Abstract
This multi‐institutional study investigated non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features (NIFTP) frequency and its diagnostic significance in Japan. We reviewed 4008 thyroid nodules resected in six institutions before NIFTP was proposed. Overall, 26 cases diagnosed as non‐invasive encapsulated follicular variant of papillary thyroid carcinoma (PTC) and 145 cases of follicular thyroid adenoma (FTA) were included. Of these nodules, 80.8% and 31.0%, respectively, were NIFTPs. In five institutions, NIFTPs were more commonly found in FTA than in PTC nodules. When NIFTP was included with PTC, the overall prevalence was 2.3%, with rates in five institutions below 5.0% (0.8%–4.4%). One NIFTP case with nuclear score 3 revealed nodal metastasis 2.5 years post‐resection, and the carcinoma cells were immunohistochemically positive for BRAF. FTAs or NIFTPs with nuclear score 2 did not metastasize. NIFTP was more common among FTA than among PTC nodules, possibly due to underdiagnosis of PTC on nuclear findings. Considering the clinical findings, molecular pathogenesis, and therapeutic strategy in Japan, NIFTP with nuclear score 2 is not different from FTA, and use of this entity terminology is not meaningful. In contrast, NIFTP with nuclear score 3 has potential for metastasis and BRAF V600E mutation. Therefore, in NIFTP cases, nuclear scores 2 and 3 should be separately reported.
Keywords: follicular thyroid adenoma, follicular thyroid carcinoma, follicular variant PTC, low‐risk neoplasm, NIFTP, papillary thyroid carcinoma
Abbreviations
- FTA
follicular thyroid adenoma
- NIEFV‐PTC
non‐invasive encapsulated follicular variant of papillary thyroid carcinoma
- NIFTP
non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features
- PTC
papillary thyroid carcinoma
INTRODUCTION
A non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features (NIFTP) is defined as a non‐invasive encapsulated/well‐demarcated follicular cell‐derived tumor with a follicular growth pattern and nuclei resembling those of papillary thyroid carcinoma (PTC). 1 This tumor type was previously diagnosed as non‐invasive encapsulated follicular variant of PTC (NIEFV‐PTC) within Western medical practice. However, due to their exceptionally indolent biological behavior, 2 , 3 , 4 they are currently categorized as low‐risk neoplasms under the term NIFTP, aiming to prevent overtreatment. 1
According to a recent meta‐analysis, 5 the prevalence of NIFTP among patients with PTC in North America and Europe is 9.3% and 9.6%, respectively. However, the prevalence rate was significantly lower (2.1%) in Asian countries. In Japan, two institutions have reported the prevalence frequency of NIFTP: 0.5% at Kuma Hospital 6 and 3.1% at Yamashita Thyroid Hospital. 7 , 8 The frequency of NIFTP varies greatly among institutions, even within the same geographic location. 9 This may be dependent on the histological interpretation of the PTC‐like nuclear features. In a high‐volume thyroid center in Japan, non‐invasive encapsulated follicular tumors with questionable PTC‐like nuclear features were diagnosed as follicular thyroid adenoma (FTA). 10 Consequently, most NIFTP cases were categorized as FTAs rather than PTCs before NIFTP was defined. To ascertain the low frequency of NIFTP in Japan, clarifying whether this observation pertains only to one institution or reflects a broader trend is essential.
Thus, this multi‐institutional study aimed to determine the frequency of NIFTP and its diagnostic significance in Japan.
MATERIALS AND METHODS
This was a collaborative study involving six Japanese institutions where pathologists specializing in thyroid diseases were employed. The protocol was approved by the institutional review boards of each institution (approval number: Kuma Hospital; 20220714‐2, Nagasaki Medical Center; 2022033, Saitama Cancer Center; 1429, Cancer Institute, Japanese Foundation for Cancer Research; 2022‐GB‐023, University of Fukui Hospital; 20220106, and Osaka Police Hospital; 1552).
We reviewed 4008 thyroid nodules resected in the above six institutions from 2006 to 2015, before NIFTP was proposed as an entity (Table 1). Among them, 2853 nodules (71.2%) were originally diagnosed as PTC, and the frequency at each institution varied from 45.1% to 85.8%. Of the PTCs, 26 (0.9%) were NIEFV‐PTCs. Overall, 194 nodules (4.8%) were diagnosed as FTA, including 145 non‐oncocytic and 49 oncocytic FTAs. A total of 171 nodules diagnosed as NIEFV‐PTC or non‐oncocytic FTA were included in the present study. The oncocytic subtype of NIFTP was not included among oncocytic FTAs. Representative hematoxylin and eosin‐stained slides were reviewed by the first author, and revised diagnoses were confirmed by a pathologist at the corresponding institution. The diagnostic criteria for NIFTP included encapsulation or clear demarcation, follicular growth pattern, <1% true papillae, <30% solid/trabecular/insular growth pattern, no psammoma bodies, nuclear features of PTC (nuclear score of 2–3), no vascular invasion, no capsular invasion, no tumor necrosis, mitotic count <3 mitosis/2 mm2, and no cytoarchitectural features of PTC subtypes other than follicular variant. 1 Follicular tumors with nuclear scores of 0 or 1 were defined as FTA. 3 The clinical data were obtained from the medical records of each institution. For a case with metastasis, immunohistochemistry was performed using Ventana OptiView BRAF V600E (VE1) mouse monoclonal antibody (Roche Diagnostics, Tokyo, Japan). We determined the statistical significance of the data using Fisher's exact probability test. A P‐value <0.05 was considered statistically significant.
Table 1.
Non‐invasive encapsulated follicular variant PTCs and follicular thyroid adenomas originally diagnosed in six Japanese institutions before the proposal of NIFTP.
| Institution | Period | Resected thyroid tumor | PTC | Non‐invasive encapsulated follicular variant PTC (/thyroid tumor, /PTC) | Follicular thyroid adenoma | ||
|---|---|---|---|---|---|---|---|
| Total | Non‐oncocytic | Oncocytic | |||||
| A | 2010–2014 | 277 | 125 (45.1%) | 7 (2.5%, 5.6%) | 32 (11.6%) | 29 (10.5%) | 3 (1.1%) |
| B | 2010–2014 | 918 | 701 (76.4%) | 1 (0.1%, 0.1%) | 28 (3.1%) | 21 (2.3%) | 7 (0.8%) |
| C | 2011–2014 | 646 | 512 (79.3%) | 0 (0%, 0%) | 23 (3.6%) | 17 (2.6%) | 6 (0.9%) |
| D | 2010–2014 | 288 | 174 (60.4%) | 2 (0.7%, 1.1%) | 34 (11.8%) | 26 (9.0%) | 8 (2.8%) |
| E | 2006–2015 | 911 | 510 (56.0%) | 11 (1.2%, 2.2%) | 16 (1.8%) | 6 (0.7%) | 10 (1.1%) |
| F | 2010 | 968 | 831 (85.8%) | 5 (0.5%, 0.6%) | 61 (6.3%) | 46 (4.8%) | 15 (1.5%) |
| Total | 4008 | 2853 (71.2%) | 26 (0.6%, 0.9%) | 194 (4.8%) | 145 (3.6%) | 49 (1.2%) | |
Abbreviations: NIFTP, non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features; PTC, papillary thyroid carcinoma.
RESULTS
The frequency of NIEFV‐PTC among PTCs at each institution varied from 0% to 5.6% (Table 1). Institution A had the highest frequency of NIEFV‐PTC, and its value was statistically significant compared to that of the other institutions (P < 0.05 to P < 0.001). The frequencies of non‐oncocytic FTAs among thyroid tumors at institutions A and D were 10.5% and 9.0%, respectively. The rates were significantly higher than those (0.7%–4.8%) of the other institutions (P < 0.005 to P < 0.001)
Table 2 shows the results of the revised diagnoses of nodules originally diagnosed as NIEFV‐PTCs or FTAs. Among the 26 nodules diagnosed as NIEFV‐PTC, five (19.2%) and 21 (80.8%) were FTAs and NIFTPs, respectively. Thirteen and eight NIFTP nodules had nuclear scores of 2 and 3, respectively. The prevalence of NIFTP in 2853 nodules diagnosed as PTCs was 0.7%. The prevalence at each institution ranged from 0% to 5.6%. Of the 145 nodules diagnosed as FTA, 45 (31.0%) were diagnosed as NIFTPs. The prevalence of NIFTP among nodules originally diagnosed as FTAs at each institute ranged from 16.7% to 41.3%. In five of the six institutions, NIFTPs were found approximately twice as often among nodules diagnosed as FTA than among those diagnosed as PTC. Most (95.6%) NIFTP nodules had a nuclear score of 2 and nuclear changes were present throughout the tumor. The remaining 100 nodules were classified as non‐oncocytic FTAs.
Table 2.
Revised diagnosis of non‐invasive encapsulated follicular variant PTC and non‐oncocytic FTA originally diagnosed in six Japanese institutions before the proposal of NIFTP.
| Original diagnosis (non‐invasive encapsulated follicular variant PTC) | Original diagnosis (non‐oncocytic FTA) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Revised diagnosis | Revised diagnosis | ||||||||
| Cases | FTA (NS 0 or 1) | NIFTP (NS 2) | NIFTP (NS 3) | NIFTP (NS 2 or 3) | Cases | FTA (NS 0 or 1) | NIFTP (NS 2) | NIFTP (NS 3) | NIFTP (NS 2 or 3) |
| A (7) | 0 | 5 | 2 | 7 (100%) | A (29) | 21 | 8 | 0 | 8 (27.6%) |
| B (1) | 0 | 1 | 0 | 1 (100%) | B (21) | 16 | 4 | 1 | 5 (23.8%) |
| C (0) | C (17) | 11 | 5 | 1 | 6 (35.3%) | ||||
| D (2) | 0 | 0 | 2 | 2 (100%) | D (26) | 20 | 6 | 0 | 6 (23.1%) |
| E (11) | 3 | 6 | 2 | 8 (72.7%) | E (6) | 5 | 1 | 0 | 1 (16.7%) |
| F (5) | 2 | 1 | 2 | 3 (60.0%) | F (46) | 27 | 19 | 0 | 19 (41.3%) |
| Total (26) | 5 | 13 | 8 | 21 (80.8%) | Total (145) | 100 | 43 | 2 | 45 (31.0%) |
Abbreviations: FTA, follicular thyroid adenoma; NIFTP, non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features; NS, nuclear score; PTC, papillary thyroid carcinoma.
Eventually, 66 NIFTP nodules were detected in patients previously diagnosed with NIEFV‐PTC or FTA, accounting for 1.6% of resected thyroid tumors. When NIFTP was included with PTC, the overall prevalence of NIFTP was estimated to be 2.3% (Table 3). The prevalence rates in five of the six participating institutions were less than 5.0% (0.8%–4.4%). The remaining institution accounted for 11.3%.
Table 3.
Prevalence of NIFTP based on revised diagnosis
| Institution | PTC (including NIFTP) | NIFTP | Prevalence (PTC = NIFTP) |
|---|---|---|---|
| A | 133 | 15 | 11.3% |
| B | 706 | 6 | 0.8% |
| C | 518 | 6 | 1.2% |
| D | 180 | 8 | 4.4% |
| E | 508 | 9 | 1.8% |
| F | 848 | 22 | 2.6% |
| Total | 2893 | 66 | 2.3% |
Abbreviations: NIFTP, non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features; PTC, papillary thyroid carcinoma.
The follow‐up periods spanned 0–16 years, with an average duration of 7.0 years. One case of NIFTP with a nuclear score of 3 demonstrated ipsilateral nodal metastasis 2.5 years following lobectomy. Histological findings closely resembled those of the primary lesion (Figure 1), and ultrasound examinations showed no neoplastic lesions in the remaining thyroid gland. Immunohistochemically, carcinoma cells from both primary and metastatic lesions were positive for BRAF (Figure 2). No cases with FTAs or NIFTPs with a nuclear score of 2 developed metastatic lesions.
Figure 1.
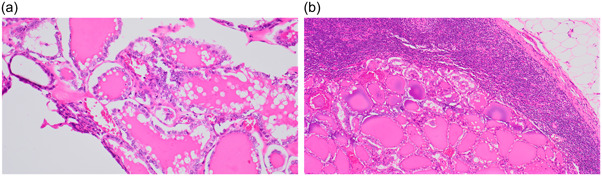
A case of non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features revealing nodal metastasis. (a) Primary site in the thyroid. (b) Metastatic site in the lymph node (hematoxylin and eosin stain, a: ×20, b: ×10).
Figure 2.
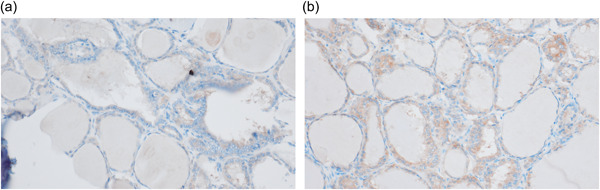
A case of non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features revealing nodal metastasis. Carcinoma cells of primary (a) and metastatic (b) lesions stained positive for BRAF V600E (immunostaining for BRAF V600E, ×20).
DISCUSSION
NIFTP was previously diagnosed as NIEFV‐PTC in Western practice. However, because of its extremely low malignant potential, the tumor was redefined as a neoplasm rather than as a carcinoma, to prevent overtreatment. 3 Nikiforov et al. reported that the prevalence of NIFTP ranged from 13.6% to 25% (mean, 18.6%) of PTCs. 3 According to a recent meta‐analysis, 5 the overall prevalence rate was 6.0%. In Asian countries, the prevalence was significantly lower (2.1%). 5 At one hospital in Japan, NIFTP accounted for 0.5% of tumors diagnosed as PTCs. 6 However, the reason for the low frequency of NIFTP in Asia remains unclear. Some speculations, such as variation in the prevalence of follicular variants among PTCs, racial and ethnic predispositions to PTC, indications for surgery, and varying thresholds for diagnosing nuclear features of PTC, have been proposed. 11 , 12 , 13 , 14 , 15 In Western countries, molecular testing using aspirated materials is common, and NIFTP with an RAS mutation is considered a surgical target, with total thyroidectomy remaining an acceptable option for some cases ultimately defined as NIFTP. 16 , 17 In contrast, in Asian countries where molecular testing is less readily available, 18 active surveillance is commonly adopted for patients with indeterminate thyroid nodules. This practice may contribute to lower resection rates and, consequently, a reduced frequency of NIFTP diagnoses. 19 , 20
In a high‐volume thyroid center in Japan, 29.5% of the tumors diagnosed as FTAs before the proposal of NIFTP as an entity were found to be NIFTPs. 10 We sought to clarify whether these findings were limited to one institution or whether this represented a trend in Japan. In the present study, we showed that NIFTPs were more commonly (approximately twice as often) found among nodules diagnosed as FTAs than among those diagnosed as PTCs before the proposal of NIFTP, except at institution A. We found that the pathologist at Institution A, who reported a high prevalence of NIFTP, considered the Western criteria for diagnosing PTC. The prevalence of NIFTPs among FTAs was 31.0%. When NIFTP nodules were included with PTC, the overall prevalence of NIFTP was estimated as 2.3%. The prevalence rates in five institutions were lower than those in Western countries. 5 Although there were differences across institutions, the data on NIFTP reported by Kuma Hospital 10 proved to be a trend in Japan.
In Japan, the pathological diagnosis of thyroid tumors is based on ‘General Rules for the Description of Thyroid Cancer’ proposed by the Japan Association of Endocrine Surgery and the Japanese Society of Thyroid Pathology. 21 , 22 , 23 This classification does not include borderline tumors, tumors of uncertain malignant potential, or NIFTPs. Therefore, questionable cases tend to be classified as benign. Non‐invasive encapsulated follicular tumors with nuclear findings characteristic of PTCs were diagnosed as NIEFV‐PTCs. In cases where the nuclear findings of PTC are inadequate or focally present, FTA is preferentially diagnosed, as shown in the present study. The trend of underdiagnosis of nuclear findings in PTC in Japan has been recognized in observer variation studies of encapsulated thyroid follicular lesions by American and Japanese pathologists. 24 This is probably due to the differences in the thresholds for assessing nuclear findings in papillary carcinoma, especially for RAS‐like PTCs. 25 The definition of the nucleus of PTC in Western clinical practice is broad and encompasses the nuclear features of classical PTC (BRAF‐like tumors) and NIFTP (RAS‐like tumors), in contrast to the narrow definition (classical) in Asian practice. 25 In cases of PTC with mild nuclear findings, Japanese pathologists tend to diagnose follicular adenomas, whereas Western pathologists tend to diagnose PTCs. 18 Therefore, in Japan, NIFTPs are more commonly found among nodules diagnosed as FTA than among nodules diagnosed as PTC. This discrepancy might explain the high prevalence of BRAF V600E mutations in PTC in most Asian countries (50%–90%) compared with the low prevalence in most Western patient cohorts (35%–50%). 25 Thompson et al. reported that the use of the nuclear scoring system to evaluate the nuclear features of PTC exhibited good to substantial interobserver agreement. 26 However, Liu et al. described that interobserver variability exists in the assessment of nuclear scores for PTC in Asian practice. 27 A more uniform evaluation of nuclear scoring for PTC remains a future challenge.
In Western countries, renaming NIEFV‐PTC to NIFTP, that is, downgrading it from a malignant to a low‐risk tumor, has been proposed to control overdiagnosis and overtreatment. 2 , 3 On the other hand, in Japan, NIFTPs with nuclear scores of 2 have been diagnosed as FTAs, that is, benign tumors. Therefore, diagnosing these as NIFTPs, that is, low‐risk neoplasms, would be an upgrade. Kakudo et al. 28 highlighted this contradictory trend and emphasized that the introduction of borderline tumor classification in Asian countries might lead to more frequent upgrading of FTAs to borderline tumors compared to the downgrading of NIEFV‐PTC from carcinoma to borderline tumors. Moreover, in Japan, even NIFTP nodules initially diagnosed as PTC through cytology have been managed with lobectomy and prophylactic central node dissection, 29 rendering it unnecessary to recklessly downgrade the nomenclature of these cases.
The present study had several limitations. First, it was a retrospective study. Second, only six institutions participated in the study. Third, not all co‐authors reviewed all cases at all institutions in detail. Fourth, a few cases for which insufficient data were available were excluded. Fifth, the sample size of NIFTP cases with a nuclear score of 3 was small. Last, molecular testing using archived specimens was not conducted because tissue samples were older than 8 years.
NIFTP has the following features: (1) extremely rare metastasis, (2) no mortality, (3) recommendation for lobectomy without radioactive iodine therapy, (4) involvement of RAS‐like molecular changes in tumorigenesis, and (5) cytology categorized as a follicular neoplasm. 1 , 30 All of these characteristics are shared with FTAs. Even FTAs categorized among benign neoplasms metastasize on rare occasions. 31 , 32 In our cases, no FTAs or NIFTPs with a nuclear score of 2 demonstrated distant metastasis, whereas one of 10 NIFTPs with a nuclear score of 3 demonstrated metastasis. The case was demonstrated to be BRAF‐positive by immunohistochemistry. While instances of NIFTPs developing lymph node metastasis are rare, it is noteworthy that most of these cases were subsequently identified as PTC with BRAF V600E mutation, encapsulated follicular growth dominant classic PTCs, or instances featuring co‐existing classic PTC. 28 We contend that NIFTPs with nuclear scores of 2 and 3 should be distinguished. The latter require differentiation from NIEFV‐PTCs, may involve BRAF mutations, and have a possibility of invasion or metastasis. 1 , 28 , 33 , 34
In conclusion, considering the above findings, it would be more reasonable to set the cutoff values for the nuclear score between 2 and 3, instead of between 1 and 2, as used by Japanese pathologists. Considering the clinical findings, molecular pathogenesis, and therapeutic strategy in Japan, the use of the term NIFTP for non‐invasive encapsulated follicular tumors with a nuclear score of 2 is not meaningful. Even if the diagnostic name NIFTP is used, an NIFTP with a nuclear score of 2 should be treated in the same manner as an FTA. In fact, these lesions could simply be categorized as FTAs. On the other hand, since NIFTP with a nuclear score of 3 may contain BRAF‐positive cases and have metastatic potential, the BRAF V600E mutation status should be evaluated by immunostaining or genetic testing, and if the mutation is present, it should be diagnosed as a non‐invasive encapsulated follicular subtype of PTC. Then, pathologists should note nuclear scores in their reports. Recently, the concepts of NIFTP and uncertain malignant potential have become widespread among Japanese pathologists, and some pathologists have begun using these diagnostic categories. This trend is welcomed to ensure compatibility with overseas academic data and to promote data sharing.
AUTHOR CONTRIBUTIONS
Conception and design of the work: MHir. Data collection, analysis, and interpretation: MHir, MI, NM, TC, YI, HY, RH, and MHig. Drafting the article: MHir. Critical revision of the article: AM and TA. Final approval of the version to be published: all authors.
CONFLICT OF INTEREST STATEMENT
None declared.
Hirokawa M, Ito M, Motoi N, Chiba T, Imamura Y, Yasuoka H, et al. Prevalence and diagnostic significance of non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features in Japan—A multi‐institutional study. Pathol Int. 2024;74:26–32. 10.1111/pin.13393
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO Classification of thyroid neoplasms. Endocr Pathol. 2022;33:27–63. [DOI] [PubMed] [Google Scholar]
- 2. Thompson LD. Ninety‐four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to noninvasive follicular thyroid neoplasm with papillary‐like nuclear features would help prevent overtreatment. Mod Pathol. 2016;29:698–707. [DOI] [PubMed] [Google Scholar]
- 3. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LDR, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, et al. American Thyroid Association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary‐like nuclear features. Thyroid. 2017;27:481–483. [DOI] [PubMed] [Google Scholar]
- 5. Rana C, Vuong HG, Nguyen TQ, Nguyen HC, Jung CK, Kakudo K, et al. The incidence of noninvasive follicular thyroid neoplasm with papillary‐like nuclear features: a meta‐analysis assessing worldwide impact of the reclassification. Thyroid. 2021;31:1502–1513. [DOI] [PubMed] [Google Scholar]
- 6. Hirokawa M, Higuchi M, Suzuki A, Hayashi T, Kuma S, Miyauchi A. Noninvasive follicular thyroid neoplasm with papillary‐like nuclear features: a single‐institutional experience in Japan. Endocr J. 2017;64:1149–1155. [DOI] [PubMed] [Google Scholar]
- 7. Bychkov A, Hirokawa M, Jung CK, Liu Z, Zhu Y, Hong SW, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary‐like nuclear features in Asian practice. Thyroid. 2017;27:983–984. [DOI] [PubMed] [Google Scholar]
- 8. Bychkov A, Jung CK, Liu Z, Kakudo K. Noninvasive follicular thyroid neoplasm with papillary‐like nuclear features in Asian practice: perspectives for surgical pathology and cytopathology. Endocr Pathol. 2018;29:276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paja M, Zafón C, Iglesias C, Ugalde A, Cameselle‐Teijeiro JM, Rodríguez‐Carnero G, et al. Rate of non‐invasive follicular thyroid neoplasms with papillary‐like nuclear features depends on pathologist's criteria: a multicentre retrospective Southern European study with prolonged follow‐up. Endocrine. 2021;73:131–140. [DOI] [PubMed] [Google Scholar]
- 10. Hirokawa M, Higuchi M, Suzuki A, Hayashi T, Kuma S, Miyauchi A. Prevalence, and diagnostic significance of noninvasive follicular thyroid neoplasm with papillary‐like nuclear features among tumors previously diagnosed as follicular adenoma: a single‐institutional study in Japan. Endocr J. 2020;67:1071–1075. [DOI] [PubMed] [Google Scholar]
- 11. Lee BWW, Bundele MM, Tan R, Fu EWZ, Chew AS, Wong JSH, et al. Noninvasive follicular thyroid neoplasm with papillary‐like nuclear features and the risk of malignancy in thyroid cytology: data from Singapore. Ann Acad Med Singapore. 2021;50:903–910. [PubMed] [Google Scholar]
- 12. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 13. Nikitski AV, Rogounovitch TI, Bychkov A, Takahashi M, Yoshiura K, Mitsutake N, et al. Genotype analyses in the Japanese and Belarusian populations reveal independent effects of rs965513 and rs1867277 but do not support the role of FOXE1 polyalanine tract length in conferring risk for papillary thyroid carcinoma. Thyroid. 2017;27:224–235. [DOI] [PubMed] [Google Scholar]
- 14. Haaga E, Kalfert D, Ludvíková M, Kholová I. Non‐invasive follicular thyroid neoplasm with papillary‐like nuclear features is not a cytological diagnosis, but it influences cytological diagnosis outcomes: a systematic review and meta‐analysis. Acta Cytol. 2022;66:85–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vuong HG, Ngo HTT, Bychkov A, Jung CK, Vu TH, Lu KB, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: a systematic review and meta‐analysis. Cancer Cytopathol. 2020;128:238–249. [DOI] [PubMed] [Google Scholar]
- 16. Nishino M. Less is more meets do more with less: exploring differences in thyroid FNA molecular testing between Asian and Western practices. Cancer Cytopathol. 2023;131:421–423. [DOI] [PubMed] [Google Scholar]
- 17. Ferris RL, Nikiforov Y, Terris D, Seethala RR, Ridge JA, Angelos P, et al. AHNS Series: Do you know your guidelines? AHNS Endocrine Section Consensus Statement: state‐of‐the‐art thyroid surgical recommendations in the era of noninvasive follicular thyroid neoplasm with papillary‐like nuclear features. Head Neck. 2018;40:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirokawa M, Auger M, Jung CK, Callegari FM. Thyroid FNA cytology: the Eastern versus Western perspectives. Cancer Cytopathol. 2023;131:415–420. 10.1002/cncy.22692 [DOI] [PubMed] [Google Scholar]
- 19. Nguyen TPX, Truong VT, Kakudo K, Vuong HG. The diversities in thyroid cytopathology practices among Asian countries using the Bethesda system for reporting thyroid cytopathology. Gland Surg. 2020;9:1735–1746. 10.21037/gs-20-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kakudo K, Higuchi M, Hirokawa M, Satoh S, Jung CK, Bychkov A. Thyroid FNA cytology in Asian practice—active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology. 2017;28:455–466. [DOI] [PubMed] [Google Scholar]
- 21.Japan association of endocrine surgery, The Japanese society of thyroid pathology. General rules for the description of thyroid cancer, the 8th edition (in Japanese). Tokyo: Kanehara‐Shuppan, 2019.
- 22. Sakamoto A, Hirokawa M, Ito M, Naganuma H, Suzuki O, Hashimoto Y, et al. Introduction of histological classification and cytology reporting format of the Japanese General Rules for the Description of Thyroid Cancer with a special focus on the differences of the WHO Histological Classification and The Bethesda System of Thyroid Cytology. Endocr J. 2021;68:621–630. [DOI] [PubMed] [Google Scholar]
- 23. Kamma H, Kameyama K, Kondo T, Imamura Y, Nakashima M, Chiba T, et al. Pathological diagnosis of general rules for the description of thyroid cancer by Japanese Society of Thyroid Pathology and Japan Association of Endocrine Surgery. Endocr J. 2022;69:139–154. [DOI] [PubMed] [Google Scholar]
- 24. Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002;26:1508–1514. [DOI] [PubMed] [Google Scholar]
- 25. Kakudo K. Different threshold of malignancy for RAS‐like thyroid tumors causes significant differences in thyroid nodule practice. Cancers. 2022;14:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson LDR, Poller DN, Kakudo K, Burchette R, Nikiforov YE, Seethala RR. An international interobserver variability reporting of the nuclear scoring criteria to diagnose noninvasive follicular thyroid neoplasm with papillary‐like nuclear features: a validation study. Endocr Pathol. 2018;29:242–249. [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, Bychkov A, Jung CK, Hirokawa M, Sui S, Hong S, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary‐like nuclear features in Asian practice. Pathol Int. 2019;69:202–210. [DOI] [PubMed] [Google Scholar]
- 28. Kakudo K, El‐Naggar AK, Hodak SP, Khanafshar E, Nikiforov YE, Nosé V, et al. Noninvasive follicular thyroid neoplasm with papillary‐like nuclear features (NIFTP) in thyroid tumor classification. Pathol Int. 2018;68:327–333. [DOI] [PubMed] [Google Scholar]
- 29. Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: Core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020;67:669–717. [DOI] [PubMed] [Google Scholar]
- 30. Cibas ES, Ali SZ. The 2017 Bethesda System for reporting thyroid cytopathology . Thyroid. 2017;27:1341–1346. [DOI] [PubMed] [Google Scholar]
- 31. Ito Y, Yabuta T, Hirokawa M, Fukushima M, Inoue H, Uruno T, et al. Distant and lymph node metastases of thyroid nodules with no pathological evidence of malignancy: A limitation of pathological examination. Endocr J. 2008;55:889–894. [DOI] [PubMed] [Google Scholar]
- 32. Lee YJ, Kim DW, Shin GW, Heo YJ, Park JY, Baek JW, et al. Unexpected lung and brain metastases 9 years after thyroid lobectomy for follicular adenoma: A case report. Front Endocrinol. 2019;10:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu B, Serrette R, Tuttle RM, Alzumaili B, Ganly I, Katabi N, et al. How many papillae in conventional papillary carcinoma? A clinical evidence‐based pathology study of 235 unifocal encapsulated papillary thyroid carcinomas, with emphasis on the diagnosis of noninvasive follicular thyroid neoplasm with papillary‐like nuclear features. Thyroid. 2019;29:1792–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fakhar Y, Khooei A, Aghaee A, Mohammadzadeh Kosari H, Wartofsky L, Zakavi SR. Bone metastasis from noninvasive follicular thyroid neoplasm with papillary‐like nuclear features (NIFTP); a case report. BMC Endocr Disord. 2021;21:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.


