Abstract
Objective:
Asthma is one of the most common comorbid conditions in pregnancy.1 While asthma has been identified as an independent risk factor for cardiovascular disease in the general population2, the influence of active asthma during pregnancy on future cardiac risk is unclear. Growing evidence has linked maternal active asthma to adverse pregnancy outcomes (APOs), such as hypertensive disorders of pregnancy (HDP), including preeclampsia which is a well-defined risk factor for future cardiovascular disease including altered cardiac structure and diastolic dysfunction.3 A thorough understanding of the relationship between pre-existing asthma and APOs may be instrumental in identifying upstream factors contributing to lifetime maternal cardiovascular risk. However, current knowledge of these relationships has been largely derived from retrospective clinical studies, which limit the precision of capturing APOs. Therefore, we investigated associations between pre-existing asthma and individual subtypes of APOs in a secondary analysis of a prospective multi-center cohort of nulliparous individuals with rigorously adjudicated pregnancy outcomes.
Study design:
We included participants from the multisite Nulliparous Outcomes in Pregnancy: Monitoring Mothers to be (nuMom2b) cohort, which recruited nulliparous individuals with a viable, singleton gestation between 60/7 and 136/7 weeks. Details of the study design have been previously described, which included medical histories in standardized interviews.4 This secondary analysis included individuals aged 18 years or older with a live birth and excluded those with a history of pre-pregnancy hypertension or diabetes. For the purposes of this analysis, we defined active asthma as a self-reported history of asthma and on current asthma treatment, including use of bronchodilator, inhaled steroid, or immune modulator, captured at the first trimester visit. The primary outcome was HDP and secondary outcomes included other APOs. Characteristics between participants who did and did not have asthma were compared. Univariate and multivariate logistic regression, described using odds ratios (ORs) and adjusted ORs (aORs) and 95% confidence intervals (95% CI), was used to determine risk of APOs. Models were adjusted for maternal age, study site, insurance type (marker of socioeconomic status), and smoking status at the first trimester visit. Race/ethnicity and body mass index (BMI) were excluded from fully adjusted models as race/ethnicity was considered as a factor reflective of the social determinants of health and BMI was conceptualized as within the casual pathway for developing HDP. The study was approved by all local institutional review boards, and participants gave written informed consent. Analyses were conducted using STATA (MP 17, College Station, TX).
Results
Of 8,741 individuals included, 1,521 (17.4%) reported a diagnosis of asthma at the first trimester visit, of whom 588 (38.7%) reported the use of any asthma medication. When comparing participants with and without asthma, a higher proportion of those with asthma reported smoking tobacco in the three months prior to pregnancy (20.7% vs 16.5%) (Table 1). Univariate logistic regression revealed that a diagnosis of asthma was associated with a significantly higher risk of HDP (OR: 1.21 [1.04, 1.42]). Following adjustment, risk of HDP remained significantly higher (aOR: 1.23 [1.06, 1.42]), specifically preeclampsia (aOR: 1.21, [1.02, 1.45]). Secondary analyses in participants with active asthma (ie additional reported use of asthma medication during or before the first trimester) demonstrated a significantly higher risk of HDP (aOR 1.32 [1.06–1.65]) including preeclampsia (aOR 1.27 [1.07–1.51]; in addition to spontaneous preterm birth (aOR 1.60 [1.30–1.96]).
Conclusions
In a diverse, nationally representative sample of nulliparous individuals,, a diagnosis of asthma was associated with a significantly higher risk of HDP. Active asthma increased the risk of both spontaneous preterm birth and HDP. This analysis supports the importance of identifying active asthma as a risk factor for APOs associated with a higher risk of future cardiovascular disease.
Concerning the biologic feasibility, inflammation is supported as a key mediator in the pathogenesis of APOs as well as in many subtypes of asthma. We hypothesize that there are overlapping processes leading to this elevated risk. For example, changes in placental vascular function implicated in preeclampsia have also been found in the placentas of patients with moderate and severe asthma.5 That no significant risk was identified for SGA may be due to being underpowered to detect a difference or may indicate that there are distinct mechanistic pathways for these APOs.
Our study is unique in that the Numom2b cohort prospectively collected participant data to ascertain asthma history and medication use in early pregnancy and followed outcomes throughout pregnancy, providing rigorous adjudication of APOs and mitigating sources of bias present in retrospective studies. As one focus of the Numom2b prospective cohort was to describe predictors of APOs, assessment of asthma severity was less highly resolved. However, we found that asthma requiring medications further amplified APO risks and though limited, a general requirement for medication serves as a reasonable surrogate of active disease and disease burden. Additionally, we acknowledge the possibility of unmeasured confounding variables; however, findings are consistent with previous publications and emphasize the need for future research. Our findings highlight maternal active asthma as a potential risk factor for APOs linked to the development of future cardiopulmonary disease and further research efforts are indicated.
Figure 1.
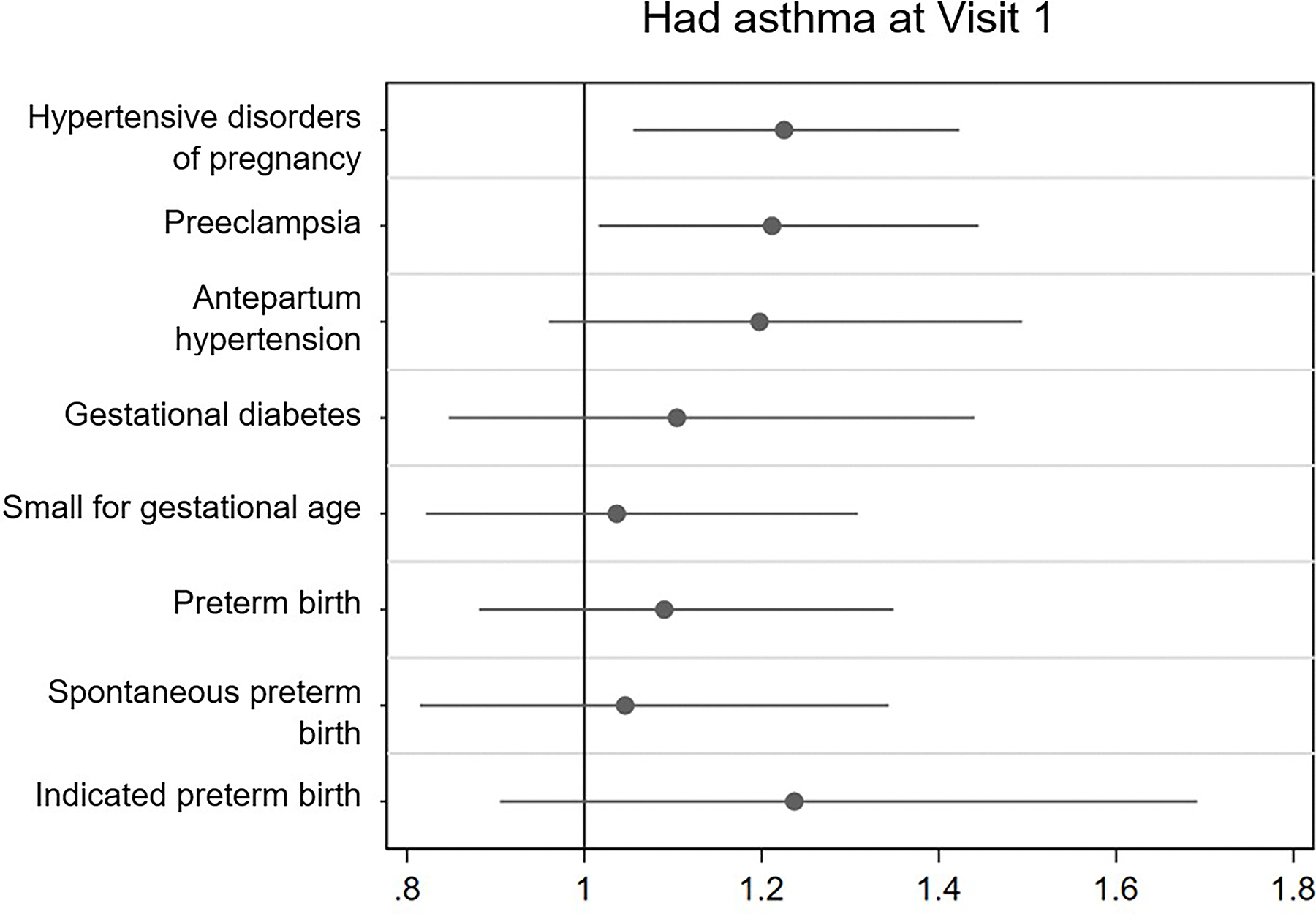
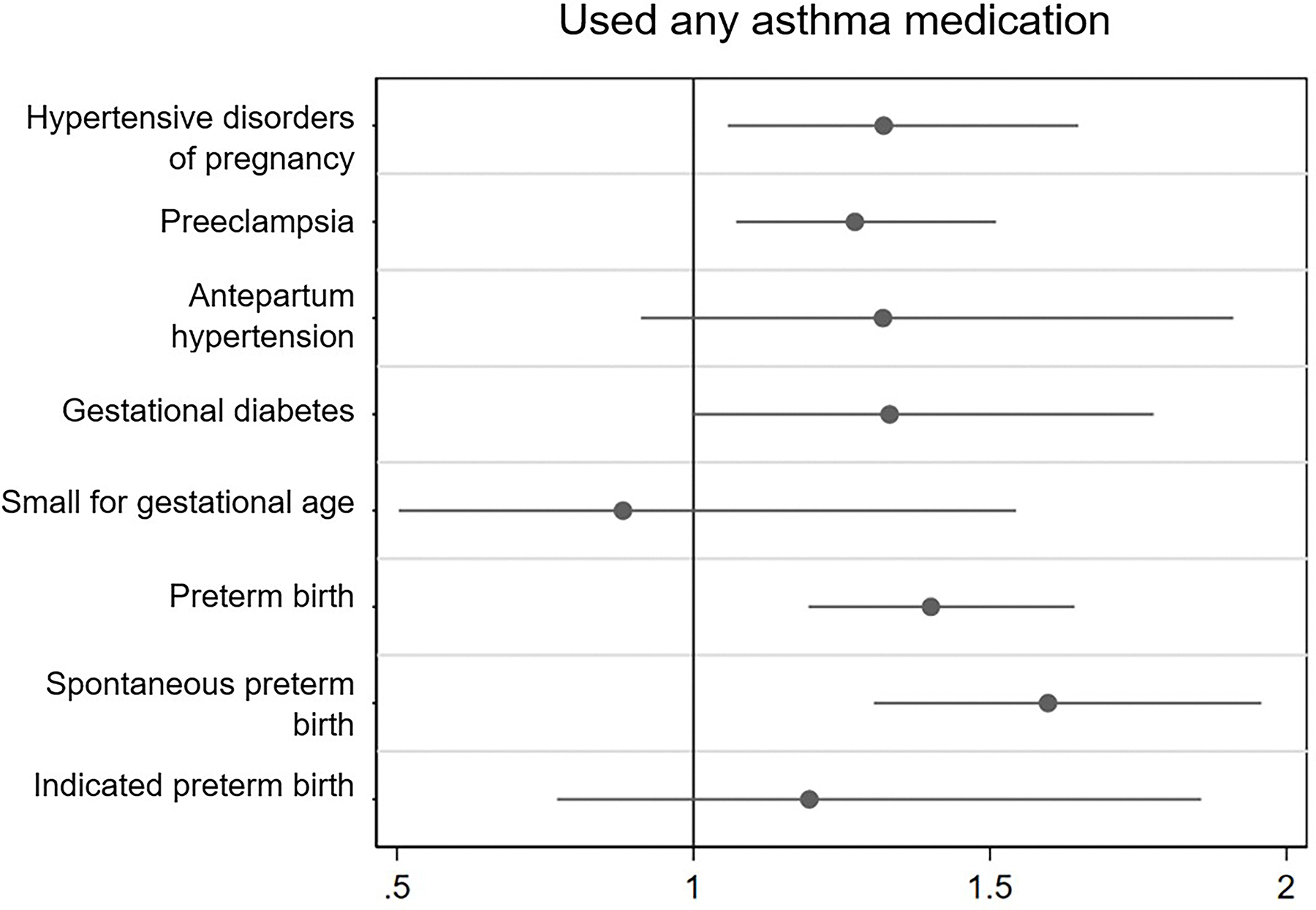
Association of asthma and use of asthma medication with adverse pregnancy outcomes and subtypes
1a. Had asthma at visit 1
1b. Used any asthma medication
Adjusted odds ratio (95% Confidence interval)
± Adjusted for maternal age, study site, insurance type, and smoking status at the first trimester visit
* Values for “Asthma at visit 1” (n=1521) (No %, Yes%, [p value]): HDP 13.5, 15.9 (0.015), preeclampsia 8.1, 9.6 (0.051), antepartum gestational hypertension 5.4, 6.3 (0.19), gestational diabetes 4.0, 4.1 (0.96), small for gestational age 4.3, 4.5 (0.66), preterm birth < 37 weeks 7.5, 8.3 (0.31), spontaneous preterm birth 5.0, 5.2 (0.69), indicated preterm birth 2.6, 3.3 (0.15). Values for “Used any asthma medication” (n=604): HDP 13.5, 17.0 (0.018), preeclampsia 8.2, 10.4 (0.063). antepartum gestational hypertension 5.3, 6.6 (0.19), gestational diabetes 4.1, 4.7 (0.45), small for gestational age 4.4, 4.3 (0.86), preterm birth < 37 weeks 7.4, 10.4 (0.006), spontaneous preterm birth 4.6, 7.4 (0.003), indicated preterm birth 2.6, 3.2 (0.35).
Table 1.
Baseline demographics among nulliparous individuals stratified by asthma statusa
| Self-Report of Prenatal Asthma | |||||
|---|---|---|---|---|---|
|
|
|||||
| No N=7,220 |
Yes N=1521 |
p-valueb | |||
|
| |||||
| Age (years) | 27 | (5) | 26 | (5) | <0.01 |
| Race/Ethnicity (n, %) | <0.01 | ||||
| Non-Hispanic White | 4,548 | 63.0% | 914 | 60.1% | |
| Non-Hispanic Black | 885 | 12.3% | 258 | 17.0% | |
| Hispanic | 1,125 | 15.6% | 224 | 14.7% | |
| Asian | 327 | 4.5% | 36 | 2.4% | |
| Others | 330 | 4.6% | 88 | 5.8% | |
| Health insurance (n, %) | 0.04 | ||||
| Public insurance | 1,791 | 24.8% | 419 | 27.5% | |
| Private insurance | 5,100 | 70.6% | 1,045 | 68.7% | |
| Others | 329 | 4.6% | 57 | 3.7% | |
| Smoked in 3 months before pregnancy (n, %) | 1,193 | 16.5% | 315 | 20.7% | <0.01 |
| Use of asthma medications | 588 | 38.7% | n/a | ||
| Study location | <0.01 | ||||
| Case Western Reserve University | 1,697 | 23.5% | 330 | 21.7% | |
| Columbia University | 629 | 8.7% | 95 | 6.2% | |
| Indiana University | 1,218 | 16.9% | 241 | 15.8% | |
| Magee-Women’s Hospital | 308 | 4.3% | 94 | 6.2% | |
| Northwestern University | 954 | 13.2% | 235 | 15.5% | |
| University of California Irvine | 1,157 | 16.0% | 218 | 14.3% | |
| University of Pennsylvania | 544 | 7.5% | 109 | 7.2% | |
| University of Utah | 713 | 9.9% | 199 | 13.1% | |
Population includes nulliparous individuals aged 18 years or older without any history of pre-pregnancy hypertension or diabetes. Individuals who reported pregnancy loss were excluded.
P-value based on t-test for maternal age and chi2 tests for race/ethnicity, insurance, and smoking status variables.
Funding:
Dr. Khan is supported by grant funding from the NIH, R01HL161514. The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be study was supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): U10 HD063036, RTI International; U10 HD063072, Case Western Reserve University; U10 HD063047, Columbia University; U10 HD063037, Indiana University; U10 HD063041, University of Pittsburgh; U10 HD063020, Northwestern University; U10 HD063046, University of California Irvine; U10 HD063048, University of Pennsylvania; and U10 HD063053, The University of Utah
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
References
- 1.Kwon HL, Triche EW, Belanger K, Bracken MB. The Epidemiology of Asthma During Pregnancy: Prevalence, Diagnosis, and Symptoms. Immunology and Allergy Clinics 2006;26:29–62. [DOI] [PubMed] [Google Scholar]
- 2.Carter P, Lagan J, Fortune C, et al. Association of Cardiovascular Disease With Respiratory Disease. Journal of the American College of Cardiology 2019;73:2166–77. [DOI] [PubMed] [Google Scholar]
- 3.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circulation Cardiovascular quality and outcomes 2017;10. [DOI] [PubMed] [Google Scholar]
- 4.Haas DM, Ehrenthal DB, Koch MA, et al. Pregnancy as a Window to Future Cardiovascular Health: Design and Implementation of the nuMoM2b Heart Health Study. Am J Epidemiol 2016;183:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifton VL, Giles WB, Smith R, et al. Alterations of placental vascular function in asthmatic pregnancies. Am J Respir Crit Care Med 2001;164:546–53. [DOI] [PubMed] [Google Scholar]


