Abstract
Pancreatitis is a common, life-threatening inflammatory disease of the exocrine pancreas. Its pathogenesis remains obscure, and no specific or effective treatment is available. Gallstones and alcohol excess are major etiologies of pancreatitis; in a small portion of patients the disease is hereditary. Pancreatitis is believed to be initiated by injured acinar cells (the main exocrine pancreas cell type), leading to parenchymal necrosis and local and systemic inflammation. The primary function of these cells is to produce, store, and secrete a variety of enzymes that break down all categories of nutrients. Most digestive enzymes, including all proteases, are secreted by acinar cells as inactive proforms (zymogens) and in physiological conditions are only activated when reaching the intestine. The generation of trypsin from inactive trypsinogen in the intestine plays a critical role in physiological activation of other zymogens. It was proposed that pancreatitis results from proteolytic autodigestion of the gland, mediated by premature/inappropriate trypsinogen activation within acinar cells. The intra-acinar trypsinogen activation is observed in experimental models of acute and chronic pancreatitis, and in human disease. On the basis of these observations, it has been considered the central pathogenic mechanism of pancreatitis - a concept with a century-old history. This review summarizes the data on trypsinogen activation in experimental and genetic rodent models of pancreatitis, particularly the more recent genetically engineered mouse models that mimic mutations associated with hereditary pancreatitis; analyzes the mechanisms mediating trypsinogen activation and protecting the pancreas against its’ damaging effects; discusses the gaps in our knowledge, potential therapeutic approaches, and directions for future research. We conclude that trypsin is not the culprit in the disease pathogenesis but, at most, a mediator of some pancreatitis responses. Therefore, the search for effective therapies should focus on approaches to prevent or normalize other intra-acinar pathologic processes, such as defective autophagy leading to parenchymal cell death and unrelenting inflammation.
Keywords: Pancreatic acinar cell, Hereditary pancreatitis, Autophagy, Endolysosomal system, Cholecystokinin, Cerulein, Cathepsin
Core Tip: Pancreatitis is a common life-threatening disease of the exocrine pancreas. Its pathogenesis remains obscure, and no specific/effective treatments are available. The current paradigm is that pancreatitis is initiated by premature, intra-acinar-cell conversion of trypsinogen to trypsin. This 130-year-old concept has only recently been tested in genetic mouse models. Our review analyzes the mechanisms mediating trypsinogen activation and protecting against its’ effects, controversies in the available data, potential therapeutic approaches, and future research directions. We conclude that intra-acinar trypsinogen activation is not the culprit but at best one of disease mediators, and possibly an epiphenomenon. This conclusion represents a paradigm shift.
INTRODUCTION
Pancreatitis is a common, life-threatening disease of the exocrine pancreas, with obscure pathogenesis and no specific or effective treatment. Pancreatitis is the 3rd most common reason for hospital admissions of those with gastrointestinal (GI) disease in the United States, the 5th most common nonmalignant GI cause of death, a heavy burden on the United States healthcare system, and a major risk factor for the deadly pancreatic cancer[1-3]. Three major forms represent the disease continuum: Acute pancreatitis (AP), recurrent AP (RAP), and chronic pancreatitis (CP). AP is mostly a one-time episode, but it can sensitize the pancreas to RAP, progressing further to CP[4,5]. The most common causes of AP are gallstones obstructing the common bile duct, and heavy alcohol and cigarette use. Other etiologies include direct trauma, genetic factors, medications, infections, and tumors. The key pathologic responses of AP are increased serum amylase and lipase, acute pancreatic inflammation, and parenchymal cell death. Tissue changes of CP include chronic inflammation, pancreas atrophy, fibrosis, and acinar-ductal metaplasia.
The established mechanistic paradigm is that AP is initiated by injured acinar cells (the main exocrine pancreas cell type), leading to inflammatory cell infiltration, parenchymal necrosis, and systemic inflammation. There is compelling evidence that mechanisms initiating and driving pancreatitis are common to all clinical forms of the disease[1-3,6,7].
The primary function of pancreatic acinar cells is to produce, store, and secrete a broad spectrum of enzymes that break down all categories of nutrients[6,8,9]. Under physiological conditions, many digestive enzymes, including all proteases, are secreted by acinar cells as inactive proforms (zymogens) and are only activated when reaching the intestine. Over a century ago, Austrian pathologist Hans Chiari proposed that premature activation of proteolytic enzymes within the pancreas causing autodigestion is the underlying mechanism of pancreatitis[10]. Trypsin is a major and potent digestive protease, and its generation from inactive trypsinogen plays a critical role in physiological activation of other zymogens in the intestine (see below). Extrapolating from this physiological process, the premature (“inappropriate”) intra-acinar conversion of trypsinogen to trypsin was postulated, and universally accepted, to be the driving pathogenic mechanism of pancreatitis. Indeed, intrapancreatic trypsinogen activation occurs in acute and chronic, experimental and human pancreatitis, and is now considered a disease hallmark[11-16]. The concept of trypsin’s central pathogenic role has been strengthened by the finding of David Whitcomb’s group[17], followed by other researchers, that hereditary pancreatitis (HP), an autosomal dominant disease, is associated with mutations in trypsinogen[13,14,16,17]. Later, alterations in other proteins mediating the “trypsin-dependent pathway” - such as chymotrypsin C and serine peptidase inhibitor Kazal type 1 (SPINK1) - were linked to increased risk of developing pancreatitis[13]. However, despite decades of intense research, the mechanisms of intrapancreatic trypsinogen activation and its pathogenic role in pancreatitis remain unclear. This review aims to summarize current information on trypsinogen activation and its role in pancreatitis; it will emphasize recent discoveries, identify gaps in our understanding of the underlying mechanisms, and discuss future research directions.
PHYSIOLOGICAL ROLE OF TRYPSIN IN DIGESTION
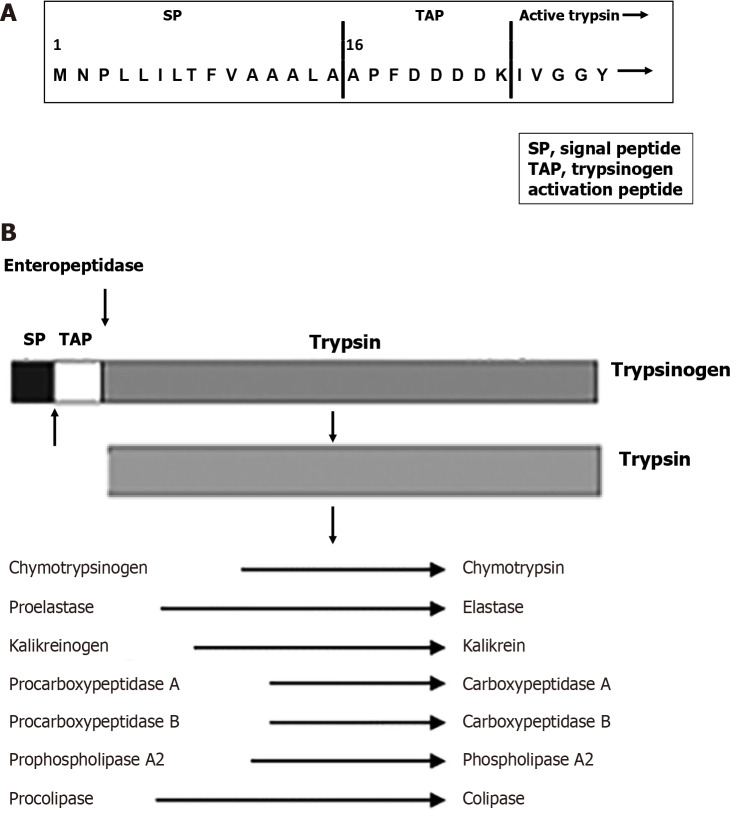
The biochemistry of trypsinogen and trypsin has been discussed in detail[13,18,19]. About 95% of human trypsinogen consists of cationic and anionic trypsinogen, the products of the serine protease 1 (PRSS1) and 2 (PRSS2) genes, respectively. The predominant mouse isoform is the cationic trypsinogen T7[13]. After synthesis and removal of the signal peptide (Figure 1A) in the endoplasmic reticulum (ER), trypsinogen (like other digestive enzymes) is packaged in the Golgi into secretory granules, which are condensed along their trafficking to become mature zymogen granules. Zymogen granules are subsequently delivered to the plasma membrane for exocytosis; the secreted digestive enzymes transit to the duodenum to meet a food bolus[20]. In a healthy pancreas, all the digestive proteases, including trypsinogen, remain inactive during their intracellular transport, secretion from acinar cells, and transit to the duodenum. After entering the small intestine, trypsinogen becomes activated by duodenal brush-border serine protease enteropeptidase (termed enterokinase), which cleaves off N-terminal trypsinogen activation peptide (TAP; 8-aminoacid-long in humans) to convert trypsinogen into trypsin (Figure 1). Trypsin’s role in digestion is central (Figure 1B), as it proteolytically activates other digestive proteases, including chymotrypsinogen, proelastase, and procarboxypeptidase B1[12,15].
Figure 1.
Trypsin structure and functions. A: N-terminal amino acid sequence of the cationic trypsinogen; B: Schematic of trypsin-mediated activation of other digestive proteases during physiological process of digestion. SP: Signal peptide; TAP: Trypsinogen activation peptide.
INTRAPANCREATIC TRYPSINOGEN ACTIVATION IN EXPERIMENTAL AND HUMAN PANCREATITIS
Methods to measure trypsinogen activation in pancreas
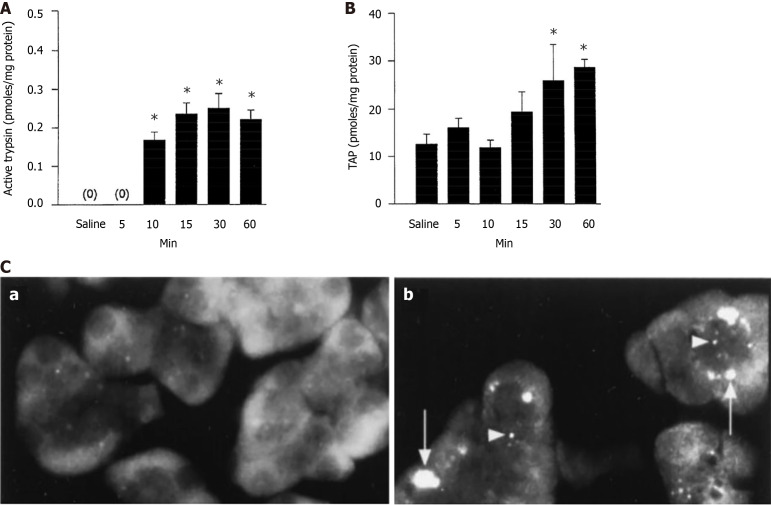
Trypsin activity is commonly measured with an enzymatic assay in tissue or cell homogenates (Figure 2A), or microscopically in live acinar cells, using a substrate the fluorescence of which increases after its’ cleavage by trypsin[21-25]. Another assay measures trypsinogen activation (but not trypsin activity) by quantifying TAP using an ELISA or immunodetection on tissue sections (Figure 2B and C). The anti-TAP antibodies do not cross-react with trypsinogen, trypsin, or other zymogen activation peptides; therefore, the amount of TAP reflects trypsinogen conversion into active trypsin[26-28]. Parallel increases in intrapancreatic trypsin activity and TAP (as illustrated in Figure 2) confirm the validity of trypsinogen activation measurement.
Figure 2.
Illustration of the main methods to measure trypsinogen activation in experimental pancreatitis. A and B: Time-course of changes in the levels of (A) trypsin activity and (B) trypsinogen activation peptide (TAP) measured in pancreas homogenates from rats subjected to i.v. infusion of 5 μg/kg cerulein (adapted from Hofbauer et al[21]), *P < 0.05 versus saline; C: Electron microscopic immunolocalization of TAP in pancreas was measured in (a) untreated rats and (b) 60 minutes after cerulein (5 μg/kg) infusion. Note intense punctate TAP immunoreactivity in small vesicles (arrowheads) and large vacuoles (arrows), which was absent in control[28]. C: Citation: Otani T, Chepilko SM, Grendell JH, Gorelick FS. Codistribution of TAP and the granule membrane protein GRAMP-92 in rat caerulein-induced pancreatitis. Am J Physiol 1998; 275: G999-G1009. Copyright© The American Physiological Society 1998. Published by American Physiological Society. Authors of American Physiological Society articles may reuse their content in reused new works (https://journals.physiology.org/publication-process#copyright).
The total amount of trypsinogen in the pancreas or acinar cells can be determined by converting trypsinogen into trypsin with enterokinase, followed by measuring generated trypsin activity with enzymatic assay. Trypsinogen levels in the pancreas can also be analyzed by immunoblot, but seeing trypsin as separate protein from trypsinogen is difficult, because the difference in molecular masses is < 1 kDa.
Rodent models of pancreatitis
Access to human pancreatitis tissue is limited because surgical treatment of pancreatitis is rare, and also too late in the disease to inform studies of early AP biology[1,2,14,15]. Therefore, the mechanisms of pancreatitis, particularly of premature trypsinogen activation, are primarily studied using animal models that reproduce the pathologies of human disease. The characteristics of these models and methods of their development have been described in detail[29-31].
Experimental pancreatitis models: The best-studied mouse and rat AP models[29-31] include those generated by intraperitoneal injections of supramaximal doses of cerulein (CER), an ortholog of cholecystokinin (CCK)-8; retrograde infusion of bile acids (e.g., taurolithocholic acid sulfate; TLCS) into the pancreatic duct; administration of high doses of L-arginine (Arg); or by feeding young female mice choline-deficient, ethionine supplemented diet. Ex-vivo/cellular AP models are developed by incubating rodent or, importantly, human acinar cells with pancreatitis inducers, such as CCK-8 or CER; these models recapitulate the early disease stages that occur within acinar cells[9,32,33]. Experimental CP models are usually induced by repeated injections of CER or Arg[29,34,35].
Genetic mouse models of pancreatitis associated with trypsinogen mutations: Gallstones and alcohol consumption account for > 80% of pancreatitis cases. Less than 1% of human disease is associated with mutations in the PRSS1 gene[13,17]. Early attempts to generate mouse models of pancreatitis caused by trypsinogen mutations were unsuccessful, possibly because of low transgene expression[36,37]; but later, HP-related genetically engineered mouse models (GEMMs) have been developed (Table 1) which reproduced a number of human disease responses[38-50]. Hegyi and Sahin-Tóth[13], Geisz and Sahin-Tóth[38], Jancsó et al[39], Geisz et al[40], Jancsó and Sahin-Tóth[43], Demcsák and Sahin-Tóth[45], Geisz et al[47], Jancsó et al[48], and Jancsó et al[49] generated mice with mutations in T7 trypsinogen related to those seen in human pancreatitis. Gui et al[41], Huang et al[42], Wan et al[44], and Wang et al[46] expressed in mice full-length mutated human PRSS1R122H (the most mutated gene in HP) or the combination of PRSS1R122H and PRSS2 genes.
Table 1.
Trypsinogen activation in hereditary pancreatitis-related genetic mouse models: Representative studies
|
Genetic mouse model
|
Genetic modification
|
Spontaneous pancreatitis
|
Increase in basal trypsin activity
|
Effects of pancreatitis inducers
|
Trypsinogen activation in CER-AP
|
Ref.
|
| PRSS1R122H | Mutated human PRSS1R122H or WT PRSS1 gene expressed under full-length elastase promoter | No | No | Severity of AP induced with CER, LPS or ethanol increased in the order: WT mouse < PRSS1 < PRSS1R122H | Increased in the order: WT mouse < PRSS1 < PRSS1R122H | [42] |
| PRSS1 | ||||||
| PRSS2 | Transgenic mice similarly expressing human PRSS2 gene | Focal areas (< 10% of total pancreas) | ND | CER-AP was more severe in PRSS2 expressing mice | ND | [44] |
| PRSS1/PRSS2 | Compound mice co-expressing WT PRSS1 and PRSS2 or PRSS1R122H and PRSS2 from their native promoters | Yes, in homozygous PRSS1R122H/PRSS2 mice, but not in PRSS1/PRSS2 mice | ND | One i.p. injection of low-dose CER induced pancreatitis in PRSS1R122H/PRSS2 but not in PRSS1/PRSS2 mice | ND | [46] |
| PRSS1R122H/PRSS2 | ||||||
| PRSS1/PRSS2+ PRSS2 | Compound PRSS1/PRSS2 or PRSS1R122H/PRSS2 mice were further crossed with mice expressing WT PRSS2 | Yes, in PRSS1R122H/PRSS2+ PRSS2 mice, but not in PRSS1/PRSS2+ PRSS2 mice | ND | ND | ND | [46] |
| PRSS1R122H/PRSS2+ PRSS2 | ||||||
| T7D23A knock-in | Heterozygous p.D23A mutation in the TAP part of T7 trypsinogen gene | Yes | Yes | ND | ND | [38] |
| T7K24R knock-in | Heterozygous p.K23R mutation in the TAP part of T7 trypsinogen gene | No | No | CER-AP was aggravated | Increased | [43] |
| Ctrb1-del | Mice deficient in CTRB1 chymotrypsin that promotes trypsinogen degradation | No | No | CER-AP was aggravated | Increased | [39,49] |
| T7K24R×Ctrb1-del | Compound mice carrying both the trypsinogen mutant T724KR and chymotrypsin Ctrb1 deletion alleles | Yes | ND | ND | ND | [49] |
CER: Cerulein; LPS: Lipopolysaccharide; ND: Not determined; TAP: Trypsinogen activation peptide.
In most cases, the expression of trypsinogen mutants mimicking those in human disease - in particular, PRSS1R122H alone - did not cause the development of spontaneous pancreatitis (Table 1). More recently, a spontaneous HP model was developed by Wang et al[46] by co-expressing human PRSS2 and PRSS1R122H from their native promoters. Notably, CER-AP was aggravated in all GEMMs examined so far (Table 1; discussed below in the section “The role of trypsin in HP-related GEMMs”).
Intrapancreatic trypsinogen activation is a universal response of pancreatitis
The first report demonstrating that experimental pancreatitis is associated with increased intrapancreatic trypsin was published almost 50 years ago[51]. Subsequent studies[21,24,28,51-78] showed that intrapancreatic trypsin increase is a signature response of experimental AP (Table 2). Trypsinogen activation in rodent and ex-vivo AP models was measured by increased trypsin enzymatic activity in the whole pancreas, acinar cell homogenates and live acinar cells, and by increases in pancreatic TAP followed by TAP release into the circulation[21,27,28,53-56,58-60,79]. TAP elevation in serum and urine was seen in patients[80-84], as well as in cats and dogs with AP[85,86]. Intrapancreatic trypsin also increased during CP progression[87,88]. These observations underscore the generality of trypsinogen activation in pancreatitis across mammalian species, including humans.
Table 2.
Trypsinogen activation in experimental AP models: Representative studies
|
Experimental pancreatitis induced by
|
Trypsin activity measured in tissue or acinar cells (ex-vivo models)
|
Ref.
|
| Cerulein | Rat pancreas | [21,28,53-55,58,59,62] |
| Mouse pancreas | [60,62,74,88,112,135,143,201] | |
| Taurocholate or TLCS | Rat pancreas | [56,61] |
| Mouse pancreas | [68,70] | |
| L-arginine | Rat pancreas | [65] |
| Mouse pancreas | [66,78] | |
| L-ornithine, L-lysine | Rat pancreas | [69,71] |
| CDE diet | Mouse pancreas | [51,52] |
| EtOH + low-dose cerulein | Mouse pancreas | [72] |
| EtOH + POA | Mouse pancreas | [73] |
| CCK/cerulein | Rat and mouse acinar cells | [21,24,64,74,129,131,136,143] |
| TLCS | Rat, mouse, and human acinar cells | [32,67,75] |
| EtOH + low-dose CCK | Rat and mouse acinar cells | [72,76] |
| NNK (tobacco compound) | Rat acinar cells | [75] |
| Agonist of the mechano receptor Piezzo1 | Mouse acinar cells | [77] |
TLCS: Taurolithocholic acid sulfate; CDE: Choline-deficient, ethionine supplemented (diet); EtOH: Ethanol; POA: Palmitoleic acid; CCK: Cholecystokinin; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.
Trypsinogen activation is an early (within minutes) event of experimental AP, occurring long before acinar cell death and inflammatory cell infiltration[24,89-91]. Pancreatic trypsin activity elicited by CER-AP in most mouse strains exhibits biphasic kinetics, with a transient peak in the first hour upon disease induction followed by a sustained increase during AP progression[60,74]. Of note, less than 1% of trypsinogen is converted to trypsin in experimental pancreatitis[11,12,28].
Trypsinogen activation in HP-related GEMMs
In contrast to experimental pancreatitis models that have been used for decades, investigation of GEMMs associated with mutations in the trypsin-dependent pathway has started only recently[13,38-50]. As illustrated in Table 1, there are numerous gaps in our current knowledge on whether and to what extent the basal pancreatic trypsin activity increases in these models (see the discussion below in “The role of trypsin in HP-related GEMMs”). Moreover, the mutants’ effect on trypsin activity was not always measured, even in models that showed worsening of CER-AP (Table 1). Little is known about the mutations’ effects on trypsin activity in acinar cells isolated from the GEMMs; and importantly, there has been no measurement of TAP levels in the pancreas, blood, or urine.
MECHANISMS OF TRYPSINOGEN ACTIVATION IN PANCREATITIS
Cathepsin B mediates trypsinogen activation in experimental pancreatitis
Cathepsin B (CTSB) is a major lysosomal cysteine protease; it is synthesized in the ER as an inactive precursor and then delivered to lysosomes after trafficking through Golgi and endosomal systems[92-94]. During trafficking, CTSB is processed into an active mature form. The activity of CTSB, as well as other lysosomal hydrolases, is maximal in acidic milieu.
An essential step in the cathepsins’ delivery to lysosomes is their segregation from digestive enzymes[95]. After being synthesized and processed in the ER, lysosomal hydrolases and digestive enzymes are delivered to the Golgi, where they are sorted into their respective pathways[95-97]. The separation, however, is not absolute, and a fraction of CTSB is also found in the secretory granules[98], with the majority of CTSB contained in endo/lysosomes.
In early 50s, scientists from the New York College of Medicine noted that substrate specificities of CTSB and enterokinase are similar, leading to a seminal study[99] which showed that CTSB can cleave TAP from trypsinogen to generate trypsin. Subsequent studies established the role of CTSB as the protease responsible for trypsinogen activation in experimental pancreatitis. They showed that trypsinogen activation in AP occurs at acidic pH, that is, at maximal CTSB activity[100]. CTSB and trypsinogen colocalize in the pancreas. Pharmacologic inhibition of CTSB with a broad-spectrum cysteine protease inhibitor E-64d or specific CTSB inhibitor CA-074Me[25,64,101,102] prevents trypsinogen activation in in-vivo and ex-vivo experimental AP models, including in human acinar cells[64,101-103]. Similarly, whole-body[47,60] and pancreas-specific[104] CTSB genetic ablation abrogates trypsinogen activation in CER-AP.
Of note, CTSB’s effect on trypsinogen is specific; it does not activate other digestive enzymes[105]. Also, lysosomal proteases other than CTSB do not cause trypsinogen activation[106].
Trypsinogen autoactivation is implicated in HP
Trypsinogen can autoactivate in the presence of a minuscule amount of trypsin, which cleaves off TAP, thus converting trypsinogen into trypsin[13,15]. This phenomenon was discovered 90 years ago by Kunitz and Northrop[107], who observed that crystalline trypsinogen contains a trace of trypsin and is gradually converted into trypsin. Trypsinogen autoactivation has been characterized biochemically in great detail (primarily by Sahin-Toth’s group) and is considered a principal mechanism of trypsin generation in HP[13,15,38,43,45,47,108].
The interrelationship between the two mechanisms of trypsinogen activation in pancreatitis
Trypsinogen autoactivation and its conversion to trypsin by CTSB are the only 2 known mechanisms of intrapancreatic trypsin generation in pancreatitis[12,13,16,45,47,50,109]. In both pathways, trypsinogen is converted to trypsin by proteolytic removal of TAP. However, in experimental pancreatitis, trypsinogen is activated solely by CTSB, with no contribution of autoactivation. Indeed, blocking CTSB prevented trypsinogen activation in experimental AP models[25,47,60,64,101,102,104], whereas blocking trypsinogen autoactivation had no effect on CER-AP[110]. Conversely, the available data indicate that CTSB does not modulate the rates of trypsinogen activation in mutations-associated GEMMs[38]. These data imply that in the currently developed experimental AP models and HP-related GEMMs, the two pathways of trypsinogen activation do not operate simultaneously[47,50]. Why this is so is unclear. As discussed below in “The role of trypsin in HP-related GEMMs”, one possible explanation for why there is no appreciable extent of autoactivation in experimental pancreatitis is a “threshold effect”: The autoactivation rate in HP-related trypsinogen mutants is much greater than in the wild type[13,45]. Another putative reason could be a different intrapancreatic localization of activated trypsinogen in experimental AP models vs HP-related GEMMs[47,50], as discussed in “The role of trypsin in HP-related GEMMs”. Interestingly, trypsinogen mutations that do not measurably increase the basal pancreatic trypsin activity enhance trypsinogen activation induced by CER-AP[42,43], indicating a possible cross-talk between autoactivation- and CTSB-mediated mechanisms (Table 1).
Where does trypsinogen activation occur in pancreatitis?
The conclusion that the acinar cell is a major site of intrapancreatic trypsin accumulation in experimental pancreatitis is based on the following evidence: Trypsinogen activation is prominent in all ex-vivo AP models on rodent and human acinar cells (Table 2); microscopy analysis shows TAP accumulation only in acinar but not in endocrine or ductal cells[28,111]; the magnitude and the kinetics of trypsinogen activation are similar between in-vivo and corresponding ex-vivo models. For example, in both mouse and cellular CER-AP, trypsin activity increases within minutes and reaches its maximum within 1 hour after AP induction[60,112].
A large amount of trypsinogen in pancreatitis is present in the interstitium[59] and could be activated by CTSB secreted by acinar cells[113,114]. Also, in pancreatitis digestive enzymes can access the extracellular space due to blockade of apical exocytosis and redirection of acinar cell secretion to the basolateral plasma membrane[115] or because of disrupted tight junctions between acinar and ductal cells[116].
A recent study revealed that trypsinogen activation in pancreatitis also occurs in activated macrophages infiltrating the pancreas[117]. In areas of pancreatic necrosis, macrophages can ingest zymogen-containing vesicles from damaged acinar cells or endocytose trypsinogen and convert it into trypsin in a CTSB-dependent manner[117]. Of note, the second phase of intrapancreatic trypsin increase in CER-AP is likely due to the contribution of this mechanism.
SITES OF TRYPSINOGEN ACTIVATION WITHIN ACINAR CELLS IN EXPERIMENTAL PANCREATITIS: THE COLOCALIZATION HYPOTHESIS
Despite intense investigation, the intra-acinar site(s) of trypsinogen activation in experimental pancreatitis have not yet been established. About 25 years ago, Michael Steer and Ashok Saluja put forward a concept termed “colocalization hypothesis” - namely, that the induction of pancreatitis is associated with perturbed trafficking of lysosomal hydrolases resulting in their colocalization with digestive enzymes within some cytoplasmic organelles where CTSB activates trypsinogen[11,12,33,97,118-120]. The hypothesis was supported by immunoelectron microscopy showing colocalization of lysosomal hydrolases and digestive enzymes within acinar cells, and confirmed by biochemical studies demonstrating that during experimental AP, CTSB activity is redistributed to heavier subcellular fractions containing zymogen granules[28,98,101,119,120].
The initial interpretation of these results was that during AP, CTSB is missorted into the secretory compartment and activates trypsinogen in zymogen granules[120]. However, subsequent studies showed that tryptic activity and TAP do not localize to zymogen granules[11,24,28,97]. The failure to identify the exact organelle(s) in which intra-acinar trypsinogen activation occurs and the mechanisms mediating “pathologic” colocalization of trypsinogen and CTSB undermine the colocalization hypothesis. In this context, recent advances are discussed in the following section on the role of endolysosomal and autophagic compartments in trypsinogen activation.
In contrast to numerous studies in experimental AP models (Table 2), we know very little about both the types of cells and the intra-acinar sites of trypsinogen activation in HP-related GEMMs.
ENDOLYSOSOMAL SYSTEM DISORDERING CAUSES INTRA-ACINAR TRYPSINOGEN ACTIVATION IN EXPERIMENTAL PANCREATITIS: A HYPOTHESIS
Intra-acinar trypsinogen activation occurs in organelles of the endolysosomal system
As stated above, studies of trypsinogen, TAP and CTSB immunolocalization in acinar cells, and subcellular CTSB redistribution indicate that in experimental pancreatitis, trypsin localizes to membrane-bound organelles in which digestive enzymes and lysosomal hydrolases colocalize; and further, that these organelles are not zymogen granules[11,12,28,97,98,101,118-120]. The organelles in which trypsinogen activation takes place are acidic. Treatments that neutralize organellar acidic pH, such as the weak cell-permeable base chloroquine, the inhibition of vacuolar H+-ATPases with Bafilomycin A1, or inhibition of the Na+/H+ exchanger with monensin, all prevent trypsinogen activation in experimental AP models[121-124].
The only acidic membrane-bound organelles in acinar cells that contain lysosomal hydrolases and can also acquire digestive enzymes are organelles of the endolysosomal system, which comprises early, recycling and late endosomes, endocytic vacuoles, lysosomes, and autolysosomes[125]. The endolysosomal system controls several processes critical for acinar cell physiology, including: Sorting out lysosomal hydrolases from the digestive enzymes, which occurs in the Golgi complex and during post-Golgi trafficking[95,96,126]; post-exocytic membrane retrieval through endocytosis, which is necessary to replenish secretory granule pools and maintain plasma membrane integrity[127,128]; digestive enzyme secretion through the so-called minor secretory pathway that uses anterograde trafficking through early and recycling endosomes[6,75,96]; autophagic elimination of nonfunctional organelles, which depends on lysosomal proteolytic capacity[129-131]. Importantly, during these physiological processes endolysosomal organelles acquire digestive enzymes.
Pancreatitis causes multiple defects in the endolysosomal system[6,129-145] some of which result in intra-acinar trypsinogen activation. Findings from the Liverpool group[134,138,141,144] indicate that endocytosis of post-exocytic structures is impaired in pancreatitis, resulting in the formation of abnormally large endocytic vacuoles containing CTSB and trypsinogen and where trypsinogen activation can occur. This is in accord with the data that cytoplasmic organelles containing TAP carry endosomal membrane markers[28,119]. Further, biochemical studies show that endosomal functions are deranged in pancreatitis and that restoring endosomal maturation prevents trypsin accumulation in ex-vivo CCK-induced pancreatitis[75,137].
Lysosomal insufficiency (see below) is another defect of the endolysosomal system in pancreatitis which can promote trypsin accumulation in acinar cells[129,130,135,136]. Because of acinar cell lysosomal insufficiency, autophagy fails to eliminate abnormal trypsin-containing organelles, resulting in trypsin accumulation in autolysosomes as evidenced by the colocalization of TAP with the autophagy marker LC3-II in pancreatitis[129,140,143,146].
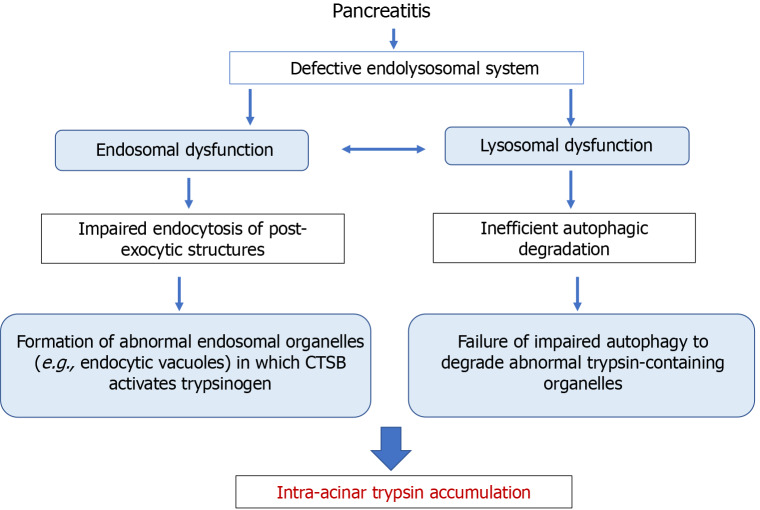
Based on these data, one can hypothesize that the intra-acinar trypsin accumulation in experimental pancreatitis is a result of two processes: The formation of abnormal endolysosomal organelles (e.g., endocytic structures[134]) in which CTSB activates trypsinogen, and the inability of impaired autophagy to eliminate these organelles (Figure 3). Detailed time-course analysis of colocalization and co-fractionation of TAP, trypsin activity, and LC3-II in CER-AP has indicated that the initial site of trypsinogen activation is not in autophagic vacuoles; however, trypsin increasingly accumulates in autolysosomes during pancreatitis progression[147]. Abnormal endocytic vacuoles[134] are likely not the sole organelle of the endolysosomal system where CTSB activates trypsinogen in pancreatitis. For example, disruption of mannose-6-phosphate mediated pathway of cathepsins’ delivery to lysosome results in the accumulation in acinar cells of abnormal (hybrid early/late) endosomes, in which CTSB activates trypsinogen[143].
Figure 3.
Putative mechanisms whereby endolysosomal and autophagy dysfunctions mediate intra-acinar trypsin accumulation in pancreatitis. CTSB: Cathepsin B.
One of the criticisms against the original colocalization hypothesis was that colocalization of CTSB and trypsinogen occurs not only in pancreatitis but also in normal pancreas[11,148,149]. In this regard, it is worth noting that colocalization of digestive enzymes with lysosomal hydrolases is not a pathologic event; it occurs in endolysosomal organelles during physiological processes. In particular, during basal autophagy autolysosomes sequester unneeded or damaged zymogen granules, resulting in colocalization of trypsinogen and CTSB[129]; this stage is normally followed by rapid cargo degradation[129,150]. In contrast, in pancreatitis autolysosomes fail to degrade sequestered cargo, resulting in the intra-acinar trypsin accumulation[6,129-143,145].
SNARE proteins regulate the endolysosomal system, autophagy, and trypsinogen activation in experimental pancreatitis
The fusogenic SNARE proteins[151-153] are key molecules involved in vesicle transport and membrane fusion, which control exocytosis, endocytosis, release of synaptic vesicles, and inflammation. They have been recently shown to regulate autophagy and trypsinogen activation in pancreatitis[133,137,139,142,145,154]. For example, intra-acinar levels of Syntaxin 2 markedly decrease in experimental and human pancreatitis[139]. Genetic ablation of Syntaxin 2 promotes the pathologic basolateral exocytosis, generation of TAP and trypsin, and inhibits autophagic degradation, thus replicating pancreatitis responses[139]. Conversely, knockdown of SNAP23 reduces basolateral exocytosis, stimulates autophagic degradation, and decreases intra-acinar trypsinogen activation in experimental pancreatitis[133,142]. These data reveal a mediatory role of SNARE proteins in linking exocytosis, autophagy, and trypsinogen activation in acinar cells.
MECHANISMS PROTECTING THE PANCREAS AGAINST TRYPSIN ACCUMULATION
Acinar cells have several mechanisms that protect them from both trypsinogen activation and resultant trypsin activity[11-16]. The protein is synthesized as inactive zymogen; both trypsinogen and the resultant trypsin are confined to membrane-bound organelles, thus protecting the cytosol; trypsin activity in these organelles is markedly reduced by their acidic pH[121-124]. Several molecules counteract or down-regulate trypsin activity, as discussed below. More recently, autophagy emerged as a major mechanism protecting the pancreas from trypsin accumulation.
SPINK1
SPINKs are a large conserved family of protease inhibitors named after Louis Kasal, who in 1948 isolated from pancreas SPINK1, a specific trypsin inhibitor and the first member of this family[155-157]. SPINK1 is a small (6.2 kDa) protein that resides in zymogen granules and is secreted with other digestive enzymes into the juice. Biochemical studies showed that complex formation with SPINK1 inhibits trypsin’s activity; the inhibition is temporary, as trypsin gradually degrades SPINK1, restoring its own proteolytic activity[158].
In early 2000s, SPINK1 gained attention because its mutations were associated with human pancreatitis[159-162]. However, the most common SPINK1 mutation p.N34S affects neither SPINK1’s ability to inhibit trypsin nor its interaction with human cationic trypsin[163-165]; and no linkage between the presence of SPINK1 and PRSS1 mutations has been found[166,167]. Although p.N34S mutation increases the risk for CP, its frequency in HP families is the same as in the general population[166,167]. Recently, the N34S variant was linked to changes in SPINK1 gene enhancer, raising the possibility of a decrease in its transcription and hence protein level[168,169]. It was also suggested that in pancreatitis, SPINK1 acts together with other genetic or environmental factors[159]. In particular, 20% of patients carrying functionally defective variants of the epithelial transient receptor potential vanilloid 6 (TRPV6) Ca2+ channels have p.N34S mutation, which may suggest their cooperative effect on trypsin activity[170,171]. In addition, SPINK1 mutations may cause its’ misfolding and subsequent degradation[164].
SPINK overexpression reduced intrapancreatic trypsin activity in CER-AP[172-174]. Yet, whether the loss of SPINK1 affects trypsin activity in the pancreas remains unknown, as mice with genetic deletion of the mouse ortholog of SPINK1 die shortly after birth[175]. Thus, the role of SPINK1 in modulating trypsin activity in pancreatitis requires further investigation. SPINK1 is a multifunctional protein regulating cancer cell proliferation, transdifferentiation, metastasis, and cancer stemness[176,177]. The contribution of trypsin-independent effects of SPINK in the pancreas cannot be excluded.
Chymotrypsin C
Chymotrypsin C (CTRC) is a secreted serine protease, a minor isoform of chymotrypsin with chymotrypsin-like activity that belongs to the elastase family[178]. CTRC acts together with trypsin to degrade trypsinogen. Trypsin first proteolytically converts chymotrypsinogen C into active CTRC, and then CTRC and trypsin act in concert to fully degrade trypsinogen[13,179-181].
An important role of CTRC in regulating intrapancreatic trypsin in human pancreatitis is supported by the finding that PRSS1 mutations enhance intrapancreatic trypsin not only by stimulating trypsinogen autoactivation but also by reducing CTRC-dependent trypsinogen degradation[180]. Paradoxically, CTRC can also directly stimulate trypsinogen autoactivation through proteolytic processing of TAP[182,183]. However, this effect is minor, and the predominant action of CTRC is trypsinogen degradation[13,180]. Interestingly, C57BL/6 mice widely used in experimental models do not express active CTRC[40] and instead express functionally analogous chymotrypsin CTRB1[49]. Restoring CTRC locus in C57BL/6 mice reduced trypsinogen activation in CER-AP, demonstrating CTRC’s protective role against intrapancreatic trypsin accumulation in experimental pancreatitis[40].
Cathepsin L
Cathepsin L (CTSL) is a lysosomal cysteine protease[184]; trypsinogen is one of its substrates. Unlike CTSB, cleavage of trypsinogen by CTSL generates a longer N-terminal oligopeptide and a truncated trypsin devoid of enzymatic activity[106]. CTSL knockouts, both whole-body and pancreas-specific, and CTSL pharmacologic inhibition all significantly increased trypsin activity in CER-AP[104,106,129], thus supporting CTSL’s protective role against intrapancreatic trypsin accumulation in experimental pancreatitis. CTSL involvement in protecting against trypsinogen autoactivation in HP-related GEMMs has not been tested.
Autophagy: A potent mechanism protecting against intrapancreatic trypsin accumulation
Recent studies have established macroautophagy/autophagy as a key acinar cell homeostatic mechanism[6,129-132,135,136,143,185]. Blocking autophagy (at various steps) by genetic means markedly increased basal trypsin activity in the pancreas[135,143,146,185-188]; remarkably, the effect was seen in all 6 examined GEMMs targeting different lysosomal/autophagic mediators (Table 3). These studies reveal a vital role of autophagy in protecting the pancreas against trypsin accumulation. They provide evidence that trypsin is generated during normal acinar cell functioning but is efficiently eliminated through autophagy. Impaired autophagic degradation/flux promotes intra-acinar trypsin accumulation in experimental pancreatitis; indeed, the pharmacologic autophagy enhancer trehalose markedly reduced intra-acinar trypsin accumulation in CER-AP and Arg-AP[78]. Of note, because of autophagy failure, acinar cells in pancreatitis accumulate not only trypsin-containing organelles but also dysfunctional mitochondria, ER, and toxic protein aggregates[6]. The role of autophagy in reducing trypsin accumulation in HP-related GEMMs has not been examined.
Table 3.
Genetic autophagy blockade increases the basal trypsin activity in the pancreas
|
Knockout mouse
|
Function of ablated protein
|
Effect of the knockout on autophagy
|
Fold increase in trypsin activity
|
Ref.
|
| Atg7Δpan | Key mediators of autophagosome formation | Blockade of autophagosome formation and autophagy | 2.5 | [186] |
| Atg5Δpan | 2.0 | [187] | ||
| VMP1Δac | 3.5 | [188] | ||
| LAMP2 KO | Major lysosomal membrane protein | Lysosomal dysfunction. Autophagy blockade | 3.0 | [135] |
| GNPTAB KO | Mediator of hydrolase delivery to lysosomes | Lysosomal and endosomal dysfunction. Autophagy blockade | 8.0 | [143] |
| TfebΔac; Tfe3-/- | Transcriptional regulator of lysosomal biogenesis, autophagy | Autophagy blockade | 2.5 | [140,146] |
EFFECTS OF OTHER PATHOLOGIC RESPONSES OF EXPERIMENTAL PANCREATITIS ON TRYPSINOGEN ACTIVATION
Aberrant Ca2+ signaling
Abnormal (global and sustained) increase in acinar cell cytosolic Ca2+ is an early pathologic response associated with many AP models[77,189-191]. 25 years ago, trypsinogen activation in CER-AP was shown to be abolished with chelators of extracellular or intracellular Ca2+ that prevent the cytosolic Ca2+ increase[192]. Later, Ca2+ influx via store-operated Ca2+ entry (SOCE) channels was shown necessary for acinar cell trypsinogen activation. Specific pharmacologic inhibitors of SOCE channels Orai1 or TRPV4, or overexpression of SARAF, a protein inhibiting Ca2+ influx, all prevented trypsinogen activation in experimental pancreatitis[191,193-195].
How exactly Ca2+ mediates trypsinogen activation in pancreatitis is not well understood. An increase in cytosolic Ca2+ per se does not cause trypsinogen activation in acinar cells, suggesting an indirect effect. One likely mechanism involves the Ca2+/calmodulin-dependent phosphatase calcineurin; its’ pharmacologic or genetic inhibition significantly reduces trypsinogen activation in experimental pancreatitis[196-199]. Another likely mechanism is that Ca2+ influx via SOCE channels mediates formation of the above-discussed abnormal endocytic vacuoles[200].
Mitochondrial dysfunction
Loss of the mitochondrial membrane potential in acinar cells, caused by persistent opening of the mitochondrial permeability transition pore (mPTP), is a universal early event in experimental pancreatitis[6]. Notably, genetic or pharmacologic mPTP blockade prevented trypsinogen activation in multiple dissimilar AP models, highlighting mitochondria’s critical regulatory role in this response[72,78,201]. The mechanisms linking mPTP opening to acinar cell trypsinogen activation (or trypsin accumulation) are unknown.
Nuclear factor kappa B activation
The master proinflammatory transcription factor nuclear factor kappa B (NF-κB) is activated in acinar cells early in pancreatitis and initiates the disease’ inflammatory response[89,202]. The interrelationship between NF-κB and trypsinogen activation has been studied for many years, with initially contradictory findings[61,89-91,102,203-207]. The preponderance of later evidence indicates, however, that the acinar cell activation of NF-κB and trypsinogen are 2 separate early pancreatitis responses that do not directly affect each other[206].
Inflammatory mediators
Neutrophil and macrophage depletion reduces intrapancreatic trypsin activity in experimental AP models, indicating that immune cells infiltrating the pancreas promote trypsinogen activation[62,208-211]. A number of inflammatory mediators produced by these cells, namely reactive oxygen species generated by neutrophil NADPH oxidase[62]; tumor necrosis factor[211]; the adhesion molecule P-selectin[210]; neutrophil-derived matrix metalloproteinase 9[209]; as well as neutrophil extracellular traps[208], all increased intrapancreatic trypsin in experimental pancreatitis. The mechanisms through which these inflammatory mediators stimulate trypsinogen activation remain unclear; however, they may act through a common pathway, e.g., by promoting macrophage infiltration of the pancreas. Indeed, as discussed above, trypsinogen activation can occur within macrophages[117].
Cholesterol dyshomeostasis
Acinar cell cholesterol metabolism is severely disrupted in experimental and human pancreatitis[143]. Notably, its normalization markedly reduced intra-acinar trypsin in CER-AP[143]. The underlying mechanisms remain to be elucidated; one possible mechanism is endolysosomal dysfunction caused by excessive cholesterol accumulation in lysosomes/late endosomes.
ROLE OF TRYPSIN IN PANCREATITIS: PARADIGM SHIFT?
For many decades, the prevailing paradigm in the field has been that the inappropriate, intrapancreatic activation of trypsinogen is the central pathogenic mechanism driving all forms of pancreatitis. Supporting this concept are the findings that human HP is associated with mutations in the PRSS1 gene[13,17,212] and that TAP levels in patients’ blood and urine correlate with the severity of pancreatitis[80-84,213,214]. However, experimental testing of this paradigm has only started in early 2000s, providing insights on which pancreatitis responses, and to what extent, are mediated by trypsinogen activation.
The role of intrapancreatic trypsin in experimental pancreatitis
Intrapancreatic trypsinogen activation is a hallmark response in various experimental models of AP that replicate the pathology of human disease induced by gallstones, alcohol consumption, and cigarette use (Table 2). The preponderance of the evidence, discussed in detail above in “Mechanisms of trypsinogen activation in pancreatitis”, shows that in experimental AP models (particularly CER-AP) trypsinogen activation is solely mediated by CTSB. Therefore, pharmacologic or genetic manipulations of CTSB have been a major tool to assess trypsin’s role in pancreatitis[47,60,64,102,104,110]. CTSB has multiple targets other than trypsin[184]; hence, the effects of these manipulations on experimental pancreatitis can be through trypsin-independent pathways. However, the findings that CTSB blockade does not affect pancreatitis responses, while preventing trypsinogen activation[47,60,64,104,110], strongly indicate that trypsin is not involved. More direct information on trypsin’s mediatory role was obtained using mice with ectopic (over)expression of active trypsin (from genetically engineered trypsinogen activated by the endogenous serine protease furin[205,215,216]) or with genetic ablation of the T7 isoform that abolished intra-acinar trypsinogen activation in experimental pancreatitis models[33,88,112,199].
The results of these and other studies have challenged the “trypsin central” paradigm of pancreatitis pathogenesis. Importantly, they have provided strong evidence that trypsin does not mediate the inflammatory response, a driver of pancreatitis and a key determinant of the disease severity: T7 and CTSB genetic ablation and CTSB pharmacologic inhibition did not reduce inflammatory cell infiltration, NF-κB activation, and the increases in cyto/chemokines in pancreas and serum evoked by CER-AP[33,47,60,102,104,112]; CTSL genetic ablation increased intrapancreatic trypsin activity but reduced inflammation in experimental AP models[106]; the furin-mediated ectopic generation of trypsin in acinar cells did not increase NF-κB activity or cytokine expression[205,215,216]; in CER-CP, the persistent NF-κB activation and T-cell infiltration in pancreas were the same between WT, T7, and CTSB knockout mice[88].
A large amount of trypsinogen in pancreatitis is present in the interstitial space[59], where the extracellularly generated (by CTSB or through autoactivation) trypsin could potentially cause acinar cell damage. To assess the effect of extracellular trypsin on pancreatitis, rats were infused with a combination of CER and enterokinase[59]. Administration of enterokinase (at relatively low doses) increased blood and lymph TAP levels in CER-AP but not in the basal condition. The enterokinase treatment did not worsen CER-induced inflammatory cell infiltration in pancreas; and the infiltration was not reduced by co-infusion of a cell-impermeable soybean trypsin inhibitor[59]. These findings suggest that extracellular trypsin, at least in CER-AP, does not promote inflammation.
The above data provide evidence that the mechanisms of the inflammatory response in experimental pancreatitis, in particular CER-AP and CER-CP models, are trypsin-independent. Studies also indicate that, in addition to inflammation, trypsin does not mediate a number of other pancreatitis responses. Both T7 and CTSB knockouts did not alter amylase release induced by CCK/CER in mouse acinar cells[60,74,112]. CTSB inhibitor, while blocking trypsinogen activation, did not prevent F-actin redistribution and activation of JNK and ERK kinases in mouse CER-AP; nor did it affect CER-induced amylase secretion in rat acinar cells[64,102]. CER-induced CP displayed the same extent of fibrosis, ductular metaplasia, and histopathology in WT, T7, and CTSB knockout mice[88].
The studies indicate, however, that trypsin mediates acinar cell death in experimental AP. T7 genetic ablation caused a 50% reduction in necrosis in CER-AP, both in vivo and ex-vivo[112]. Extracellular trypsin generated with enterokinase increased necrosis and hemorrhage in CER-AP[59,217]. Exogenous trypsin stimulated acinar cell death ex-vivo by promoting ferroptosis[218], a form of regulated necrosis[219]. Finally, there is strong correlation between the amount of TAP in blood and urine and parenchymal necrosis in pancreatitis patients[81,213,214]. Studies in CTSB deficient mice generated contradictory results, reporting no effect[47,104] or reduction of necrosis[60] in CER-AP. However, CTSB mediates acinar cell apoptosis in pancreatitis[74,220]; and in AP models, apoptosis causes secondary necrosis[221,222] complicating the use of CTSB knockout for the analysis of trypsin’s effect on acinar cell death.
Taken together, the data indicate that trypsinogen activation is not the primary driver of experimental pancreatitis. There are several caveats to this conclusion. The involvement of trypsin in pancreatitis responses was mainly studied in CER-AP, so the findings must be expanded to other, dissimilar AP and CP models. Another caveat is the absence of information on the role of trypsin in major organelles’ disordering (e.g., mitochondria or autophagy) that mediates pancreatitis pathologies. But the key limitation is that we do not understand the mechanisms through which trypsin affects pancreatitis responses, particularly necrosis.
The finding that trypsin, a potent protease with broad substrate recognition[223], may not cause extensive acinar cell damage in experimental AP indicates the decisive role of protective mechanisms, as discussed above in “Mechanisms protecting the pancreas against trypsin accumulation”. One can speculate that trypsin would be especially dangerous in the cytosol and thus an important role of protective mechanisms is to prevent or counteract its’ escape from membrane-bound organelles.
Involvement of PAR2 in the effects of trypsin in experimental AP
The extracellular trypsin (e.g., in the interstitium) may affect acinar cells in pancreatitis by activating, via site-specific proteolysis, protease-activated receptor-2 (PAR2) on the plasma membrane[224]. The effect of PAR2 on the severity of experimental pancreatitis is, however, model-dependent: PAR2 genetic ablation decreased acinar cell injury in TLCS-AP but had an opposite effect in CER-AP[224-228]. The mechanisms underlying these differences are poorly understood, and the impact of PAR2 activation on pancreatitis remains unclear. PAR2 is also present on ductal cells. Its activation by extracellular trypsin reduces pancreatic ductal bicarbonate secretion, which could contribute to the development of CP[229].
The role of trypsin in HP-related GEMMs
HP-related GEMMs employ mutations and other genetic manipulations in trypsinogen and other proteins in the trypsin-dependent pathway of pancreatitis that promote trypsinogen autoactivation, impede trypsinogen degradation, or inhibit trypsin enzymatic activity[13]. As noted above, the development of these genetic models started only recently, and there are many gaps in the analysis of their effects on trypsinogen activation and its role in disease development.
First, the effects of genetic modifications on steady-state intrapancreatic trypsin activity have been measured in only a few of the GEMMs (Table 1); moreover, there was no measurement of TAP. These measures are critical because the available data show that trypsinogen autoactivation in these GEMMs does not necessarily increase trypsin activity in the pancreas. For example, inserting in mice the full-length human PRSS1R122H gene, the most mutated gene in HP, did not increase the basal intrapancreatic trypsin activity[42]. Moreover, it did not cause spontaneous pancreatitis[42] despite the data that this mutation facilitates trypsinogen autoactivation[41,48,180]. A GEMM that did generate both intrapancreatic trypsin activity and spontaneous pancreatitis (Table 1) is the “artificial” T7D23A mutant with a 50-fold increased autoactivation rate[38]. However, even for this model very few parameters of pancreatitis have been reported. Thus, the lack of critical data so far precludes a conclusion about trypsin’s causative or mediatory role in developing spontaneous pancreatitis in HP-related GEMMs. A finding that GEMMs that display marked increases in the basal intrapancreatic trypsin invariably develop spontaneous pancreatitis would provide strong evidence supporting such role for trypsin; conversely, an absence of positive correlation would argue against it.
The second conclusion from the available data is that CER-AP is worsened and CER-induced trypsin activity is greater in all HP-related GEMMs examined so far (Table 1), even if they show no increase in basal intrapancreatic trypsin and no spontaneous pancreatitis[37,39,41-44,46,49,216]. For example, in T7K24R mice analogous to the HP-associated human p.K23R mutant, CER-AP exhibits about 3-fold higher intrapancreatic trypsin activity and increased cyto/chemokine levels and inflammatory cell infiltration, as compared to WT[43]. Similar effects were observed in PRSS1R122H mice; furthermore, a severe CP developed in these mice 70 days after one episode of CER-AP[41]. The results using “enzymatically dead” PRSS1R122H construct[41] suggested that the intensification of CER-AP in this GEMM requires trypsin activity.
These and similar data indicate that HP-related mutations and other genetic manipulations sensitize the pancreas to the damage induced by experimental pancreatitis stressors. The sensitization mechanism(s) remain to be determined; in fact, it is not straightforward to understand how the CER-induced/CTSB-mediated trypsinogen activation can be augmented by increased autoactivation of mutated trypsinogen in HP-related GEMMs subjected to CER-AP. One can speculate that protective mechanisms in HP-related GEMMs are able to counteract increased trypsinogen activation in the basal condition, but they become impaired or overwhelmed when these mice are subjected to experimental pancreatitis. In a hypothetical scenario, efficient autophagy protects against increased basal trypsin activity in HP-related GEMMs; in WT CER-AP, autophagy is impaired and fails to prevent trypsin accumulation[129,130]; and in HP-related GEMMs subjected to CER-AP, the impaired autophagy is even less efficient in coping with mutated/auto activated trypsinogen or with increased trypsinogen load (as in compound GEMMs expressingPRSS1R122H plus PRSS2[46]).
Another possible sensitization scenario is a “threshold effect” whereby too much active trypsin overwhelms intact pancreas protective mechanisms[45]. In fact, spontaneous pancreatitis developed in transgenic mice co-expressing human PRSS2 and PRSS1R122H but not PRSS1R122H alone[46]. T7K24R mice did not develop spontaneous pancreatitis; but the T7K24RxCtrb1-del strain, in which chymotrypsin CTRB1 that promotes trypsinogen degradation (see “Mechanisms protecting the pancreas against trypsin accumulation”) is also genetically ablated, did[48,49]. Consistent with “threshold effect” extensive pancreas damage was only caused by a high but not moderate level of ectopic/furin-mediated trypsin activity[205,215].
CONCLUSION
In summary, the intrapancreatic trypsinogen activation is a universal response in experimental (in-vivo and ex-vivo) pancreatitis models that reproduce pathologies of the vast majority of human pancreatitis cases of various etiology. Acinar cell is the primary site of intrapancreatic trypsin accumulation in experimental pancreatitis. It also occurs in activated macrophages infiltrating the pancreas; there could be a contribution from interstitial trypsinogen activation. In experimental pancreatitis, intra-acinar trypsinogen activation is CTSB mediated and occurs in acidic organelles, likely of endolysosomal system. The data on trypsin generation in HP-related GEMMs are much more limited; they indicate that, different from experimental pancreatitis, it is mediated by trypsinogen autoactivation.
The role of trypsin in pancreatitis remains a matter of intense research and much debate, and we have only started answering the question posited in the title of this review. However, compelling evidence leads to the conclusion that CTSB-mediated trypsinogen activation does not drive experimental pancreatitis. The preponderance of the results indicates that increase in trypsin does not mediate the inflammatory response, but it promotes parenchymal cell death in experimental AP and CP models. Due to the paucity of available data, the question about the pathogenic (causative or mediatory) role of trypsinogen autoactivation in the disease development in HP-related GEMMs remains open.
A number of mechanisms counteract or reduce intra-acinar trypsin levels and/or activity which could elicit damage to the pancreas. They protect against basal trypsin activity generated during normal acinar cell functioning; one, recently emerged, defense mechanism is autophagy that degrades trypsin-containing organelles. One can think that pancreas damage ensues when the protective mechanisms become impaired or overwhelmed - which is the case with autophagy in experimental pancreatitis. To what extent the damage is mediated by the increase in intra-acinar trypsin remains, however, to be determined. One may further speculate that trypsin becomes pathogenic when it is not contained within membraned structures; or when its activity raises over a threshold level. Future research should elucidate these issues.
In addition to detailed characterization of the mechanisms protecting the pancreas against trypsin accumulation, future research should explore the involvement of extracellular/interstitial trypsinogen activation. Of note, in contrast to experimental pancreatitis models, even the intra-acinar site(s) where trypsin’s increased activity occurs have not been examined in HP-related GEMMs. But perhaps the 2 most important research directions are characterization of downstream molecular targets of trypsin in pancreas and acinar cells, in particular those mediating cell death; and elucidation of conceivable interrelationships (synergy?) between trypsin-independent pathways driving the disease, such as mitochondrial dysfunction, and trypsin.
ACKNOWLEDGEMENTS
The authors thank Dr. Miklos Sahin-Toth for insightful discussions.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association, 253581; American Pancreatic Association, 8183255210.
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Miao MS S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
Contributor Information
Anna S Gukovskaya, Department of Medicine, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA 90073, United States; Department of Medicine, VA Greater Los Angeles Healthcare System, Los Angeles, CA 90073, United States. agukovsk@ucla.edu.
Markus M Lerch, Department of Medicine, Ludwig Maximilian University Hospital, Munich 81377, Germany.
Julia Mayerle, Department of Medicine II, Ludwig Maximilian University of Munich, Munich 81377, Germany.
Matthias Sendler, Department of Medicine A, University of Greifswald, Greifswald 17475, Germany.
Baoan Ji, Department of Cancer Biology, Mayo Clinic, Jacksonville, FL 32224, United States.
Ashok K Saluja, Department of Surgery, University of Miami Miller School of Medicine, Miami, FL 33136, United States.
Fred S Gorelick, Departments of Cell Biology and Internal Medicine, Yale University School of Medicine and VA West Haven, New Haven, CT 06519, United States.
Ilya Gukovsky, Department of Medicine, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA 90073, United States; Department of Medicine, VA Greater Los Angeles Healthcare System, Los Angeles, CA 90073, United States.
References
- 1.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479–496. doi: 10.1038/s41575-019-0158-2. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022;162:621–644. doi: 10.1053/j.gastro.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machicado JD, Yadav D. Epidemiology of Recurrent Acute and Chronic Pancreatitis: Similarities and Differences. Dig Dis Sci. 2017;62:1683–1691. doi: 10.1007/s10620-017-4510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcomb DC. Central role of the sentinel acute pancreatitis event (SAPE) model in understanding recurrent acute pancreatitis (RAP): Implications for precision medicine. Front Pediatr. 2022;10:941852. doi: 10.3389/fped.2022.941852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gukovskaya AS, Gorelick FS, Groblewski GE, Mareninova OA, Lugea A, Antonucci L, Waldron RT, Habtezion A, Karin M, Pandol SJ, Gukovsky I. Recent Insights Into the Pathogenic Mechanism of Pancreatitis: Role of Acinar Cell Organelle Disorders. Pancreas. 2019;48:459–470. doi: 10.1097/MPA.0000000000001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habtezion A, Gukovskaya AS, Pandol SJ. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology. 2019;156:1941–1950. doi: 10.1053/j.gastro.2018.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low JT, Shukla A, Thorn P. Pancreatic acinar cell: new insights into the control of secretion. Int J Biochem Cell Biol. 2010;42:1586–1589. doi: 10.1016/j.biocel.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Williams JA. Regulation of acinar cell function in the pancreas. Curr Opin Gastroenterol. 2010;26:478–483. doi: 10.1097/MOG.0b013e32833d11c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiari H. Über die Selbstverdauung des menschlichen Pankreas. Zeitschrift für Heilkunde. 1896;17:69–96. [Google Scholar]
- 11.Lerch MM, Gorelick FS. Early trypsinogen activation in acute pancreatitis. Med Clin North Am. 2000;84:549–563, viii. doi: 10.1016/s0025-7125(05)70239-x. [DOI] [PubMed] [Google Scholar]
- 12.Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- 13.Hegyi E, Sahin-Tóth M. Genetic Risk in Chronic Pancreatitis: The Trypsin-Dependent Pathway. Dig Dis Sci. 2017;62:1692–1701. doi: 10.1007/s10620-017-4601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sendler M, Mayerle J, Lerch MM. Molecular Basis of Diseases of the Exocrine Pancreas. In: Coleman WB, Tsongalis GJ. Essential Concepts in Molecular Pathology (Second Edition). Amsterdam: Elsevier, 2020: 367-379. [Google Scholar]
- 15.Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Tóth M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology. 2019;156:1951–1968.e1. doi: 10.1053/j.gastro.2018.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sendler M, Lerch MM. The Complex Role of Trypsin in Pancreatitis. Gastroenterology. 2020;158:822–826. doi: 10.1053/j.gastro.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 18.Scheele G, Bartelt D, Bieger W. Characterization of human exocrine pancreatic proteins by two-dimensional isoelectric focusing/sodium dodecyl sulfate gel electrophoresis. Gastroenterology. 1981;80:461–473. [PubMed] [Google Scholar]
- 19.Chen JM, Ferec C. Genes, cloned cDNAs, and proteins of human trypsinogens and pancreatitis-associated cationic trypsinogen mutations. Pancreas. 2000;21:57–62. doi: 10.1097/00006676-200007000-00052. [DOI] [PubMed] [Google Scholar]
- 20.Youngs G. Hormonal control of pancreatic endocrine and exocrine secretion. Gut. 1972;13:154–161. doi: 10.1136/gut.13.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, Steer ML. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998;275:G352–G362. doi: 10.1152/ajpgi.1998.275.2.G352. [DOI] [PubMed] [Google Scholar]
- 22.Kawabata S, Miura T, Morita T, Kato H, Fujikawa K, Iwanaga S, Takada K, Kimura T, Sakakibara S. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem. 1988;172:17–25. doi: 10.1111/j.1432-1033.1988.tb13849.x. [DOI] [PubMed] [Google Scholar]
- 23.Krüger B, Lerch MM, Tessenow W. Direct detection of premature protease activation in living pancreatic acinar cells. Lab Invest. 1998;78:763–764. [PubMed] [Google Scholar]
- 24.Krüger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halangk W, Krüger B, Ruthenbürger M, Stürzebecher J, Albrecht E, Lippert H, Lerch MM. Trypsin activity is not involved in premature, intrapancreatic trypsinogen activation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G367–G374. doi: 10.1152/ajpgi.00315.2001. [DOI] [PubMed] [Google Scholar]
- 26.Hurley PR, Cook A, Jehanli A, Austen BM, Hermon-Taylor J. Development of radioimmunoassays for free tetra-L-aspartyl-L-lysine trypsinogen activation peptides (TAP) J Immunol Methods. 1988;111:195–203. doi: 10.1016/0022-1759(88)90127-5. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-del Castillo C, Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Jehanli A, Patel G, Hermon-Taylor J, Warshaw AL. Generation and possible significance of trypsinogen activation peptides in experimental acute pancreatitis in the rat. Pancreas. 1992;7:263–270. doi: 10.1097/00006676-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Otani T, Chepilko SM, Grendell JH, Gorelick FS. Codistribution of TAP and the granule membrane protein GRAMP-92 in rat caerulein-induced pancreatitis. Am J Physiol. 1998;275:G999–G1009. doi: 10.1152/ajpgi.1998.275.5.G999. [DOI] [PubMed] [Google Scholar]
- 29.Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193. doi: 10.1053/j.gastro.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 30.Saluja AK, Dudeja V. Relevance of animal models of pancreatic cancer and pancreatitis to human disease. Gastroenterology. 2013;144:1194–1198. doi: 10.1053/j.gastro.2013.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorelick FS, Lerch MM. Do Animal Models of Acute Pancreatitis Reproduce Human Disease? Cell Mol Gastroenterol Hepatol. 2017;4:251–262. doi: 10.1016/j.jcmgh.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lugea A, Waldron RT, Mareninova OA, Shalbueva N, Deng N, Su HY, Thomas DD, Jones EK, Messenger SW, Yang J, Hu C, Gukovsky I, Liu Z, Groblewski GE, Gukovskaya AS, Gorelick FS, Pandol SJ. Human Pancreatic Acinar Cells: Proteomic Characterization, Physiologic Responses, and Organellar Disorders in ex Vivo Pancreatitis. Am J Pathol. 2017;187:2726–2743. doi: 10.1016/j.ajpath.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saluja A, Dudeja V, Dawra R, Sah RP. Early Intra-Acinar Events in Pathogenesis of Pancreatitis. Gastroenterology. 2019;156:1979–1993. doi: 10.1053/j.gastro.2019.01.268. [DOI] [PubMed] [Google Scholar]
- 34.Neuschwander-Tetri BA, Burton FR, Presti ME, Britton RS, Janney CG, Garvin PR, Brunt EM, Galvin NJ, Poulos JE. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci. 2000;45:665–674. doi: 10.1023/a:1005423122127. [DOI] [PubMed] [Google Scholar]
- 35.Otsuki M, Yamamoto M, Yamaguchi T. Animal models of chronic pancreatitis. Gastroenterol Res Pract. 2010;2010:403295. doi: 10.1155/2010/403295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selig L, Sack U, Gaiser S, Klöppel G, Savkovic V, Mössner J, Keim V, Bödeker H. Characterisation of a transgenic mouse expressing R122H human cationic trypsinogen. BMC Gastroenterol. 2006;6:30. doi: 10.1186/1471-230X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Athwal T, Huang W, Mukherjee R, Latawiec D, Chvanov M, Clarke R, Smith K, Campbell F, Merriman C, Criddle D, Sutton R, Neoptolemos J, Vlatković N. Expression of human cationic trypsinogen (PRSS1) in murine acinar cells promotes pancreatitis and apoptotic cell death. Cell Death Dis. 2014;5:e1165. doi: 10.1038/cddis.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geisz A, Sahin-Tóth M. A preclinical model of chronic pancreatitis driven by trypsinogen autoactivation. Nat Commun. 2018;9:5033. doi: 10.1038/s41467-018-07347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jancsó Z, Hegyi E, Sahin-Tóth M. Chymotrypsin Reduces the Severity of Secretagogue-Induced Pancreatitis in Mice. Gastroenterology. 2018;155:1017–1021. doi: 10.1053/j.gastro.2018.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisz A, Jancsó Z, Németh BC, Hegyi E, Sahin-Tóth M. Natural single-nucleotide deletion in chymotrypsinogen C gene increases severity of secretagogue-induced pancreatitis in C57BL/6 mice. JCI Insight. 2019;4:e129717. doi: 10.1172/jci.insight.129717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gui F, Zhang Y, Wan J, Zhan X, Yao Y, Li Y, Haddock AN, Shi J, Guo J, Chen J, Zhu X, Edenfield BH, Zhuang L, Hu C, Wang Y, Mukhopadhyay D, Radisky ES, Zhang L, Lugea A, Pandol SJ, Bi Y, Ji B. Trypsin activity governs increased susceptibility to pancreatitis in mice expressing human PRSS1R122H. J Clin Invest. 2020;130:189–202. doi: 10.1172/JCI130172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang H, Swidnicka-Siergiejko AK, Daniluk J, Gaiser S, Yao Y, Peng L, Zhang Y, Liu Y, Dong M, Zhan X, Wang H, Bi Y, Li Z, Ji B, Logsdon CD. Transgenic Expression of PRSS1(R122H) Sensitizes Mice to Pancreatitis. Gastroenterology. 2020;158:1072–1082.e7. doi: 10.1053/j.gastro.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jancsó Z, Sahin-Tóth M. Mutation That Promotes Activation of Trypsinogen Increases Severity of Secretagogue-Induced Pancreatitis in Mice. Gastroenterology. 2020;158:1083–1094. doi: 10.1053/j.gastro.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan J, Haddock A, Edenfield B, Ji B, Bi Y. Transgenic expression of human PRSS2 exacerbates pancreatitis in mice. Gut. 2020;69:2051–2052. doi: 10.1136/gutjnl-2019-320399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demcsák A, Sahin-Tóth M. Rate of Autoactivation Determines Pancreatitis Phenotype in Trypsinogen Mutant Mice. Gastroenterology. 2022;163:761–763. doi: 10.1053/j.gastro.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Wan J, Wang L, Pandol SJ, Bi Y, Ji B. Wild-Type Human PRSS2 and PRSS1(R122H) Cooperatively Initiate Spontaneous Hereditary Pancreatitis in Transgenic Mice. Gastroenterology. 2022;163:313–315.e4. doi: 10.1053/j.gastro.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geisz A, Tran T, Orekhova A, Sahin-Tóth M. Trypsin Activity in Secretagogue-induced Murine Pancreatitis Is Solely Elicited by Cathepsin B and Does Not Mediate Key Pathologic Responses. Gastroenterology. 2023;164:684–687.e4. doi: 10.1053/j.gastro.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jancsó Z, Morales Granda NC, Demcsák A, Sahin-Tóth M. Mouse model of PRSS1 p.R122H-related hereditary pancreatitis highlights context-dependent effect of autolysis-site mutation. Pancreatology. 2023;23:131–142. doi: 10.1016/j.pan.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jancsó Z, Demcsák A, Sahin-Tóth M. Modelling chronic pancreatitis as a complex genetic disease in mice. Gut. 2023;72:409–410. doi: 10.1136/gutjnl-2022-327601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee B, Husain SZ, Gukovsky I. Genetically Engineered Mouse Models Shine New Light on Decades-old Story of Trypsin in Pancreatitis. Gastroenterology. 2023;164:524–526. doi: 10.1053/j.gastro.2023.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao KN, Tuma J, Lombardi B. Acute hemorrhagic pancreatic necrosis in mice. Intraparenchymal activation of zymogens, and other enzyme changes in pancreas and serum. Gastroenterology. 1976;70:720–726. [PubMed] [Google Scholar]
- 52.Niederau C, Liddle RA, Ferrell LD, Grendell JH. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J Clin Invest. 1986;78:1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi H, Kimura T, Mimura K, Nawata H. Activation of proteases in cerulein-induced pancreatitis. Pancreas. 1989;4:565–571. doi: 10.1097/00006676-198910000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Bialek R, Willemer S, Arnold R, Adler G. Evidence of intracellular activation of serine proteases in acute cerulein-induced pancreatitis in rats. Scand J Gastroenterol. 1991;26:190–196. doi: 10.3109/00365529109025030. [DOI] [PubMed] [Google Scholar]
- 55.Lüthen R, Niederau C, Grendell JH. Intrapancreatic zymogen activation and levels of ATP and glutathione during caerulein pancreatitis in rats. Am J Physiol. 1995;268:G592–G604. doi: 10.1152/ajpgi.1995.268.4.G592. [DOI] [PubMed] [Google Scholar]
- 56.Nakae Y, Naruse S, Kitagawa M, Hirao S, Yamamoto R, Hayakawa T. Activation of trypsinogen in experimental models of acute pancreatitis in rats. Pancreas. 1995;10:306–313. doi: 10.1097/00006676-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Grady T, Mah'Moud M, Otani T, Rhee S, Lerch MM, Gorelick FS. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol. 1998;275:G1010–G1017. doi: 10.1152/ajpgi.1998.275.5.G1010. [DOI] [PubMed] [Google Scholar]
- 58.Grady T, Saluja A, Kaiser A, Steer M. Edema and intrapancreatic trypsinogen activation precede glutathione depletion during caerulein pancreatitis. Am J Physiol. 1996;271:G20–G26. doi: 10.1152/ajpgi.1996.271.1.G20. [DOI] [PubMed] [Google Scholar]
- 59.Hartwig W, Jimenez RE, Werner J, Lewandrowski KB, Warshaw AL, Fernández-del Castillo C. Interstitial trypsinogen release and its relevance to the transformation of mild into necrotizing pancreatitis in rats. Gastroenterology. 1999;117:717–725. doi: 10.1016/s0016-5085(99)70466-x. [DOI] [PubMed] [Google Scholar]
- 60.Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1197–G1208. doi: 10.1152/ajpgi.2001.280.6.G1197. [DOI] [PubMed] [Google Scholar]
- 62.Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, Holland S, Pandol SJ. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–984. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- 63.Lu Z, Karne S, Kolodecik T, Gorelick FS. Alcohols enhance caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G501–G507. doi: 10.1152/ajpgi.00388.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Acker GJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol. 2002;283:G794–G800. doi: 10.1152/ajpgi.00363.2001. [DOI] [PubMed] [Google Scholar]
- 65.Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G238–G245. doi: 10.1152/ajpgi.00471.2005. [DOI] [PubMed] [Google Scholar]
- 66.Dawra R, Sharif R, Phillips P, Dudeja V, Dhaulakhandi D, Saluja AK. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1009–G1018. doi: 10.1152/ajpgi.00167.2006. [DOI] [PubMed] [Google Scholar]
- 67.Fischer L, Gukovskaya AS, Penninger JM, Mareninova OA, Friess H, Gukovsky I, Pandol SJ. Phosphatidylinositol 3-kinase facilitates bile acid-induced Ca(2+) responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G875–G886. doi: 10.1152/ajpgi.00558.2005. [DOI] [PubMed] [Google Scholar]
- 68.Laukkarinen JM, Van Acker GJ, Weiss ER, Steer ML, Perides G. A mouse model of acute biliary pancreatitis induced by retrograde pancreatic duct infusion of Na-taurocholate. Gut. 2007;56:1590–1598. doi: 10.1136/gut.2007.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rakonczay Z Jr, Hegyi P, Dósa S, Iványi B, Jármay K, Biczó G, Hracskó Z, Varga IS, Karg E, Kaszaki J, Varró A, Lonovics J, Boros I, Gukovsky I, Gukovskaya AS, Pandol SJ, Takács T. A new severe acute necrotizing pancreatitis model induced by L-ornithine in rats. Crit Care Med. 2008;36:2117–2127. doi: 10.1097/CCM.0b013e31817d7f5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology. 2010;138:715–725. doi: 10.1053/j.gastro.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biczó G, Hegyi P, Dósa S, Shalbuyeva N, Berczi S, Sinervirta R, Hracskó Z, Siska A, Kukor Z, Jármay K, Venglovecz V, Varga IS, Iványi B, Alhonen L, Wittmann T, Gukovskaya A, Takács T, Rakonczay Z Jr. The crucial role of early mitochondrial injury in L-lysine-induced acute pancreatitis. Antioxid Redox Signal. 2011;15:2669–2681. doi: 10.1089/ars.2011.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shalbueva N, Mareninova OA, Gerloff A, Yuan J, Waldron RT, Pandol SJ, Gukovskaya AS. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology. 2013;144:437–446.e6. doi: 10.1053/j.gastro.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang W, Haynes AC, Mukherjee R, Wen L, Latawiec D, Tepikin AV, Criddle DN, Prinjha RK, Smithers N, Sutton R. Selective inhibition of BET proteins reduces pancreatic damage and systemic inflammation in bile acid- and fatty acid ethyl ester- but not caerulein-induced acute pancreatitis. Pancreatology. 2017;17:689–697. doi: 10.1016/j.pan.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Sendler M, Maertin S, John D, Persike M, Weiss FU, Krüger B, Wartmann T, Wagh P, Halangk W, Schaschke N, Mayerle J, Lerch MM. Cathepsin B Activity Initiates Apoptosis via Digestive Protease Activation in Pancreatic Acinar Cells and Experimental Pancreatitis. J Biol Chem. 2016;291:14717–14731. doi: 10.1074/jbc.M116.718999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Messenger SW, Thomas DD, Cooley MM, Jones EK, Falkowski MA, August BK, Fernandez LA, Gorelick FS, Groblewski GE. Early to Late Endosome Trafficking Controls Secretion and Zymogen Activation in Rodent and Human Pancreatic Acinar Cells. Cell Mol Gastroenterol Hepatol. 2015;1:695–709. doi: 10.1016/j.jcmgh.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mareninova OA, Gretler SR, Lee GE, Pimienta M, Qin Y, Elperin JM, Ni J, Razga Z, Gukovskaya AS, Gukovsky I. Ethanol inhibits pancreatic acinar cell autophagy through upregulation of ATG4B, mediating pathological responses of alcoholic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2023;325:G265–G278. doi: 10.1152/ajpgi.00053.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romac JM, Shahid RA, Swain SM, Vigna SR, Liddle RA. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat Commun. 2018;9:1715. doi: 10.1038/s41467-018-04194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, Gretler S, Lugea A, Malla SR, Dawson D, Ruchala P, Whitelegge J, French SW, Wen L, Husain SZ, Gorelick FS, Hegyi P, Rakonczay Z Jr, Gukovsky I, Gukovskaya AS. Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models. Gastroenterology. 2018;154:689–703. doi: 10.1053/j.gastro.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Naruse S, Kitagawa M, Ishiguro H, Nakae Y, Hayakawa T. Urinary excretion of trypsinogen activation peptide (TAP) in taurocholate-induced pancreatitis in rats. Pancreas. 2001;22:24–27. doi: 10.1097/00006676-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 80.Gudgeon AM, Heath DI, Hurley P, Jehanli A, Patel G, Wilson C, Shenkin A, Austen BM, Imrie CW, Hermon-Taylor J. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet. 1990;335:4–8. doi: 10.1016/0140-6736(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 81.Tenner S, Fernandez-del Castillo C, Warshaw A, Steinberg W, Hermon-Taylor J, Valenzuela JE, Hariri M, Hughes M, Banks PA. Urinary trypsinogen activation peptide (TAP) predicts severity in patients with acute pancreatitis. Int J Pancreatol. 1997;21:105–110. doi: 10.1007/BF02822381. [DOI] [PubMed] [Google Scholar]
- 82.Nakae Y, Naruse S, Kitagawa M, Ishiguro H, Kato M, Hayakawa S, Kondo T, Hayakawa T. Molecular forms of serum pancreatic stone protein in acute pancreatitis. Int J Pancreatol. 1999;25:17–21. doi: 10.1385/IJGC:25:1:17. [DOI] [PubMed] [Google Scholar]
- 83.Khan Z, Vlodov J, Horovitz J, Jose RM, Iswara K, Smotkin J, Brown A, Tenner S. Urinary trypsinogen activation peptide is more accurate than hematocrit in determining severity in patients with acute pancreatitis: a prospective study. Am J Gastroenterol. 2002;97:1973–1977. doi: 10.1111/j.1572-0241.2002.05953.x. [DOI] [PubMed] [Google Scholar]
- 84.Lempinen M, Stenman UH, Finne P, Puolakkainen P, Haapiainen R, Kemppainen E. Trypsinogen-2 and trypsinogen activation peptide (TAP) in urine of patients with acute pancreatitis. J Surg Res. 2003;111:267–273. doi: 10.1016/s0022-4804(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 85.Mansfield CS, Jones BR, Spillman T. Assessing the severity of canine pancreatitis. Res Vet Sci. 2003;74:137–144. doi: 10.1016/s0034-5288(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 86.Allen HS, Steiner J, Broussard J, Mansfield C, Williams DA, Jones B. Serum and urine concentrations of trypsinogen-activation peptide as markers for acute pancreatitis in cats. Can J Vet Res. 2006;70:313–316. [PMC free article] [PubMed] [Google Scholar]
- 87.Miyamoto T, Nakamura H, Nagashio Y, Asaumi H, Harada M, Otsuki M. Overexpression of Smad6 exacerbates pancreatic fibrosis in murine caerulein-induced chronic pancreatic injuries. Pancreas. 2010;39:385–391. doi: 10.1097/MPA.0b013e3181bb9603. [DOI] [PubMed] [Google Scholar]
- 88.Sah RP, Dudeja V, Dawra RK, Saluja AK. Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology. 2013;144:1076–1085.e2. doi: 10.1053/j.gastro.2013.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 90.Hietaranta AJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Relationship between NF-kappaB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun. 2001;280:388–395. doi: 10.1006/bbrc.2000.4120. [DOI] [PubMed] [Google Scholar]
- 91.Tando Y, Algül H, Schneider G, Weber CK, Weidenbach H, Adler G, Schmid RM. Induction of IkappaB-kinase by cholecystokinin is mediated by trypsinogen activation in rat pancreatic lobules. Digestion. 2002;66:237–245. doi: 10.1159/000068364. [DOI] [PubMed] [Google Scholar]
- 92.Kominami E, Tsukahara T, Hara K, Katunuma N. Biosyntheses and processing of lysosomal cysteine proteinases in rat macrophages. FEBS Lett. 1988;231:225–228. doi: 10.1016/0014-5793(88)80736-1. [DOI] [PubMed] [Google Scholar]
- 93.Rowan AD, Mason P, Mach L, Mort JS. Rat procathepsin B. Proteolytic processing to the mature form in vitro. J Biol Chem. 1992;267:15993–15999. [PubMed] [Google Scholar]
- 94.Brix K, Dunkhorst A, Mayer K, Jordans S. Cysteine cathepsins: cellular roadmap to different functions. Biochimie. 2008;90:194–207. doi: 10.1016/j.biochi.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 95.Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332 ( Pt 3):593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Acker GJ, Perides G, Steer ML. Co-localization hypothesis: a mechanism for the intrapancreatic activation of digestive enzymes during the early phases of acute pancreatitis. World J Gastroenterol. 2006;12:1985–1990. doi: 10.3748/wjg.v12.i13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saluja A, Hashimoto S, Saluja M, Powers RE, Meldolesi J, Steer ML. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol. 1987;253:G508–G516. doi: 10.1152/ajpgi.1987.253.4.G508. [DOI] [PubMed] [Google Scholar]
- 99.Greenbaum LM, Hirshkowitz A, Shoichet I. The activation of trypsinogen by cathepsin B. J Biol Chem. 1959;234:2885–2890. [PubMed] [Google Scholar]
- 100.Figarella C, Miszczuk-Jamska B, Barrett AJ. Possible lysosomal activation of pancreatic zymogens. Activation of both human trypsinogens by cathepsin B and spontaneous acid. Activation of human trypsinogen 1. Biol Chem Hoppe Seyler. 1988;369 Suppl:293–298. [PubMed] [Google Scholar]
- 101.Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304–310. doi: 10.1016/s0016-5085(97)70108-2. [DOI] [PubMed] [Google Scholar]
- 102.Van Acker GJ, Weiss E, Steer ML, Perides G. Cause-effect relationships between zymogen activation and other early events in secretagogue-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1738–G1746. doi: 10.1152/ajpgi.00543.2006. [DOI] [PubMed] [Google Scholar]
- 103.Liang T, Dolai S, Xie L, Winter E, Orabi AI, Karimian N, Cosen-Binker LI, Huang YC, Thorn P, Cattral MS, Gaisano HY. Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J Biol Chem. 2017;292:5957–5969. doi: 10.1074/jbc.M117.777433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen W, Imasaka M, Iwama H, Nishiura H, Ohmuraya M. Double deficiency of cathepsin B and L in the mouse pancreas alters trypsin activity without affecting acute pancreatitis severity. Pancreatology. 2022;22:880–886. doi: 10.1016/j.pan.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 105.Lindkvist B, Fajardo I, Pejler G, Borgström A. Cathepsin B activates human trypsinogen 1 but not proelastase 2 or procarboxypeptidase B. Pancreatology. 2006;6:224–231. doi: 10.1159/000091961. [DOI] [PubMed] [Google Scholar]
- 106.Wartmann T, Mayerle J, Kähne T, Sahin-Tóth M, Ruthenbürger M, Matthias R, Kruse A, Reinheckel T, Peters C, Weiss FU, Sendler M, Lippert H, Schulz HU, Aghdassi A, Dummer A, Teller S, Halangk W, Lerch MM. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology. 2010;138:726–737. doi: 10.1053/j.gastro.2009.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kunitz M, Northrop JH. The isolation of crystalline trypsinogen and its conversion into crystalline trypsin. Science. 1934;80:505–506. doi: 10.1126/science.80.2083.505. [DOI] [PubMed] [Google Scholar]
- 108.Kereszturi E, Sahin-Tóth M. Intracellular autoactivation of human cationic trypsinogen mutants causes reduced trypsinogen secretion and acinar cell death. J Biol Chem. 2009;284:33392–33399. doi: 10.1074/jbc.M109.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Halangk W, Lerch MM. Early events in acute pancreatitis. Gastroenterol Clin North Am. 2004;33:717–731. doi: 10.1016/j.gtc.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 110.Demcsák A, Geisz A, Sahin-Tóth M. Engineering mouse cationic trypsinogen for rapid and selective activation by cathepsin B. Sci Rep. 2019;9:9188. doi: 10.1038/s41598-019-45631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lüthen R, Owen RL, Sarbia M, Grendell JH, Niederau C. Premature trypsinogen activation during cerulein pancreatitis in rats occurs inside pancreatic acinar cells. Pancreas. 1998;17:38–43. doi: 10.1097/00006676-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Dawra R, Sah RP, Dudeja V, Rishi L, Talukdar R, Garg P, Saluja AK. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217.e2. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kukor Z, Mayerle J, Krüger B, Tóth M, Steed PM, Halangk W, Lerch MM, Sahin-Tóth M. Presence of cathepsin B in the human pancreatic secretory pathway and its role in trypsinogen activation during hereditary pancreatitis. J Biol Chem. 2002;277:21389–21396. doi: 10.1074/jbc.M200878200. [DOI] [PubMed] [Google Scholar]
- 114.Lyo V, Cattaruzza F, Kim TN, Walker AW, Paulick M, Cox D, Cloyd J, Buxbaum J, Ostroff J, Bogyo M, Grady EF, Bunnett NW, Kirkwood KS. Active cathepsins B, L, and S in murine and human pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G894–G903. doi: 10.1152/ajpgi.00073.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gaisano HY, Lutz MP, Leser J, Sheu L, Lynch G, Tang L, Tamori Y, Trimble WS, Salapatek AM. Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J Clin Invest. 2001;108:1597–1611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fallon MB, Gorelick FS, Anderson JM, Mennone A, Saluja A, Steer ML. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology. 1995;108:1863–1872. doi: 10.1016/0016-5085(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 117.Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, Partecke LI, Döring P, Gukovsky I, Gukovskaya AS, Wagh PR, Lerch MM, Mayerle J. Cathepsin B-Mediated Activation of Trypsinogen in Endocytosing Macrophages Increases Severity of Pancreatitis in Mice. Gastroenterology. 2018;154:704–718.e10. doi: 10.1053/j.gastro.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Steer ML. Frank Brooks memorial Lecture: The early intraacinar cell events which occur during acute pancreatitis. Pancreas. 1998;17:31–37. doi: 10.1159/000026152. [DOI] [PubMed] [Google Scholar]
- 119.Gorelick FS, Otani T. Mechanisms of intracellular zymogen activation. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:227–240. doi: 10.1053/bega.1999.0021. [DOI] [PubMed] [Google Scholar]
- 120.Steer ML. Early events in acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:213–225. doi: 10.1053/bega.1999.0020. [DOI] [PubMed] [Google Scholar]
- 121.Niederau C, Grendell JH. Intracellular vacuoles in experimental acute pancreatitis in rats and mice are an acidified compartment. J Clin Invest. 1988;81:229–236. doi: 10.1172/JCI113300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lerch MM, Saluja AK, Dawra R, Saluja M, Steer ML. The effect of chloroquine administration on two experimental models of acute pancreatitis. Gastroenterology. 1993;104:1768–1779. doi: 10.1016/0016-5085(93)90658-y. [DOI] [PubMed] [Google Scholar]
- 123.Waterford SD, Kolodecik TR, Thrower EC, Gorelick FS. Vacuolar ATPase regulates zymogen activation in pancreatic acini. J Biol Chem. 2005;280:5430–5434. doi: 10.1074/jbc.M413513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kolodecik T, Gorelick F, Thrower E. Genetic and pharmacologic manipulation of vacuolar ATPase; effects on zymogen activation in pancreatic acini. Open Access Anim Physiol. 2009;2009:1–11. doi: 10.2147/oaap.s7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scott CC, Vacca F, Gruenberg J. Endosome maturation, transport and functions. Semin Cell Dev Biol. 2014;31:2–10. doi: 10.1016/j.semcdb.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 126.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 127.Kamalesh K, Scher N, Biton T, Schejter ED, Shilo BZ, Avinoam O. Exocytosis by vesicle crumpling maintains apical membrane homeostasis during exocrine secretion. Dev Cell. 2021;56:1603–1616.e6. doi: 10.1016/j.devcel.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Azarnia Tehran D, Maritzen T. Endocytic proteins: An expanding repertoire of presynaptic functions. Curr Opin Neurobiol. 2022;73:102519. doi: 10.1016/j.conb.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 129.Mareninova OA, Hermann K, French SW, O'Konski MS, Pandol SJ, Webster P, Erickson AH, Katunuma N, Gorelick FS, Gukovsky I, Gukovskaya AS. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gukovskaya AS, Gukovsky I. Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G993–G1003. doi: 10.1152/ajpgi.00122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mareninova OA, Jia W, Gretler SR, Holthaus CL, Thomas DDH, Pimienta M, Dillon DL, Gukovskaya AS, Gukovsky I, Groblewski GE. Transgenic expression of GFP-LC3 perturbs autophagy in exocrine pancreas and acute pancreatitis responses in mice. Autophagy. 2020;16:2084–2097. doi: 10.1080/15548627.2020.1715047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gukovskaya AS, Gukovsky I, Algül H, Habtezion A. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology. 2017;153:1212–1226. doi: 10.1053/j.gastro.2017.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang X, Sheu L, Tamori Y, Trimble WS, Gaisano HY. Cholecystokinin-regulated exocytosis in rat pancreatic acinar cells is inhibited by a C-terminus truncated mutant of SNAP-23. Pancreas. 2001;23:125–133. doi: 10.1097/00006676-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 134.Sherwood MW, Prior IA, Voronina SG, Barrow SL, Woodsmith JD, Gerasimenko OV, Petersen OH, Tepikin AV. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci U S A. 2007;104:5674–5679. doi: 10.1073/pnas.0700951104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mareninova OA, Sendler M, Malla SR, Yakubov I, French SW, Tokhtaeva E, Vagin O, Oorschot V, Lüllmann-Rauch R, Blanz J, Dawson D, Klumperman J, Lerch MM, Mayerle J, Gukovsky I, Gukovskaya AS. Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Cell Mol Gastroenterol Hepatol. 2015;1:678–694. doi: 10.1016/j.jcmgh.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mareninova OA, Dillon DL, Wightman CJM, Yakubov I, Takahashi T, Gaisano HY, Munson K, Ohmuraya M, Dawson D, Gukovsky I, Gukovskaya AS. Rab9 Mediates Pancreatic Autophagy Switch From Canonical to Noncanonical, Aggravating Experimental Pancreatitis. Cell Mol Gastroenterol Hepatol. 2022;13:599–622. doi: 10.1016/j.jcmgh.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Messenger SW, Jones EK, Holthaus CL, Thomas DDH, Cooley MM, Byrne JA, Mareninova OA, Gukovskaya AS, Groblewski GE. Acute acinar pancreatitis blocks vesicle-associated membrane protein 8 (VAMP8)-dependent secretion, resulting in intracellular trypsin accumulation. J Biol Chem. 2017;292:7828–7839. doi: 10.1074/jbc.M117.781815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chvanov M, De Faveri F, Moore D, Sherwood MW, Awais M, Voronina S, Sutton R, Criddle DN, Haynes L, Tepikin AV. Intracellular rupture, exocytosis and actin interaction of endocytic vacuoles in pancreatic acinar cells: initiating events in acute pancreatitis. J Physiol. 2018;596:2547–2564. doi: 10.1113/JP275879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dolai S, Liang T, Orabi AI, Holmyard D, Xie L, Greitzer-Antes D, Kang Y, Xie H, Javed TA, Lam PP, Rubin DC, Thorn P, Gaisano HY. Pancreatitis-Induced Depletion of Syntaxin 2 Promotes Autophagy and Increases Basolateral Exocytosis. Gastroenterology. 2018;154:1805–1821.e5. doi: 10.1053/j.gastro.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang S, Ni HM, Chao X, Wang H, Bridges B, Kumer S, Schmitt T, Mareninova O, Gukovskaya A, De Lisle RC, Ballabio A, Pacher P, Ding WX. Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis. Autophagy. 2019;15:1954–1969. doi: 10.1080/15548627.2019.1596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Faveri F, Chvanov M, Voronina S, Moore D, Pollock L, Haynes L, Awais M, Beckett AJ, Mayer U, Sutton R, Criddle DN, Prior IA, Wileman T, Tepikin AV. LAP-like non-canonical autophagy and evolution of endocytic vacuoles in pancreatic acinar cells. Autophagy. 2020;16:1314–1331. doi: 10.1080/15548627.2019.1679514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dolai S, Takahashi T, Qin T, Liang T, Xie L, Kang F, Miao YF, Xie H, Kang Y, Manuel J, Winter E, Roche PA, Cattral MS, Gaisano HY. Pancreas-specific SNAP23 depletion prevents pancreatitis by attenuating pathological basolateral exocytosis and formation of trypsin-activating autolysosomes. Autophagy. 2021;17:3068–3081. doi: 10.1080/15548627.2020.1852725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mareninova OA, Vegh ET, Shalbueva N, Wightman CJ, Dillon DL, Malla S, Xie Y, Takahashi T, Rakonczay Z Jr, French SW, Gaisano HY, Gorelick FS, Pandol SJ, Bensinger SJ, Davidson NO, Dawson DW, Gukovsky I, Gukovskaya AS. Dysregulation of mannose-6-phosphate-dependent cholesterol homeostasis in acinar cells mediates pancreatitis. J Clin Invest. 2021;131 doi: 10.1172/JCI146870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Voronina S, Chvanov M, De Faveri F, Mayer U, Wileman T, Criddle D, Tepikin A. Autophagy, Acute Pancreatitis and the Metamorphoses of a Trypsinogen-Activating Organelle. Cells. 2022;11 doi: 10.3390/cells11162514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang L, Diao J. VAMP8 phosphorylation regulates lysosome dynamics during autophagy. Autophagy Rep. 2022;1:79–82. doi: 10.1080/27694127.2022.2031378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang S, Ni HM, Chao X, Ma X, Kolodecik T, De Lisle R, Ballabio A, Pacher P, Ding WX. Critical Role of TFEB-Mediated Lysosomal Biogenesis in Alcohol-Induced Pancreatitis in Mice and Humans. Cell Mol Gastroenterol Hepatol. 2020;10:59–81. doi: 10.1016/j.jcmgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Malla SR, Krueger B, Wartmann T, Sendler M, Mahajan UM, Weiss FU, Thiel FG, De Boni C, Gorelick FS, Halangk W, Aghdassi AA, Reinheckel T, Gukovskaya AS, Lerch MM, Mayerle J. Early trypsin activation develops independently of autophagy in caerulein-induced pancreatitis in mice. Cell Mol Life Sci. 2020;77:1811–1825. doi: 10.1007/s00018-019-03254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Willemer S, Bialek R, Adler G. Localization of lysosomal and digestive enzymes in cytoplasmic vacuoles in caerulein-pancreatitis. Histochemistry. 1990;94:161–170. doi: 10.1007/BF02440183. [DOI] [PubMed] [Google Scholar]
- 149.Tooze J, Hollinshead M, Hensel G, Kern HF, Hoflack B. Regulated secretion of mature cathepsin B from rat exocrine pancreatic cells. Eur J Cell Biol. 1991;56:187–200. [PubMed] [Google Scholar]
- 150.Kaizuka T, Morishita H, Hama Y, Tsukamoto S, Matsui T, Toyota Y, Kodama A, Ishihara T, Mizushima T, Mizushima N. An Autophagic Flux Probe that Releases an Internal Control. Mol Cell. 2016;64:835–849. doi: 10.1016/j.molcel.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 151.Gaisano HY. A hypothesis: SNARE-ing the mechanisms of regulated exocytosis and pathologic membrane fusions in the pancreatic acinar cell. Pancreas. 2000;20:217–226. doi: 10.1097/00006676-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 152.Wang T, Li L, Hong W. SNARE proteins in membrane trafficking. Traffic. 2017;18:767–775. doi: 10.1111/tra.12524. [DOI] [PubMed] [Google Scholar]
- 153.Khvotchev M, Soloviev M. SNARE Modulators and SNARE Mimetic Peptides. Biomolecules. 2022;12 doi: 10.3390/biom12121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pickett JA, Thorn P, Edwardson JM. The plasma membrane Q-SNARE syntaxin 2 enters the zymogen granule membrane during exocytosis in the pancreatic acinar cell. J Biol Chem. 2005;280:1506–1511. doi: 10.1074/jbc.M411967200. [DOI] [PubMed] [Google Scholar]
- 155.Kazal LA, Spicer DS, Brahinsky RA. Isolation of a crystalline trypsin inhibitor-anticoagulant protein from pancreas. J Am Chem Soc. 1948;70:3034–3040. doi: 10.1021/ja01189a060. [DOI] [PubMed] [Google Scholar]
- 156.Laskowski M Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- 157.Suzuki M, Minowa K, Nakano S, Isayama H, Shimizu T. Genetic Abnormalities in Pancreatitis: An Update on Diagnosis, Clinical Features, and Treatment. Diagnostics (Basel) 2020;11 doi: 10.3390/diagnostics11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Laskowski M, Wu FC. Temporary inhibition of trypsin. J Biol Chem. 1953;204:797–805. [PubMed] [Google Scholar]
- 159.Pfützer RH, Barmada MM, Brunskill AP, Finch R, Hart PS, Neoptolemos J, Furey WF, Whitcomb DC. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–623. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 160.Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 161.Liu J, Lu SY, Wang YG, Wei ZY, Zhang HX. SPINK1 Gene is Significantly Associated With Pancreatitis: A Comprehensive Meta-Analysis. Pancreas. 2017;46:1373–1380. doi: 10.1097/MPA.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 162.Muller N, Sarantitis I, Rouanet M, de Mestier L, Halloran C, Greenhalf W, Férec C, Masson E, Ruszniewski P, Lévy P, Neoptolemos J, Buscail L, Rebours V. Natural history of SPINK1 germline mutation related-pancreatitis. EBioMedicine. 2019;48:581–591. doi: 10.1016/j.ebiom.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nagel F, Palm GJ, Geist N, McDonnell TCR, Susemihl A, Girbardt B, Mayerle J, Lerch MM, Lammers M, Delcea M. Structural and Biophysical Insights into SPINK1 Bound to Human Cationic Trypsin. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23073468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Király O, Wartmann T, Sahin-Tóth M. Missense mutations in pancreatic secretory trypsin inhibitor (SPINK1) cause intracellular retention and degradation. Gut. 2007;56:1433–1438. doi: 10.1136/gut.2006.115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morales Granda NC, Toldi V, Miczi M, Lassoued M, Szabó A. Inhibition of mouse trypsin isoforms by SPINK1 and effect of human pancreatitis-associated mutations. Pancreatology. 2023;23:358–366. doi: 10.1016/j.pan.2023.04.043. [DOI] [PubMed] [Google Scholar]
- 166.Chen JM, Mercier B, Audrezet MP, Ferec C. Mutational analysis of the human pancreatic secretory trypsin inhibitor (PSTI) gene in hereditary and sporadic chronic pancreatitis. J Med Genet. 2000;37:67–69. doi: 10.1136/jmg.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schneider A. Serine protease inhibitor kazal type 1 mutations and pancreatitis. Clin Lab Med. 2005;25:61–78. doi: 10.1016/j.cll.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 168.Boulling A, Masson E, Zou WB, Paliwal S, Wu H, Issarapu P, Bhaskar S, Génin E, Cooper DN, Li ZS, Chandak GR, Liao Z, Chen JM, Férec C. Identification of a functional enhancer variant within the chronic pancreatitis-associated SPINK1 c.101A>G (p.Asn34Ser)-containing haplotype. Hum Mutat. 2017;38:1014–1024. doi: 10.1002/humu.23269. [DOI] [PubMed] [Google Scholar]
- 169.Pu N, Masson E, Cooper DN, Génin E, Férec C, Chen JM. Chronic Pancreatitis: The True Pathogenic Culprit within the SPINK1 N34S-Containing Haplotype Is No Longer at Large. Genes (Basel) 2021;12 doi: 10.3390/genes12111683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Masamune A, Kotani H, Sörgel FL, Chen JM, Hamada S, Sakaguchi R, Masson E, Nakano E, Kakuta Y, Niihori T, Funayama R, Shirota M, Hirano T, Kawamoto T, Hosokoshi A, Kume K, Unger L, Ewers M, Laumen H, Bugert P, Mori MX, Tsvilovskyy V, Weißgerber P, Kriebs U, Fecher-Trost C, Freichel M, Diakopoulos KN, Berninger A, Lesina M, Ishii K, Itoi T, Ikeura T, Okazaki K, Kaune T, Rosendahl J, Nagasaki M, Uezono Y, Algül H, Nakayama K, Matsubara Y, Aoki Y, Férec C, Mori Y, Witt H, Shimosegawa T. Variants That Affect Function of Calcium Channel TRPV6 Are Associated With Early-Onset Chronic Pancreatitis. Gastroenterology. 2020;158:1626–1641.e8. doi: 10.1053/j.gastro.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 171.Hamada S, Masson E, Chen JM, Sakaguchi R, Rebours V, Buscail L, Matsumoto R, Tanaka Y, Kikuta K, Kataoka F, Sasaki A, Le Rhun M, Audin H, Lachaux A, Caumont B, Lorenzo D, Billiemaz K, Besnard R, Koch S, Lamireau T, De Koninck X GREPAN (Genetic REsearch on PANcreatitis) Study Group, Génin E, Cooper DN, Mori Y, Masamune A, Férec C. Functionally deficient TRPV6 variants contribute to hereditary and familial chronic pancreatitis. Hum Mutat. 2022;43:228–239. doi: 10.1002/humu.24315. [DOI] [PubMed] [Google Scholar]
- 172.Liddle RA. Susceptibility to pancreatitis related to PSTI/SPINK1 expression. Gastroenterol Clin North Am. 2004;33:807–816. doi: 10.1016/j.gtc.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 173.Nathan JD, Romac J, Peng RY, Peyton M, Macdonald RJ, Liddle RA. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–727. doi: 10.1053/j.gastro.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 174.Ohmuraya M, Sugano A, Hirota M, Takaoka Y, Yamamura K. Role of Intrapancreatic SPINK1/Spink3 Expression in the Development of Pancreatitis. Front Physiol. 2012;3:126. doi: 10.3389/fphys.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ohmuraya M, Hirota M, Araki M, Mizushima N, Matsui M, Mizumoto T, Haruna K, Kume S, Takeya M, Ogawa M, Araki K, Yamamura K. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 2005;129:696–705. doi: 10.1016/j.gastro.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 176.Ozaki N, Ohmuraya M, Hirota M, Ida S, Wang J, Takamori H, Higashiyama S, Baba H, Yamamura K. Serine protease inhibitor Kazal type 1 promotes proliferation of pancreatic cancer cells through the epidermal growth factor receptor. Mol Cancer Res. 2009;7:1572–1581. doi: 10.1158/1541-7786.MCR-08-0567. [DOI] [PubMed] [Google Scholar]
- 177.Lin TC. Functional Roles of SPINK1 in Cancers. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22083814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tomomura M, Tomomura A. Caldecrin: A pancreas-derived hypocalcemic factor, regulates osteoclast formation and function. World J Biol Chem. 2015;6:358–365. doi: 10.4331/wjbc.v6.i4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Szmola R, Sahin-Tóth M. Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: identity with Rinderknecht's enzyme Y. Proc Natl Acad Sci U S A. 2007;104:11227–11232. doi: 10.1073/pnas.0703714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Szabó A, Sahin-Tóth M. Increased activation of hereditary pancreatitis-associated human cationic trypsinogen mutants in presence of chymotrypsin C. J Biol Chem. 2012;287:20701–20710. doi: 10.1074/jbc.M112.360065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Szabó A, Radisky ES, Sahin-Tóth M. Zymogen activation confers thermodynamic stability on a key peptide bond and protects human cationic trypsin from degradation. J Biol Chem. 2014;289:4753–4761. doi: 10.1074/jbc.M113.538884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nemoda Z, Sahin-Tóth M. Chymotrypsin C (caldecrin) stimulates autoactivation of human cationic trypsinogen. J Biol Chem. 2006;281:11879–11886. doi: 10.1074/jbc.M600124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Németh BC, Wartmann T, Halangk W, Sahin-Tóth M. Autoactivation of mouse trypsinogens is regulated by chymotrypsin C via cleavage of the autolysis loop. J Biol Chem. 2013;288:24049–24062. doi: 10.1074/jbc.M113.478800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Reinheckel T, Deussing J, Roth W, Peters C. Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L. Biol Chem. 2001;382:735–741. doi: 10.1515/BC.2001.089. [DOI] [PubMed] [Google Scholar]
- 185.Gukovskaya AM, Mareninova OM, Ding WX, Habtezion A, Gukovsky I. Models of Pancreatitis Caused by Genetic Blockage of Autophagy/Lysosomal Pathway. Pancreapedia Exocrine Pancreas Knowl Base. 2021 [Google Scholar]
- 186.Antonucci L, Fagman JB, Kim JY, Todoric J, Gukovsky I, Mackey M, Ellisman MH, Karin M. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci U S A. 2015;112:E6166–E6174. doi: 10.1073/pnas.1519384112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Diakopoulos KN, Lesina M, Wörmann S, Song L, Aichler M, Schild L, Artati A, Römisch-Margl W, Wartmann T, Fischer R, Kabiri Y, Zischka H, Halangk W, Demir IE, Pilsak C, Walch A, Mantzoros CS, Steiner JM, Erkan M, Schmid RM, Witt H, Adamski J, Algül H. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626–638.e17. doi: 10.1053/j.gastro.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 188.Wang S, Chao X, Jiang X, Wang T, Rodriguez Y, Yang L, Pacher P, Ni HM, Ding WX. Loss of acinar cell VMP1 triggers spontaneous pancreatitis in mice. Autophagy. 2022;18:1572–1582. doi: 10.1080/15548627.2021.1990672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gerasimenko JV, Gerasimenko OV, Petersen OH. The role of Ca2+ in the pathophysiology of pancreatitis. J Physiol. 2014;592:269–280. doi: 10.1113/jphysiol.2013.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huang W, Booth DM, Cane MC, Chvanov M, Javed MA, Elliott VL, Armstrong JA, Dingsdale H, Cash N, Li Y, Greenhalf W, Mukherjee R, Kaphalia BS, Jaffar M, Petersen OH, Tepikin AV, Sutton R, Criddle DN. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut. 2014;63:1313–1324. doi: 10.1136/gutjnl-2012-304058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Swain SM, Romac JM, Shahid RA, Pandol SJ, Liedtke W, Vigna SR, Liddle RA. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. J Clin Invest. 2020;130:2527–2541. doi: 10.1172/JCI134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol. 1999;276:G835–G842. doi: 10.1152/ajpgi.1999.276.4.G835. [DOI] [PubMed] [Google Scholar]
- 193.Wen L, Voronina S, Javed MA, Awais M, Szatmary P, Latawiec D, Chvanov M, Collier D, Huang W, Barrett J, Begg M, Stauderman K, Roos J, Grigoryev S, Ramos S, Rogers E, Whitten J, Velicelebi G, Dunn M, Tepikin AV, Criddle DN, Sutton R. Inhibitors of ORAI1 Prevent Cytosolic Calcium-Associated Injury of Human Pancreatic Acinar Cells and Acute Pancreatitis in 3 Mouse Models. Gastroenterology. 2015;149:481–92.e7. doi: 10.1053/j.gastro.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Son A, Ahuja M, Schwartz DM, Varga A, Swaim W, Kang N, Maleth J, Shin DM, Muallem S. Ca(2+) Influx Channel Inhibitor SARAF Protects Mice From Acute Pancreatitis. Gastroenterology. 2019;157:1660–1672.e2. doi: 10.1053/j.gastro.2019.08.042. [DOI] [PubMed] [Google Scholar]
- 195.Waldron RT, Chen Y, Pham H, Go A, Su HY, Hu C, Wen L, Husain SZ, Sugar CA, Roos J, Ramos S, Lugea A, Dunn M, Stauderman K, Pandol SJ. The Orai Ca(2+) channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J Physiol. 2019;597:3085–3105. doi: 10.1113/JP277856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Husain SZ, Grant WM, Gorelick FS, Nathanson MH, Shah AU. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1594–G1599. doi: 10.1152/ajpgi.00500.2006. [DOI] [PubMed] [Google Scholar]
- 197.Awla D, Zetterqvist AV, Abdulla A, Camello C, Berglund LM, Spégel P, Pozo MJ, Camello PJ, Regnér S, Gomez MF, Thorlacius H. NFATc3 regulates trypsinogen activation, neutrophil recruitment, and tissue damage in acute pancreatitis in mice. Gastroenterology. 2012;143:1352–1360.e7. doi: 10.1053/j.gastro.2012.07.098. [DOI] [PubMed] [Google Scholar]
- 198.Muili KA, Ahmad M, Orabi AI, Mahmood SM, Shah AU, Molkentin JD, Husain SZ. Pharmacological and genetic inhibition of calcineurin protects against carbachol-induced pathological zymogen activation and acinar cell injury. Am J Physiol Gastrointest Liver Physiol. 2012;302:G898–G905. doi: 10.1152/ajpgi.00545.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol. 2013;29:523–530. doi: 10.1097/MOG.0b013e328363e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Voronina S, Collier D, Chvanov M, Middlehurst B, Beckett AJ, Prior IA, Criddle DN, Begg M, Mikoshiba K, Sutton R, Tepikin AV. The role of Ca2+ influx in endocytic vacuole formation in pancreatic acinar cells. Biochem J. 2015;465:405–412. doi: 10.1042/BJ20140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mukherjee R, Mareninova OA, Odinokova IV, Huang W, Murphy J, Chvanov M, Javed MA, Wen L, Booth DM, Cane MC, Awais M, Gavillet B, Pruss RM, Schaller S, Molkentin JD, Tepikin AV, Petersen OH, Pandol SJ, Gukovsky I, Criddle DN, Gukovskaya AS, Sutton R NIHR Pancreas Biomedical Research Unit. Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: inhibition prevents acute pancreatitis by protecting production of ATP. Gut. 2016;65:1333–1346. doi: 10.1136/gutjnl-2014-308553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rakonczay Z Jr, Hegyi P, Takács T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 203.Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-kappaB in rat pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;280:C465–C472. doi: 10.1152/ajpcell.2001.280.3.C465. [DOI] [PubMed] [Google Scholar]
- 204.Baumann B, Wagner M, Aleksic T, von Wichert G, Weber CK, Adler G, Wirth T. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest. 2007;117:1502–1513. doi: 10.1172/JCI30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ji B, Gaiser S, Chen X, Ernst SA, Logsdon CD. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. J Biol Chem. 2009;284:17488–17498. doi: 10.1074/jbc.M109.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gukovsky I, Gukovskaya A. Nuclear factor-κB in pancreatitis: Jack-of-all-trades, but which one is more important? Gastroenterology. 2013;144:26–29. doi: 10.1053/j.gastro.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Neuhöfer P, Liang S, Einwächter H, Schwerdtfeger C, Wartmann T, Treiber M, Zhang H, Schulz HU, Dlubatz K, Lesina M, Diakopoulos KN, Wörmann S, Halangk W, Witt H, Schmid RM, Algül H. Deletion of IκBα activates RelA to reduce acute pancreatitis in mice through up-regulation of Spi2A. Gastroenterology. 2013;144:192–201. doi: 10.1053/j.gastro.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 208.Abdulla A, Awla D, Thorlacius H, Regnér S. Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. J Leukoc Biol. 2011;90:975–982. doi: 10.1189/jlb.0411195. [DOI] [PubMed] [Google Scholar]
- 209.Awla D, Abdulla A, Syk I, Jeppsson B, Regnér S, Thorlacius H. Neutrophil-derived matrix metalloproteinase-9 is a potent activator of trypsinogen in acinar cells in acute pancreatitis. J Leukoc Biol. 2012;91:711–719. doi: 10.1189/jlb.0811443. [DOI] [PubMed] [Google Scholar]
- 210.Hartman H, Abdulla A, Awla D, Lindkvist B, Jeppsson B, Thorlacius H, Regnér S. P-selectin mediates neutrophil rolling and recruitment in acute pancreatitis. Br J Surg. 2012;99:246–255. doi: 10.1002/bjs.7775. [DOI] [PubMed] [Google Scholar]
- 211.Sendler M, Dummer A, Weiss FU, Krüger B, Wartmann T, Scharffetter-Kochanek K, van Rooijen N, Malla SR, Aghdassi A, Halangk W, Lerch MM, Mayerle J. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut. 2013;62:430–439. doi: 10.1136/gutjnl-2011-300771. [DOI] [PubMed] [Google Scholar]
- 212.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144:1292–1302. doi: 10.1053/j.gastro.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schmidt J, Fernández-del Castillo C, Rattner DW, Lewandrowski K, Compton CC, Warshaw AL. Trypsinogen-activation peptides in experimental rat pancreatitis: prognostic implications and histopathologic correlates. Gastroenterology. 1992;103:1009–1016. doi: 10.1016/0016-5085(92)90036-x. [DOI] [PubMed] [Google Scholar]
- 214.Foitzik T, Fernandez-del Castillo C, Lewandrowski KB, Rattner DW, Herfarth C, Warshaw AL. [Trypsinogen activation peptides in acute pancreatitis. Experimental data and clinical implications] Chirurg. 1994;65:186–189. [PubMed] [Google Scholar]
- 215.Gaiser S, Daniluk J, Liu Y, Tsou L, Chu J, Lee W, Longnecker DS, Logsdon CD, Ji B. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379–1388. doi: 10.1136/gut.2010.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhan X, Wan J, Zhang G, Song L, Gui F, Zhang Y, Li Y, Guo J, Dawra RK, Saluja AK, Haddock AN, Zhang L, Bi Y, Ji B. Elevated intracellular trypsin exacerbates acute pancreatitis and chronic pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2019;316:G816–G825. doi: 10.1152/ajpgi.00004.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hartwig W, Kolvenbach M, Hackert T, Fortunato F, Schneider L, Büchler MW, Werner J. Enterokinase induces severe necrosis and rapid mortality in cerulein pancreatitis: characterization of a novel noninvasive rat model of necro-hemorrhagic pancreatitis. Surgery. 2007;142:327–336. doi: 10.1016/j.surg.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 218.Liu K, Liu J, Zou B, Li C, Zeh HJ, Kang R, Kroemer G, Huang J, Tang D. Trypsin-Mediated Sensitization to Ferroptosis Increases the Severity of Pancreatitis in Mice. Cell Mol Gastroenterol Hepatol. 2022;13:483–500. doi: 10.1016/j.jcmgh.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Talukdar R, Sareen A, Zhu H, Yuan Z, Dixit A, Cheema H, George J, Barlass U, Sah R, Garg SK, Banerjee S, Garg P, Dudeja V, Dawra R, Saluja AK. Release of Cathepsin B in Cytosol Causes Cell Death in Acute Pancreatitis. Gastroenterology. 2016;151:747–758.e5. doi: 10.1053/j.gastro.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
- 222.Odinokova IV, Sung KF, Mareninova OA, Hermann K, Gukovsky I, Gukovskaya AS. Mitochondrial mechanisms of death responses in pancreatitis. J Gastroenterol Hepatol. 2008;23 Suppl 1:S25–S30. doi: 10.1111/j.1440-1746.2007.05271.x. [DOI] [PubMed] [Google Scholar]
- 223.Di Cera E. Serine proteases. IUBMB Life. 2009;61:510–515. doi: 10.1002/iub.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Suhaj P, Olejar T, Matej R. PAR2: The Cornerstone of Pancreatic Diseases. Physiol Res. 2022;71:583–596. doi: 10.33549/physiolres.934931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Namkung W, Han W, Luo X, Muallem S, Cho KH, Kim KH, Lee MG. Protease-activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology. 2004;126:1844–1859. doi: 10.1053/j.gastro.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 226.Sharma A, Tao X, Gopal A, Ligon B, Andrade-Gordon P, Steer ML, Perides G. Protection against acute pancreatitis by activation of protease-activated receptor-2. Am J Physiol Gastrointest Liver Physiol. 2005;288:G388–G395. doi: 10.1152/ajpgi.00341.2004. [DOI] [PubMed] [Google Scholar]
- 227.Singh VP, Bhagat L, Navina S, Sharif R, Dawra RK, Saluja AK. Protease-activated receptor-2 protects against pancreatitis by stimulating exocrine secretion. Gut. 2007;56:958–964. doi: 10.1136/gut.2006.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Laukkarinen JM, Weiss ER, van Acker GJ, Steer ML, Perides G. Protease-activated receptor-2 exerts contrasting model-specific effects on acute experimental pancreatitis. J Biol Chem. 2008;283:20703–20712. doi: 10.1074/jbc.M801779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim MH, Choi BH, Jung SR, Sernka TJ, Kim S, Kim KT, Hille B, Nguyen TD, Koh DS. Protease-activated receptor-2 increases exocytosis via multiple signal transduction pathways in pancreatic duct epithelial cells. J Biol Chem. 2008;283:18711–18720. doi: 10.1074/jbc.M801655200. [DOI] [PMC free article] [PubMed] [Google Scholar]