miscarriage, and gestational hypertension, and vice versa. Heterogeneity testing using the MR-Egger method confirmed the null causal hypothesis, with odds ratios (OR) approximating 1, and P-values exceeding 0.05. Notably, the results from the IVW analysis (OR 1.410, CI 1.039-1.915, P-value 0.028) indicate statistically significant evidence supporting a causal relationship between chronic periodontitis and LBW. However, caution is advised in interpreting the causal relationship, considering the non-significant P-values obtained from other methods.
Conclusion
Within the limitations of this MR study, the findings do not support the influence of periodontitis on LBW, PTB, stillbirth, miscarriage, and GH, nor vice versa.
Keywords: Mendelian randomisation, Periodontitis, Adverse pregnancy outcomes, Low birthweight, Pre-term birth, Stillbirth, Miscarriage, Gestational hypertension
Introduction
Periodontitis, an inflammatory condition impacting the supporting tissues around teeth, including the gingiva, periodontal ligament, and alveolar bone, culminates in tooth loosening, representing its ultimate clinical outcome.1 Presently, approximately 50% of the global adult population grapples with periodontitis, and around 23.6% contend with severe forms of the ailment. It has evolved into a prominent public health challenge, casting a wide influence on global oral health.2 The pathogenesis of periodontitis involves a complex interplay of factors, with a notable aspect being the compromised immune and metabolic responses provoked by periodontal pathogens.3,4 Gingivitis serves as the initial stage characterised by reversible inflammation, progressing to involve bone, gums, and periodontal ligaments, ultimately exposing deep structures to oral microorganisms.5 The host's inflammatory response to bacteria instigates immune-mediated cellular reactions, causing degradation of periodontal tissues and, ultimately, tooth loss.6 According to the 1999 classification, periodontitis encompasses aggressive (acute) and chronic forms.7 Aggressive periodontitis, distinguished by early onset and rapid progression, is less prevalent (around 0.1% prevalence), while chronic periodontitis, the more common phenotype, tends to affect the elderly more frequently.8,9
Adverse pregnancy outcomes (APOs) refer to unsuccessful pregnancies resulting in offspring with abnormal appearance or function, encompassing pre-term birth, low birth weight, abortion, and more.10 In recent years, the incidence of APOs has surged, becoming a primary cause of perinatal infant and child mortality. This phenomenon poses a substantial societal and familial burden, evolving into a serious public health concern.11,12 Past studies have implicated smoking, malnutrition, and unhealthy lifestyles as APOs risk factors.13, 14, 15 Notably, the connection between local and systemic inflammatory immune responses and APOs has garnered increased attention.12,16 Periodontitis, in particular, is thought to impact pregnancy outcomes by allowing periodontal pathogens and their metabolites to enter the bloodstream, triggering a host immune inflammatory response.17 As early as 1996, Offenbacher et al. reported a 7.5 times higher probability of pre-term birth in pregnant women with periodontal disease, linking APOs with periodontal disease and indicating periodontal infection as a risk factor for low birth weight infants.18
Nevertheless, the current clinical evidence concerning the association between APOs and periodontitis remains contentious.19,20 Epidemiology-based studies exhibit significant flaws, including methodological inconsistencies that hinder comparability and undermine the credibility of conclusive findings.21 Moreover, shared risk factors such as smoking, alcohol consumption, and stress complicate observational studies, acting as confounding variables and potentially leading to spurious associations.22 Notably, the causal link between smoking, alcohol consumption, and periodontitis has been established by scholars.23 Therefore, unravelling the potential causal relationship between APOs and periodontitis holds paramount importance for enhancing the management of both conditions. To address this, we conducted a 2-way Mendelian randomisation (MR) study investigating the association between periodontitis and APOs, accompanied by sensitivity analyses to account for pleiotropic single-nucleotide polymorphisms (SNPs) linked to potential confounders.
MR serves as a statistical model utilising genetic variation as instrumental variables (IVs).24 In recent years, propelled by the swift progress in genome-wide association analysis data, MR has gained widespread adoption in causal association analysis.25,26 The fundamental tenet of MR analysis hinges on leveraging the relationship between genes and phenotypes to discern potential causal links.27 This method draws upon Mendel's second law of genetics, whereby genetic variation is randomly distributed across individuals and populations, mirroring an experimental process akin to a randomised controlled trial.28 In contrast to traditional epidemiology, MR analysis boasts advantages such as circumventing confounding factors, enhancing the credibility of causal inferences, validating observational study outcomes, having a broader trial scope, and saving time and effort compared to traditional observational studies, all while negating concerns related to reverse causality logic.29,30
Materials and method
Study design
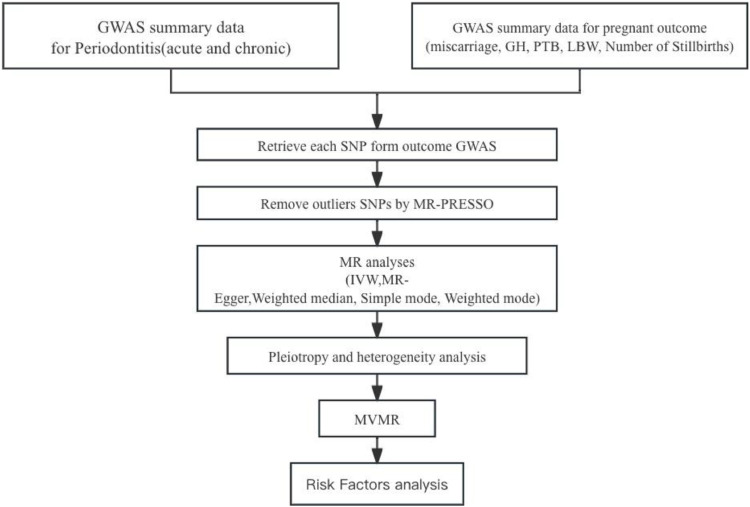
In this study, utilising Genome-Wide Association Study (GWAS) summary data for the exposure variable, periodontitis, and ensuring its independence from the GWAS summary data for the outcome variable, APOs, we conducted a two-sample MR analysis to explore the causal effects of periodontitis on APOs. The MR analysis adhered to the following three assumptions: (1) strong correlation between instrumental variables and exposure variable; (2) independence of instrumental variables from observed or unobserved confounding factors; (3) instrumental variables solely affect the outcome through exposure. The specific procedures are outlined in Figure 1.
Figure 1.
Study design. GH, gestational hypertension; IVW, inverse variance weighted; LBW, low birth weight; PTB, pre-term birth.
Selection of genetic instruments
To fulfil the assumptions of the MR analysis, this study employed a stringent criterion of P < 5 × 10−8 to select SNPs associated with periodontitis. Independence was set at 10,000 kb, with an r2 threshold of <0.001. To minimise confounding effects, we utilised Pheno Scanner (http://www.phenoscanner.medschl.cam.ac.uk/phenoscanner) to estimate potential associations between instrumental variables and confounding factors. This software provides information on SNP phenotypes. Additionally, some MR sensitivity analyses required a minimum of three SNPs related to the exposure, so if the number of available SNPs for analysis was fewer than three, the threshold P-value was adjusted to 5 × 10–6. After removing SNPs without proxy SNPs available in the outcome GWAS, we harmonised the exposure and outcome data before evaluating the association between periodontitis and APOs. (Table)
Table.
Genome-Wide Association Study summary data and expression quantitative trait loci studies’ data information.
| GWAS ID | Traits | N of cases | N of controls | Sample size | Sex | Population |
|---|---|---|---|---|---|---|
| Finn-b-K11 | Acute periodontitis | 367 | 195,395 | 195,762 | Males and females | European |
| Finn-b-K11 | Chronic periodontitis | 3046 | 195,395 | 198,441 | Males and females | European |
| Ebi-a-GCST90018786 | Abortion | 7069 | 250,492 | 257,561 | NA | European |
| finn-b-O15_ | Gestational [pregnancy-induced] hypertension | 4255 | 114,735 | 118,990 | Males and females | European |
| finn-b-O15 | Preterm labour and delivery | 5480 | 98,626 | 104,106 | Males and females | European |
| ukb-b-6412 | Number of stillbirths | NA | NA | 78,879 | NA | European |
| finn-b-P16 | Disorders related to short gestation and low birth weight, not elsewhere classified | 258 | 218,490 | 218,748 | Males and females | European |
Two-sample MR analysis
This study employed the IVW method as the primary analytical approach to determine causal relationships. The IVW method calculates weighted averages, with the reciprocal of each instrument's variance serving as the weight, assuming all instruments are valid. Cochran's Q test was used to assess heterogeneity, and if present, the IVW method's random-effects model was applied; otherwise, the fixed-effects model was the primary outcome. Additionally, we utilised the MR-Egger regression method, weighted median estimation (WME), simple mode, and weighted mode. The MR-Egger intercept method was employed to detect horizontal pleiotropy. The leave-one-out analysis involved systematically excluding each SNP and recalculating the results using the remaining SNPs. If a specific SNP's removal did not result in a statistically significant difference compared to the overall result, it indicated that the SNP did not exert nonspecific effects on the estimation.
Statistical analysis
Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were employed to estimate the relative risk attributed to the presence of the exposure. TwoSampleMR package in R software was utilised for the two-sample MR analysis and related sensitivity analyses. All statistical analyses and visualisations were conducted using R version 4.2.3. A significance level of P < 0.05 was considered to indicate statistically significant differences.
Results
The causal effect of periodontitis on APOs
Our study employed MR analysis to investigate the causal relationships between different exposure factors and various outcomes. In this study, we utilised multiple analytical methods, including IVW, MR Egger, WM, simple mode, and weighted mode methods, to comprehensively assess these relationships.
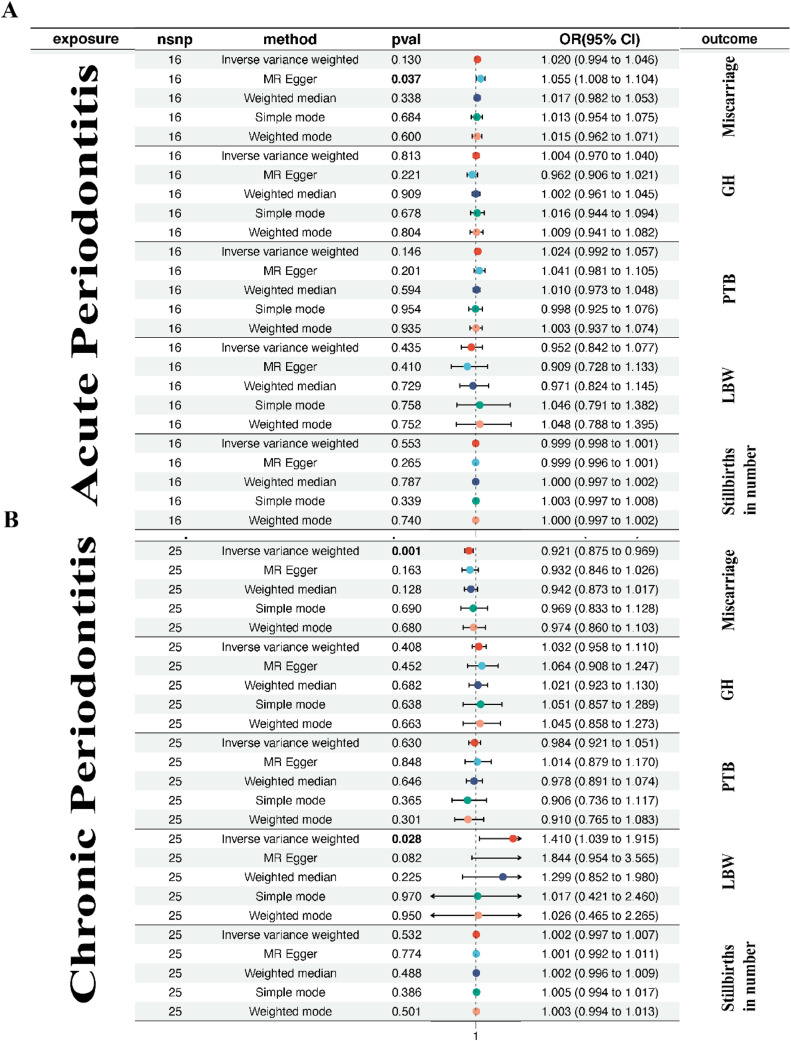
Firstly, for the outcome of LBW, we observed inconsistent results among different analysis methods. Specifically, the OR value estimated by the IVW method was 0.952 with a 95% CI of 0.842 to 1.077 and a P-value of 0.435 for acute periodontitis, while for chronic periodontitis, it was 1.410 with a 95% CI of 1.039 to 1.915 and a P-value of 0.028. The MR-Egger analysis yields an OR of 1.844 with a CI of 0.954 to 3.566 and a P-value of 0.082. The weighted median OR is 1.300 with a CI of 0.852 to 1.980 and a P-value of 0.225. For simple mode, the OR is 1.017 with a CI of 0.421 to 2.460 and a P-value of 0.970. Lastly, weighted mode shows an OR of 1.026 with a CI of 0.465 to 2.265 and a P-value of 0.950. In summary, while the IVW analysis provides statistically significant evidence supporting a causal link, other methods and their non-significant P-values suggest caution in interpreting causality. Similarly, for outcomes including PTB, miscarriage, number of stillbirths, and GH, we observed inconsistent results among different analysis methods (Figure 2). Our study suggests that different analytical methods exhibit inconsistency when assessing the causal relationships between periodontitis and various outcomes. This inconsistency may be influenced by sample size, data characteristics, and analytical methods. Therefore, further research is needed to validate and interpret these relationships for a more comprehensive understanding of the impact of periodontitis on health outcomes.
Figure 2.
The causal effect of periodontitis (A: acute periodontitis; B: chronic periodontitis) on adverse pregnant outcomes (miscarriage, GH, PTB, LBW, number of stillbirths) were investigated using the IVW method, with summary statistics for periodontitis from the FinnGen and adverse pregnant outcomes from FinnGen, UK Biobank, EMBL-EBI.
The causal effect of APOs on periodontitis
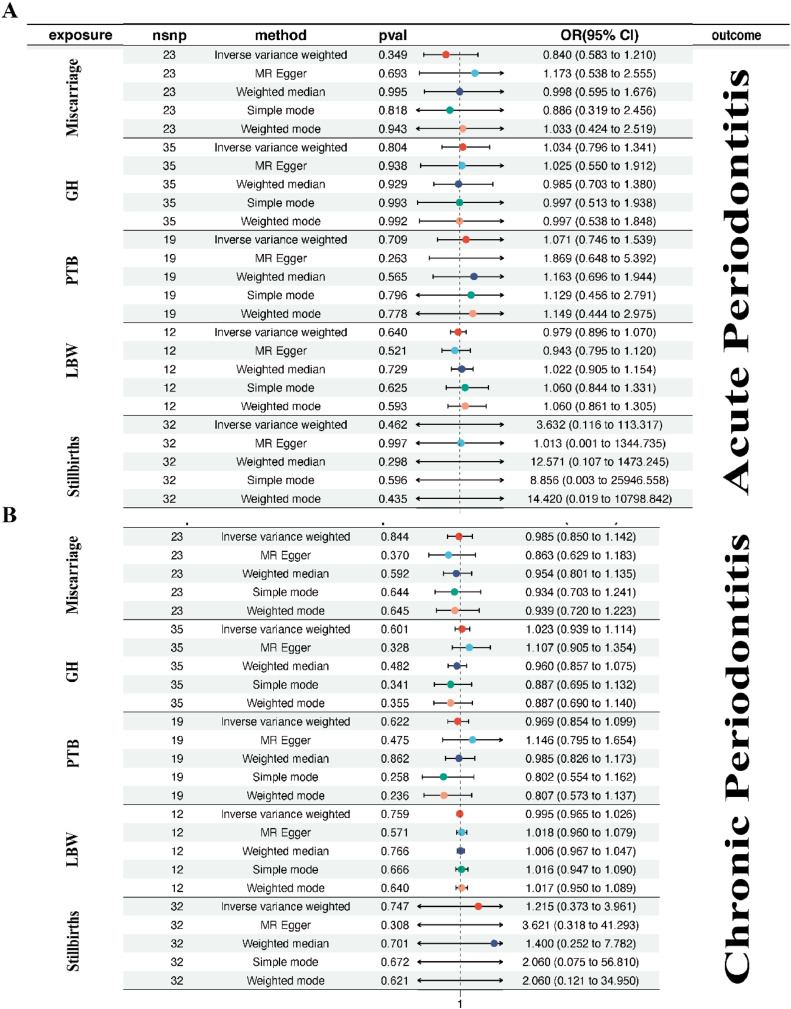
For the exposure factor of GH with the outcome being acute periodontitis, different analysis methods yielded diverse results. The causal OR estimated by various methods were as follows: IVW method reported an OR of 1.034 with a 95% CI from 0.796 to 1.341, and a P-value of 0.804; MR Egger method showed an OR of 1.025 with a 95% CI from 0.550 to 1.912, and a P-value of 0.938; weighted median method indicated an OR of 0.985 with a 95% CI from 0.703 to 1.380, and a P-value of 0.929; simple mode method provided an OR of 0.997 with a 95% CI from 0.513 to 1.938, and a P-value of 0.993; weighted mode method resulted in an OR of 0.997 with a 95% CI from 0.538 to 1.848, and a P-value of 0.992. Similarly, for the exposure factor of GH with the outcome being chronic periodontitis, different analysis methods again yielded varied results. Specifically, the IVW method estimated a causal OR of 1.023 with a 95% CI from 0.939 to 1.114, and a P-value of 0.601. MR Egger method showed an OR of 1.107 with a 95% CI from 0.905 to 1.354, and a P-value of 0.328; weighted median method indicated an OR of 0.960 with a 95% CI from 0.857 to 1.075, and a P-value of 0.482; simple mode method provided an OR of 0.887 with a 95% CI from 0.695 to 1.132, and a P-value of 0.341; weighted mode method resulted in an OR of 0.887 with a 95% CI from 0.690 to 1.140, and a P-value of 0.355. Similar inconsistent results were observed for other exposure factors such as the number of stillbirths, LBW, miscarriage, and PTB (Figure 3). Overall, these findings suggest that the causal relationships between exposure factors and outcomes are inconsistent across different analysis methods, likely influenced by various factors. Therefore, further research is needed to confirm these relationships and explore potential biological mechanisms. Additionally, it is crucial to acknowledge assumptions and limitations in MR analysis, and interpret these results cautiously, considering other types of studies to gain a more comprehensive understanding.
Figure 3.
The causal effect of adverse pregnant outcomes (miscarriage, GH, PTB, LBW, stillbirth) on periodontitis (A: acute periodontitis; B: chronic periodontitis) were investigated using the IVW method, with summary statistics for periodontitis from the FinnGen and adverse pregnant outcomes from FinnGen, UK Biobank, EMBL-EBI.
Discussion
This is the first study investigating the bidirectional causal relationship between periodontitis and APOs using multiple complementary MR methods. Our two-sample MR analyses did not observe evidence supporting the genetically predicted association between periodontitis and APOs in individuals of European descent. Similarly, the reverse MR analysis found no evidence suggesting a genetic predisposition to APOs related to periodontitis. Our MR analysis results are inconsistent with existing observational studies. While these studies have been debated, scholars have generally not questioned the correlation between these two diseases, considering it bidirectional. On one hand, factors such as vomiting during early pregnancy, hormonal fluctuations, changes in dietary habits, and late-pregnancy gastric reflux are believed to increase susceptibility to oral diseases, including periodontitis.31,32 On the other hand, key pathogens of periodontal disease disrupt the host's immune regulation and disturb the oral ecological balance, leading to an increased risk of APOs through immune system dysregulation.33,34 To further understand the relationship between periodontitis and APOs, scholars have conducted animal model experiments. Bobetsis et al35 reported that periodontal pathogens and their pathogenic components can spread through the bloodstream to the placenta-foetus, triggering ectopic infection and tissue damage or inducing inflammation at the infection site, resulting in elevated levels of inflammatory factors and mediators. Moreover, inflammatory factors and mediators produced at the site of periodontitis can also reach the placenta-foetus through the bloodstream, causing intrauterine inflammation. Another possibility is that pathogens, cytokines, and mediators in the blood enter and stimulate the liver to produce acute-phase reactants, activating a systemic inflammatory response. The generated inflammatory mediators enter the placenta-foetus through the bloodstream, exacerbating intrauterine inflammation. With the advancement of sequencing technologies, increasing microbial evidence has been discovered. In 2014, Aagaard et al36 proposed the existence of a unique microbiota in the placenta, and the placental microbiome is most similar to the human oral microbiome. Microbial communities present in the placenta and foetal organs are considered crucial for establishing and initiating foetal immune function before birth.37 Some omics analyses have indicated significant changes in the composition of the placental microbial community in women with APOs compared to normal pregnancies.38 However, several studies have questioned the existence of placental and amniotic membrane microbial communities, arguing that previous research on the placental microbiome did not adequately control for contamination and lacked rigorous and appropriate negative controls.39,40 Another controversial issue is the uncertainty of clinical interventions for APOs prevention in the context of periodontitis. Bobetsis et al35 reported a total of 15 randomised controlled trials globally assessing the impact of nonsurgical periodontal interventions during mid-pregnancy on APOs. The results showed that most periodontal interventions only improved periodontitis-related parameters without a significant impact on APOs occurrence. A plausible explanation is that periodontal treatment during pregnancy cannot eliminate oral pathogens that have already entered the placenta-foetus in early pregnancy, or periodontal treatment fails to alleviate the exposure pathway of key pathogens causing intrauterine infections, thus improving periodontal health indicators do not effectively alleviate APOs symptoms. However, our MR study does not support any causal links in either direction. A reasonable explanation could be the presence of numerous confounding factors between the two, such as the systemic health problems exhibited by the majority of periodontitis patients, especially comorbidities sharing inflammatory pathways, resulting in the observed association between periodontitis and APOs.
In general, while the IVW analysis furnishes statistically significant evidence supporting a causal relationship between chronic periodontitis and LBW, caution is warranted in interpreting causation due to other methods and their nonsignificant P-values. The MR-Egger analysis explores bias that may arise from pleiotropy, and alternative methods provide additional perspectives on the robustness of the results. Integrating these findings in the context of the entire study is crucial. Despite inconsistent results from different analytical methods, the point estimates for both hypotheses (periodontitis affecting PTB, stillbirth, miscarriage, and GH, and vice versa) generally hovered around and below 1, with highly overlapping CIs. Therefore, we consider it unlikely that periodontitis and APOs (LBW, PTB, stillbirth, miscarriage, and GH) have a causal relationship. This study has limitations. Firstly, MR based on aggregated genetic statistics limits the scope of the analysis. Additionally, the partial Clinical Periodontal Index (CPI) often underestimates severe periodontitis and overestimates less severe periodontitis, erroneously determining the numerator of the MR Wald ratio estimator.41 Overall, the study results emphasise the need for caution when considering the impact of different factors and methods in causal inference. The inconsistency of different analytical methods may partially stem from differences in confounding factors, sample size, data quality, and selected statistical techniques. Therefore, these results require further research for validation and interpretation. Future studies could consider using larger samples, more comprehensive control of confounding factors, and more sophisticated statistical models to gain a more comprehensive understanding of the relationship between exposure factors and different outcomes.
Conclusion
In conclusion, our study indeed fails to provide sufficient evidence indicating a causal relationship between periodontitis and APOs, and vice versa. Therefore, despite numerous prospective studies suggesting a positive correlation between periodontal disease and various APOs, the underlying evidence remains weak. Future meticulously designed explanatory studies are essential to validate this relationship and, if present, determine its magnitude.
Conflict of interest
None disclosed.
Acknowledgments
Acknowledgements
The authors acknowledge and thank the investigators of original GWAS studies for sharing summary data used in this study.
Author contributions
Study design: Kun Chen. Data analysis and interpretation: Liying Tang and Kun Chen. Manuscript writing and critical revision: Liying Tang and Kun Chen. Data acquisition and curation: Liying Tang and Kun Chen.
Ethics statement
The present study is based on summary-level data that have been made publicly available. In all original studies, ethical approval had been obtained.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75(1):7–23. doi: 10.1111/prd.12221. [DOI] [PubMed] [Google Scholar]
- 2.Trindade D, Carvalho R, Machado V, Chambrone L, Mendes JJ, Botelho J. Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta-analysis of epidemiological studies. J Clin Periodontol. 2023;50(5):604–626. doi: 10.1111/jcpe.13769. [DOI] [PubMed] [Google Scholar]
- 3.Teles F, Collman RG, Mominkhan D, Wang Y. Viruses, periodontitis, and comorbidities. Periodontol 2000. 2022;89(1):190–206. doi: 10.1111/prd.12435. [DOI] [PubMed] [Google Scholar]
- 4.Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000. 2018;76(1):85–96. doi: 10.1111/prd.12147. [DOI] [PubMed] [Google Scholar]
- 5.Page RC. Gingivitis. J Clin Periodontol. 1986;13(5):345–355. doi: 10.1111/j.1600-051x.1986.tb01471.x. [DOI] [PubMed] [Google Scholar]
- 6.Garaicoa-Pazmino C, Fretwurst T, Squarize CH, et al. Characterization of macrophage polarization in periodontal disease. J Clin Periodontol. 2019;46(8):830–839. doi: 10.1111/jcpe.13156. [DOI] [PubMed] [Google Scholar]
- 7.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45(S20):S149–S161. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 8.Susin C, Haas AN, Albandar JM. Epidemiology and demographics of aggressive periodontitis. Periodontol 2000. 2014;65(1):27–45. doi: 10.1111/prd.12019. [DOI] [PubMed] [Google Scholar]
- 9.Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000. 2020;82(1):257–267. doi: 10.1111/prd.12323. [DOI] [PubMed] [Google Scholar]
- 10.Smith GCS. Adverse pregnancy outcomes and pre-pregnancy mental health care. Lancet Psychiatry. 2023;10(10):734–735. doi: 10.1016/S2215-0366(23)00236-5. [DOI] [PubMed] [Google Scholar]
- 11.Crump C, Sundquist J, McLaughlin MA, et al. Adverse pregnancy outcomes and long term risk of ischemic heart disease in mothers: national cohort and co-sibling study. BMJ. 2023;380 doi: 10.1136/bmj-2022-072112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M, Wambua S, Lee SI, et al. Autoimmune diseases and adverse pregnancy outcomes: an umbrella review. Lancet. 2023;402:S84. doi: 10.1016/S0140-6736(23)02128-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Wang S, Tuo L, et al. Relationship between maternal vitamin D levels and adverse outcomes. Nutrients. 2022;14(20):4230. doi: 10.3390/nu14204230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamadneh S, Hamadneh J. Active and passive maternal smoking during pregnancy and birth outcomes: a study from a developing country. Ann Glob Health. 2021;87(1):122. doi: 10.5334/aogh.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maas VYF, Poels M, Lamain-de Ruiter M, et al. Associations between periconceptional lifestyle behaviours and adverse pregnancy outcomes. BMC Pregnancy Childbirth. 2021;21(1):492. doi: 10.1186/s12884-021-03935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joo EH, Kim YR, Kim N, Jung JE, Han SH, Cho HY. Effect of endogenic and exogenic oxidative stress triggers on adverse pregnancy outcomes: preeclampsia, fetal growth restriction, gestational diabetes mellitus and preterm birth. Int J Mol Sci. 2021;22(18):10122. doi: 10.3390/ijms221810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu B, Han YW. Oral bacteria, oral health, and adverse pregnancy outcomes. Periodontol 2000. 2022;89(1):181–189. doi: 10.1111/prd.12436. [DOI] [PubMed] [Google Scholar]
- 18.Offenbacher S, Katz V, Fertik G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67(10 Suppl):1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 19.Varadan M, Ramamurthy J. Association of periodontal disease and pre-term low birth weight infants. J Obstetr Gynecol India. 2015;65(3):167–171. doi: 10.1007/s13224-014-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jajoo NS, Shelke AU, Bajaj RS, Patil PP, Patil MA. Association of periodontitis with pre term low birth weight—a review. Placenta. 2020;95:62–68. doi: 10.1016/j.placenta.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Figuero E, Han YW, Furuichi Y. Periodontal diseases and adverse pregnancy outcomes: mechanisms. Periodontol 2000. 2020;83(1):175–188. doi: 10.1111/prd.12295. [DOI] [PubMed] [Google Scholar]
- 22.Bobetsis YA, Ide M, Gürsoy M, Madianos PN. Periodontal diseases and adverse pregnancy outcomes. Present and future. Periodontol 2000. 2023;00:1–28. doi: 10.1111/prd.12486. [DOI] [PubMed] [Google Scholar]
- 23.Baumeister S, Freuer D, Nolde M, et al. Testing the association between tobacco smoking, alcohol consumption, and risk of periodontitis: a Mendelian randomization study. J Clin Periodontol. 2021;48(11):1414–1420. doi: 10.1111/jcpe.13544. [DOI] [PubMed] [Google Scholar]
- 24.Birney E. Mendelian Randomization. Cold Spring Harb Perspect Med. 2021;12(4) doi: 10.1101/cshperspect.a041302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma C, Wu M, Gao J, et al. Periodontitis and stroke: a Mendelian randomization study. Brain Behav. 2023;13(2):e2888. doi: 10.1002/brb3.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Zheng Q, Xu M, Zeng C, Deng X. Association between circulating 25-hydroxyvitamin D metabolites and periodontitis: results from the NHANES 2009–2012 and Mendelian randomization study. J Clin Periodontol. 2023;50(2):252–264. doi: 10.1111/jcpe.13736. [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Baird D, Borges M-C, et al. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep. 2017;4(4):330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J, Holmes M.V. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. 2019;10(4):486–496. doi: 10.1002/jrsm.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekula P, Del Greco M F, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253–3265. doi: 10.1681/ASN.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Güncü G, Tözüm T, Çaglayan F. Effects of endogenous sex hormones on the periodontium—review of literature. Aust Dent J. 2005;50(3):138–145. doi: 10.1111/j.1834-7819.2005.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 32.Boggess KA. Maternal oral health in pregnancy. Obstetr Gynecol. 2008;111(4):976–986. doi: 10.1097/AOG.0b013e31816a49d3. [DOI] [PubMed] [Google Scholar]
- 33.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 35.Bobetsis YA, Graziani F, Gürsoy M, Madianos PN. Periodontal disease and adverse pregnancy outcomes. Periodontol 2000. 2020;83(1):154–174. doi: 10.1111/prd.12294. [DOI] [PubMed] [Google Scholar]
- 36.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra A, Lai GC, Yao LJ, et al. Microbial exposure during early human development primes fetal immune cells. Cell. 2021;184(13):3394–3409.e20. doi: 10.1016/j.cell.2021.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince AL, Ma J, Kannan PS, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol. 2016;214(5):627.e1–627.e16. doi: 10.1016/j.ajog.2016.01.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy KM, Bellissimo CJ, Breznik JA, et al. Over-celling fetal microbial exposure. Cell. 2021;184(24):5839–5841. doi: 10.1016/j.cell.2021.10.026. [DOI] [PubMed] [Google Scholar]
- 40.de Goffau MC, Lager S, Sovio U, et al. Human placenta has no microbiome but can contain potential pathogens. Nature. 2019;572(7769):329–334. doi: 10.1038/s41586-019-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baelum V, Fejerskov O, Manji F, Wanzala P. Influence of CPITN partial recordings on estimates of prevalence and severity of various periodontal conditions in adults. Community Dent Oral Epidemiol. 1993;21(6):354–359. doi: 10.1111/j.1600-0528.1993.tb01098.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.