Abstract
Purpose
Polycystic ovary syndrome (PCOS) significantly affects women. This study investigated the association between serum anti‐Müllerian hormone (AMH) levels and menstrual cycle disorders, and AMH for PCOS in a general cohort of young Japanese women.
Methods
We measured serum AMH levels in 528 healthy female students at two universities in Japan between 2014 and 2020. We investigated the association between serum AMH levels and hormone levels, menstrual cycle, and body mass index.
Results
The mean (±standard deviation) AMH level was 4.78 ± 2.88 ng/mL. Correlations were observed between serum AMH and luteinizing hormone (LH) or LH/follicle‐stimulating hormone (FSH) levels in women with irregular menstruation (LH: r = 0.542, p < 0.001; LH/FSH: r = 0.584, p < 0.001). The optimal serum AMH cutoff value that predicted LH ≥7.1 IU/L and LH/FSH ≥1.21 (PCOS diagnostic criteria revised by Japan Society of Obstetrics and Gynecology) in women with menstrual irregularities was 5.30 ng/mL (area under the curve: 0.815, sensitivity: 84.2%, specificity: 70.3%).
Conclusions
Serum AMH can be measured during annual health checkups and may be a useful biomarker for early and arcuate diagnosis and intervention in women with PCOS.
Keywords: anti‐Müllerian hormone, menstrual irregularity, polycystic ovary syndrome, preconception care, puberty
This study shows the association between serum anti‐Müllerian hormone (AMH) levels and menstrual cycle disorders and AMH's usefulness for diagnosing PCOS in a general cohort of young Japanese women. Serum AMH can be measured during annual health checkups and may be a useful biomarker for early intervention in women with PCOS.
1. INTRODUCTION
Anti‐Müllerian hormone (AMH) is a widely used ovarian reserve marker. 1 , 2 , 3 AMH is secreted by the granulosa cells of small follicles, and data from adult women have shown that serum AMH levels are elevated in polycystic ovary syndrome (PCOS), 4 , 5 , 6 , 7 in which the number of small follicles increases. PCOS is one of the most prevalent endocrine disorders affecting women of reproductive age. In addition to causing infertility due to ovulation dysfunction, PCOS can lead to metabolic disturbances, such as insulin resistance. PCOS is also associated with an increased risk of endometrial cancer, perinatal complications, and depressive symptoms, posing a significant threat to women's health throughout their life stages. 8 , 9 Therefore, early management is essential, and there is a growing emphasis on diagnosing and managing PCOS in young women.
Considering the importance of career development for young women, preconception care is gaining significance. 10 Being aware of one's ovarian reserve and fertility before desiring pregnancy can provide valuable insights for life planning. Additionally, knowing the possibility of PCOS is crucial for overall health management, including prevention of the aforementioned conditions, irrespective of future pregnancy plans. Irregular menstruation, the main symptom of PCOS, is believed to have continued since puberty, but for several years after menarche, abnormal menstrual cycles are observed even in normal cohorts, making it difficult to differentiate girls with PCOS from those without. Moreover, the presence of polycystic ovaries and high androgen‐like symptoms, such as acne, which are also present in normal cohorts, further complicate the diagnosis of PCOS in young women. 11 , 12
In December 2023, the Japanese Society of Obstetrics and Gynecology (JSOG) revised the diagnostic criteria for PCOS, allowing AMH to replace the follicle count as an adjunct diagnosis to ovarian findings. However, few studies have focused on young women at a critical time in preconception care. 13 Therefore, in this study, we aimed to investigate the association between serum AMH levels and menstrual irregularities in young Japanese women and to use this information for PCOS diagnosis and subsequent health management.
2. MATERIALS AND METHODS
2.1. Subjects
Data on serum AMH levels, height, body weight, and presence or absence of irregular menstruation were corrected from women over the age of 20 years who were non‐medical students at two universities in Aichi, Japan and had taken a lecture about fertility from 2014 to 2020. Blood samples were collected on the same day as the lecture from students who gave consent, regardless of their menstrual cycle. Serum was separated from whole blood and stored at −80°C until assay analysis. The height and weight of the subjects were extracted from their health checkup results. Menstrual irregularities were assessed using a questionnaire during blood sampling.
2.2. Measurement of serum hormone levels
AMH levels were measured by chemiluminescent enzyme immunoassay (CLEIA) with LUMIPULSE®, estradiol (E2) and testosterone were measured by electrochemiluminescence immunoassay (ECLIA), and follicle‐stimulating hormone (FSH) and luteinizing hormone (LH) were measured by chemiluminescent immunoassay (CLIA), all of which are widely used clinically in Japan and outsourced to SRL, Inc. (Tokyo, Japan). If the serum AMH level was <2 ng/mL or >8 ng/mL, the students were recommended to go to a health care center or hospital for additional testing, and the basal hormone levels of FSH, LH, and testosterone were measured during menstruation. If the serum AMH level was between 2 and 8 ng/mL, the remaining serum was used to measure the serum E2 level. If the E2 level was less than 40 pg/mL, the same sample was used to measure LH, FSH, and testosterone levels, assuming that the date of blood collection was during the early follicular phase and used the hormone levels as basal hormone levels for later analysis.
2.3. Statistical analysis
IBM SPSS Statistics 29.0.1.0 (IBM, Armonk, NY) was used for statistical analysis. The χ2 test was used to examine the association between serum AMH levels and the prevalence of menstrual irregularities. The Mann–Whitney U test was used for two‐group comparisons, the Kruskal–Wallis test was used for three‐group comparison, and Spearman's rank correlation coefficient was used to evaluate the correlation between each basal hormone level and AMH levels. A receiver operating characteristics (ROC) curve was used to evaluate the effectiveness of serum AMH in PCOS diagnosis, and the shortest distance on the ROC curve between the optimal sensitivity and specificity ([1 − sensitivity] 2 + [1 − specificity] 2) was used to determine the optimal cutoff value. Statistical significance was defined as a p‐value <0.05.
2.4. Ethical considerations
This study was approved by the ethics committees of the universities and our institute (2013‐02634071). We obtained informed consent from all students.
3. RESULTS
3.1. Association between serum AMH levels and menstrual irregularities
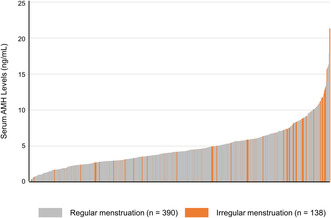
A total of 528 female students participated in the study, including 179 (33.9%) who had their basal hormone levels measured (Figure 1). The median age of the participants was 21 years (range: 20–27) (Table 1). The serum AMH level (mean ± standard deviation [SD]) was 4.78 ± 2.88 ng/mL, and 26.1% of the students presented with menstrual irregularities. The proportion of students with abnormal menstruation was 49.2% in the group with AMH levels ≥8 ng/mL, which was significantly higher than in the group with AMH < 2 ng/mL (22.4%, p < 0.01) or 2 ng/mL to <8 ng/mL (22.9%, p < 0.01) (Table 1 and Figure 2). The serum AMH levels did not differ significantly among the three groups: lean, standard, and obese (Figure 3). The serum AMH levels were significantly higher in the irregular menstruation group than in the regular menstruation group (p < 0.01), but age, height, weight, and body mass index (BMI) were not significantly different between the two groups (Table 2). An investigation into the parameters related to AMH values and PCOS was conducted due to the observation that students with irregular menstrual cycles had higher AMH values than those with regular menstrual cycles.
FIGURE 1.
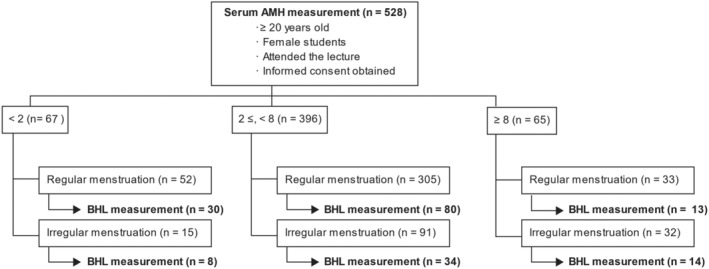
Inclusion criteria and subjects. AMH, Anti‐Müllerian hormone; BHL, Basal hormone levels.
TABLE 1.
Characteristics of subjects.
| Subjects, n | 528 |
| Age, median (range) (years) | 21 (20–27) |
| Irregular menstruation, n (%) | 138 (26.1) |
| Serum AMH, mean ± SD (ng/mL) | 4.78 ± 2.88 |
| < 2.0 ng/mL, n (%) | 67 (12.7) |
| Irregular menstruation, n (%) | 15 (22.4)a |
| ≥ 2.0, <8.0 ng/mL, n (%) | 396 (75.0) |
| Irregular menstruation, n (%) | 91 (22.9)a |
| ≥ 8.0 ng/mL, n (%) | 65 (12.3) |
| Irregular menstruation, n (%) | 32 (49.2)b |
Note: There was a statistical significance between a and b at p < 0.01 by χ 2 test.
Abbreviations: AMH, anti‐Müllerian hormone; SD, standard deviation.
FIGURE 2.
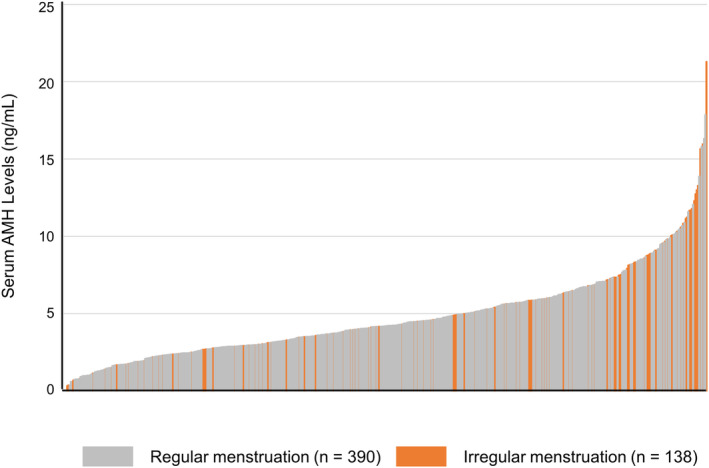
Graph showing AMH levels for all subjects. The orange and gray bars indicate serum AMH levels for those with irregular and regular menstruation, respectively. n = 528. AMH: Anti‐Müllerian hormone.
FIGURE 3.
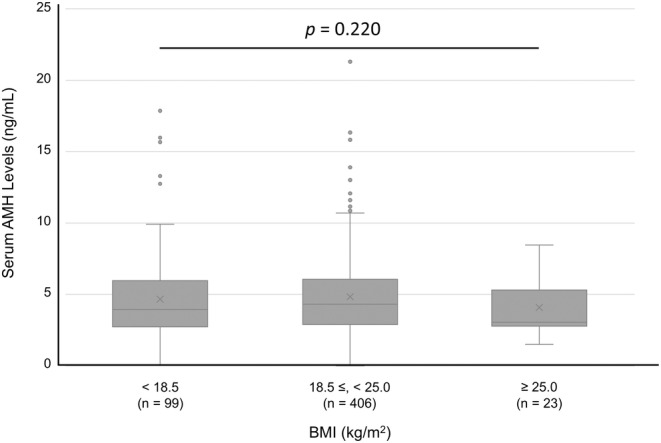
Serum AMH levels for each group classified by BMI. Variables are presented as the mean ± SD. The Kruskal–Wallis test was applied. AMH, Anti‐Müllerian hormone; BMI, Body mass index; SD, Standard deviation; n. s., Not significant.
TABLE 2.
Comparison of regular and irregular menstruation groups in all participants.
| Menstrual cycle | Regular n = 390 | Irregular n = 138 | p |
|---|---|---|---|
| Serum AMH level (ng/mL) | 4.08 (2.80–5.68) | 4.93 (3.07–7.51) | <0.01 |
| Age (years) | 21 (20–21) | 20 (20–21) | 0.22 |
| Height (cm) | 157.9 (154.1–160.8) | 157.8 (154.8–160.1) | 0.88 |
| Body Weight (kg) | 50.4 (46.7–55.6) | 49.5 (46.6–53.5) | 0.18 |
| BMI (kg/m2) | 20.6 (19.0–22.1) | 20.0 (18.8–21.8) | 0.08 |
Note: Variables are presented as the median (interquartile range). The Mann–Whitney U‐test was applied.
Abbreviations: AMH, anti‐Müllerian hormone; BMI, body mass index.
3.2. Irregular menstruation and serum AMH levels in relation to basic hormone levels
In subgroups where basal hormone levels were measured, serum LH levels and LH/FSH ratios were significantly higher in the irregular menstruation group than in the regular menstruation group (median [interquartile range], LH: 5.22 [3.14–8.20] vs. 3.77 [2.51–5.22], p < 0.01; LH/FSH: 0.97 [0.55–1.52] vs. 0.67 [0.43–0.93], p < 0.01) (Table 3). Moderate correlations were found between serum AMH and serum LH levels (r = 0.542, p < 0.001) and between serum AMH and serum LH/FSH ratio (r = 0.584, p < 0.001) in the irregular menstruation group. In contrast, there was no correlation between serum LH and serum AMH levels (r = 0.152, p = 0.093) and a weak correlation between the serum LH/FSH ratio and serum AMH levels (r = 0.313, p < 0.001) in the regular menstruation group. Furthermore, no significant correlations were observed between serum FSH and serum AMH levels in the irregular menstruation group (r = −0.242, p = 0.072). Regarding serum testosterone and serum AMH levels, only weak correlations were identified in both groups (irregular menstruation group: r = 0.432, p < 0.001; regular menstruation group: r = 352, p < 0.001) (Figure 4 and Figure S1).
TABLE 3.
Association between menstrual cycle and serum hormone levels.
| Menstrual cycle | Regular n = 123 | Irregular n = 56 | p |
|---|---|---|---|
| Serum AMH level (ng/mL) | 3.71 (2.17–5.66) | 4.92 (2.94–7.61) | 0.01 |
| LH (IU/L) | 3.77 (2.51–5.22) | 5.22 (3.14–8.20) | <0.01 |
| FSH (IU/L) | 5.50 (4.48–6.71) | 5.50 (4.79–6.35) | 0.68 |
| LH/FSH | 0.67 (0.43–0.93) | 0.97 (0.55–1.52) | <0.01 |
| Testosterone (ng/mL) | 0.24 (0.17–0.34) | 0.29 (0.18–0.40) | 0.10 |
Note: Variables are presented as the median (interquartile range). The Mann–Whitney U‐test was applied.
Abbreviations: AMH, anti‐Müllerian hormone; FSH, follicle‐stimulating hormone; LH, luteinizing hormone.
FIGURE 4.
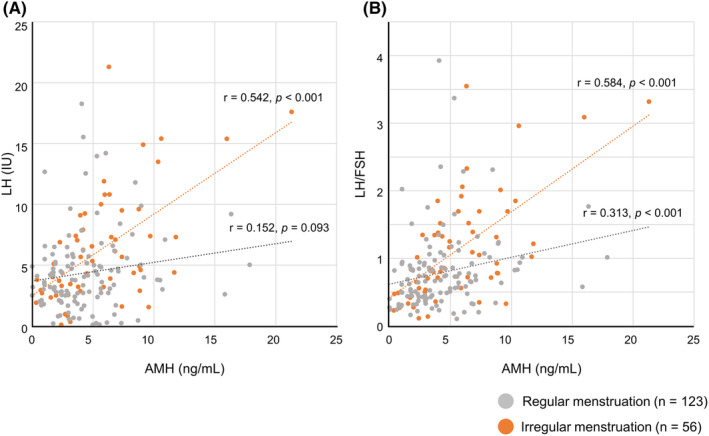
Correlations between serum AMH levels and hormone levels. AMH, Anti‐Müllerian hormone; FSH, Follicle‐stimulating hormone; LH, Luteinizing hormone.
3.3. Serum AMH level as a predictor of PCOS
To investigate whether serum AMH levels can be used for screening PCOS, we generated an ROC curve of serum AMH for LH levels >7.1 IU/L and an LH/FSH ratio >1.21, one of the three points included in the diagnostic criteria of PCOS proposed by JSOG, in the group with menstrual irregularities (Figure 5). The area under the curve was 0.815. The optimal serum AMH cutoff value was 5.30 ng/mL, with a sensitivity of 84.2% and specificity of 70.3%, respectively.
FIGURE 5.
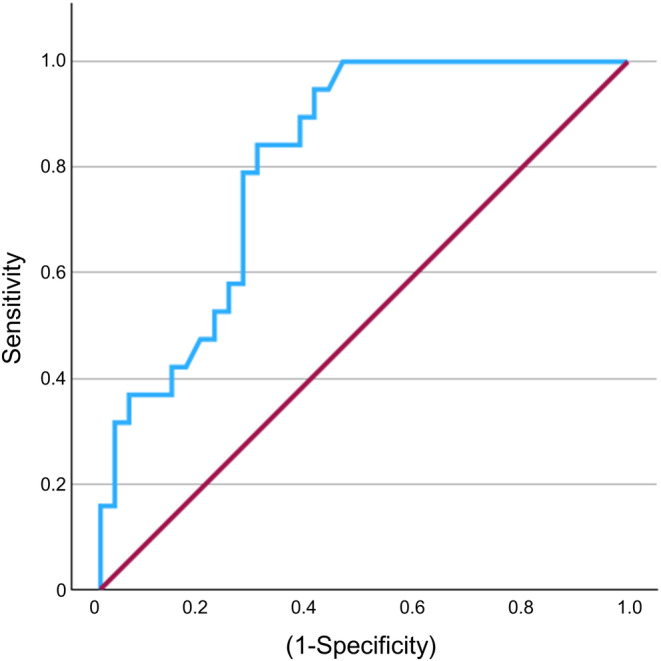
Ability of serum AMH levels to diagnosis PCOS. The receiver operating characteristic curve demonstrates serum AMH cutoff values for LH >7.1 IU/L and LH/FSH ratio >1.21 in the group with menstrual irregularities (n = 56). AMH, Anti‐Müllerian hormone; LH, Luteinizing hormone; FSH, Follicle‐stimulating hormone.
4. DISCUSSION
In this study, we demonstrated that serum AMH levels were higher in women with irregular menstruation compared to those without, suggesting that PCOS, a condition in which AMH levels are elevated, may be more involved in irregular menstruation in young women than in those with a decline in ovarian reserve. Consistent with this, the AMH levels in this cohort were associated with abnormal basic hormone levels and elevated LH and LH/FSH, indicative of PCOS. Meta‐analyses have reported that AMH is useful as an adjunct to the diagnosis of PCOS, 14 and the JSOG revised the diagnostic criteria for PCOS in December 2023, 15 allowing AMH to replace the antral follicle count as an adjunct diagnosis to ovarian findings. The new diagnostic criteria were established based on the results of a national case survey. The mean age at AMH measurement in the 20–29 age group was 27.1 ± 1.9 (mean ± SD) years, suggesting that the majority of patients were in their late twenties and had a desire to raise a child. In recent years, although several meta‐analyses have reported elevated serum AMH levels in patients with PCOS, 4 , 5 , 6 , 7 most studies have focused on women of reproductive age around 30 years, especially those undergoing fertility treatment, and few studies have focused on younger women. 13 This revision also states that it is important to identify high‐risk cases of adolescent PCOS. However, the criteria for adult polycystic ovarian morphology (PCOM) cannot be applied to adolescent girls within 8 years of menarche, and transvaginal or transrectal ultrasound is impractical for adolescent girls.
Many women do not see a gynecologist until they realize that they are infertile after trying to conceive. Additionally, some women do not see a gynecologist because they do not wish to raise a child, despite having irregular menstrual periods. PCOS not only increases the risk of infertility due to impaired ovulation but also influences future cancer development and perinatal complications such as hypertension due to pregnancy and gestational diabetes mellitus. 16 , 17 If PCOS can be diagnosed at the time of puberty, not only can the risk of endometrial cancer be reduced through therapeutic intervention, but the risk of complications can also be reduced by managing glucose intolerance and obesity before pregnancy and being prepared for perinatal complications when they occur. A better understanding of fertility at a younger age can increase awareness of one's reproductive health and help in lifestyle management. The diagnosis of PCOS in young individuals is still debated, suggesting the use of criteria other than those applied to adults. Several guidelines from specialized medical societies recommend using indicators such as menstrual cycle irregularities and excessive androgen levels as diagnostic criteria. However, these guidelines do not recommend the use of AMH measurements for PCOS diagnosis due to the diversity of measurement methods. 11 , 18 Nevertheless, the accumulation of evidence, as presented in this report, raises the possibility that AMH measurements could serve as a useful adjunctive diagnostic tool.
In this study, there were no significant differences in serum AMH levels among the three groups of patients with BMI < 18.5 kg/m2, 18.5–25.0 kg/m2, and ≥25 kg/m2. Consistent with our findings, systematic reviews of the association between obesity and serum AMH levels reported no association between obesity and serum AMH levels. 19 , 20
Several meta‐analyses have reported the usefulness of serum AMH for the diagnosis of PCOS, but the cutoff values vary widely in the literature. For example, Iliodromiti et al. reported a cutoff serum AMH level of 4.7 ng/mL for PCOS diagnosis based on the Rotterdam criteria 21 via summary ROC analysis in a meta‐analysis of 683 cases in 10 references; however, there is a wide distribution in the literature, ranging from 3.50–8.40 ng/mL. 22 Indeed, Teede et al. reported cutoff values of 10–57 pmol/L (1.40–7.98 ng/mL). 4 One of the main reasons for the variation in cutoff values is the difference in the age of the participants, with few studies on young women. In the present study, where the age of the subjects was limited to 20–27 years, the cutoff value for serum AMH was 5.3, which is lower than the cutoff of 6.1–7.25 ng/mL reported in a systematic review in adolescents up to 29 years. 13 By contrast, the revised diagnostic criteria from JSOG set an AMH cutoff value of 4.4 ng/mL for women aged 20–29 years when measured by LUMIPULSE®. The slightly higher value observed in this study reflects differences in the study population and methodology. While the revised JSOG diagnostic criteria prioritize sensitivity, 23 our findings suggest that AMH cutoff values are useful in predicting elevated LH levels and LH/FSH ratios, which is consistent with a more severe cutoff level in contrast to the JOSG criteria.
This study has some limitations. First, the number of samples for which basal hormone levels could be measured was small compared to the number of samples for which AMH could be measured; therefore, an investigation into the relationship between serum AMH levels and basic hormone levels may be insufficient. Second, we were unable to perform ultrasound examinations on all students, which is one of the diagnostic criteria for PCOS according to the JSOG. 15
We used the JSOG criteria rather than the Rotterdam criteria 21 as the clinical presentation of Japanese women with PCOS differs from that of Europeans and Americans, with lower incidences of hyperandrogenemia and obesity. There is no literature on the usefulness of serum AMH for the diagnosis of PCOS in the general Japanese population of young women. This study is one of the rare studies in a young Japanese cohort and may provide powerful data for examining serum AMH levels and their association with PCOS in young Japanese women. We know that basal hormone levels vary throughout the menstrual cycle, whereas serum AMH levels are stable and can be measured during annual health checkups. In addition, by measuring AMH levels during health checkups, it is possible to identify not only patients with PCOS but also those with premature ovarian insufficiency (POI) and women at high risk for POI with low AMH levels. This will provide these women with the opportunity to make decisions about future pregnancies based on their own ovarian reserve.
In conclusion, we found that in a general cohort of young women, serum AMH levels were higher in women with irregular menstruation than in those with regular menstruation, and that high serum AMH levels and PCOS might be associated with irregular menstruation. Overall, serum AMH levels may motivate young women to visit gynecologists and allow early intervention for patients with PCOS because they can be measured during annual health checkups.
FUNDING INFORMATION
Author M.G was supported by JSPS KAKENHI (grant No. 20 K11508) and author A.I was supported by Pfizer Health Research Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Akira Iwase is the Editor‐in‐Chief of Reproductive Medicine and Biology and a co‐author of this article, and Satoko Osuka is an Editorial Board member of Reproductive Medicine and Biology and a co‐author of this article. They were excluded from editorial decision‐making related to the acceptance and publication of this article. Editorial decision‐making was handled independently by Another Editor to minimize bias.
Supporting information
Figure S1.
ACKNOWLEDGMENTS
We thank all members of the Kajiyama Laboratory for their valuable discussions. We also wish to thank Editage (www.editage.com) for providing the language editing.
Miyake N, Osuka S, Ohsawa I, Tonoike T, Uno T, Tsuzuki K, et al. Association between anti‐Müllerian hormone levels and polycystic ovary syndrome in a general cohort of young women in Japan. Reprod Med Biol. 2024;23:e12615. 10.1002/rmb2.12615
REFERENCES
- 1. Visser JA, Schipper I, Laven JS, Themmen AP. Anti‐Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol. 2012;8(6):331–341. [DOI] [PubMed] [Google Scholar]
- 2. Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti‐Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688–701. [DOI] [PubMed] [Google Scholar]
- 3. Iwase A, Osuka S, Goto M, Murase T, Nakamura T, Takikawa S, et al. Clinical application of serum anti‐Müllerian hormone as an ovarian reserve marker: a review of recent studies. J Obstet Gynaecol Res. 2018;44(6):998–1006. [DOI] [PubMed] [Google Scholar]
- 4. Teede H, Misso M, Tassone EC, Dewailly D, Ng EH, Azziz R, et al. Anti‐Müllerian hormone in PCOS: a review informing international guidelines. Trends Endocrinol Metab. 2019;30(7):467–478. [DOI] [PubMed] [Google Scholar]
- 5. Pérez‐López FR, Ornat L, López‐Baena MT, Santabárbara J, Savirón‐Cornudella R, Pérez‐Roncero GR. Circulating kisspeptin and anti‐mullerian hormone levels, and insulin resistance in women with polycystic ovary syndrome: a systematic review, meta‐analysis, and meta‐regression. Eur J Obstet Gynecol Reprod Biol. 2021;260:85–98. [DOI] [PubMed] [Google Scholar]
- 6. Lv B, Xing C, He B. Effects of bariatric surgery on the menstruation‐ and reproductive‐related hormones of women with obesity without polycystic ovary syndrome: a systematic review and meta‐analysis. Surg Obes Relat Dis. 2022;18(1):148–160. [DOI] [PubMed] [Google Scholar]
- 7. Anand S, Kumar A, Prasad A, Trivedi K. Updated meta‐analysis on the diagnostic accuracy of serum anti‐Müllerian hormone in poly cystic ovary syndrome involving 13 509 subjects. J Obstet Gynaecol Res. 2022;48(8):2162–2174. [DOI] [PubMed] [Google Scholar]
- 8. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harada M. Pathophysiology of polycystic ovary syndrome revisited: current understanding and perspectives regarding future research. Reprod Med Biol. 2022;21(1):e12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO . Preconception care: maximizing the gains for maternal and child health. 2013. Available from: https://www.who.int/publications/i/item/WHO‐FWC‐MCA‐13.02. [Google Scholar]
- 11. Peña AS, Witchel SF, Hoeger KM, Oberfield SE, Vogiatzi MG, Misso M, et al. Adolescent polycystic ovary syndrome according to the international evidence‐based guideline. BMC Med. 2020;18(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33(5):445–447. [DOI] [PubMed] [Google Scholar]
- 13. Tsukui Y, Kitahara Y, Hasegawa Y, Kobayashi M, Osuka S, Iwase A. Anti‐Müllerian hormone levels in the diagnosis of adolescent polycystic ovarian syndrome: a systematic review and meta‐analysis. Endocr J. 2022;69(8):897–906. [DOI] [PubMed] [Google Scholar]
- 14. Iwase A, Asada Y, Sugishita Y, Osuka S, Kitajima M, Kawamura K, et al. Anti‐Müllerian hormone for screening, diagnosis, evaluation, and prediction: a systematic review and expert opinions. J Obstet Gynaecol Res. 2024;50(1):15–39. [DOI] [PubMed] [Google Scholar]
- 15. JSOG . Results of a National Case Study on polycystic ovary syndrome and new diagnostic criteria (2024) in Japan. 2024. Available from: https://www.jsog.or.jp/medical/5122/. [Google Scholar]
- 16. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod Update. 2014;20(5):748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–592. [DOI] [PubMed] [Google Scholar]
- 18. Teede HJ, Tay CT, Laven JJE, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endocrinol. 2023;189(2):G43–G64. [DOI] [PubMed] [Google Scholar]
- 19. Moslehi N, Shab‐Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta‐analysis. Menopause. 2018;25(9):1046–1055. [DOI] [PubMed] [Google Scholar]
- 20. Oldfield AL, Kazemi M, Lujan ME. Impact of obesity on anti‐Müllerian hormone (AMH) levels in women of reproductive age. J Clin Med. 2021;10(14):3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rotterdam ESHRE/ASRM‐Sponsored PCOS consensus workshop group . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. [DOI] [PubMed] [Google Scholar]
- 22. Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti‐Müllerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta‐analysis of extracted data. J Clin Endocrinol Metab. 2013;98(8):3332–3340. [DOI] [PubMed] [Google Scholar]
- 23. Noguchi H, Iwasa T, Iwase A, Kanasaki H, Kimura F, Kugu K, et al. Cut‐off value for anti‐Müllerian hormone in the diagnostic criteria for polycystic ovary syndrome in the Japanese population. J Obstet Gynaecol Res. 2024;50(8):1368–1382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.


