Central Illustration
Key Words: biomass, disease modification, pulmonary arterial hypertension
Highlights
-
•
We introduce a novel paradigm focusing on excess biomass as a fundamental but modifiable endophenotype in PAH.
-
•
This framework will stimulate PAH-modifying therapeutics and promote new insights on disease inception.
Summary
Fibroproliferative remodeling of distal pulmonary arterioles is a cornerstone characteristic of pulmonary arterial hypertension (PAH). Data from contemporary quantitative imaging suggest that anabolic synthesis of macromolecular substrate, defined here as biomass, is the proximate event that causes vascular remodeling via pathogenic changes to DNA, collagen, cytoskeleton, and lipid membranes. Modifying biomass is achievable but requires tilting the balance in favor of endogenous degradation over synthetic pathways in order to advance the first-ever disease-modifying PAH pharmacotherapy. Viewing PAH pathobiology through the lens of biomass represents an opportunity to decipher novel determinants of disease inception and inform interventions that induce reverse remodeling.
Idiopathic pulmonary arterial hypertension (PAH) is a vasculopathy of distal pulmonary arterioles that increases pulmonary vascular resistance, leading to right heart failure and accelerated mortality.1 Currently approved medical therapies for PAH target vasodilatory signaling pathways; however, abnormal vasoreactivity is central to disease pathophysiology in a minority of PAH patients. Yet, even in patients who do not demonstrate vasoreactivity during invasive testing, pharmacotherapies with vasoactive properties remain the mainstay approach. Indeed, averting downward clinical decline is uncommon in the longitudinal management of PAH patients despite maximal vasodilatory treatment, and, thus, advancing disease-modifying therapeutics that durably reprogram underlying disease pathobiology is a major unmet need. Sotatercept traps bioactive growth differentiation factors and is the first activin signaling inhibitor approved for patient care. By therapeutically modifying pathways distinct from vasodilatory signaling, this progress sets the stage for sustained reduction in pathogenic vascular remodeling that drives PAH.
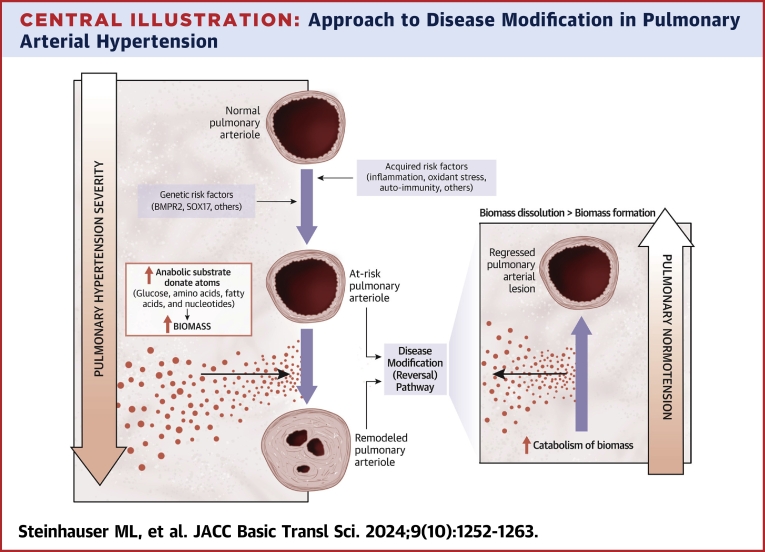
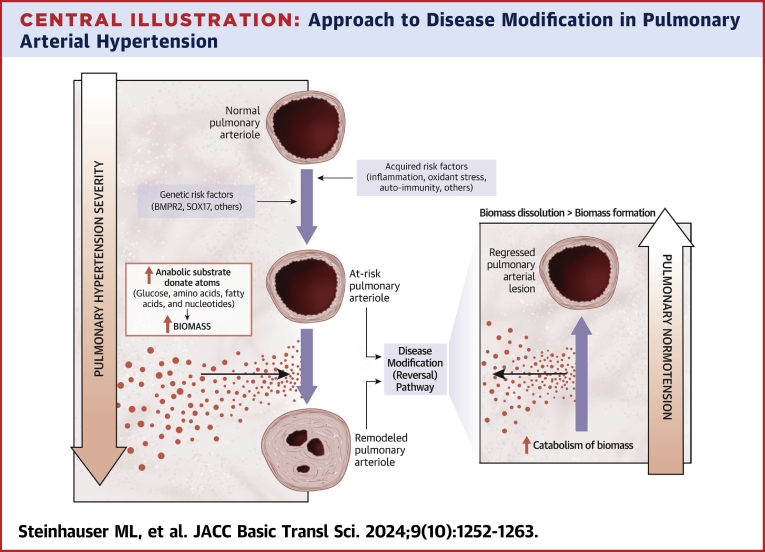
Compared to normal, the pulmonary arterioles in advanced PAH are dramatically remodeled by (peri)vascular fibrosis and increased cellularity. The expansion of smooth muscle cells, fibroblasts, pericytes, and inflammatory cells in the deep adventitial and medial layers of the vessel wall is mirrored at the luminal interface by the proliferation of endothelial cells and the formation of stereotypical plexiform lesions that may completely obliterate the lumen. In short, the matter comprising the remodeled vessel is increased by >10-fold (Figure 1). In this paper, we introduce the term biomass to PAH, which we define as the collective extracellular, cellular, and subcellular material that comprises the remodeled pulmonary vessel. In turn, we speculate that the glucose, fatty acid, amino acid, and nucleotide macromolecular substrate that form the elemental basis for biomass are a root cause of fibroproliferative remodeling in PAH. We posit that true disease modification in PAH will require reducing obliterative biomass, which is integral to pathological remodeling in PAH, providing rationale to establish a conceptual framework for how consideration of biomass may inform disease-modifying therapeutics (Central Illustration).
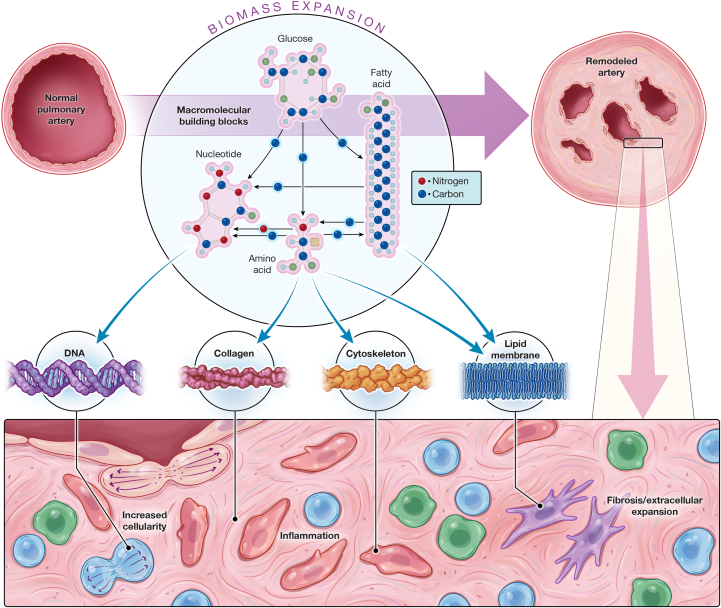
Figure 1.
Expansion of Biomass as a Driver of PAH Pathobiology
Pulmonary arterial hypertension (PAH) is defined by fibroproliferative remodeling and plexiform growth, resulting from fibrosis and expansion of extracellular matrix, proliferation of endothelial cells and mesenchymal cells, and infiltration of immune and inflammatory cells. This massive increase in the cellular and extracellular material that comprises the vessel wall and occupies the vessel lumen represents the collective pathological biomass. (Circle inset) If expansion of cellular biomass is reduced to the atomic level, critical elemental components including the carbon and nitrogen backbone of biomass can be sourced from a variety of substrates and interconnected metabolic pathways. Although macromolecular building blocks can be imported into cells to directly supply anabolic production of biomass, including key components such as DNA (nucleotides), collagen (amino acids), cytoskeleton (amino acids), and lipid (fatty acids), there are also multiple paths by which atoms can flux from one building block type to another because the catabolic breakdown of molecules can in turn supply substrate for alternative biosynthetic pathways. The tricarboxylic acid (TCA) cycle represents a useful example of this concept. The TCA cycle is supplied by catabolism of amino acids, glucose, and fatty acids. Aside from its central role in energy production, it also serves as a hub in the trafficking of substrate to diverse biosynthetic pathways, including protein synthesis and de novo nucleotide synthesis. In this manner, the new DNA synthesized during cell division may contain atoms derived from nucleotides, fatty acids, amino acids, and glucose.
Central Illustration.
Approach to Disease Modification in Pulmonary Arterial Hypertension
Metabolic Reprogramming in PAH to Support Biomass
Pathologic fibrosis, cellular proliferation, and changes in cell state, such as phenotype switching through endothelial-mesenchymal transitions or inflammatory cell activation, all contribute to vascular remodeling in PAH, each driven by distinct cellular and molecular pathways. However, anabolic production of biomass is the proximate molecular event upstream of each of these pathological processes. In this way, the conceptual framework of biomass emphasizes the opportunity to target the basest substrate for macroscopic and ultrastructural microscopic changes to the blood vessel architecture that are observed on gross inspection and histological analysis, respectively. Proliferating cells replicate not just their genomes but also their entire cellular infrastructure. Vascular fibrosis involves anabolic synthesis of extracellular matrix proteins such as collagen. Even changes in cell state involve anabolic processes that may include the RNA, lipid, and protein synthesis that drives cell type–specific programs, requisite remodeling of cellular cytoskeleton and membranes, and the production of specialized organelles. Therefore, understanding the metabolic basis for biomass may provide common targets relevant across pathological cell types, disease stages, and PAH from different disease drivers.
A myriad of interconnected biochemical pathways determines the ultimate macromolecular composition of any tissue, including but not limited to: 1) information flow from DNA to biosynthetic translation of specific cellular and extracellular proteins; and 2) synthesis of, and enzymatic modifications to, fatty acids and lipids, driving formation of the full range of structural and signaling lipid species. However, a common prerequisite for anabolic metabolism that drives biomass is “building blocks” or substrates for synthesis of the dominant macromolecular cellular and extracellular constituents, including amino acids (protein), nucleotides (RNA and DNA), and fatty acids (lipid). Cells generally import these substrates directly, recycle them from the catabolic breakdown of macromolecules, or synthesize them de novo including through co-optation of intermediates from glycolytic or fatty acid catabolism. In the following paragraphs, we provide examples of each of these categoric approaches to cellular production of substrate for macromolecular biosynthesis.
The simplest path to acquisition of biosynthetic substrate by individual cells is direct importation, which links cellular metabolism to tissue and systemic metabolism. Polar molecules, including amino acids and nucleotides, are imported into cells by facilitated diffusion through membrane transporters.2 Fatty acids, which are substrate not just for structural membrane lipids but also for lipid signaling species, may directly diffuse across plasma membranes at low rates or traverse more efficiently via specific fatty acid transporters, such as CD36.3,4 Many of these transporters are transcriptionally upregulated and/or increased in the plasma membranes of cells in PAH vessels or in response to pathological stimuli of relevance to PAH.5,6
The availability of biosynthetic substrates in systemic circulation may be modified by diet and nutritional states, increasingly recognized as potential contributors to PAH. Obesity is a state of nutritional excess that may predispose to PAH.7, 8, 9 Obesity drives pathobiological pathways of known relevance to PAH, including inflammation and insulin resistance.10 Although it is conceivable that nutritional excess might directly fuel PAH remodeling by providing anabolic substrates, there is not a one-to-one relationship between dietary intake and resultant levels in circulation. Secondary disease effects on metabolically active tissues outside of the pulmonary vasculature, such as skeletal muscle, may account for steady-state differences in circulating metabolite biomarkers of PAH, including amino acid and lipid species.10,11 Indeed, even in states of extreme nutritional deprivation, the core building blocks of DNA, RNA, protein, and lipids are present in circulation.12 Moreover, it is critical to distinguish systemic metabolism and metabolic function from cellular metabolic processes in the pulmonary vasculature, which may further differ across the various cells comprising PAH lesions. It will be important to dissect whether emerging metabolite predictors of PAH (eg, N2,N2-dimethylguanosine; N1-methylinosine; malate; fumarate; and acetylphosphate, among others13,14) are simply biomarkers of the disease or if they participate in disease pathogenesis either as signaling molecules or as substrate for anabolic growth.
Phagocytes engulf dead cells and scavenge debris, which in turn can be broken down to release amino acids for anabolic growth, a process most established in atherosclerotic disease of systemic arteries.15 However, the consumption of extracellular material may extend beyond specialized leukocytes because endothelial cells also have the capacity to engulf dead cells, at least in vitro.16 Engulfment of extracellular microvesicles (eg, exosomes) is an additional conduit for importation of extracellular materials inclusive of vesicular membranes and any interior payload. Although much attention has been paid to the signaling properties of messenger RNAs and microRNAs contained in exosomes, vesicles may also carry triglycerides, which are both a potential source of energy and recycled anabolic substrate.17 In cancer, another endocytic process called macropinocytosis imports protein as a source of amino acids to fuel growth, a process also observed in endothelial cells.18,19 Therefore, multiple potential mechanisms may support internalization and catabolic breakdown of macromolecules from the microenvironment to supply substrate to increase biomass, even though the degree to which such mechanisms drive PAH pathobiology is not known.
The pathways of fatty acid beta oxidation, glycolysis, and the citric acid cycle are central to carbon catabolism that supports cellular energetic demands. Complete catabolism of glucose by glycolysis, the tricarboxylic acid (TCA) cycle, and oxidative phosphorylation maximizes energy production. Similarly, beta oxidation of fatty acids produces acetyl coenzyme A, which can also supply the TCA cycle and oxidative phosphorylation. However, at multiple nodes in the catabolic consumption of fatty acids and glucose, intermediates can be diverted to alternative metabolic fates.20,21 Glycolytic intermediates can supply the pentose phosphate pathway and contribute to de novo synthesis of nucleotides. Acetyl coenzyme A from glycolytic metabolism (or fatty acid oxidation) can be used for de novo fatty acid synthesis. TCA intermediates can be diverted for synthesis of amino acids, a subset of which in turn can also serve as substrate for de novo nucleotide synthesis. In short, catabolic pathways that are so critical for energy production are paradoxically also a potential source of substrate for anabolic synthesis of macromolecular building blocks.
The co-opting of glycolytic metabolites for biomass is the leading hypothesis to explain the Warburg phenomenon in proliferating cancer cells whereby glycolysis is increased under normoxic conditions without a matched increase in oxidative phosphorylation.22 Warburg metrics are also demonstrable in endothelial and smooth muscle cellular models of PAH with stable isotope tracers under normoxic conditions, including: 1) increased glucose uptake and glycolysis; 2) increased lactate production; and 3) flux of constituent glucose atoms to support fibroproliferative biomass.23,24 However, proving such metabolic reprogramming in vivo is more challenging. Even in tumor studies in which there is requisite tissue for bulk metabolic flux analyses, it is difficult to fully account for the metabolic activities of noncancerous tumor cells. Indeed, cellular heterogeneity is a particularly important confounder in the complex architecture of the PAH lung where “normal” lung parenchymal cells far outnumber disease-driving cells in the pulmonary arterioles. Pivotal studies have addressed this by correlating glucose avidity detected at tissue scale resolution with fluorodeoxyglucose (FDG) positron emission tomography to up-regulation of putative metabolic regulators in PAH lesions.5,25 Furthermore, abnormal FDG positron emission tomography uptake patterns have been observed in the right ventricle in PAH patients and experimental models in vivo (reviewed in detail by Thenappan et al26). Functional improvements derived from targeting of metabolic regulatory nodes further advance the metabolic basis for PAH25,27; however, it is difficult to prove that functional benefits are a direct consequence of the reversal of metabolic reprogramming in part because of the dual roles of metabolic pathways in energy production and supply of anabolic substrate. In addition, there are limited tools available to directly probe and quantify metabolic functions of individual cells, as opposed to the tissue scale insight achievable with positron emission tomography, and therefore little direct understanding of the metabolic functionalities and differences between the various constituent cell types of PAH vascular lesions in vivo, including endothelial cells, mesenchymal cells, and immune cells. As such, there is an unmet need for new approaches to interrogate the intersection between metabolism, energy balance, biomass dynamics, and pathological remodeling in the pulmonary vasculature in vivo.
Imaging Mass Spectrometry Reveals Metabolic Reprogramming in the Pulmonary Vasculature In Vivo
We developed a new approach to studying metabolic reprogramming of individual cells in PAH in vivo using state-of-the-art imaging mass spectrometry.28 Multi-isotope imaging mass spectrometry (MIMS) allows for multiplexed and quantitative tracking of nonradioactive stable isotope tracers at suborganelle resolution in tissues collected after in vivo tracer administration.29 Translational human studies are feasible if the tissue is either easily accessible by minimally invasive sampling (eg, blood cells and subcutaneous fat) or can be collected during an invasive procedure that is part of usual clinical care.30, 31, 32 In essence, MIMS brings functional metabolic imaging as achievable with positron emission tomography from tissue scale down to the suborganelle level and does so with the quantitative power of mass spectrometry.33,34
MIMS enables quantitation and visualization of biomass dynamics per se. For example, through multiplexed MIMS imaging of amino acid and glucose tracers in an inflammatory PAH rodent model, we discovered heightened glucose and proline avidity of individual endothelial and medial cells in PAH lesions relative to control vessels.28 Moreover, within PAH lesions, the endothelium demonstrates relatively greater glucose and proline avidity relative to medial cells. One important aspect of this finding is that it definitively localizes the type of metabolic/functional readout achievable with positron emission tomography to the pathological cells and remodeled vascular tissue itself. However, beyond the gain in imaging resolution, stable isotope-tagged glucose is seamlessly incorporated into downstream glucose metabolic pathways, including its co-optation for biomass.35 The use of glucose to supply anabolic substrate for biomass was further demonstrated by tracking of glucose label into the extracellular collagen fibrils identified through correlative electron microscopy, a finding only explainable by the divergence of glucose metabolites to support collagen biosynthesis, an unambiguous component of pathological biomass in PAH.
Aside from what is arguably the most direct evidence of glucose reprogramming in remodeled PAH vessels, in vivo, the application of MIMS to PAH is important because it provides a method to quantify biomass turnover at subcellular resolution, in the same way that MIMS has been used in substrate tracer pulse-chase experiments to measure turnover dynamics of specific constituents of fat tissues, both cellular (adipocytes) and subcellular (lipid droplets).29,31,36 MIMS can also be coupled with other analytical methods. Although MIMS is a destructive analysis, only the surface atomic layers of a tissue section are consumed, leaving additional material for orthogonal analyses. Moreover, tissue sections used for MIMS are an order of magnitude thinner than traditional histologic preparations, allowing for interrogation of adjacent sections with other modalities, such as the identification of specific immune/inflammatory subtypes with immunofluorescence microscopy. We also predict that multiomics analyses of cells concurrently analyzed by MIMS will be achievable as the requisite technologies become more sensitive. Beyond revealing nuanced cellular identities and phenotypes, merging MIMS with spatial transcriptomics or spatial proteomics may provide an opportunity to discover novel molecular programs driving biomass that can be therapeutically exploited. Furthermore, understanding how new experimental interventions modulate biomass dynamics with MIMS holds promise for testing and prioritizing candidate disease-modifying interventions.
Therapeutic Targeting of Biomass
We have described how PAH metabolic reprogramming may resemble cancer cells. However, there are important limitations to using the biology of cancer to explain pathological biomass in PAH because pulmonary vascular cells do not exhibit malignant transformation. Without the unlimited cellular proliferation and invasive growth seen in cancer, there is a ceiling on total biomass expansion of any individual arteriole—the sum of the limited degree of outward growth (so-called “positive vascular remodeling”) and the medial occlusion of the lumen (so-called “negative or invaginated vascular remodeling”). Once the lumen of the vessel is completely obliterated by pathological remodeling, the vascular geometry limits additional expansion of total biomass burden. Consequently, the principle of conservation of mass necessitates that the rate of biomass formation must be matched to the rate of biomass dissolution once a vessel has reached such a point of advance remodeling. This does not exclude the possibility of ongoing fibrotic remodeling (ie, replacement of one biomass component [eg, functional cells] with another more pathological component [eg, fibrosis]). However, this model suggests that the dynamics of biomass in any given vessel are likely to be very different depending on the degree of obstructive biomass deposition that has already occurred. Consequently, optimal therapeutic strategies to address pathological biomass may differ depending on disease stage.
We propose that there are several nonmutually exclusive ways in which viewing the problem of PAH through the lens of biomass holds promise to accelerate the development of effective disease-modifying therapeutics.1 By linking quantitative measurements of cellular and subcellular biomass dynamics obtained with MIMS to molecular data collected with orthogonal methods such as spatial transcriptomics, novel biomass regulators and candidate drug targets may emerge.2 We advance a framework around biomass dynamics that leads to different potential biomass-centric approaches depending on disease stage (Figure 2) whereby targeting anabolic biomass expansion might be more efficacious in early disease, whereas the stimulation of catabolic dissolution of obliterative biomass might be required in advanced disease.3 The consideration of differential biomass dynamics at different disease stages may also provide insight into therapeutic efficacy, therapeutic resistance, and rationale design of combination therapies. In the following paragraphs, we explore these concepts using preclinical data and emerging therapeutics as potential examples of biomass-centric therapies.
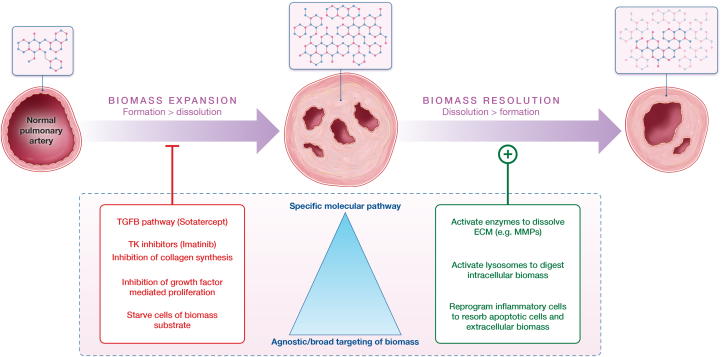
Figure 2.
Conceptual Framework for Therapeutic Targeting of Biomass for PAH
The ultimate goal of pulmonary arterial hypertension (PAH) therapeutics is restoration of normal blood flow and durable disease modification, which will require reducing the pathological biomass that obliterates the lumen of pulmonary arteries in PAH patients. This figure demonstrates 2 theoretic paths to reducing pathological biomass contextualized by examples of potential targets: (left) the inhibition of biomass expansion or (right) the augmentation of biomass dissolution (right). Once a lesion is established (middle vessel), disease modification will require shifting the biomass turnover equation such that dissolution exceeds formation. The dynamics of biomass turnover under pathological conditions are not fully elucidated, and, therefore, an unanswered question is whether the background rate of biomass dissolution is sufficiently high such that lesion regression would be feasible in a practical time frame if new biomass deposition was effectively neutralized. Current therapeutic development is largely focused on developing strategies that target fibroproliferative growth (ie, biomass expansion). An alternative and largely untapped approach could be activation of the intracellular and/or extracellular mechanisms involved in the disposal of biomass. For the extracellular matrix (ECM), this would involve secreted proteases (eg, matrix metalloproteinase [MMP]). For intracellular biomass, this would involve canonical systems such as proteosomal degradation and/or lysosomal digestion of macromolecules. This could also involve reprogramming of immune cells to remove cells and extracellular material. TGFB = transforming growth factor beta; TK = tyrosine kinase.
Inhibition of fibroproliferative growth pathways
In our view, any drug that inhibits the fibroproliferative pathology underlying arterial remodeling ostensibly targets biomass (Figure 2). Therefore, such drugs that have already been tested in human PAH patients can be viewed as candidate modulators of biomass and provide a framework to consider progress and potential barriers to successful disease modification. Sotatercept is a putative modifier of biomass through its effects on proliferative growth, although evidence of this remains limited to experimental models rather than data from clinical samples. The drug is a ligand trap for growth differentiation factors 8/11 and activin A, which effectively reorients signaling through activin receptors away from progrowth Smad2/3 signaling while preserving bone morphogenetic protein–mediated SMAD1/5/8 signaling. Preclinical studies demonstrated efficacy in PAH animal models with attenuation of cell proliferation, obstructive vasculopathy, and pulmonary vascular resistance.37 The randomized controlled STELLAR trial established improvements in multiple disease metrics in human PAH patients, including the 6-minute walk distance, pulmonary vascular resistance, and circulating natriuretic peptides, after 24 weeks of treatment.38,39
Imatinib is a tyrosine kinase inhibitor repurposed from the cancer field for therapeutic development in PAH based on evidence that tyrosine kinases targeted by the drug contribute to proliferative growth in PAH.40 As seen with sotatercept, imatinib is efficacious in more than 1 animal model of PAH,40,41 and a signal of efficacy has been demonstrated in translational human trials.42, 43, 44 Moreover, in the rat monocrotaline model of PAH, antiremodeling effects of imatinib correlate with a reduction in FDG glucose avidity in the lungs, which could be consistent with attenuation of glucose flux to biomass.25 Even though definitive evidence of reverse remodeling remains limited to experimental (rather than clinical) data, demonstration of improvement in PAH disease metrics with 2 different drugs targeting aspects of fibroproliferative growth underscores the potential therapeutic power of inhibiting pathological biomass expansion.
The experience to date with these 2 seemingly promising antiproliferative drugs is counterbalanced by signs of some clinically relevant off-target effects. Imatinib-treated PAH patients developed intracranial hemorrhage at a rate of approximately 2% in a 24-week randomized controlled trial, a toxicity signal that increased to approximately 4% in an open-label extension study.44 Although bleeding events primarily affected patients who were also receiving systemic anticoagulant therapies, a signal of bleeding risk exists outside of PAH. In the larger experience with imatinib in cancer, treatment has been associated with various forms of ocular bleeding, including in the conjunctiva and retina.45,46 Although the underlying mechanisms are not known and many affected patients were prescribed the anticoagulant drug warfarin, a potential vascular mechanism to account for this effect cannot be excluded. No such single or unifying toxicity has yet been elucidated for sotatercept. However, unusual telangiectasias with longer-term treatment is also suggestive of vascular toxicity and raises the question of whether more serious vascular abnormalities will emerge with greater study.47 The overall experience to date with sotatercept and imatinib underscores the potential for off-target effects when targeting fundamental growth pathways in the vasculature.
Starving pathological cells of metabolic substrate for biomass
Another approach to targeting biomass could be to deprive pathological cells of substrate for macromolecular biosynthesis and biomass, a strategy considered for cancer therapeutics. Such a strategy could theoretically be accomplished by targeting substrate uptake or the mechanisms that control shunting of substrate to anabolic synthesis of biomass. The most advanced example of a metabolic drug in PAH involves targeting pyruvate dehydrogenase kinase (PDK), which is an inhibitor of pyruvate dehydrogenase, a gatekeeping enzyme for mitochondrial glucose catabolism.48 If viewed through the lens of metabolic reprogramming and biomass, increased flux of glycolytic products into mitochondria could limit the use of glycolytic intermediates for de novo synthesis of nucleotides, which are important for proliferative growth.21 Indeed, inhibition of PDK with dichloroacetate reduces proliferative remodeling and FDG glucose lung uptake in PAH models25 while improving hemodynamic metrics in PAH models and in a subset of PAH patients treated as part of a small open-label study.49 Although the depth of human data for PDK targeting is limited, the possibility of intrinsic compensatory mechanisms has been raised as 1 explanation for inconsistent clinical responses, underscoring how the interconnectivity and redundancy of metabolic pathways may equally obscure mechanisms of benefit and therapeutic resistance in nonresponders.
Glutamine is a promising metabolic target rooted in cancer biology. Cancer cells are often described as addicted to glutamine, with its diverse metabolic fates supporting proliferation through: 1) incorporation of glutamine into new protein; 2) conversion to other amino acids, including proline, which can be used for collagen synthesis and critical extracellular matrix among other fates; 3) anaplerotic flux into the TCA cycle to support energy needs; and 4) anaplerotic flux into the TCA cycle to supply substrate for biosynthetic reactions including de novo nucleotide synthesis. In line with its fundamental role in cellular growth, experimental models of PAH and human patients display evidence of increased glutamine uptake and metabolism.50,51 The development of cancer therapeutics that inhibit uptake of glutamine by the SLC1A5 transporter or proximal steps in intracellular glutamine metabolism sets the stage for drug repurposing for PAH. Indeed, targeting of the proximal conversion of glutamine to glutamate with a glutaminase inhibitor improved pathological remodeling and hemodynamic indexes in preclinical PAH models.51 Even though the early experience with the CB-839 glutaminase inhibitor in cancer patients suggests only rare serious adverse events, the more common dose-related toxicities (eg, fatigue, nausea, and liver function abnormalities) that may be viewed as tolerable for cancer therapeutics may be limiting in a chronic disease like PAH.52 This underscores the challenge of targeting anabolic processes without disrupting homeostatic maintenance of nondiseased tissues.
Targeting pre-existing biomass with a dissolution strategy
We propose that true disease modification will require addressing the problem of pre-existing pathological biomass, particularly for patients presenting with advanced disease. This will require driving the rate of biomass dissolution in excess of the rate of new biomass formation (Figure 2). An open question is whether the endogenous rate of biomass turnover is sufficiently high to achieve net resorption if new biomass production was effectively neutralized. However, in the vascular remodeling that characterizes atherosclerotic disease of systemic arteries, there is little evidence that neutralization of a disease driver is sufficient to tip this balance because even the achievement of low levels of low-density lipoprotein cholesterol stabilize but do not dramatically reverse obstructive plaques.53 This has focused attention in the atherosclerosis field on activating macrophages to scavenge and remove dead cells, cellular debris, and lipids.15 It may be the case that such a strategy will also be required in PAH to achieve true disease modification, particularly in advanced disease.
Achievement of disease modification through biomass dissolution will have to contend with multiple different biomass components, most notably the excess cells and extracellular matrix that dominate remodeled vessels. The elucidation of Specialized enzymes, such as those in the matrix metalloproteinase (MMP) family, may be required to dissolve extracellular fibrotic material for subsequent clearance. MMPs are elucidated by inflammatory processes, may contribute to associated tissue damage when their activity is uncontrolled, and are augmented in PAH animal models and patients; however, genetic (or inflammation-induced) loss of function of matrix metalloproteinase-8 worsens vascular remodeling from chronic hypoxia, supporting the concept that some MMPs may also fulfill adaptive functions.54 Pathological cellularity will also need to be reduced through the induction of cell death (eg, apoptosis) and subsequent clearance. As such, we speculate that true biomass dissolution, including the degradation and disposal of the extracellular matrix and of cellular debris, will require the coordinated action of phagocytic cells, such as macrophages.
Inflammation is a feature of diseased pulmonary arteries and a known disease driver, underscoring the challenge of harnessing the immune system to resolve pathological biomass. A deficiency in cells with adaptive immunomodulatory properties, such as regulatory T cells known to be depleted in PAH patients and experimental models, promotes maladaptive inflammation and disease progression.55 Similarly, although macrophages participate in pathological sterile inflammatory responses, their nonselective targeting may also promote PAH. For example, the depletion of macrophages exclusively in male mice through inducible, macrophage-specific expression of diphtheria toxin increases susceptibility to hypoxia-induced pulmonary hypertension.56 However, it is not just the number of macrophages but rather their cellular phenotype that is important. Indeed, the macrophage is a plastic cell that takes on diverse context-dependent properties, including the traditional proinflammatory (M1) vs anti-inflammatory (M2) dichotomy, the imbalance of which is associated with PAH pathology.56,57 Therefore, it may be through phenotype switching of the resident immune and inflammatory cells that executing a program of biomass dissolution becomes possible.
Any dissolution strategy may ultimately converge on lysosomes, the specialized waste disposal organelles of the cell. Lysosomes are important to autophagy pathways that degrade intracellular constituents and for digestion of materials imported by phagocytosis or endocytosis. However, autophagy can also be both adaptive and maladaptive depending on the context and cell type. For example, in cancer, autophagy may both drive the death of cancer cells or promote cell survival and proliferation.58 A similar dichotomy may apply to PAH. Lysosomal activity is enhanced in PAH lesions in which it may fuel pathologic antiapoptotic and proproliferative activity. Lysosomes may also facilitate ubiquitin-mediated degradation of BMPR2—a protective protein in PAH—which is reversable with administration of the lysosomal inhibitor chloroquine.59 By contrast, genetic targeting of the autophagy mediator, microtubule-associated protein-1 light chain-3B, exacerbates experimental PAH, suggesting a protective role for autophagy and lysosomes.60 Therefore, driving lysosomal degradation of biomass in advanced PAH lesions may ultimately require a cell and context-specific strategy. Moreover, given that lysosomes are also integral to macromolecular recycling pathways, an effective dissolution strategy will have to avoid simply fueling biomass synthesis with recycled substrate. Although flow through an obliterative lesion cannot be normalized without displacing or removal of pathological biomass, it is important to note that there are no definitive examples even in the preclinical literature of unambiguous biomass dissolution, and, therefore, our framework for disease modification through biomass dissolution is speculative.
Targeting orthogonal determinants of biomass with combination therapy
An important theme in our consideration of biomass is that of redundancy, which plays out at multiple different levels as follows: multiple cell types contribute to the occlusive vasculopathy in PAH, different molecular and biochemical cellular pathways drive the endophenotypes of fibrosis and cell proliferation, and multiple interconnected metabolic pathways can supply substrate for synthesis of the macromolecules that constitute biomass. In the cancer field, molecularly targeted combination strategies may achieve therapeutic synergy through identification of a mechanism of resistance exposed by 1 agent that can be neutralized with a second drug. As such, true therapeutic vulnerability and successful disease modification may only emerge through consideration of combination therapies targeting different determinants of pathological biomass.
Considerations for the right ventricle
Abnormal right ventricular (RV) function, including hypertrophic remodeling and impaired lusitropy, is an important determinant of outcome in PAH.24 Prior reports have identified a distinct metabolic profile related to RV function and outcomes in PAH patients, including heightened FDG glucose uptake in the right ventricle by positron emission tomography and distinct systemic metabolite profiles, suggesting dysregulation of critical metabolic pathways related to carbohydrate, fatty acid, and amino acid metabolism.61 Data on subcellular substrate use across RV cell types is lacking but well positioned to provide wider insight on key drivers of biomass that may underpin RV dysfunction independent of, or in response to, changes in RV afterload. This is an important consideration because metabolic regulators may have different (and potentially opposing) functions in RV vs pulmonary vascular cells, and it is possible that specific RV-targeted therapies will be required to achieve disease resolution.
Arguments against the hypothesis
We have advanced the premise that viewing the pathobiology of pulmonary vascular disease through the lens of biomass will illuminate underlying mechanisms and guide a rational approach to disease-modifying therapeutics. The underlying heterogeneity of PAH disease drivers may mean that the concepts put forth here are less applicable in subsets of PAH patients. Currently approved pharmacotherapies for PAH, which emerged from successful clinical trials, by and large modulate vascular tone through pulmonary vasodilation without detectable evidence of reverse remodeling. Thus, it is important to emphasize that fibroproliferative changes to the ultrastructure of pulmonary arterioles do not necessarily imply decrement to vascular function. This is undoubtedly the case in calcium-channel blocker–sensitive PAH, in which a hypercontractile phenotype drives elevation in pulmonary artery pressure but can be treated effectively with high-dose diltiazem or another within-class drug. The genetics of PAH represents another important source of disease heterogeneity. A minority of PAH patients carry 1 of the disease-causing variants in genes such as BMPR2 or SOX17, among others. Although it is notable that genetic variants linked to PAH are incompletely penetrant, highlighting the importance of environmental and/or epistatic modifiers, such putative monogenic drivers of hereditary PAH are the likely causal molecular event upstream of biomass synthesis that causes PAH and may be modifiable therapeutic targets in the gene editing era. Although uncommon, these scenarios nonetheless provide important examples that would direct focus away from biomass as the fundamental target of disease modification in PAH. Furthermore, the extent to which targeting biomass could induce disease modification in each affected cell type and across different PAH clinical stages is not known and is an important additional area for consideration. Aside from the uncommon cases of heritable, monogenic PAH, a single inciting molecular driver of either biomass expansion and/or PAH pathobiology more broadly is not known. The discovery of such factor(s) hold promise to not only reveal the most proximate drivers of biomass expansion and dynamics but will also be critical to solidifying our advancement of the biomass framework for therapeutic discovery.
Conclusions
Plexigenic and fibroproliferative remodeling is the cornerstone feature of PAH. Recent data leveraging nanoscale molecular imaging direct focus on the accumulation of biomass driven by macromolecular biosynthesis as an upstream step in the wider pathophysiologic cascade that results in early mortality. Targeting the accumulation of biomass itself may take 2 general forms: the inhibition of biomass accumulation to enable endogenous antiremodeling pathways or directed activation of biomass resorption, either of which holds promise to modify disease in PAH. In doing so, opportunities to clarify the origins of PAH are likely to emerge, advancing strategies focusing on anticipatory therapeutics for this highly morbid disease as well as other cardiopulmonary diseases characterized by dysregulated cellular metabolism.
Funding Support and Author Disclosures
Dr Steinhauser has received University of Pittsburgh School of Medicine seed funds; and is supported by National Institutes of Health grant DP2CA216362. Dr Maron has received personal fees from Actelion Pharmaceuticals, Tenax, and Regeneron; has received grants from Deerfield Company; has a patent PCT/US2019/059890 pending to None, a patent #9,605,047 issued to None, a patent PCT/US2020/066886 pending to None, and a patent BWH 2023-152-29618-0438P02 pending to None; has received 5R01HL139613-03 grant, National Institutes of Health grant R01HL163960, National Institutes of Health grant R01HL153502, and National Institutes of Health grant R01HL155096-01; and is an investigator at the University of Maryland-Institute for Health Computing, which is supported by funding from Montgomery County, Maryland, and The University of Maryland Strategic Partnership (MPowering the State, a formal collaboration between the University of Maryland, College Park, and the University of Maryland, Baltimore).
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Maron B.A., Abman S.H., Elliott C.G., et al. Pulmonary arterial hypertension: diagnosis, treatment, and novel advances. Am J Respir Crit Care Med. 2021;203:1472–1487. doi: 10.1164/rccm.202012-4317SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hediger M.A., Clémençon B., Burrier R.E., Bruford E.A. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med. 2013;34:95–107. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abumrad N., Harmon C., Ibrahimi A. Membrane transport of long-chain fatty acids: evidence for a facilitated process. J Lipid Res. 1998;39:2309–2318. [PubMed] [Google Scholar]
- 4.Coburn C.T., Knapp F.F., Febbraio M., Beets A.L., Silverstein R.L., Abumrad N.A. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 5.Zhao L., Ashek A., Wang L., et al. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation. 2013;128:1214–1224. doi: 10.1161/CIRCULATIONAHA.113.004136. [DOI] [PubMed] [Google Scholar]
- 6.Ang S.O., Chen H., Hirota K., et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 7.Taraseviciute A., Voelkel N.F. Severe pulmonary hypertension in postmenopausal obese women. Eur J Med Res. 2006;11:198–202. [PubMed] [Google Scholar]
- 8.Poms A.D., Turner M., Farber H.W., Meltzer L.A., McGoon M.D. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest. 2013;144:169–176. doi: 10.1378/chest.11-3241. [DOI] [PubMed] [Google Scholar]
- 9.Frank R.C., Min J., Abdelghany M., et al. Obesity is associated with pulmonary hypertension and modifies outcomes. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemnes A.R., Luther J.M., Rhodes C.J., et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight. 2019;4(1) doi: 10.1172/jci.insight.123611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pi H., Xia L., Ralph D.D., et al. Metabolomic signatures associated with pulmonary arterial hypertension outcomes. Circ Res. 2023;132:254–266. doi: 10.1161/CIRCRESAHA.122.321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinhauser M.L., Olenchock B.A., O’Keefe J., et al. The circulating metabolome of human starvation. JCI Insight. 2018;3(16) doi: 10.1172/jci.insight.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhathli E., Julian T., Girach Z.U.A., et al. Mendelian randomization study with clinical follow-up links metabolites to risk and severity of pulmonary arterial hypertension. J Am Heart Assoc. 2024;13(6) doi: 10.1161/JAHA.123.032256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes C.J., Ghataorhe P., Wharton J., et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation. 2017;135:460–475. doi: 10.1161/CIRCULATIONAHA.116.024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlach B.D., Ampomah P.B., Yurdagul A., et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 2021;33(12):2445–2463.e8. doi: 10.1016/j.cmet.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez-Ortiz Z.G., Pendergraft W.F., Prasad A., et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat Immunol. 2013;14:917–926. doi: 10.1038/ni.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty S.E., Grijalva A., Xu X., Ables E., Nomani A., Ferrante A.W. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363:989–993. doi: 10.1126/science.aaw2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Commisso C., Davidson S.M., Soydaner-Azeloglu R.G., et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim B., Li J., Jang C., Arany Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017;36:2321–2333. doi: 10.15252/embj.201796436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deberardinis R.J., Sayed N., Ditsworth D., Thompson C.B. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A., El Kasmi K.C., Plecitá-Hlavatá L., et al. Hallmarks of pulmonary hypertension: mesenchymal and inflammatory cell metabolic reprogramming. Antioxid Redox Signal. 2018;28:230–250. doi: 10.1089/ars.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M., Riddle S., Zhang H., et al. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation. 2016;134:1105–1121. doi: 10.1161/CIRCULATIONAHA.116.023171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Saavedra D., Sanders L., Freeman S., et al. Stable isotope metabolomics of pulmonary artery smooth muscle and endothelial cells in pulmonary hypertension and with TGF-beta treatment. Sci Rep. 2020;10:413. doi: 10.1038/s41598-019-57200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsboom G., Wietholt C., Haney C.R., et al. Lung 1⁸F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:670–679. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thenappan T., Ormiston M.L., Ryan J.J., Archer S.L. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360 doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acharya A.P., Tang Y., Bertero T., et al. Simultaneous pharmacologic inhibition of yes-associated protein 1 and glutaminase 1 via inhaled poly(lactic-co-glycolic) acid-encapsulated microparticles improves pulmonary hypertension. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wertheim B.M., Wang R.S., Guillermier C., et al. Proline and glucose metabolic reprogramming supports vascular endothelial and medial biomass in pulmonary arterial hypertension. JCI Insight. 2023;8(4) doi: 10.1172/jci.insight.163932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinhauser M.L., Bailey A.P., Senyo S.E., et al. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature. 2012;481:516–519. doi: 10.1038/nature10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinhauser M.L., Lechene C.P. Quantitative imaging of subcellular metabolism with stable isotopes and multi-isotope imaging mass spectrometry. Semin Cell Dev Biol. 2013;24:661–667. doi: 10.1016/j.semcdb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillermier C., Fazeli P.K., Kim S., et al. Imaging mass spectrometry demonstrates age-related decline in human adipose plasticity. JCI Insight. 2017;2 doi: 10.1172/jci.insight.90349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H., Zhang C.H., Ammanamanchi N., et al. Control of cytokinesis by β-adrenergic receptors indicates an approach for regulating cardiomyocyte endowment. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aaw6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gyngard F., Trakimas L., Steinhauser M.L. High-fidelity quantification of cell cycle activity with multi-isotope imaging mass spectrometry. Methods Mol Biol. 2021;2158:257–268. doi: 10.1007/978-1-0716-0668-1_19. [DOI] [PubMed] [Google Scholar]
- 34.Narendra D.P., Guillermier C., Gyngard F., Huang X., Ward M.E., Steinhauser M.L. Coupling APEX labeling to imaging mass spectrometry of single organelles reveals heterogeneity in lysosomal protein turnover. J Cell Biol. 2020;219 doi: 10.1083/jcb.201901097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Guillermier C., De Raedt T., et al. Imaging mass spectrometry reveals tumor metabolic heterogeneity. iScience. 2020;23 doi: 10.1016/j.isci.2020.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S.M., Lun M., Wang M., et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20:1049–1058. doi: 10.1016/j.cmet.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yung L.M., Yang P., Joshi S., et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci Transl Med. 2020;12(543) doi: 10.1126/scitranslmed.aaz5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humbert M., McLaughlin V., Gibbs J.S.R., et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021;384:1204–1215. doi: 10.1056/NEJMoa2024277. [DOI] [PubMed] [Google Scholar]
- 39.Hoeper M.M., Badesch D.B., Ghofrani H.A., et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478–1490. doi: 10.1056/NEJMoa2213558. [DOI] [PubMed] [Google Scholar]
- 40.Schermuly R.T., Dony E., Ghofrani H.A., et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe K., Toba M., Alzoubi A., et al. Tyrosine kinase inhibitors are potent acute pulmonary vasodilators in rats. Am J Respir Cell Mol Biol. 2011;45:804–808. doi: 10.1165/rcmb.2010-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghofrani H.A., Seeger W., Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412–1413. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 43.Ghofrani H.A., Morrell N.W., Hoeper M.M., et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. 2010;182:1171–1177. doi: 10.1164/rccm.201001-0123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoeper M.M., Barst R.J., Bourge R.C., et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127:1128–1138. doi: 10.1161/CIRCULATIONAHA.112.000765. [DOI] [PubMed] [Google Scholar]
- 45.Radaelli F., Vener C., Ripamonti F., et al. Conjunctival hemorrhagic events associated with imatinib mesylate. Int J Hematol. 2007;86:390–393. doi: 10.1007/BF02983993. [DOI] [PubMed] [Google Scholar]
- 46.Breccia M., Gentilini F., Cannella L., et al. Ocular side effects in chronic myeloid leukemia patients treated with imatinib. Leuk Res. 2008;32:1022–1025. doi: 10.1016/j.leukres.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Humbert M., McLaughlin V., Gibbs J.S.R., et al. Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur Respir J. 2023;61 doi: 10.1183/13993003.01347-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMurtry M.S., Bonnet S., Wu X., et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 49.Michelakis E.D., Gurtu V., Webster L., et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med. 2017;9(413) doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 50.Egnatchik R.A., Brittain E.L., Shah A.T., et al. Dysfunctional BMPR2 signaling drives an abnormal endothelial requirement for glutamine in pulmonary arterial hypertension. Pulm Circ. 2017;7:186–199. doi: 10.1086/690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertero T., Oldham W.M., Cottrill K.A., et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harding J.J., Telli M., Munster P., et al. A phase I dose-escalation and expansion study of telaglenastat in patients with advanced or metastatic solid tumors. Clin Cancer Res. 2021;27:4994–5003. doi: 10.1158/1078-0432.CCR-21-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bittencourt M.S., Cerci R.J. Statin effects on atherosclerotic plaques: regression or healing? BMC Med. 2015;13:260. doi: 10.1186/s12916-015-0499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dieffenbach P.B., Mallarino Haeger C., Rehman R., et al. A novel protective role for matrix metalloproteinase-8 in the pulmonary vasculature. Am J Respir Crit Care Med. 2021;204:1433–1451. doi: 10.1164/rccm.202108-1863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C.N., Hajji N., Yeh F.C., et al. Restoration of Foxp3. Am J Respir Crit Care Med. 2023;208:879–895. doi: 10.1164/rccm.202301-0181OC. [DOI] [PubMed] [Google Scholar]
- 56.Zawia A., Arnold N.D., West L., et al. Altered macrophage polarization induces experimental pulmonary hypertension and is observed in patients with pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2021;41:430–445. doi: 10.1161/ATVBAHA.120.314639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vergadi E., Chang M.S., Lee C., et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Towers C.G., Wodetzki D., Thorburn A. Autophagy and cancer: modulation of cell death pathways and cancer cell adaptations. J Cell Biol. 2020;219(1) doi: 10.1083/jcb.201909033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long L., Yang X., Southwood M., et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 2013;112:1159–1170. doi: 10.1161/CIRCRESAHA.111.300483. [DOI] [PubMed] [Google Scholar]
- 60.Lee S.J., Smith A., Guo L., et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:649–658. doi: 10.1164/rccm.201005-0746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farha S., Comhair S., Hou Y., et al. Metabolic endophenotype associated with right ventricular glucose uptake in pulmonary hypertension. Pulm Circ. 2021;11 doi: 10.1177/20458940211054325. [DOI] [PMC free article] [PubMed] [Google Scholar]