Key Points
-
•
One-year fixed-duration Ven-Obi results in durable treatment-free remissions, with a 6-year PFS rate of 53% and TTNT rate of 65%, in CLL.
-
•
End-of-treatment minimal residual disease status is associated with PFS and OS.
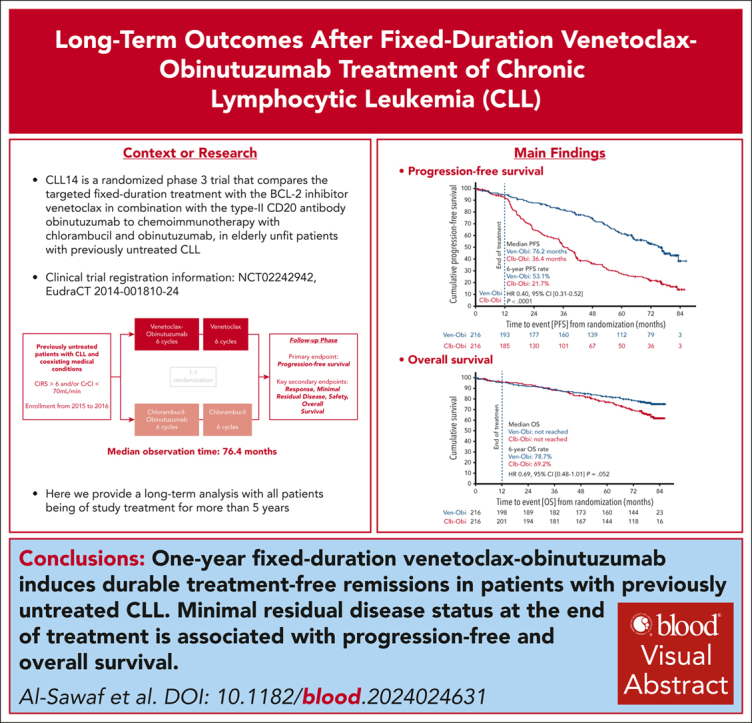
Visual Abstract
Abstract
In the CLL14 study, patients with previously untreated chronic lymphocytic leukemia (CLL) and coexisting conditions were randomized to 12 cycles of venetoclax-obinutuzumab (Ven-Obi, n = 216) or chlorambucil-obinutuzumab (Clb-Obi, n = 216). Progression-free survival (PFS) was the primary end point. Key secondary end points included time-to-next-treatment (TTNT), rates of undetectable minimal residual disease (uMRD), overall survival (OS), and rates of adverse events. Patient reported outcomes of time until definitive deterioration (TUDD) in quality of life (QoL) were analyzed. At a median observation time of 76.4 months, PFS remained superior for Ven-Obi compared with Clb-Obi (median, 76.2 vs 36.4 months; hazard ratio [HR], 0.40; 95% confidence interval [CI], 0.31-0.52; P < .0001). Likewise, TTNT was longer after Ven-Obi (6-year TTNT, 65.2% vs 37.1%; HR, 0.44; 95% CI, 0.33-0.58; P < .0001). In the Ven-Obi arm, presence of del(17p), unmutated immunoglobulin heavy-chain variable region, and lymph node size of ≥5 cm were independent prognostic factors for shorter PFS. The 6-year OS rate was 78.7% in the Ven-Obi and 69.2% in the Clb-Obi arm (HR, 0.69; 95% CI, 0.48-1.01; P = .052). A significantly longer TUDD in global health status/QoL was observed in the Ven-Obi than in the Clb-Obi arm (median, 82.1 vs 65.1 months; HR, 0.70; 95% CI, 0.51-0.97). Follow-up–adjusted second primary malignancies incidence rates were 2.3 and 1.4 per 1000 patient-months in the Ven-Obi and Clb-Obi arm, respectively. The sustained long-term survival and QoL benefits support the use of 1-year fixed-duration Ven-Obi in CLL. This trial was registered at www.ClinicalTrials.gov as #NCT02242942.
Al-Sawaf and colleagues report the 6-year results of the CLL14 study, a multicenter open label phase 3 trial for older treatment-naive patients with chronic lymphocytic leukemia and coexisting conditions, comparing 1-year fixed-duration venetoclax-obinutuzumab (VO) with chlorambucil-obinutuzumab. Five years after all patients completed therapy, 67% of patients who received VO have not required more therapy and 53% have not progressed, rates double that seen with the previous standard therapy, with the greatest incremental improvements seen in those with poor prognosis genetics. Patients who received VO have better quality of life, better global health status, and improved survival, with no significant excess of second cancers.
Introduction
Fixed-duration, targeted combination treatment of chronic lymphocytic leukemia (CLL) has become an established treatment for managing first-line and relapsed/refractory CLL.1, 2, 3 By targeting B-cell lymphoma 2 (BCL2) with the BCL2–homology 3 mimetic agent venetoclax (Ven), and combining this with CD20 antibodies such as rituximab or obinutuzumab (Obi), it is possible to induce rapid remissions with high rates of undetectable minimal residual disease (uMRD) in patients with previously treated CLL as well as those with untreated CLL.4, 5, 6, 7, 8 This approach provides the opportunity to discontinue treatment while maintaining treatment-free remissions of several years in many patients.9,10 Because of the clinical and biological heterogeneity of CLL, the outcomes and disease dynamics vary after fixed-duration treatment.11,12 Hence, careful study of duration of remissions as well as prognostic factors and biomarkers in relation to response are necessary within prospective long-term follow-ups.
The phase 3 CLL14 study explored the fixed-duration combination of Ven with the type-2 CD20 antibody Obi (Ven-Obi) compared with chemoimmunotherapy with chlorambucil plus Obi (Clb-Obi) in patients with previously untreated CLL and coexisting conditions.6 The primary analysis of CLL14 showed that 6 cycles of Ven-Obi combination followed by 6 cycles of Ven monotherapy, induces deep remissions with uMRD levels of <10−4 after end of treatment in 76% of patients, compared with uMRD levels in 35% of patients after Clb-Obi. This finding translated to a significant extension of progression-free survival (PFS) with Ven-Obi compared with Clb-Obi.11 All patients treated within CLL14 have been off study treatment since 2017 and ongoing follow-up provides unique insights into long-term outcomes after Ven-Obi treatment completion.
Here, we a report on the 6-year results from the CLL14 study, demonstrating a sustained prolongation of PFS and time-to-next-treatment (TTNT) after Ven-Obi compared with Clb-Obi. We explore the correlation between different MRD levels and survival and the long-term impact of treatment on health-related quality of life. Using the long-term observation window, we elucidate the independent prognostic impact of baseline biomarkers in multivariable models, thereby identifying patients with high- and low-risk disease in the context of fixed-duration Ven-Obi treatment.
Methods
Study design and conduct
The study design and eligibility criteria have been published previously.6 Patients with previously untreated CLL and coexisting conditions were randomized 1:1 to 12 cycles of Ven with 6 cycles of Obi, or 12 cycles of Clb with 6 cycles of Obi. The study protocol is included in the supplemental Information, available on the Blood website. Follow-up is ongoing, all patients are off study treatment. The study was registered at US and European Union clinical trial registries (ClinicalTrials.gov identifier: NCT02242942; and EU clinical trial registries: EudraCT 2014-001810-24, respectively) and approved by ethical review boards responsible for each study site. The study was performed according to the principles of the Declaration of Helsinki. All patients provided written informed consent to participate.
End points and assessments
The primary end point was investigator-assessed PFS with progressive disease determined by the study investigators according to International Workshop on Chronic Lymphocytic Leukemia guidelines.13 Secondary end points included MRD rates in the peripheral blood and bone marrow at various time points (see Protocol).
Patient-reported outcomes (PROs) were analyzed based on European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire, focusing on scales previously associated with study treatment.14 Metrics included time until definitive deterioration (TUDD)15 in global health status/quality of life as well as in functioning and symptom scales and mean change from baseline.
Adverse events (AEs) were reported until 28 days after the last dose of study treatment. Grade 3 or 4 AEs were to be reported for up to 6 months after last dose of study drug. Grade ≥3 infections were reported for 2 years after last dose of study treatment, unless the patient received another line of antileukemic therapy after disease progression. After disease progression, investigators were only required to report study treatment–related serious AEs and second primary malignancies (SPM) per protocol.
Statistical analyses
Kaplan-Meier estimates were used to analyze the time-to-event data of PFS, defined as time from randomization to first occurrence of progressive disease or death of any cause; TTNT, defined as time from randomization to first occurrence of next antileukemic therapy or death of any cause; and overall survival (OS), defined as time from randomization to death of any cause. Comparisons were done by a 2-sided log-rank test and Cox proportional hazards regression model (stratified by Binet stage and geographic region). Further details on the statistical analyses, including quality of life and SPM analyses, are provided in supplemental Methods.
The study was initially designed on the basis of an assumed hazard ratio (HR) for progressive disease or death of 0.65, with a 2-sided statistical significance level of .05 and 170 events providing power of ∼80%. As per study protocol, the primary analysis of the primary and secondary end points was performed with a data cutoff date on 17 August 2018, with 107 events, following the recommendation of the independent data and safety monitoring board .6 There was no alpha spending allocated to all subsequent analyses (including the current analysis), that is, P values are considered descriptive.
Analyses were performed using SPSS version 28 (SPSS, Chicago, IL), SAS version 9.4 (SAS Institute, Cary, NS), and R version 3.6.1 (R Foundation, Vienna, Austria).
Results
Patient characteristics and follow-up
In total, 432 patients were enrolled; 216 were randomized to the Ven-Obi arm and 216 were randomized to the Clb-Obi arm (supplemental Figure 1). The median age of patients at enrollment was 72 years, median cumulative illness rating scale score was 8, and median creatinine clearance was 66.3 mL/min. In total, 59.8% of patients had unmutated immunoglobulin heavy-chain variable region (IGHV) status, and 11.8% of patients had del(17p) and/or TP53 mutation, with most of those patients having concomitant del(17p) and TP53 mutation. International Prognostic Index high or very-high risk CLL was present in 64.3% of patients, and 26.4% and 9.3% had intermediate or low-risk disease, respectively (supplemental Tables 1 and 2).
At the data cutoff on 14 November 2022, all patients had been off study treatment for at least 5 years. The median age of the patient population at this data cutoff was 77 years (range, 45-96). The median observation time was 76.4 months (interquartile range, 52.5-80.5).
PFS
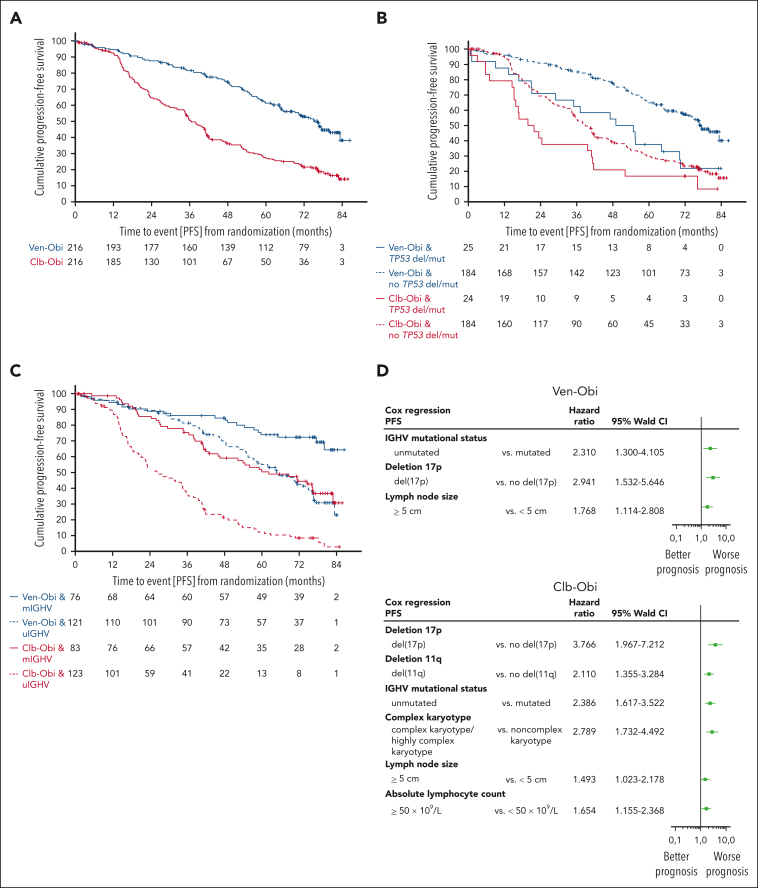
With all patients being off treatment for >5 years, PFS remained significantly superior for Ven-Obi than for Clb-Obi (median, 76.2 vs 36.4 months; HR, 0.4; 95% confidence interval [CI], 0.31-0.52; P < .0001; Figure 1 A). At 6 years after randomization, the estimated investigator-assessed PFS rate was 53.1% (95% CI, 45.9-60.3) after Ven-Obi and 21.7% (95% CI, 15.8-27.6) after Clb-Obi. Overall, 101 PFS events occurred in the Ven-Obi arm and 161 in the Clb-Obi arm. Of those PFS events, 67 were disease progressions (66.3% of PFS events) in the Ven-Obi arm and 141 (87.6% of PFS events) in the Clb-Obi arm.
Figure 1.
PFS. (A) PFS according to Ven-Obi (blue) and Clb-Obi (red) arm. (B) PFS according to presence (solid line) or absence (dashed line) of TP53 deletion/mutation. (C) PFS according to mutated- (solid line) or unmutated- (dashed line) IGHV status. (D) Multivariable models for PFS for the Ven-Obi (upper panel) and Clb-Obi arm (lower panel). del/mut, deletion and/or mutation; mIGHV, mutated IGHV; uIGHV, unmutated IGHV.
Patients with del(17p) and/or TP53 mutation had a longer PFS when treated with Ven-Obi than with Clb-Obi (median PFS, 51.9 vs 20.8 months; HR, 0.56; 95% CI, 0.30-1.06; Figure 1B). In the Ven-Obi arm, of 18 (21 in the Clb-Obi arm) PFS events in the del(17p) and/or TP53 mutation group, 14 (16 in the Clb-Obi arm) were progressive diseases and 4 (5 in the Clb-Obi arm) were deaths (unrelated to progressive disease). Patients with del(17p) and/or TP53 mutation had a shorter PFS than patients without del(17p) and/or TP53 mutation, both in the Ven-Obi arm (median PFS in patients without del(17p) and/or TP53 mutation, 76.6 months; HR, 2.29; 95% CI, 1.37-3.83) and in the Clb-Obi arm (median PFS in patients without del(17p) and/or TP53 mutation, 38.9 months; HR, 1.66; 95% CI, 1.05-2.63).
Patients with unmutated-IGHV status had a shorter PFS than patients with mutated-IGHV status, both in the Ven-Obi arm (median PFS in patients with mutated-IGHV status, not reached; HR, 2.66; 95% CI, 1.63-4.34) and in the Clb-Obi arm (median PFS in patients with mutated-IGHV status, 62.2 months; HR, 3.07; 95% CI, 2.15-4.38). Among patients with an unmutated-IGHV status, PFS was significantly longer in the Ven-Obi arm than in the Clb-Obi arm (median PFS, 64.8 vs 26.9 months; HR, 0.30; 95% CI, 0.22-0.42; Figure 1C). In the Ven-Obi arm, of 71 PFS events in the unmutated-IGHV group (Clb-Obi: 105 PFS events), 49 (Clb-Obi: 92) were disease progressions and 22 (Clb-Obi: 13) were deaths, of which 21 were unrelated to progressive disease (Clb-Obi: 13).
In the Ven-Obi arm, patients with an unmutated-IGHV status and without concomitant del(17p) and/or TP53 mutation had a median PFS of 69.0 months and a 6-year PFS rate of 47.2%, whereas for patients with a mutated-IGHV status and with concomitant del(17p) and/or TP53 mutation the median PFS was not reached and the 6-year PFS rate was 75.0% (supplemental Figure 2).
Presence of del(17p), unmutated-IGHV status, and lymph node size of ≥5cm were identified as independent negative prognostic factors in the Ven-Obi arm (Figure 1D). In the Clb-Obi arm, presence of del(17p), presence of del(11q), unmutated-IGHV status, complex karyotype (≥3 aberrations), lymph node size of ≥5 cm, and absolute lymphocyte count of ≥50 × 109/L were identified as independent negative prognostic factors (Figure 1D).
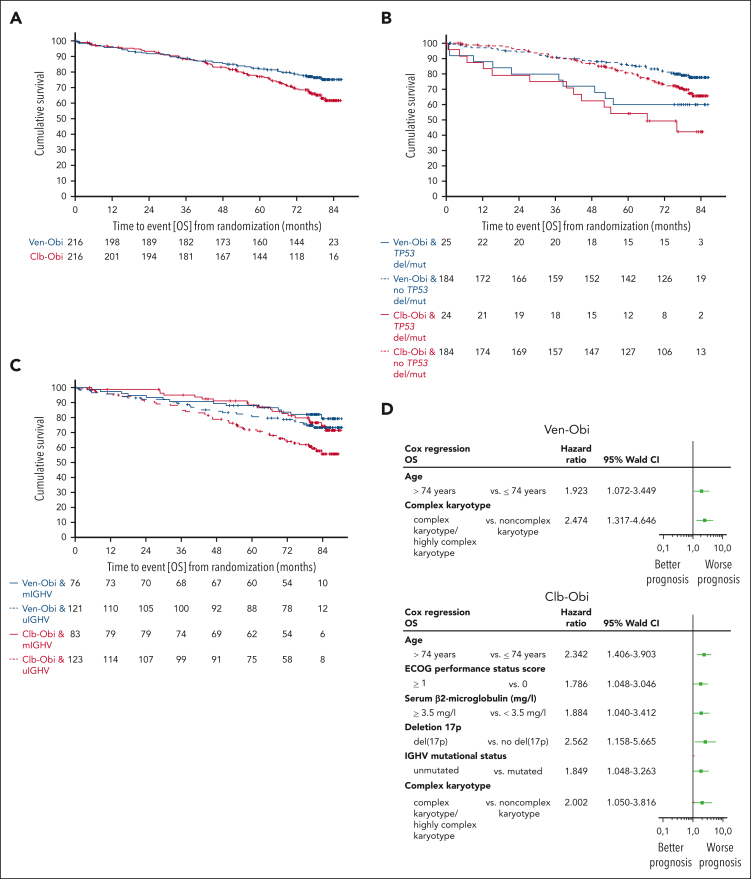
OS
At 6 years after randomization, the estimated OS rate was 78.7% in the Ven-Obi arm and 69.2% in the Clb-Obi arm (HR, 0.69; 95% CI, 0.48-1.01; P = .052; Figure 2A). Of 48 deaths in the Ven-Obi arm, 9 (18.8%) were related to CLL progression, whereas 26 (37.1%) of 70 deaths in the Clb-Obi arm were associated with CLL progression (supplemental Table 3). Patients with del(17p) and/or TP53 mutation had a shorter OS than patients without del(17p) and/or TP53 mutation in the Ven-Obi arm (6-year OS rate, 60.0% vs 81.9%; HR, 2.31; 95% CI, 1.15-4.65) and in the Clb-Obi arm (6-year OS rate, 49.2% vs 72.9%; HR, 2.50; 95% CI, 1.36-4.59; Figure 2B). In the Ven-Obi arm, patients with unmutated IGHV did not have a significantly shorter OS than patients with mutated IGHV (6-year OS rate, 77.7% vs 82.1%; HR, 1.43; 95% CI, 0.75-2.70). In contrast, in the Clb-Obi arm, patients with unmutated IGHV had a significantly shorter OS than patients with mutated-IGHV status (6-year OS rate, 63.0% vs 79.7%; HR, 2.00; 95% CI, 1.17-3.41; Figure 2C). For patients with unmutated-IGHV status, OS was significantly longer with Ven-Obi than with Clb-Obi (HR, 0.58; 95% CI, 0.36-0.92).
Figure 2.
OS. (A) OS according to Ven-Obi (blue) and Clb-Obi (red) arm. (B) OS according to presence (solid line) or absence (dashed line) of TP53 deletion/mutation. (C) OS according to mutated- (solid line) or unmutated- (dashed line) IGHV status. (D) Multivariable models for OS for the Ven-Obi (upper panel) and Clb-Obi arm (lower panel). ECOG, Eastern Cooperative Oncology Group. mIGHV, mutated IGHV; uIGHV unmutated IGHV; del/mut, deletion and/or mutation.
A multivariable analysis suggested age (≥75 years) and complex karyotype as independent negative prognostic factors for OS in the Ven-Obi arm, and age, Eastern Cooperative Oncology Group performance status, β2 microglobulin, presence of del(17p), unmutated-IGHV status, and complex karyotype in the Clb-Obi arm (Figure 2D).
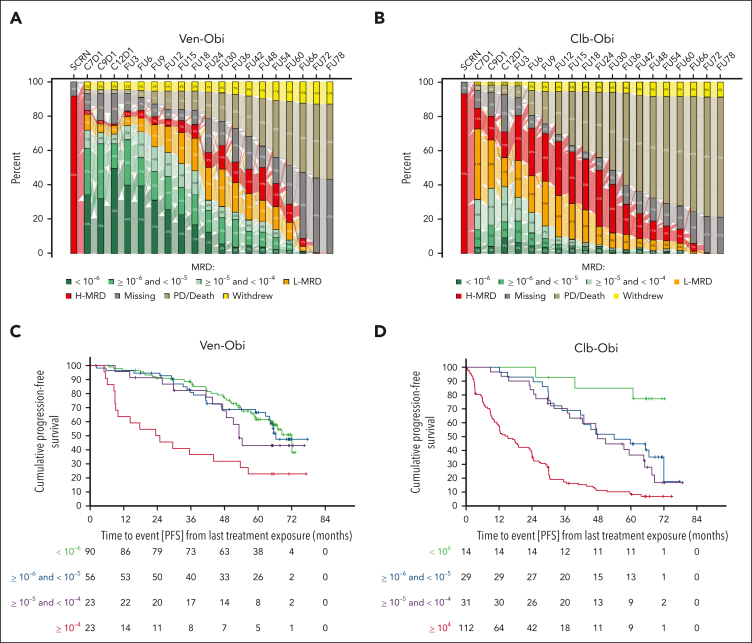
MRD rates
At follow-up month 60, that is 5 years after end of therapy, 17 (7.9% of the intention-to-treat population) patients in the Ven-Obi arm still had uMRD (<10−4 by next-generation sequencing in the peripheral blood), 22 (10.2%) had low MRD (≥10−4 and <10−2) and 23 (10.6%) high MRD (≥ 10−2; Figure 3A), compared with 4 (1.9%) uMRD, 9 (4.2%) low MRD, and 18 (8.3%) high MRD in the Clb-Obi arm (Figure 3B). Of those patients with available MRD samples at follow-up month 60, 17 of 62 (27.4%) had MRD levels of <10−4 in the Ven-Obi arm and 4 of 31 (12.9%) in the Clb-Obi arm at follow-up month 60. Common features among patients with maintained MRD levels of <10−4 were a mutated-IGHV status (15 of 17 [88.2%] patients in the Ven-Obi arm) and sole del(13q) (12 of 17 [70.6%] patients in the Ven-Obi arm; Table 1).
Figure 3.
MRD. (A) MRD assessments in the peripheral blood by next-generation sequencing in the Ven-Obi arm. (B) MRD assessments in the peripheral blood by next-generation sequencing in the Clb-Obi arm. (C) Landmark PFS analysis according to MRD status in the Ven-Obi arm. (D) Landmark PFS analysis according to MRD status in the Clb-Obi arm.
Table 1.
Baseline patient characteristics of patients with sustained uMRD levels
| Characteristic | Clb-Obi |
Ven-Obi |
All other patients [ITT] |
|---|---|---|---|
| (N = 4) | (N = 17) | (N = 411) | |
| Age (y) | |||
| Median (range) | 72.5 (67-85) | 73 (54-83) | 72 (41-89) |
| ≥75, n (%) | 2 (50.0) | 5 (29.4) | 143 (34.8) |
| Male sex, n (%) | 3 (75.0) | 10 (58.8) | 276 (67.2) |
| Binet stage, n (%) | |||
| A | 2 (50.0) | 3 (17.6) | 85 (20.7) |
| B | 0 (0.0) | 8 (47.1) | 148 (36.0) |
| C | 2 (50.0) | 6 (35.3) | 178 (43.3) |
| B-symptoms present, n (%) | 1 (25.0) | 9 (52.9) | 205 (49.9) |
| Total CIRS score | |||
| Median (range) | 4 (1-12) | 9 (4-16) | 8 (0-28) |
| >6, n (%) | 2 (50.0) | 16 (94.1) | 345 (83.9) |
| Serum β2-microglobulin (mg/L) | |||
| Median (range) | 4.8 (2.7-8.7) | 3.6 (1.5-7.7) | 4.1 (1.0-14.2) |
| >3.5, n (%) | 3 (75.0) | 9 (52.9) | 236/388 (60.8) |
| Estimated creatinine clearance (mL/min) | |||
| Median (range) | 57.7 (34.0-69.2) | 65.0 (35.6-152.6) | 66.6 (25.1-295.6) |
| <70, n (%) | 4 (100.0) | 10 (58.8) | 234/407 (57.5) |
| Cytogenetic subgroups as per hierarchy,∗n (%) | |||
| Deletion in 17p | 0 (0.0) | 0 (0.0) | 31/418 (7.4) |
| Deletion in 11q | 1 (25.0) | 1 (5.9) | 74/418 (17.7) |
| Trisomy in 12 | 0 (0.0) | 1 (5.9) | 76/418 (18.2) |
| No abnormalities | 1 (25.0) | 3 (17.6) | 92/418 (22.0) |
| Deletion in 13q∗ alone | 2 (50.0) | 12 (70.6) | 145/418 (34.7) |
| IGHV mutational status,∗n (%) | |||
| Mutated | 2 (50.0) | 15 (88.2) | 142/387 (36.7) |
| Unmutated | 2 (50.0) | 2 (11.8) | 240/387 (62.0) |
| Nonevaluable | 0 (0.0) | 0 (0.0) | 5/387 (1.3) |
| TP53 mutational status, n (%) | |||
| Mutated | 1 (25.0) | 2 (11.8) | 39/400 (9.8) |
| Unmutated | 3 (75.0) | 15 (88.2) | 361/400 (90.3) |
| TP53 deleted and/or mutated, n (%) | 1 (25.0) | 2 (11.8) | 46/396 (11.6) |
| CLL-IPI risk group, n (%) | |||
| Low | 1 (25.0) | 4 (23.5) | 30/360 (8.3) |
| Intermediate | 1 (25.0) | 4 (23.5) | 90/360 (25.0) |
| High | 1 (25.0) | 7 (41.2) | 202/360 (56.1) |
| Very high | 1 (25.0) | 2 (11.8) | 38/360 (10.6) |
| Complex karyotype group, n (%) | |||
| NCKT | 4 (100.0) | 14/15 (93.3) | 315/378 (83.3) |
| CKT/HCKT | 0 (0.0) | 1/15 (6.7) | 63/378 (16.7) |
CIRS, cumulative illness rating scale; CKT, complex karyotype; CLL-IPI, chronic lymphocytic leukemia international prognostic index; HCKT, highly complex karyotype; ITT, intention-to-treat; NCKT, noncomplex karyotype.
Statistically significant difference in all patients being uMRD at FU60 (pooled cohort of Ven-Obi and Clb-Obi) as compared to the remaining study population.
A PFS landmark analysis from last treatment exposure indicated a 6-year PFS rate in the Ven-Obi arm with MRD of <10−6, ≥10−6 and <10−5, and ≥10−5 and <10−4 at end of treatment of 61.5%, 66.4%, and 43.0%, respectively, whereas patients with MRD of ≥10−4 had a 6-year PFS rate of 22.7% (Figure 3C). In the Clb-Obi arm, 6-year PFS rates for patients with MRD of <10−6, ≥10−6 and <10−5, and ≥10−5 and <10−4 were 85.1%, 44.8%, and 36.7%, respectively, and for MRD of >10−4, 9.2% (Figure 3D). End-of-treatment MRD status was associated with OS for patients treated in the Ven-Obi arm: the 6-year OS rate was 59.1% for patients with MRD of ≥10−4 compared with 86.6% for patients with MRD of <10−4 at the end of treatment (HR, 3.42; 95% CI, 1.65-7.06). On the contrary, in the Clb-Obi arm, 6-year OS rates were 67.5% and 78.9% (HR, 1.69; 95% CI, 0.96-2.96), respectively (supplemental Figure 3).
An association between end-of-treatment MRD status and PFS was observed in patients with mutated- as well as unmutated-IGHV status: in the Ven-Obi arm, the 6-year PFS rate for patients with MRD of ≥10−4 and mutated-IGHV status was 42.9%, whereas the 6-year PFS rate for patients with MRD of <10−4 and mutated-IGHV status was 80.8% (HR, 3.30; 95% CI, 1.07-10.16). For patients with unmutated-IGHV status treated with Ven-Obi the 6-year PFS rates were 10.0% and 48.5% (HR, 3.76; 95% CI, 1.83-7.73; supplemental Figure 4A), respectively. Similarly, in the Clb-Obi arm, patients with MRD of ≥10−4 had a shorter PFS than patients with MRD of <10−4, both with mutated (HR, 3.84; 95% CI, 2.06-7.15) and unmutated IGHV (HR, 4.29; 95% CI, 2.56-7.21). However, patients with unmutated IGHV and MRD of <10−4 had a longer median PFS of 42.8 months compared with patients with mutated IGHV and MRD of ≥10−4 with a median PFS of 27.2 months, although this difference was not statistically significant (HR, 0.68; 95% CI, 0.39-1.18; supplemental Figure 4B).
End-of-treatment MRD status also discriminated outcomes in patients with del(17p) and/or TP53 mutation. In the Ven-Obi arm, patients with MRD of <10−4 and del(17p) and/or TP53 mutation had a median PFS of 52.3 months, which was not significantly shorter than patients with MRD of <10−4 and without del(17p) and/or TP53 mutation, with a median PFS of 68.5 months (HR, 1.88; 95% CI, 0.96-3.67; supplemental Figure 5A). In the Clb-Obi arm, only 5 patients had MRD of <10−4 and del(17p) and/or TP53 mutation, with a median PFS of 66.6 months, which was not significantly shorter than patients with MRD of <10−4 and without del(17p) and/or TP53 mutation, with a median PFS of 59.8 months (HR, 0.54; 95% CI, 0.13-2.24; supplemental Figure 5B).
Although the majority of patients with MRD of <10−4 in the peripheral blood at the end of treatment had also concordant MRD of <10−4 in the bone marrow, 4 patients (2 in the Ven-Obi arm, and 2 in the Clb-Obi arm) had MRD of >10−2 in the bone marrow despite MRD of <10−4 in the peripheral blood (supplemental Table 4). All patients with peripheral blood MRD of <10−6 had concordant bone marrow MRD < 10−4 status.
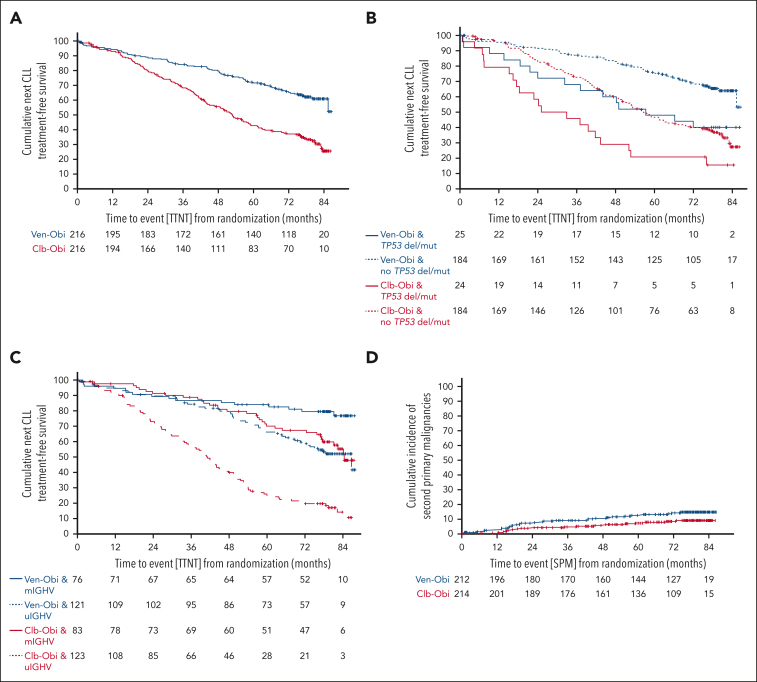
TTNT
TTNT, defined as initiation of a next line of antileukemic treatment or death, was significantly longer after Ven-Obi than after Clb-Obi treatment (median TTNT, not reached vs 52.9 months; 6-year TTNT rate, 65.2% vs 37.1%; HR, 0.44; 95% CI, 0.33-0.58; P < .0001; Figure 4 A). In both arms, patients with higher-risk disease required earlier initiation of next-line treatment. Patients with del(17p) and/or TP53 mutation had a shorter TTNT than patients without del(17p) and/or TP53 mutation (Ven-Obi: median TTNT, 57.3 months vs not reached; HR, 2.25; 95% CI, 1.28-3.97; Clb-Obi: median TTNT, 29.0 vs 56.6 months; HR, 2.13; 95% CI, 1.32-3.43; Figure 4B). Patients with unmutated IGHV had a shorter TTNT than patients with mutated-IGHV status (Ven-Obi: median TTNT, 85.4 months vs not reached; HR, 2.45; 95% CI, 1.40-4.28; Clb-Obi: median TTNT, 40.6 vs 83.1 months; HR, 3.53; 95% CI, 2.37-5.25; Figure 4C). Thirty-nine patients in the Ven-Obi arm, and 103 in the Clb-Obi arm received a next line of therapy.
Figure 4.
TTNT and second primary malignancy incidence. (A) TTNT, defined as time to initiation of next line of antileukemic treatment or death from any cause, according to Ven-Obi (blue) or Clb-Obi (red) arm. (B) TTNT according to presence (solid line) or absence (dashed line) of TP53 deletion/mutation. (C) TTNT according to mutated- (solid line) and unmutated- (dashed line) IGHV status. (D) Cumulative incidence of SPM according to Ven-Obi (blue) and Clb-Obi (red) arm.
The 6-year TTNT rate with deaths before the next line of treatment censored was 79.0% in the Ven-Obi arm and 48.1% in the Clb-Obi arm (HR, 0.29; 95% CI, 0.20-0.42; supplemental Figure 6).
In the Ven-Obi arm, 23 (59.0%) of 39 patients who received a next line of therapy were treated with Bruton tyrosine kinase inhibitors (BTKis), 7 (17.9%) received other targeted agents, and 9 (23.1%) received chemoimmunotherapy. Of 103 patients with initiation of a subsequent therapy in the Clb-Obi arm, 55 (53.4%) received BTKis, 15 (14.6%) received BCL2 inhibitors, and 31 (30.1%) received chemoimmunotherapy.
PROs
At 6 years after randomization, the completion rate for PRO questionnaires was high at 89.3% in the Ven-Obi arm and 92.9% in the Clb-Obi arm. Among patients included in the PRO population, there were 197 patients in the Ven-Obi arm and 198 in the Clb-Obi arm. A significantly longer TUDD in global health status/quality of life scale score was observed in the Ven-Obi arm compared with the Clb-Obi arm (median TUDD, 82.1 vs 65.1 months; HR, 0.70; 95% CI, 0.51-0.97), indicating sustained health-related quality of life after Ven-Obi compared with Clb-Obi therapy (supplemental Figure 7A). A significantly longer TUDD in fatigue was observed in the Ven-Obi arm than in the Clb-Obi arm (median TUDD, 82.9 vs 64.4 months; HR, 0.65; 95% CI, 0.47-0.90), suggesting less fatigue after Ven-Obi than Clb-Obi (supplemental Figure 7B). No statistically significant differences between treatment arms were observed for TUDD in the other analyzed domains. In the Ven-Obi arm, beside the 6-year TUDD rates of 57.6% (Clb-Obi: 47.6%) and 58.6% (Clb-Obi: 45.4%) in global health status/quality of life scale score and fatigue, respectively, the highest TUDD rates were observed for domains of insomnia and emotional function (6-year rates, 54.4% and 54.5%, respectively), which were comparable with that of the Clb-Obi arm (50.6% and 51.3%, respectively; supplemental Table 5). The mean change from baseline analysis further demonstrated largely maintained scales of fatigue or insomnia, in both the Ven-Obi and Clb-Obi arm (supplemental Figure 8).
Safety
During the observation period (median, 76.8 months in the Ven-Obi arm, and 75.8 months in the Clb-Obi arm), serious AEs were reported in 133 of 212 (62.7%) treated patients in the Ven-Obi arm and in 101 of 214 (47.2%) treated patients in the Clb-Obi arm. The majority of AEs occurred during the treatment phase in both study arms, whereas 9.9% of AEs in the Ven-Obi arm and 6.9% in the Clb-Obi arm occurred after treatment (Table 2). As per study protocol, AE reporting (except related serious AEs and SPMs) was not required once a next line of treatment was started, therefore fewer events might have been reported in the Clb-Obi arm because of the higher number of next-line therapies and deaths.
Table 2.
Posttreatment treatment-related AEs
| Clb-Obi (N = 214) |
Ven-Obi (N = 212) |
|
|---|---|---|
| Total no. of patients with at least 1 AE | 10 (4.7%) | 17 (8.0%) |
| Neutropenia | 2 (0.9%) | 6 (2.8%) |
| Maximum CTC grade 3 | 1 (0.5%) | 3 (1.4%) |
| Maximum CTC grade 4 | 1 (0.5%) | 3 (1.4%) |
| Febrile neutropenia | 1 (0.5%) | 2 (0.9%) |
| Maximum CTC grade 1 | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 4 | 1 (0.5%) | 1 (0.5%) |
| Pneumonia | 1 (0.5%) | 1 (0.5%) |
| Maximum CTC grade 3 | 1 (0.5%) | 1 (0.5%) |
| Sepsis | 1 (0.5%) | 1 (0.5%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 5 | 0 (0.0%) | 1 (0.5%) |
| Anemia | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Atrial fibrillation | 1 (0.5%) | 1 (0.5%) |
| Maximum CTC grade 2 | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Headache | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Basal cell carcinoma | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 2 | 1 (0.5%) | 0 (0.0%) |
| Bladder neoplasm | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| Calculus bladder | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| Candida infection | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| COVID-19 | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| Dyspnea | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Hemoptysis | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 2 | 1 (0.5%) | 0 (0.0%) |
| Hepatitis viral | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| Infected neoplasm | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 4 | 0 (0.0%) | 1 (0.5%) |
| Infection | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 4 | 0 (0.0%) | 1 (0.5%) |
| Malignant melanoma | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Ophthalmic herpes zoster | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Penile cancer | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Plasma cell myeloma | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| Pneumonia streptococcal | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Pneumonitis | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Prostate cancer | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| Sinusitis | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Sinusitis Aspergillus | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Splenomegaly | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 4 | 0 (0.0%) | 1 (0.5%) |
| Staphylococcal bacteremia | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| Streptococcal infection | 1 (0.5%) | 0 (0.0%) |
| Maximum CTC grade 3 | 1 (0.5%) | 0 (0.0%) |
| T-cell lymphoma | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 4 | 0 (0.0%) | 1 (0.5%) |
| Thrombocytopenia | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
| Toxicity of various agents | 0 (0.0%) | 1 (0.5%) |
| Maximum CTC grade 3 | 0 (0.0%) | 1 (0.5%) |
Investigator-assessed relatedness.
CTC, common terminology criteria.
SPMs, excluding nonmelanoma skin cancers, were reported in 30 (14.2%) patients in the Ven-Obi and 18 (8.4%) in the Clb-Obi arm. The 5-year and 6-year cumulative SPM incidence rates were 12.6% and 14.2%, respectively, in the Ven-Obi arm, and 7.4% and 8.5%, respectively, in the Clb-Obi arm. The differences between the Ven-Obi and Clb-Obi arm were not statistically significant (P = .071; Figure 4 D). The follow-up-adjusted incidence rates of SPMs were 2.3 cases per 1000 patient-months in the Ven-Obi arm and 1.4 in the Clb-Obi arm. In the Ven-Obi arm the most common SPMs were solid organ tumors in 17 patients and melanoma in 8 patients (supplemental Table 6). Eleven patients with solid organ tumors and 4 patients with melanomas were reported in the Clb-Obi arm. Three patients experienced secondary hematological malignancies in the Ven-Obi arm (1 T-cell lymphoma, and 2 myelodysplastic syndromes) and 3 in the Clb-Obi arm (acute myeloid leukemia, mantle cell lymphoma, and plasma cell myeloma). Four patients died because of their SPMs in the Ven-Obi arm and 6 in the Clb-Obi arm. Two Richter transformations were reported in the Ven-Obi arm and 4 in the Clb-Obi arm.
Discussion
Fixed-duration targeted treatment comprising the BCL-2 inhibitor Ven in combination with either an anti-CD20 antibody (rituximab in the relapsed/refractory CLL setting,7 or Obi in the first-line setting,6) or the BTKi ibrutinib,16,17 have become a standard of care in the treatment of CLL. Given the limited duration of active treatment, long-term follow-up is required to elucidate disease dynamics and confirm the long-term feasibility, safety, and efficacy of a targeted, fixed-duration approach. This most recent data cutoff provides multiple novel observations, including the identification of IGHV as an independent prognostic factor in the context of Ven-Obi, the TTNT periods in multiple subgroups, and differential quality of life outcomes.
This report provides long-term data on patients with previously untreated CLL and coexisting conditions, commonly understood as patients who are unfit, who received first-line therapy with either Ven-Obi or Clb-Obi.6 With all patients being off study treatment for >5 years, this, to the best of our knowledge, longest follow-up data confirms sustained remissions in >50% of patients treated with Ven-Obi with no additional toxicity reported. Similar to previous analyses,9,11,18 presence of del(17p) and/or TP53 mutations was associated with a significantly shorter PFS and OS in patients receiving Ven-Obi, highlighting the prognostic relevance of this finding. However, the median TTNT, which typically indicates the time until patients experience a symptomatic progressive disease requiring initiation of a next line of treatment, was still not reached with >6 years of observation, which suggests treatment-free disease control for multiple years, even in presence of some high-risk features.
In line with the data on Ven-Obi reported from the randomized CLL13/GAIA study8 as well as the recent follow-up data on Ven-Ibrutinib (Ven-Ibru) from the randomized GLOW study,19 the current follow-up demonstrates an independent prognostic impact of the IGHV status on PFS. However, across all mentioned studies, the 3-year PFS rate was ≥80%, indicating the clinical feasibility of fixed-duration treatment. Indeed, median TTNT in patients with unmutated-IGHV status treated with Ven-Obi was 85.4 months, suggesting a substantial off-treatment period even in presence of unmutated IGHV. The majority of patients with mutated-IGHV status (n = 76) continue to benefit from ongoing remissions, as observed by a 6-year PFS rate of 72.4% and a 6-year TTNT rate of 79.5%. This rate finding is comparable with, or higher than, 6-year PFS rates reported with fludarabine, cyclophosphamide and rituximab (FCR) in fit patients with mutated IGHV in the MDACC 300-patient study (75%, n = 88) or the CLL8 study (56%, n = 117).20,21
With longer observation time, the number of patients with uMRD in the peripheral blood has dropped further in both arms, with an MRD of <10−4 rate of 8% in the Ven-Obi arm at 6 years. However, because rates are reported based on the intention-to-treat population, as recommended by International Workshop on Chronic Lymphocytic Leukemia guidelines,13 cases with missing samples, prior progressive disease , or death due to comorbidities may bias the rates and result in overestimation of MRD-positive cases. When adjusting the denominator to only include patients alive and with available samples, 27% of patients in the Ven-Obi arm had MRD of <10−4. Interestingly, most of these patients had a mutated-IGHV status and sole del(13q), suggesting that patients with these low-risk markers are most likely to experience long-term remissions with sustained uMRD. Although end-of-treatment MRD levels of <10−6 in the peripheral blood were not associated with a longer PFS than MRD of <10−4, and therefore did not provide additional prognostic information in the Ven-Obi arm, it was observed that all patients with MRD of <10−6 had MRD of <10−4 in the bone marrow, as assessed by allele-specific oligonucleotide polymerase chain reaction (ASO-PCR). This suggests that bone marrow assessments can be dispensed with when higher sensitivity assays are used for peripheral blood measurements.
A further observation in the current analysis was a significant difference in patient reported outcomes of patients treated with Ven-Obi vs Clb-Obi, as indicated by a longer time until definite deterioration in global health quality of life and fatigue.22,23 These are clinically relevant PROs that correlate with the symptom burden of CLL.14,24 The observed difference suggests improved CLL-related symptom control after fixed-duration Ven-Obi and further supports the use of a targeted fixed-duration treatment in the first-line setting. The current analysis also clarifies the previously reported higher cumulative incidence of SPMs in the Ven-Obi arm: the follow-up-adjusted incidence rates in the Ven-Obi and Clb-Obi arm did not differ significantly, thereby suggesting that patients treated with Ven-Obi might not have a higher risk of developing SPMs than after Clb-Obi treatment. Previously, both CLL13 and the MURANO trials have not demonstrated an enrichment in SPMs.8,12
Although this report provides clinically relevant insights into the long-term efficacy of fixed-duration targeted therapy of CLL, several open questions remain: given the availability of continuous BTKis and, more recently, the oral fixed-duration regimen of Ven-Ibru, in light of missing randomized comparisons, it remains unclear, which group of patients should preferably be treated with continuous BTKis, Ven-Ibru or, as discussed in this report, Ven-Obi. Studies like CLL17 (ClinicalTrials.gov identifier: NCT04608318) will contribute to better patient stratification, but until sufficient prospective data are available, the feasibility and toxicity profile of each regimen as well as patient preferences are likely to be key decision drivers. Moreover, although this report confirms the short OS for patients with detectable MRD after Ven-Obi therapy, it remains unclear whether treatment modulation would provide any clinical benefits, because such options were not part of the study protocol. The recently published FLAIR study demonstrated the efficacy of MRD-guided ibrutinib plus Ven over chemoimmunotherapy with FCR, however, the benefit over a fixed-duration or continuous targeted therapy remains unclear .17 This question will eventually be addressed by studies like the ongoing MAJIC study (ClinicalTrials.gov identifier: NCT05057494), which prospectively compares MRD-guided Ven-Obi and Ven-acalabrutinib,25 or the planned CLL18 study, which will compare fixed-duration Ven-Obi to fixed-duration Ven-pirtobrutinib and MRD-guided Ven-pirtobrutinib.
In conclusion, this report confirms the long-term safety and efficacy of 1-year fixed-duration Ven-Obi in patients with previously untreated CLL. Given the prospective mature follow-up in a representative patient population, the study corroborates the use of fixed-duration, targeted treatment in patients with previously untreated CLL.
Conflict-of-interest disclosure: O.A.-S. reports advisory board participation with Ascentage, AstraZeneca, AbbVie, Gilead, Janssen, and Roche; received speaker honoraria from Adaptive, AstraZeneca, AbbVie, BeiGene, Gilead, Janssen, and Roche; and received research funding from BeiGene, AbbVie, Janssen, and Roche). S.R. reports honoraria from Merck Shapre and Dohme (MSD). S.O., Y.M.C., J.D, M.B., and Y.J. report employment with Genentech/Hoffmann-la Roche. A.-M.F. reports personal fees from Celgene, Janssen, and Hoffmann-La Roche. E.T. reports advisory board participation with AbbVie, Janssen-Cilag, and BeiGene; received speaker honoraria from AstraZeneca, AbbVie, BeiGene, Gilead, Janssen, and Roche; and received research funding from Roche, AbbVie, and Gilead. C.S. received speaker honoraria from AstraZeneca and AbbVie; and reports advisory board participation with Janssen-Cilag). M.R. reports grants from F. Hoffman-La Roche Ltd; and personal fees from F. Hoffmann-La Roche Ltd and AbbVie. K.-A.K. reports grants from F. Hoffmann-La Roche Ltd and AbbVie, during the conduct of the study; and received personal fees from F. Hoffmann-La Roche Ltd and AbbVie. C.U.N. reports grants and/or consultancy fees from AbbVie, Janssen, AstraZeneca, BeiGene, Genmab, Octapharma, MSD, Lilly, Takeda, CSL Behring, and Novo Nordisk Foundation. B.C. reports employment with AbbVie. C.P.P. received speaker honoraria from AstraZeneca and Pfizer; reports research funding from Gilead Sciences; and reports advisory board participation with AbbVie. R.W. reports advisory board participation with AbbVie, BeiGene, and Janssen; reports speaker honoraria from AbbVie and Janssen; and received research funding from BioOra, and Janssen. J.P. reports employment with Genentech/Hoffmann-la Roche. B.E. reports grants and personal fees from F. Hoffmann-La Roche Ltd, AbbVie, AstraZeneca, BeiGene, and Janssen; reports personal fees from Celgene, Novartis, ArQule, Gilead, Oxford Biomedica (United Kingdom), Adaptive Biotechnologies, and Hexal. S.S. reports grants, personal fees, and nonfinancial support from AbbVie, AstraZeneca, Celgene, Gilead, GlaxoSmithKline, Hoffmann La-Roche Ltd, Janssen, Novartis, Pharmacyclics, Sunesis, and Verastem. Y.J. reports employment with Genentech/Hoffmann-La Roche; and reports stock in Genentech. M.H. received honoraria from Roche, Janssen, AbbVie, Gilead Sciences, and AstraZeneca; reports advisory or consulting role with Janssen, AbbVie, Gilead Sciences, Genentech/Roche, and AstraZeneca; participates in speakers bureau of Janssen, AbbVie, Gilead Sciences, Roche/Genentech, and AstraZeneca; reports research funding from Roche, AbbVie, Janssen, Gilead Sciences, and AstraZeneca; and received travel, accommodations, and expenses funds from Roche and Janssen. K.F reports advisory board participation with AstraZeneca and AbbVie; received speaker honoraria from AbbVie and Roche; and received research funding from AbbVie and Roche. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank Michele Porro Lurà, Konrad Bilski, and Corinne Rabault (Basel, Switzerland) and Hasan Alhasani (AbbVie Inc, Chicago, IL) for invaluable support in the conception and conduct of the trial. The authors thank all the patients, their families, and their nurses and physicians for their participation in the trial.
This study was supported by F. Hoffmann-La Roche and AbbVie. It was also supported in part by the Deutsche Forschungsgemeinschaft (German Research Foundation), project no. 524342988 (O.A.-S.) and SFB 1530 project C04 (K.F.).
Authorship
Contribution: O.A.-S., S.R., C.Z., and K.F. conducted the analyses, interpreted the data, and wrote the manuscript; S.O., Y.M.C., and Y.J. managed and reviewed the data; A.-M.F. supervised the clinical safety analyses; E.T., C.S., and S.S. provided the fluorescence in situ hybridization, TP53, and IGHV data; M.R. supervised the MRD analyses in CLL14; K.-A.K. supervised the flow cytometry analysis in CLL14; B.S.M., D.J., N.E., and J.D. conducted and supervised the quality of life analyses; and L.S., C.U.N., A.S., J.L., R.W., D.S., A.K., M.B., E.D.R., B.E., and M.H. reviewed the data and the manuscript.
Footnotes
The MRD, the clinical, and the PROs data generated during this study are available, for academic noncommercial purposes, through the corresponding authors, Othman Al-Sawaf (othman.al-sawaf@uk-koeln.de) and Kirsten Fischer (kirsten.fischer@uk-koeln.de). Project proposals with the intent-to-achieve aims will be evaluated by the German CLL Study Group (GCLLSG) and the funders Roche and AbbVie. The study protocol will be provided in the appendix of this publication. The statistical analysis plan and informed consent form will be made available upon request.
Presented in part at the annual meeting of the European Haematology Association, Frankfurt, Germany, 9 June 2023; the 17th International Conference on Malignant Lymphoma (ICML), Lugano, Switzerland, 15 June 2023; and the 20th International Workshop on Chronic Lymphocytic Leukemia, Boston, MA, 7 October 2023.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Contributor Information
Othman Al-Sawaf, Email: othman.al-sawaf@uk-koeln.de.
Kirsten Fischer, Email: kirsten.fischer@uk-koeln.de.
Supplementary Material
References
- 1.Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679–1705. doi: 10.1002/ajh.26367. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA. Treatment of chronic lymphocytic leukemia. N Engl J Med. 2020;383(5):460–473. doi: 10.1056/NEJMra1908213. [DOI] [PubMed] [Google Scholar]
- 3.Shadman M. Diagnosis and treatment of chronic lymphocytic leukemia: a review. JAMA. 2023;329(11):918–932. doi: 10.1001/jama.2023.1946. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 5.Montero J, Haq R. Adapted to survive: targeting cancer cells with BH3 mimetics. Cancer Discov. 2022;12(5):1217–1232. doi: 10.1158/2159-8290.CD-21-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236. doi: 10.1056/NEJMoa1815281. [DOI] [PubMed] [Google Scholar]
- 7.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi: 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 8.Eichhorst B, Niemann CU, Kater AP, et al. First-line venetoclax combinations in chronic lymphocytic leukemia. N Engl J Med. 2023;388(19):1739–1754. doi: 10.1056/NEJMoa2213093. [DOI] [PubMed] [Google Scholar]
- 9.Al-Sawaf O, Zhang C, Tandon M, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188–1200. doi: 10.1016/S1470-2045(20)30443-5. [DOI] [PubMed] [Google Scholar]
- 10.Kater AP, Seymour JF, Hillmen P, et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;37(4):269–277. doi: 10.1200/JCO.18.01580. [DOI] [PubMed] [Google Scholar]
- 11.Al-Sawaf O, Zhang C, Lu T, et al. Minimal residual disease dynamics after venetoclax-obinutuzumab treatment: extended off-treatment follow-up from the randomized CLL14 study. J Clin Oncol. 2021;39(36):4049–4060. doi: 10.1200/JCO.21.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seymour JF, Kipps TJ, Eichhorst BF, et al. Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood. 2022;140(8):839–850. doi: 10.1182/blood.2021015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 14.Al-Sawaf O, Gentile B, Devine J, et al. Health-related quality of life with fixed-duration venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: results from the randomized, phase 3 CLL14 trial. Am J Hematol. 2021;96(9):1112–1119. doi: 10.1002/ajh.26260. [DOI] [PubMed] [Google Scholar]
- 15.Bonnetain F, Dahan L, Maillard E, et al. Time until definitive quality of life score deterioration as a means of longitudinal analysis for treatment trials in patients with metastatic pancreatic adenocarcinoma. Eur J Cancer. 2010;46(15):2753–2762. doi: 10.1016/j.ejca.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Kater Arnon P, Owen C, Moreno C, et al. Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. NEJM Evid. 2022;1(7) doi: 10.1056/EVIDoa2200006. [DOI] [PubMed] [Google Scholar]
- 17.Munir T, Cairns DA, Bloor A, et al. Chronic Lymphocytic Leukemia Therapy Guided by Measurable Residual Disease. N Engl J Med. 2024;390(4):326–337. doi: 10.1056/NEJMoa2310063. [DOI] [PubMed] [Google Scholar]
- 18.Al-Sawaf O, Zhang C, Jin HY, et al. Transcriptomic profiles and 5-year results from the randomized CLL14 study of venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab in chronic lymphocytic leukemia. Nat Commun. 2023;14(1):2147. doi: 10.1038/s41467-023-37648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemann CU, Munir T, Moreno C, et al. Fixed-duration ibrutinib–venetoclax versus chlorambucil–obinutuzumab in previously untreated chronic lymphocytic leukaemia (GLOW): 4-year follow-up from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(12):1423–1433. doi: 10.1016/S1470-2045(23)00452-7. [DOI] [PubMed] [Google Scholar]
- 20.Thompson PA, Bazinet A, Wierda WG, et al. Sustained remissions in CLL after frontline FCR treatment with very-long-term follow-up. Blood. 2023;142(21):1784–1788. doi: 10.1182/blood.2023020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 22.Anota A, Hamidou Z, Paget-Bailly S, et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24(1):5–18. doi: 10.1007/s11136-013-0583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnetain F, Dahan L, Maillard E, et al. Time until definitive quality of life score deterioration as a means of longitudinal analysis for treatment trials in patients with metastatic pancreatic adenocarcinoma. Eur J Cancer. 2010;46(15):2753–2762. doi: 10.1016/j.ejca.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Holtzer-Goor KM, Schaafsma MR, Joosten P, et al. Quality of life of patients with chronic lymphocytic leukaemia in the Netherlands: results of a longitudinal multicentre study. Qual Life Res. 2015;24(12):2895–2906. doi: 10.1007/s11136-015-1039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan CE, Davids MS, Hermann R, et al. MAJIC: a phase III trial of acalabrutinib + venetoclax versus venetoclax + obinutuzumab in previously untreated chronic lymphocytic leukemia or small lymphocytic lymphoma. Future Oncol. 2022;18(33):3689–3699. doi: 10.2217/fon-2022-0456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.