Abstract
Background
Depression is common in persons with dementia and is often under‐detected and under‐treated. It is critical to understand which available tools accurately detect depression in the context of dementia.
Methods
We updated our systematic review completed in 2015. The search strategy of our original review was replicated in Medline, Embase, and PsycINFO. Studies describing the use of a tool to identify depression in persons with dementia, compared to a criterion standard, and reporting diagnostic accuracy outcomes were included in the review update. Pooled prevalence estimates of major depression and pooled estimates of diagnostic accuracy outcomes (i.e., sensitivity [SN], specificity [SP]) for tools were calculated.
Results
Three studies were included of the 8980 returned from the database search and were added to the prior 20 articles from the 2015 review. The Cornell Scale for Depression in Dementia (CSDD), Geriatric Depression Scale (GDS)−15 item, Neuropsychiatric Inventory‐Depression items (NPI‐D), and Depression in Old Age Scale (DIA‐S) were evaluated in the three studies. Two new studies were added to the existing pooled prevalence estimate of major depression (29%, 95% confidence interval [CI] = 21.6%–36.5%, n = 17) and pooled diagnostic accuracy estimate for the CSDD at the best cut‐off (SN = 0.83, 95% CI = 0.74–0.90; SP = 0.81, 95% CI = 0.69–0.89). New pooled diagnostic accuracy estimates were completed for the CSDD (cut‐off ≥12) (SN = 0.61, 95% CI = 0.42–0.77; SP = 0.83, 95% CI = 0.76–0.88), GDS‐15 (best cut‐off) (SN = 0.65, 95% CI = 0.40–0.83; SP = 0.72, 95% CI = 0.55–0.85), and Montgomery Asberg Depression Rating Scale (MADRS) (best cut‐off) (SN = 0.77, 95% CI = 0.67–0.85; SP = 0.68, 95% CI = 0.60‐0.75).
Conclusions
The CSDD continues to have the most evidence for depression case finding in persons living with dementia. The CSDD and Hamilton Depression Rating Scale have the highest sensitivities and may be recommended for use over other common tools like the GDS‐15 and MADRS. Newly identified tools like the NPI‐D and DIA‐S require further study before they can be recommended for use in practice.
Keywords: dementia, depression, detection, systematic review
Key points
We updated our existing systematic review identifying tools to detect depression in persons with dementia compared to a criterion standard.
The Cornell Scale for Depression in Dementia continues to have the most evidence and adequate sensitivity and specificity for use in identifying depression in the context of dementia.
Two tools, the NPI‐D and DIA‐S, not previously identified in the original systematic review were identified and had adequate sensitivity in detecting depression in the context of depression.
It is critical for clinicians to understand which tools accurately detect depression in the context of dementia so that depression can be diagnosed, treatment can be initiated, and care can be improved for persons canwith dementia.
1. INTRODUCTION
Depression commonly occurs in persons with dementia and is often under‐detected. 1 A total of 37%–41% of persons with dementia experience depressive symptoms. 2 Depression is common across clinical settings for persons with dementia including long‐term care. 3 , 4 Although depression is a common co‐morbidity in persons with dementia it also appears to increase the risk of dementia in those without dementia (odds ratio: 2.64 [95% confidence interval, CI: 2.43, 2.86]) representing a possible risk factor or prodrome for dementia. 5
Depression is challenging to detect in persons with dementia, due to overlapping symptoms between depression, bereavement, dementia or other behaviors (e.g., apathy). 6 Severity of cognitive impairment impacts detection of depression, as communication, recall, and insight can vary or be impaired. 7 Use of accurate tools can facilitate detection by aligning questions with clinical criterion, involving care‐partners, and using consistent questions. Depression can be insidious, and can present subtly with changes in appetite, energy, or isolation. 6 Given the challenges with discerning depressive symptoms, the use of tools is pivotal to ensure accurate detection and thus appropriate management. There is a need to identify accurate and easy to use tools to detect depression in persons with dementia to improve care for this population.
The original systematic review 7 , 8 completed on May 27, 2015, sought to evaluate the diagnostic accuracy of screening tools for depression in persons with dementia. Twenty studies that evaluated eight unique depression screening tools were identified. The Cornell Scale for Depression in Dementia (CSDD), Geriatric Depression Scale (GDS)‐30 item, Hamilton Depression Rating Scale (HDRS), GDS‐15 item, and Montgomery Asberg Depression Rating Scale (MADRS) were the most commonly studied tools. Criterion standard assessments of depression were completed based on the Diagnostic and Statistical Manual of Mental Disorders (DSM) (versions III, III‐R, IV), International Classification of Diseases (ICD) 10th Revision, Research Diagnostic Criteria (RDC), or Provisional Diagnostic Criteria for Depression in Alzheimer's Disease (PDC‐dAD). The pooled prevalence estimate for major depression was calculated (30.3%, 95% CI = 22.1%–38.5%) and pooled estimates of diagnostic accuracy outcomes were completed at the best cut‐offs for the CSDD (sensitivity [SN] = 0.84, 95% CI = 0.73–0.91; specificity [SP] = 0.80, 95% CI = 0.65–0.90), GDS‐30 (SN = 0.62, 95% CI = 0.45–0.76; SP = 0.81, 95% CI = 0.75–0.85), and HDRS (SN = 0.86, 95% CI = 0.63–0.96; SP = 0.84, 95% CI = 0.76–0.90). 7
This review was updated to ensure we had the best possible evidence to inform clinical implementation and clinical practice guidelines as well as ensure the best evidence is available for accurate identifications of patients with depression in the setting of dementia as compared to a criterion standard. 9
2. METHODS
The search strategy, inclusion criteria, data extraction, and review procedures detailed in the original review publication were replicated for the review update. 7 The original review search strategy using four search concepts (dementia, depression, older adult, diagnostic accuracy) was used to search MEDLINE, Embase, and PsycINFO on June 15, 2023. Two independent reviewers screened eligible studies, completed risk of bias assessments, and extracted data from included studies.
The active machine learning feature in Covidence 10 was used to display the most relevant articles at the level of title/abstract review. Articles that described the diagnostic accuracy of a tool to identify index cases of depression in persons with dementia were included at the level of title/abstract review. Diagnostic accuracy studies comparing depression tools with a criterion standard (e.g., Diagnostic and Statistical Manual of Mental Disorders) in outpatients with dementia were included at the level of full text review.
Risk of bias for included studies was assessed using the Quality Assessment for Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool. 11 The following data were extracted from included studies: participant characteristics, setting characteristics, depression prevalence and assessment information, depression tool characteristics, criterion standard characteristics, and diagnostic accuracy outcomes (sensitivity, specificity, likelihood ratios).
The methods for deriving the pooled prevalence and diagnostic accuracy outcomes described in the original review publication were replicated to update existing diagnostic accuracy estimates and new estimates with four or more studies. 7 , 12 , 13 , 14 Analyzes were done across the individual studies best reported cut‐off, meaning the cut‐off reported by each article with the highest sensitivity and specificity. Where able we also did analyzes comparing sensitivity and specificity at the same cut‐off. Diagnostic accuracy estimates were completed for comparisons when there were three or more studies. To replicate the modeling completed in the original review, we estimated pooled prevalence using a Mantel‐Haenszel weighted DerSimonian and Laird model with the midas command in Stata. 12 , 15 Meta‐analyzes of diagnostic accuracy outcomes were estimated using a bivariate random‐effects model. 15 , 16 Forest plots were produced to graphically display the diagnostic accuracy results. Between study heterogeneity was assessed using the I 2 statistic and p‐value of Cochran's Q‐statistic 13 for pooled prevalence. The metadta command in Stata, using a bivariate random‐effects model, was used to estimate pooled diagnostic accuracy outcomes with three studies. 16 In pooled diagnostic accuracy estimates with three studies, the I 2 statistic 17 was used to assess between study heterogeneity. All analyzes were completed using Stata version 17.0. 18 This study is reported as per the PRISMA‐DTA statement. 19 Ethical approval was not required due to the nature of the study.
3. RESULTS
A total of 8980 studies were retrieved from the databases searched. A total of 374 articles of the 6780 screened at the level of title/abstract were reviewed in full text. Three articles met the review inclusion criteria (Figure S1). Agreement between reviewers was 95.6% at the level of title/abstract review and 93.3% at the level of full text review.
3.1. New studies added to review
Four depression screening tools, the CSDD, GDS‐15, Neuropsychiatric Inventory‐Depression items (NPI‐D), and Depression in Old Age Sale (DIA‐S) were identified in the three newly identified studies. One study recruited 46 participants with dementia from outpatient clinics (median age: 66.5, 73.9% female, mean Mini Mental Status Exam [MMSE] score of 17.6, 69.6% Alzheimer's disease) and looked only at the CSDD. 20 The second study evaluated the CSDD, GDS‐15, and NPI‐D in a sample of 136 participants (mean age: 76.7, 66.9% female, mean MMSE score of 11.5, 66.2% Alzheimer's disease) with dementia recruited from outpatient clinics. 21 The third study evaluated the GDS‐15 and DIA‐S but did not describe the characteristics of the 148 participants from the dementia sub‐group of the study sample 22 (Table 1 and Table S1). All studies used the DSM as the criterion standard to assess for major depression. The prevalence of major depression in the two reporting study samples was 21.7% 20 and 18.4%. 21
Table 1.
Index tool and criterion standard descriptions, evaluation locations, rater descriptions, and diagnostic accuracy outcomes (i.e., sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, area under the curve) of index tool compared to criterion standard at all reported cut‐offs.
| Study (first author, year, country) | Index test score [mean (SD)] | Is the index tool self‐ or clinician‐rated? | Index tool | Reference standard | Cut‐off | SN | SP | LR+ | LR− | AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| Huynh, 2022, Vietnam 20 | 7.0 (2.0–11.0) [median (IQR)] | NR | CSDD‐Vietnamese Version | DSM‐5 | ≥8 | 0.90 | 0.69 | 2.90 | 0.14 | 0.86 |
| ≥12 | 0.70 | 0.89 | 6.36 | 0.34 | ||||||
| ≥13 | 0.70 | 0.92 | 8.75 | 0.33 | ||||||
| ≥14 | 0.50 | 0.97 | 16.67 | 0.52 | ||||||
| ≥17 | 0.40 | 1.00 | – | 0.60 | ||||||
| Mougias, 2017, Greece, a , 21 | 4.9 (4.0) | Self‐rated | GDS‐15 | DSM‐IV | ≥7 | 0.77 | 0.87 | 5.77 | 0.26 | 0.87 |
| 5.1 (4.6) | Clinician‐rated | CSDD | DSM‐IV | ≥6 | 0.88 | 0.79 | 4.25 | 0.15 | 0.92 | |
| 2.4 (3.5) | Caregiver‐rated | NPI‐D | DSM‐IV | ≥0 | 0.88 | 0.68 | 2.72 | 0.18 | 0.81 | |
| Wunner, 2022, Germany 22 | NR | Self‐rated | GDS‐15 | DSM‐5 | ≥5 | 0.73 | 0.74 | 2.8 | 0.4 | 0.77 |
| NR | Self‐rated | DIA‐S | DSM‐5 | ≥3 | 0.86 | 0.63 | 2.3 | 0.2 | 0.83 | |
| ≥4 | 0.68 | 0.72 | 2.4 | 0.4 |
Note: Bold indicates the author identified optimal cut‐off.
Abbreviations: AUC, area under the curve; CSDD, Cornell Scale for Depression in Dementia; DIA‐S, Depression in Old Age Scale; DSM‐IV, Diagnostic and Statistical Manual of Mental Disorders‐Fourth Edition; DSM‐5, Diagnostic and Statistical Manual of Mental Disorders‐Fifth Edition; GDS‐15, Geriatric Depression Scale‐15 item; IQR, interquartile range; NPI‐D, Neuropsychiatric Inventory‐Depression Items; NR, not reported; SD, standard deviation; SN, sensitivity; SP, specificity.
Mougias, 2017 did not clearly state which version of the GDS was being evaluated in the study. Review authors emailed the study authors, but a response was not received. Based on the version of the GDS cited by Mougias, 2017 and the cut‐off reported, reviewers inferred that the study authors were evaluating the 15‐item version of the GDS.
The diagnostic accuracy outcomes for the CSDD from the two reporting studies 20 , 21 were added to existing pooled estimates. Diagnostic accuracy outcomes for the GDS‐15 could not be pooled with existing review evidence because not all study participants completed the GDS‐15 assessment (n = 104/136) 21 and the prevalence of depression was not reported for the dementia sub‐group of the study sample. 22 Reviewers were unable to back‐calculate the true positive, true negative, false positive, and false negative values from the sensitivity, specificity, and prevalence reported.
The NPI and DIA‐S for depression screening in dementia were not identified in the original review.
Neuropsychiatric Inventory: The NPI is a caregiver‐rated tool that rates thefrequency, severity, and distress of 10 behavioral areas. 23 At a cut‐off of ≥0, the NPI had an SN of 0.88, SP of 0.68, and area under the curve (AUC) of 0.81 (Table 1).
Depression in Old Age Scale: The DIA‐S is a self‐rated depression screening tool developed for use with clinical older adult populations. 23 At a cut‐off of ≥3, the DIA‐S had an SN of 0.86, SP of 0.63, and AUC of 0.83.
3.2. Risk of bias
The three included studies had a low risk of bias related to the applicability of the study to the review question (Table S2). Bias resulted from not enrolling a consecutive or random sample of participants, 20 , 22 lack of blinding of the index test rater to the criterion standard results, 21 , 22 not pre‐specifying a threshold cut‐off, 20 , 21 , 22 lack of blinding for the criterion standard rater to the index test results, 21 , 22 and not specifying the time between administering the index and criterion standard tests. 21 , 22
3.3. Pooled depression prevalence estimate
The pooled depression prevalence estimate was completed with 15 studies with unique study populations from the original review and the two 20 , 21 newly identified studies reporting prevalence estimates from the review update. The pooled prevalence for depression was found to be 29% ([n = 17], 95% CI = 21.6%–36.5%, I 2 = 96.7%, p < 0.001).
3.4. Pooled diagnostic accuracy estimates
The results of the updated and newly completed pooled diagnostic accuracy estimates for the CSDD, GDS‐15, and MADRS are displayed in Table 2.
Table 2.
Meta‐analysis findings for updated and new comparisons completed for the CSDD, GDS‐15, and MADRS.
| Tool, cut‐off | Study IDa (first author, year) | Total sample size (n) | Total depression (n) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| CSDD, Best reported (n = 12) | |||||
| da Gloria Portugal et al., 2011 | 71 | 51 | 0.80 | 0.65 | |
| Huynh et al., 2022 | 46 | 10 | 0.70 | 0.92 | |
| Jeon et al., 2014 | 46 | 13 | 0.69 | 0.58 | |
| Knapskog et al., 2011 | 55 | 13 | 0.92 | 0.52 | |
| Korner et al., 2006 | 51 | 38 | 0.95 | 0.92 | |
| Leontjevas et al., 2012 | 101 | 18 | 0.94 | 0.49 | |
| Lim et al., 2012 | 121 | 59 | 0.87 | 0.98 | |
| Maixner et al., 1995 | 115 | 23 | 0.65 | 0.85 | |
| Mougias et al., 2017 | 136 | 25 | 0.88 | 0.79 | |
| Porta‐Etessam et al., 2011 | 1239 | 67 | 0.57 | 0.83 | |
| Vida et al., 1993 | 34 | 10 | 0.90 | 0.75 | |
| Wongpakaran et al., 2013 | 35 | 13 | 0.92 | 0.95 | |
| Pooled estimate: Sensitivity = 0.83 (95% CI = 0.74–0.90, I 2 = 71.71%, p < 0.001), Specificity = 0.81 (95% CI = 0.69–0.89, I 2 = 91.14%, p < 0.001) | |||||
| CSDD, ≥6 (n = 5) | |||||
| Knapskog et al., 2011 | 55 | 13 | 0.85 | 0.59 | |
| Leontjevas et al., 2012 | 101 | 18 | 1.00 | 0.43 | |
| Lim et al., 2012 | 121 | 59 | 0.91 | 0.96 | |
| Mougias et al., 2017 | 136 | 25 | 0.88 | 0.79 | |
| Vida et al., 1993 | 34 | 10 | 0.90 | 0.67 | |
| Pooled estimate: Sensitivity = 0.90 (95% CI = 0.82–0.95, I 2 = 0%, p = 0.89), Specificity = 0.74 (95% CI = 0.50–0.89, I 2 = 93.17%, p < 0.001) | |||||
| CSDD, ≥8 (n = 5) | |||||
| Huynh et al., 2022 | 46 | 10 | 0.90 | 0.69 | |
| Knapskog et al., 2011 | 55 | 13 | 0.62 | 0.74 | |
| Leontjevas et al., 2012 | 101 | 18 | 0.83 | 0.58 | |
| Lim et al., 2012 | 121 | 59 | 0.82 | 1.00 | |
| Vida et al., 1993 | 34 | 10 | 0.80 | 0.83 | |
| Pooled estimate: Sensitivity = 0.79 (95% CI = 0.69–0.87, I 2 = 0%, p = 0.44), Specificity = 0.81 (95% CI = 0.60–0.93, I 2 = 89.12%, p < 0.001) | |||||
| CSDD, ≥12 (n = 4) | |||||
| da Gloria Portugal et al., 2011 | 71 | 51 | 0.78 | 0.69 | |
| Huynh et al., 2022 | 46 | 10 | 0.70 | 0.89 | |
| Porta‐Etessam et al., 2011 | 1239 | 67 | 0.56 | 0.83 | |
| Vida et al., 1993 | 34 | 10 | 0.40 | 0.96 | |
| Pooled estimate: Sensitivity = 0.61 (95% CI = 0.42–0.77, I 2 = 66.10%, p = 0.03), Specificity = 0.83 (95% CI = 0.76–0.88, I 2 = 50.83%, p = 0.11) | |||||
| GDS‐15, Best reported (n = 3) | |||||
| Burke et al., 1991 | 72 | 10 | 0.60 | 0.63 | |
| Korner et al., 2006 | 47 | 36 | 0.81 | 0.73 | |
| Li et al., 2015 | 45 | 13 | 0.38 | 0.88 | |
| Pooled estimate: Sensitivity = 0.65 (95% CI = 0.40–0.83, I 2 = 58.47%), Specificity = 0.72 (95% CI = 0.55–0.85, I 2 = 52.54%) | |||||
| MADRS, Best reported (n = 3) | |||||
| da Gloria Portugal et al., 2011 | 71 | 51 | 0.75 | 0.75 | |
| Knapskog et al., 2011 | 55 | 13 | 0.85 | 0.67 | |
| Leontjevas et al., 2012 | 101 | 18 | 0.78 | 0.66 | |
| Pooled estimate: Sensitivity = 0.77 (95% CI = 0.67–0.85, I 2 = 0%), Specificity = 0.68 (95% CI = 0.60–0.75, I 2 = 0%) | |||||
Abbreviations: CI, confidence interval; CSDD, Cornell Scale for Depression in Dementia; GDS, Geriatric Depression Scale‐15 item; MADRS, Montgomery Asberg Depression Rating Scale; n, number of participants.
Complete references available for each study available in Supporting Information Material.
Cornell Scale for Depression in Dementia: Two of the new studies 20 , 21 were added to the existing pooled estimate for the CSDD best reported cut‐off ([n = 12] SN = 0.83 [95% CI = 0.74–0.90, I 2 = 71.71%, p < 0.001]; SP = 0.81 [95% CI = 0.69–0.89, I 2 = 91.14%, p < 0.001]; AUC = 0.89 [95% CI = 0.86–0.92]) (Figure 1). Updates to the existing pooled estimates for the CSDD at cut‐offs of ≥6 ([n = 5 21 , 24 , 25 , 26 , 27 ] SN = 0.90 [95% CI = 0.82–0.95, I 2 = 0.00, p = 0.89]; SP = 0.74 [95% CI = 0.50‐0.89, I 2 = 93.17, p < 0.001]) and ≥8 ([n = 5 20 , 24 , 25 , 26 , 27 ] SN = 0.79 [95% CI = 0.69–0.87, I 2 = 0%, p = 0.44]; SP = 0.81 [95% CI = 0.60–0.93, I 2 = 89.12, p < 0.001]) were completed. A new pooled estimate was completed for the CSDD at the ≥12 cut‐off ([n = 4 20 , 27 , 28 , 29 ] SN = 0.61 [95% CI = 0.42–0.77, I 2 = 66.10%, p = 0.03]; SP = 0.83 [95% CI = 0.76–0.88, I 2 = 50.83%, p = 0.11]).
Figure 1.
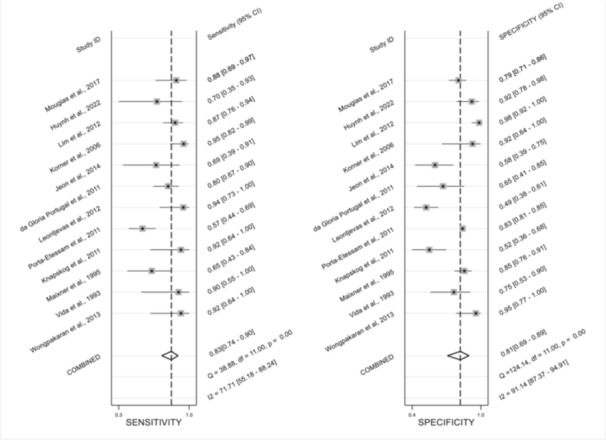
Forest plots of the pooled sensitivity and specificity for the CSDD at the best cut‐off (n = 12).
Geriatric Depression Scale: Three studies 30 , 31 , 32 identified in the original review were pooled in an estimate for the GDS‐15 best cut‐off (SN = 0.65 [95% CI = 0.40–0.83, I 2 = 58.47%]; SP = 0.72 [95% CI = 0.55–0.85, I 2 = 52.54%]). Three studies 24 , 25 , 28 identified in the original review were pooled in an estimate for the MADRS best cut‐off (SN = 0.77 [95% CI = 0.67–0.85, I 2 = 0%]; SP = 0.68 [95% CI = 0.60–0.75, I 2 = 0%]).
4. DISCUSSION
The pooled diagnostic accuracy estimates for the CSDD were updated from the original review publication to include data from two newly identified studies and pooled diagnostic accuracy estimates for the CSDD at a new cut‐off was added. Pooled estimates for the GDS‐15 and MADRS were not completed in the original review because three studies were considered inadequate to complete comparisons with meta‐analytic methods available in Stata at that time. The new Stata command, metadta, fits different models based on the number of studies thus enabling further comparisons. 33
The CSDD was created to assess major depression in persons with dementia and includes both patient and informant interview components. 34 Of the estimates pooled for specific cut‐offs for the CSDD, the updated pooled estimate at the ≥6 had the highest sensitivity of the cut‐off specific pooled estimates (SN = 0.90) and maintained the lowest specificity (SP = 0.74) with no significant heterogeneity. Given this, the cut‐off of ≥6 would be considered best for clinical practice with a high sensitivity indicating a low risk of false negatives.
The CSDD cut‐off of ≥12 had the highest specificity (SP = 0.83) for CSDD cut‐off specific pooled estimates compared previously to a cut‐off of ≥8. The GDS‐15 (SN = 0.65) and MADRS (SN = 0.77) both had lower sensitivities compared to the CSDD (SN = 0.83) at the best cut‐off. In the setting of case finding, depression tools with a higher sensitivity may be favored.
In updated CSDD estimates, heterogeneity was not identified for cut‐off specific sensitivity estimates at ≥6 and ≥8. Significant heterogeneity was identified in the new pooled sensitivity estimate for the ≥12 cut‐off. A moderate degree of heterogeneity was identified in both the pooled sensitivity and specificity estimates for the GDS‐15 at the best cut‐off, while the analysis failed to identify heterogeneity in the pooled sensitivity and specificity estimates for the MADRS at the best cut‐off. There was insufficient evidence to complete cut‐off specific analyzes for the GDS‐15 and MADRS. Heterogeneity in pooled diagnostic accuracy estimates of best cut‐offs could likely be attributed to the use of different cut‐offs in the comparison. Differences in the study populations including dementia type and severity could not readily be explored due to a lack of reporting on participant characteristics in studies.
Two new tools, the NPI‐depression items and DIA‐S, were identified in the review update. Both the NPI‐depression items (cut‐off ≥0, SN = 0.88) and DIA‐S (cut‐off ≥3, SN = 0.86) had reasonable sensitivities for identifying depression in persons with dementia. The NPI may be used to assess solely depression in persons with dementia or assess depression as part of global neuropsychiatric symptom assessments. 23 The DIA‐S is intended for use across healthcare settings and is easy to use and interpret. 35
A lack of reporting of depression prevalence for study samples in two newly identified articles 21 , 22 prohibited the inclusion of the GDS‐15 findings in pooled diagnostic accuracy estimates. It is possible that relevant publications may have been missed in the review update despite the use of the comprehensive search strategy detailed in the original review publication.
The CSDD was designed specifically for depression in persons with dementia. At the cut‐off of ≥6 there is a high sensitivity with no discernable heterogeneity across five studies, indicating that this tool is ideal for use to detect depressive symptoms for persons with dementia. The CSDD tool uses an interview with the person with dementia and their care‐partner; it is likely this combination plus the tools focus on the components of criterion for depression leads to high accuracy. The current Canadian guidelines for behaviors and psychological symptoms in persons with dementia recommend the use of this tool. 9
5. CONCLUSION
The review update identified three new studies comparing four depression tools to a criterion standard in persons with dementia. Two more tools, the NPI and DIA‐S were evaluated and had adequate sensitivity for depression case finding in dementia. Diagnostic accuracy analyzes were updated to include the newly identified evidence and additional analyzes were completed for the GDS‐15 and MADRS using meta‐analytic methods not previously available. The CSDD has a high sensitivity and adequate specificity for depression case finding in persons with dementia and continues to have the most evidence. Recent guidelines 9 have been made to reflect these findings recommending the use of the CSDD.
AUTHOR CONTRIBUTIONS
Kayla Atchison: Data curation; formal analysis; methodology; project administration; writing—original draft; writing—review and editing. Alaia Nazir: Project administration; writing—review and editing. Pauline Wu: Data curation; project administration; writing—review and editing. Dallas Seitz: Conceptualization; formal analysis; writing—review and editing. Jennifer A Watt: Conceptualization; formal analysis; writing—review and editing. Zahra Goodarzi: Conceptualization; data curation; formal analysis; methodology; project administration; supervision; writing—review and editing. All authors have read and approved the final version of the manuscript Dr. Goodarzi had full access to all the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Zahra Goodarzi, Zahra Goodarzi affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This project was funded by the Canadian Institutes of Health Research, Project Number 10031338. Kayla Atchison had some hours reimbursed by the Public Health Association of Canada.
Atchison K, Nazir A, Wu P, Seitz D, Watt JA, Goodarzi Z. Depression detection in dementia: A diagnostic accuracy systematic review and meta analysis update. Health Sci Rep. 2024;7:e70058. 10.1002/hsr2.70058
Impact statement: We certify that this work is confirmatory of recent novel clinical research by updating and adding to the following relevant research: “Goodarzi ZS, Mele BS, Roberts DJ, Holroyd‐Leduc J. Depression case finding in individuals with dementia: a systematic review and meta‐analysis. Journal of the American Geriatrics Society. 2017 May;65(5):937‐48.” The present research adds updated pooled diagnostic accuracy estimates for the Cornell Scale for Depression in Dementia at the best cut‐off, ≥6, and ≥8. New pooled diagnostic accuracy estimates were completed for the Cornell Scale for Depression in Dementia at the ≥12 cut‐off, the Geriatric Depression Scale‐15 item (best cut‐off), and the Montgomery Asberg Depression Rating Scale (best cut‐off) which provides a better understanding of the clinical utility of these tools. Two tools, the Neuropsychiatric Inventory‐depression items and Depression in Old Age Scale, not previously identified as being validated using a criterion standard were identified and showed adequate sensitivity to identify depression in the context of dementia. The Cornell Scale for Depression in Dementia has the most evidence and highest sensitivity with adequate specificity and, therefore, is supported for use.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information Materials.
REFERENCES
- 1. Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry. 2011;24:461‐472. [DOI] [PubMed] [Google Scholar]
- 2. Leung DKY, Chan WC, Spector A, Wong GHY. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2021;36:1330‐1344. [DOI] [PubMed] [Google Scholar]
- 3. Hoben M, Heninger A, Holroyd‐Leduc J, Knopp‐Sihota J, Estabrooks C, Goodarzi Z. Depressive symptoms in long term care facilities in Western Canada: a cross sectional study. BMC Geriatr. 2019;19:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canadian Institute for Health Information . Analysis in Brief: Depression Among Seniors in Residential Care [online]. Accessed July 30. Available at: https://secure.cihi.ca/free_products/ccrs_depression_among_seniors_e.pdf
- 5. Snowden MB, Atkins DC, Steinman LE, et al. Longitudinal association of dementia and depression. Am J Geriatr Psychiatry. 2015;23:897‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watt JA, Goodarzi Z, Veroniki AA, et al. Comparative efficacy of interventions for reducing symptoms of depression in people with dementia: systematic review and network meta‐analysis. BMJ. 2021;372:n532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goodarzi ZS, Mele BS, Roberts DJ, Holroyd‐Leduc J. Depression case finding in individuals with dementia: a systematic review and meta‐analysis. J Am Geriatr Soc. 2017;65:937‐948. [DOI] [PubMed] [Google Scholar]
- 8. Erratum. J Am Geriatr Soc. 2018;66:1441. [DOI] [PubMed] [Google Scholar]
- 9. Canadian Coalition for Seniors' Mental Health . Canadian Clinical Practice Guidelines for Assessing and Managing Behavioural and Psychological Symptoms of Dementia (BPSD). 2024. Available at: https://ccsmh.ca/wp-content/uploads/2024/05/DIGITAL_CCSMH_BPSD-Clinical-Guidelines_May2024_ENG.pdf.
- 10. Covidence systematic review software [computer program]. Veritas Health Innovation. Melbourne, Australia. Available at: www.covidence.org [Google Scholar]
- 11. Whiting PF. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529‐536. [DOI] [PubMed] [Google Scholar]
- 12. DerSimonian R. Meta‐analysis in the design and monitoring of clinical trials. Stat Med. 1996;15:1237‐1248. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JP, Thomas J, Chandler J, et al. (Eds.). Cochrane Handbook for Systematic Reviews of Interventions, Version 6.5. Cochrane. [online]. Accessed October 23, 2016. Available at: http://handbook.cochrane.org, 2024.
- 15. Dwamena B. MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components S456880, Boston College Department of Economics, 2007.
- 16. Nyaga VN. METADTA: Stata module to perform fixed‐ and random‐effects meta‐analysis and meta‐regression of diagnostic accuracy studies. Statistical Software Components S458794, Boston College Department of Economics, 2020.
- 17. Zhou Y, Dendukuri N. Statistics for quantifying heterogeneity in univariate and bivariate meta‐analyses of binary data: the case of meta‐analyses of diagnostic accuracy. Stat Med. 2014;33:2701‐2717. [DOI] [PubMed] [Google Scholar]
- 18.StataCorp. Stata Statistical Software: Release 17 [computer program]. Version 16 2019. StataCorp LLC. 2023.
- 19. McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta‐analysis of diagnostic test accuracy studies: the PRISMA‐DTA statement. JAMA. 2018;319:388‐396. [DOI] [PubMed] [Google Scholar]
- 20. Huynh TT, Nguyen NTT, Nguyen TDP, Tran TC. Vietnamese version of cornell scale for depression in dementia at an outpatient memory clinic: a reliability and validity study. Dement Geriatr Cogn Dis Extra. 2022;12:34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mougias AA, Politis A, Mougias MA, et al. Assessing depression in Greek dementia patients: which scale to use? Psychiatriki. 2017;28:203‐210. [DOI] [PubMed] [Google Scholar]
- 22. Wunner C, Stemmler M, Masuch J, Gosch M, Waller C, Singler K. Screening for depression in old age: a comparison of the geriatric depression scale and the depression in old age scale. Zeitschrift für Gerontologie und Geriatrie. 2022;55:44‐50. [DOI] [PubMed] [Google Scholar]
- 23. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308. [DOI] [PubMed] [Google Scholar]
- 24. Knapskog A‐B, Barca ML, Engedal K. A comparison of the validity of the cornell scale and the MADRS in detecting depression among memory clinic patients. Dementia Geriatr Cognit Disord. 2012;32:287‐294. [DOI] [PubMed] [Google Scholar]
- 25. Leontjevas R, Gerritsen DL, Vernooij‐Dassen MJFJ, Smalbrugge M, Koopmans RTCM. Comparative validation of proxy‐based montgomery‐asberg depression rating scale and cornell scale for depression in dementia in nursing home residents with dementia. Am J Geriatr Psychiatry. 2012;20:985‐993. [DOI] [PubMed] [Google Scholar]
- 26. Lim HK, Hong SC, Won WY, Hahn C, Lee CU. Reliability and validity of the Korean version of the cornell scale for depression in dementia. Psychiatry Investig. 2012;9:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vida S, Des Rosiers P, Carrier L, Gauthier S. Depression in Alzheimer's disease: receiver operating characteristic analysis of the cornell scale for depression in dementia and the hamilton depression scale. J Geriatr Psychiatry Neurol. 1994;7:159‐162. [DOI] [PubMed] [Google Scholar]
- 28. da Glória Portugal M, Coutinho ESF, Almeida C, et al. Validation of montgomery‐asberg rating scale and cHornell scale for depression in dementia in Brazilian elderly patients. Int Psychogeriatr. 2012;24:1291‐1298. [DOI] [PubMed] [Google Scholar]
- 29. Porta‐Etessam J, Tobaruela‐González JL, Rabes‐Berendes C. Depression in patients with moderate Alzheimer disease: a prospective observational cohort study. Alzheimer Dis Assoc Disord. 2011;25:317‐325. [DOI] [PubMed] [Google Scholar]
- 30. Burke KC. Comparing age at onset of major depression and other psychiatric disorders by birth cohorts in five US community populations. Arch Gen Psychiatry. 1991;48:789‐795. [DOI] [PubMed] [Google Scholar]
- 31. Kørner A, Lauritzen L, Abelskov K, et al. The geriatric depression scale and the cornell scale for depression in dementia. A validity study. Nord J Psychiatry. 2006;60:360‐364. [DOI] [PubMed] [Google Scholar]
- 32. Li Z, Jeon Y‐H, Low L‐F, et al. Validity of the geriatric depression scale and the collateral source version of the geriatric depression scale in nursing homes. Int Psychogeriatr. 2015;27:1495‐1504. [DOI] [PubMed] [Google Scholar]
- 33. Nyaga VN, Arbyn M. Metadta: a Stata command for meta‐analysis and meta‐regression of diagnostic test accuracy data–a tutorial. Arch Public Health. 2022;80:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry. 1988;23:271‐284. [DOI] [PubMed] [Google Scholar]
- 35. Heidenblut S, Zank S. Screening for depression with the depression in old age scale (DIA‐S) and the geriatric depression scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych. 2014;27:41‐49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information Materials.


