Abstract
ABSTRACT
Objective
To evaluate the impact of intraoperative neuromonitoring (IONM) on stroke and operative mortality after coronary and/or valvular operations.
Methods
This was an observational study of coronary and/or valvular heart operations from 2010 to 2021. Baseline characteristics and postoperative outcomes were compared by the use or non-use of IONM, which included both electroencephalography and somatosensory-evoked potentials. Propensity-score matching was employed to assess the association of IONM usage with operative mortality and stroke.
Results
A total of 19 299 patients underwent a cardiac operation, of which 589 (3.1%) had IONM. Patients with IONM were more likely to have had baseline cerebrovascular disease (60% vs 22%). Patients with IONM had increased operative mortality (5.3% vs 2.5%) and stroke (4.9% vs 1.9%). Moreover, stroke and mortality were highly correlated, with 14% of strokes resulting in death, while only 2% of non-strokes resulted in death (p<0.001). The unadjusted Kaplan-Meier survival estimate was significantly lower among the group with IONM (p<0.001, log-rank). After propensity matching, however, there was no difference in operative mortality or stroke across each group: 3.6% vs 5.3% for mortality and 3.7% vs 5.4% for stroke. In the propensity-matched cohort, the Kaplan-Meier survival estimates were not significantly different across each group (p=0.419, log-rank).
Conclusions
Adjusting for baseline risk, there was no significant difference in adverse outcomes across each group. IONM may serve as a biomarker of cerebral ischaemia, and empirical adjustments based on changes may provide benefits for neurologic outcomes in high-risk patients. The efficacy of IONM during cardiac surgery should be prospectively validated.
Keywords: STROKE, Coronary Artery Bypass, Heart Valve Prosthesis Implantation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Although existing data have demonstrated the effectiveness of dual-modality intraoperative neuromonitoring (IONM) in reducing adverse neurologic outcomes after aortic arch surgery and carotid endarterectomy, IONM during routine adult cardiac surgery has not yet been adopted as the clinical standard of care, despite suggestions by the American Heart Association.
WHAT THIS STUDY ADDS
This study suggests that IONM may be safely used in the intraoperative setting, and it may provide actionable neurologic data for intervening and possibly improving neurologic outcomes after cardiac surgery.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
While further data are needed to establish IONM as the standard of care, these results should support the expanded utilisation of IONM during routine adult cardiac surgery.
Introduction
Postoperative stroke is a devastating complication in patients undergoing cardiac surgery, and it continues to be a leading cause of morbidity and mortality.1 The incidence of postoperative stroke remains 1–5%2 due to high rates of atherosclerosis, pre-existing cerebrovascular disease and elevated age of the patient population.3 Stroke is associated with a greater likelihood of in-hospital mortality, increased hospital cost and length of stay, risk of cognitive decline and major disability.2 The aetiology of stroke is related to cerebral thromboembolism and/or hypoperfusion during surgery.4 Embolic strokes may be attributed to atherosclerotic plaques, cardiac thrombi from arrhythmias or debris from surgical instrumentation.5 Conversely, stroke might also be related to cerebral hypoperfusion before, during or after cardiopulmonary bypass. Even as the operative mortality after cardiac surgery continues to decline,6 stroke persists as a major postoperative morbidity.7
Intraoperative neuromonitoring (IONM) can detect adverse neurologic events in real-time and may lead to interventions that might improve neurologic outcomes after cardiac surgery. Dual-modality IONM with electroencephalography (EEG) and somatosensory evoked potentials (SSEPs) has been shown to be more sensitive at detecting adverse neurologic events than either modality alone.8,11 Although several studies have suggested the effectiveness of dual-modality IONM in reducing adverse neurologic outcomes after aortic arch surgery12 13 and carotid endarterectomy,10 14 15 IONM during routine adult cardiac surgery has not yet been adopted as the clinical standard of care, despite preliminary data suggesting possible neurologic benefit and suggestions by Scientific Statement from the American Heart Association (AHA) on strategies to reduce stroke during surgery.2 16 17
The primary aim of this study is to evaluate the efficacy of IONM on postoperative stroke after coronary and/or valvular operations. Secondarily, this study sought to evaluate the impact of IONM on other short-term morbidity and long-term survival after cardiac surgery.
Patients and methods
Patient population and study design
This was an observational study, using a prospectively maintained institutional database of all cardiac operations performed at a single institution between 2010 and 2021. This study adhered to STROBE guidelines for cohort studies. Definitions and terminology were consistent with the Society of Thoracic Surgeons database. All patients who underwent coronary and/or valvular heart operations were included for analysis. Aortic arch replacements that were conducted under hypothermic circulatory arrest were excluded. Patients were subsequently dichotomised into two groups, according to the use or non-use of dual-modality IONM (including both SSEP and EEG). Baseline characteristics and postoperative outcomes were compared across each group. The primary aim of this study was to evaluate the association between IONM usage and stroke. Following the standardised definitions of the Society of Thoracic Surgeons database, stroke was defined as a neurologic deficit of abrupt onset caused by a disturbance in blood supply to the brain that did not resolve within 24 hours. Transient ischaemic attacks were therefore not included in this definition.
Intraoperative neuromonitoring
During this study’s timeframe at this institution, the use of IONM was determined by surgeon discretion; however, in general, IONM was indicated for use in patients with a history of stroke or severe carotid artery stenosis (≥50% stenosis in one or both carotid arteries). All patients who underwent a cardiac operation with the use of IONM had both SSEP and EEG monitoring, as previously described.12 18
To generate SSEPs, subdermal needle electrode pairs were used to independently stimulate the left and right median or ulnar nerves at the wrist, and the left and right posterior tibial nerves at the ankle. Constant current stimulation was used, at intensities sufficient to evoke a consistent and supramaximal response. To record the thalamocortical and cortical potentials, or N20-P30 SSEPs, scalp electrodes were placed at P4/Fz and P3/Fz (according to the International 10–20 System.19 The dorsal column nucleus, or brainstem potential, was recorded using an electrode, which was referenced to Fz and localised on the mastoid. The peripheral potential generated in the brachial plexus was recorded using electrodes placed at the bilateral erb’s point and referenced to each other. EEG was recorded using electrodes placed on the scalp (according to the International 10–20 System).19 EEG was recorded using eight channels: F3-P3, P3-O1, F3-T3, T3-O1, F4-P4, P4-O2, F4-T4 and T4-O2.
SSEP and EEG changes were monitored throughout the operation by a dedicated and certified neurophysiologist. Significant SSEP changes were defined as a persistent and consistent ≥50% decrease in the cortical amplitude and/or ≥10% prolongation of latency from baseline values in ≥2 averaged trials. To be considered a significant EEG change, the EEG recording must display a ≥50% decrease in the amplitude of the fast frequency or a ≥50% increase in theta or delta activity.
Statistical methods and analysis
Primary stratification was between the IONM group and the group without IONM. Differences between baseline demographic, clinical and operative variables were compared across each group (table 1). Short-term postoperative outcomes were compared across each group (table 2). Next, a propensity-score analysis was conducted to compare differences in postoperative outcomes across each group (table 3). The matched cohort was generated via 1:1 greedy matching, using a calliper of 0.2 of the SD of the logit propensity score. After matching, standardised mean differences (SMDs) were calculated to assess covariate balance, with <10% being considered well-balanced and <15% being considered acceptably balanced. Short-term postoperative outcomes were compared across each group of the propensity-matched cohort (table 4). Finally, unadjusted survival estimates were generated using Kaplan-Meier methods and compared using log-rank statistics, before and after propensity-score matching (figures1 2). All statistical analyses were performed using STATAV.15.0 (Stata Corp, College Station, Texas, USA). All tests were two-sided with an α level of 0.05 considered to indicate statistical significance.
Table 1. Baseline characteristics, comparing patients with IONM and patients without IONM.
| Variable | No IONM (n=18 710) | IONM (n=589) | P value |
| Age (years) | 65.8±11.9 | 64.5±14.0 | 0.010 |
| Sex (female) | 5984 (32.0) | 188 (31.9) | 0.974 |
| Caucasian race | 17 484 (93.5) | 554 (94.1) | 0.555 |
| Body mass index (kg/m2) | 29.9±6.3 | 28.5±6.1 | <0.001 |
| Diabetes mellitus | 7336 (39.2) | 200 (34.0) | 0.010 |
| Chronic dialysis use | 498 (2.7) | 20 (3.4) | 0.278 |
| Chronic lung disease | 4340 (23.2) | 175 (29.7) | <0.001 |
| Peripheral vascular disease | 3083 (16.5) | 160 (27.2) | <0.001 |
| Cerebrovascular disease | 4025 (21.5) | 356 (60.4) | <0.001 |
| Preoperative haematocrit (%) | 38.5±5.7 | 37.0±6.1 | <0.001 |
| Preoperative creatinine (mg/dL) | 1.2±1.0 | 1.2±0.9 | 0.461 |
| Congestive heart failure (≤14 days) | 4951 (26.5) | 182 (30.9) | 0.016 |
| Ejection fraction | 52.2±12.2 | 52.2±12.2 | 0.993 |
| Redo surgery | 1870 (10.0) | 124 (21.1) | <0.001 |
| Surgical status | 0.045 | ||
| Elective | 9292 (49.7) | 283 (48.1) | |
| Urgent | 8755 (46.8) | 295 (50.1) | |
| Emergent/salvage | 663 (3.5) | 11 (1.9) | |
| Cardiopulmonary bypass time (min) | 121±56.6 | 146±73.3 | <0.001 |
| Ischaemic time (min) | 91.1±45.1 | 112±56.3 | <0.001 |
IONMintraoperative neurophysiologic monitoring
Table 2. Postoperative outcomes, comparing patients with IONM and patients without IONM.
| Variable | No IONM (n=18 710) | IONM (n=589) | P value |
| Operative mortality (STS definition) | 462 (2.5) | 31 (5.3) | <0.001 |
| ICU length of stay (hours) | 45.2 (26.0–74.9) | 48.5 (27.2–95.9) | <0.001 |
| Stroke | 354 (1.9) | 29 (4.9) | <0.001 |
| Prolonged ventilation (>24 hours) | 2016 (10.8) | 95 (16.1) | <0.001 |
| New dialysis requirement | 544 (2.9) | 18 (3.1) | 0.833 |
| Re-exploration for bleeding | 640 (3.4) | 38 (6.5) | <0.001 |
| Postoperative blood product transfusion | 6607 (35.3) | 274 (46.5) | <0.001 |
ICUintensive care unitIONMintraoperative neurophysiologic monitoringSTSSociety of Thoracic Surgeons
Table 3. Baseline characteristics, comparing patients with IONM and patients without IONM, after propensity-score matching.
| Variable | No IONM (n=589) | IONM (n=589) | SMD |
| Age (years) | 63.9±13.8 | 64.5±14.0 | 0.047 |
| Sex (female) | 196 (33.23) | 188 (31.9) | 0.029 |
| Caucasian race | 558 (94.7) | 554 (94.1) | 0.028 |
| Body mass index (kg/m2) | 28.5±5.9 | 28.5±6.1 | 0.002 |
| Diabetes mellitus | 210 (35.7) | 200 (34.0) | 0.035 |
| Chronic dialysis use | 22 (3.7) | 20 (3.4) | 0.020 |
| Chronic lung disease | 179 (30.4) | 175 (29.7) | 0.015 |
| Peripheral vascular disease | 168 (28.5) | 160 (27.2) | 0.033 |
| Cerebrovascular disease | 350 (59.4) | 356 (60.4) | 0.023 |
| Preoperative haematocrit (%) | 37.0±6.1 | 37.0±6.1 | 0.006 |
| Preoperative creatinine (mg/dL) | 1.23±1.0 | 1.21±0.9 | 0.020 |
| Congestive heart failure (≤14 days) | 179 (30.4) | 182 (30.9) | 0.011 |
| Ejection fraction | 52.5±11.6 | 52.2±12.2 | 0.022 |
| Redo surgery | 128 (21.7) | 124 (21.1) | 0.019 |
| Surgical status | |||
| Elective | 299 (50.8) | 283 (48.1) | 0.054 |
| Urgent | 280 (47.5) | 295 (50.1) | 0.051 |
| Emergent/salvage | 10 (1.7) | 11 (1.9) | 0.013 |
IONMintraoperative neurophysiologic monitoringSMDstandardised mean difference
Table 4. Postoperative outcomes, comparing patients with IONM and patients without IONM, after propensity-score matching.
| Variable | No IONM (n=589) | IONM (n=589) | P value |
| Operative mortality (STS definition) | 21 (3.6) | 31 (5.3) | 0.156 |
| ICU length of stay (hours) | 47.3 (26.3–77.6) | 48.5 (27.2–95.9) | 0.075 |
| Stroke | 22 (3.7) | 32 (5.4) | 0.164 |
| Prolonged ventilation (>24 hours) | 27 (4.6) | 18 (3.1) | 0.171 |
| New dialysis requirement | 19 (3.2) | 38 (6.5) | 0.010 |
| Re-exploration for bleeding | 234 (39.7) | 274 (46.5) | 0.019 |
| Postoperative blood product transfusion | 20 (3.4) | 29 (4.9) | 0.189 |
ICUintensive care unitIONMintraoperative neurophysiologic monitoringSTSSociety of Thoracic Surgeons
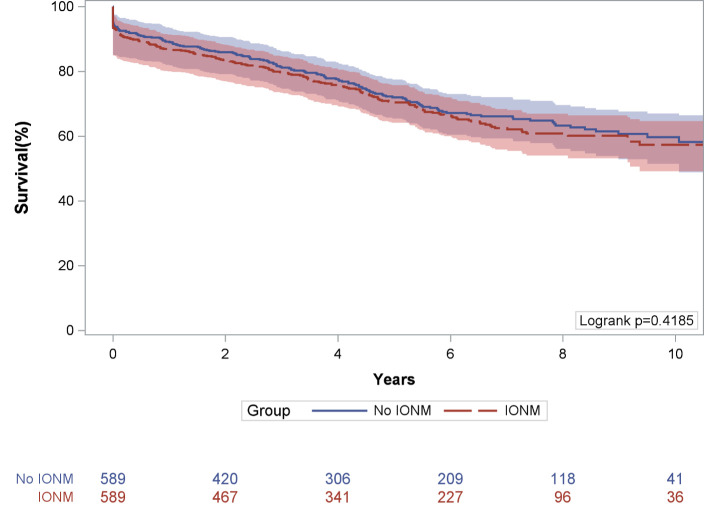
Figure 1. Kaplan-Meier survival estimates, compared across patients with IONM and patients without IONM. IONM, intraoperative neurophysiologic monitoring.
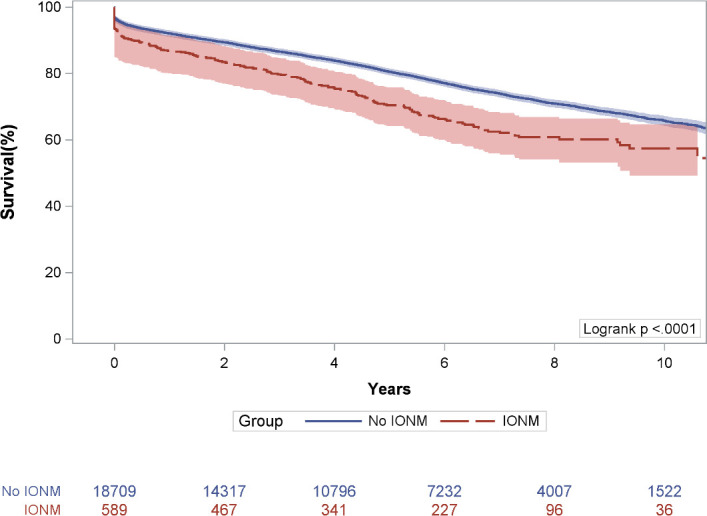
Figure 2. Kaplan-Meier survival estimates, compared across patients with IONM and patients without IONM, after propensity-score matching. IONM, intraoperative neurophysiologic monitoring.
Results
Baseline demographic, clinical and operative variables
A total of 19 299 patients who underwent cardiac procedures were identified, of which 589 (3.1%) had IONM. Table 1 includes the baseline characteristics for the entire cohort analysed according to the use or non-use of IONM. Patients with IONM were younger and had lower body mass index than patients without IONM. Patients with IONM had more comorbidities, including chronic lung disease, peripheral vascular disease, cerebrovascular disease and congestive heart failure, but were less likely to have had diabetes. Patients with IONM were also more likely to have had a redo sternotomy but were less likely to have had an emergent/salvage surgery. Finally, patients with IONM had significantly longer cardiopulmonary bypass time and longer ischaemic time.
Postoperative outcomes
Table 2 presents short-term postoperative outcomes across each group. Patients with IONM were more likely to have had a stroke and were more likely to have had operative mortality. Patients with IONM also had longer intensive care unit LOS, were more likely to have had prolonged mechanical ventilation, were more likely to be re-explored for excessive bleeding and were more likely to require postoperative blood product transfusions. However, the incidence of new-onset renal failure requiring haemodialysis was similar across each group. Of note, stroke and mortality were highly correlated, with 14% of strokes resulting in death, while only 2% of non-strokes resulted in death (p<0.001).
Propensity-score matched analysis
Based on the baseline characteristics listed in table 1, propensity-score matching yielded 589 pairs. Table 3 presents demographic, clinical and operative data for the matched cohort, analysed by IONM usage. After matching, the groups were well balanced across all baseline covariates, with SMD being <0.05.
Table 4 presents short-term postoperative outcomes across each group for the propensity-matched cohort. After matching, there was no difference in operative mortality or stroke across each group. Patients with IONM were more likely to have had new-onset renal failure requiring haemodialysis and re-exploration for bleeding after matching. All other postoperative outcomes were similar between the two groups, including intensive care unit length of stay, prolonged mechanical ventilation (>24 hours) and postoperative blood product transfusions.
Long-term survival
Average (±SD) and median (IQR) follow-up for the entire cohort were 5.0±3.7 and 4.8 (2.2–7.5) years, respectively. In the total cohort, 2.5% of patients were lost to follow-up.
Figure 1 presents Kaplan-Meier survival estimates stratified by IONM versus no IONM. The unadjusted Kaplan-Meier survival estimate was significantly lower among the group with IONM, compared with the group without IONM (figure 1, p<0.001, log-rank). For the group with IONM, survival was 86.7% (95% CI 83.9 to 89.4) at 1 year, 70.4% (95% CI 66.4 to 74.2) at 5 years and 57.3% (95% CI 51.7 to 62.8) at 10 years. For the group without IONM, survival was 92.1% (95% CI 91.7 to 92.5) at 1 year, 80.5% (95% CI 79.8 to 81.1) at 5 years and 65.8% (95% CI 64.7 to 66.8) at 10 years.
Figure 2 presents the survival curves for the propensity-matched cohort. In the propensity-matched cohort, the Kaplan-Meier survival estimates were not significantly different across each group (p=0.419, log-rank). For the group with IONM, survival was 86.7% (95% CI 83.9 to 89.4) at 1 year, 70.4% (95% CI 66.4 to 74.2) at 5 years and 57.3% (95% CI 51.7 to 62.8) at 10 years. For the group without IONM, survival was 89.1% (95% CI 86.5 to 91.5) at 1 year, 71.9% (95% CI 67.8 to 76.0) at 5 years and 59.7% (95% CI 54.1 to 65.2) at 10 years.
Discussion
IONM may detect cerebral ischaemic insults during cardiac surgery and may therefore inform perioperative decision-making to prevent adverse neurologic events. The present study explores the impact of IONM on the incidence of stroke after coronary and/or valvular operations. A few notable findings are evident from this observational analysis. First, IONM use was associated with increased postoperative stroke in the unmatched cohort, which may reflect higher baseline risk in the IONM group compared with the group without IONM. Second, after propensity score matching, there was no association between IONM use and postoperative stroke, which highlights the potential of IONM in tailoring interventions to reverse neurologic insult during cardiac surgery. Third, survival was similarly better in the group without IONM compared with the group with IONM in the unmatched cohort; however, this survival difference did not persist in the propensity-matched cohort. By implication, IONM may be associated with improved outcomes after cardiac surgery, which is likely to be mediated by its benefit for neurologic outcomes in high-risk patients.
Postoperative stroke is a major adverse complication associated with cardiac surgery. Patients with postoperative stroke have a six to nine times higher risk of death compared with patients without stroke.20 The frequency of this complication is reported to be as high as 5% in patients undergoing coronary operation and almost 16% in patients undergoing valve surgery or those with pre-existing cerebrovascular disease.21 Thromboembolism and hypoperfusion are the main aetiologies of intraoperative stroke during coronary and/or valvular operations.22 Ascending aortic atherosclerosis is documented in >50% of patients undergoing coronary artery bypass grafting, and of concern as surgical manipulation can dislodge atherosclerotic plaques.2 Furthermore, hypoperfusion and a decrease in mean arterial pressure are important predictors of watershed strokes,23 which are more likely to require intensive long-term care than any other postoperative stroke.24
The advantage of multimodal brain monitoring (ie, dual utilisation of both SSEP and EEG) is that it can evaluate the function of various nervous system structures, which in turn can guide decision-making during all phases of cardiac surgery. IONM has been widely evaluated for use during carotid endarterectomy, where postoperative stroke secondary to thrombotic occlusions that impede cerebral perfusion is a feared neurologic complication.10 14 During aortic arch reconstruction, the concern of stroke stems from the hypothermic circulatory arrest which harbours increased risk of cerebral hypoperfusion.25 26 Prior findings suggest that dual-modality neuromonitoring is more predictive of postoperative stroke than SSEP or EEG alone.15 16 EEG can detect cerebral ischaemia and permanent EEG changes have been found to be significant postoperative stroke predictors.18 However, anaesthesia can suppress cortical activity and EEG signals, which emphasises the advantage of SSEP, that have the benefit of resistance to anaesthetic agents and mild temperature variations.27 SSEP also evaluates the integrity of the somatosensory pathway and is a more sensitive detector of subcortical ischaemia compared with EEG.28 Each neuromonitoring modality brings its own array of benefits that complement the others’ deficiencies, allowing clinicians to tailor informed interventions during surgery to prevent neurologic insult.
In the present study, IONM use was associated with postoperative stroke in the unmatched cohort, comprising all patients who had IONM for coronary and/or valvular operations. As there is no established standard of care regarding IONM,29 the IONM was used at surgeon discretion. In general, however, IONM was indicated for use in patients with specific risk factors for perioperative stroke, such as a history of stroke or severe carotid artery stenosis.30 Patients with IONM had 2.7 times higher odds of stroke compared with patients without IONM, which may suggest that IONM was appropriately chosen for patients who have a high-risk profile of stroke. This highlights the clinical importance of accurately assessing patient-specific risk factors of stroke as these factors can be informative to surgeons to use IONM during surgery to assess neurologic insult among this high-risk patient population. In contrast to our observations, a prior study by Zanatta et al found that the incidence of postoperative stroke was lower for patients who received IONM, compared with patients who did not receive IONM.16 However, the two groups in their study were significantly different across numerous baseline characteristics with patients who did not receive IONM being of older age and having a higher number of combined procedures, which suggests possible confounding bias as these factors increase the risk of major neurologic complications. Additionally, in this prior study, IONM use was determined based on anaesthesiologist availability rather than surgeon’s discretion, which resulted in substantial differences in participant selection between the two studies.
In the propensity-matched analysis, IONM use was no longer associated with postoperative stroke. Thus, when the higher baseline risk of stroke in the IONM group is accounted for, the group with IONM had a similar likelihood of postoperative stroke compared with the group without IONM. Similarly, while overall long-term survival was significantly lower in the unmatched cohort for patients who had IONM, the survival advantage did not persist after propensity score matching. By implication, when accounting for baseline risk, neurologic and mortality outcomes become similar across each group, which may suggest that IONM use enables patients with higher baseline risk to achieve comparable results to patients with lower baseline risk. That is, this study’s propensity-matched findings could reflect the intraoperative interventions in the IONM group that were used to reverse neurologic insults. IONM with SSEP and EEG15 has been shown to be a significant biomarker for cerebral ischaemia and stroke, during carotid endarterectomy,11 cerebral aneurysm clipping,31 endovascular procedures32 and cardiac surgeries.18 Most importantly, empirical interventions during surgery have the reduced risk of postoperative stroke, suggesting the value of IONM.33 In fact, empirical interventions like increasing mean arterial pressure, checking flow rate in bypass, evaluating other physiologic variables and increasing postoperative care in the intensive care unit are performed but not consistently documented, thus evaluating the efficacy of IONM challenging in retrospective studies. This led to the Scientific Statement from the AHA, on Considerations for Reduction of Risk of Perioperative Stroke in Adult Patients Undergoing Cardiac and Thoracic Aortic Operations to suggest neuromonitoring where available.2 However, further studies are warranted to determine the benefit of IONM use and protocol-guided interventions for reducing strokes after cardiac surgery—possibly as a randomised controlled trial.
Various manoeuvres were performed to correct intraoperative EEG and SSEP abnormalities. These manoeuvres were designed to augment cerebral perfusion, enhance cerebral oxygen delivery and/or reduce cerebral metabolic demand. The patient’s head position was optimised if there was neck rotation or facial plethora; the partial pressure of arterial carbon dioxide was increased to ≥40 mm Hg; the mean arterial pressure was pharmacologically elevated to >60 mm Hg; the pump flow was increased to 2.5 L/min/m2; the fraction of inspired oxygen was increased; red blood cell transfusions were administered, especially if haematocrit was <20%; the heart was repositioned to improve jugular venous drainage; CPB cannula was repositioned if suboptimal cerebral perfusion was suspected; anaesthesia was deepened as necessary; and the temperature was maintained between 32°C and 36°C. For coronary artery bypass grafting, conversion to an off-pump approach would be considered if there were IONM abnormalities prior to cross-clamping in conjunction with atheromatous changes detected on epiaortic ultrasound. Anaortic inflow configurations were also considered feasible. Once the heart was adequately rested after the cross-clamp was removed, the patient was allowed to be pulsatile while maintaining hypertension. Finally, for all IONM abnormalities that persisted at the end of the case, we advocate immediate head and neck CT angiogram with brain perfusion to look for any large vessel occlusion and to determine whether endovascular neurointervention may be indicated. Establishing a protocol-driven algorithm for intervening on IONM abnormalities is critical for reversing neurologic injury detected during cardiac surgery.
Limitations
There are several important limitations to this study. First, there are inherent limitations due to the retrospective design and the use of medical records to review IONM reports and empirical interventions in response to IONM changes. Second, while the propensity-matched analysis suggests that IONM may mitigate the risk of stroke, it cannot be determined if the reduction in the incidence of postoperative stroke was due to intraoperative interventions in response to IONM abnormalities or due to nature of the matched analysis, the balancing of patient-specific risk factors for stroke between the two groups resulted in the observed stroke reduction. Third, the single-centre design allowed for standardisation and consistency in procedures; however, all surgeries were performed by a small group of surgeons in a single, high-volume institution thereby limiting the generalisability of the findings.
Conclusion
In this study of the role of IONM during cardiac surgery, IONM use was associated with postoperative stroke and operative mortality, suggesting its potential role as a neurophysiologic biomarker. However, these associations were not present after propensity score matching for risk factors associated with stroke. While IONM may mitigate adverse neurologic outcomes after cardiac surgery, these results suggest that IONM may be safely used in the intraoperative setting. Prospective clinical studies should be performed to confirm the results for wider acceptance and implementation.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Presented at: Presented as an oral presentation at the Eastern Cardiothoracic Surgical Society (ECTSS), 60th Annual Meeting, 6–9 October 2022.
Ethics approval: This study was approved by the institutional review board of the University of Pittsburgh on 4/17/2019 (STUDY18120143), with written consent being waived.
Contributor Information
James Brown, Email: brownja7@upmc.edu.
Nidhi Iyanna, Email: iyanna.nidhi@medstudent.pitt.edu.
Sarah Yousef, Email: yousefs@upmc.edu.
Derek Serna-Gallegos, Email: sernagallegosdr@upmc.edu.
Jianhui Zhu, Email: zhuj@upmc.edu.
Pyongsoo Yoon, Email: yoonpd2@upmc.edu.
David Kaczorowski, Email: kaczorowskidj2@upmc.edu.
Johannes Bonatti, Email: bonattijo@upmc.edu.
Danny Chu, Email: chud@upmc.edu.
Jeffrey Balzer, Email: balzjr@upmc.edu.
Kathirvel Subramaniam, Email: subramaniamk@upmc.edu.
Parthasarathy D Thirumala, Email: thirumalapd@upmc.edu.
Ibrahim Sultan, Email: sultani@upmc.edu.
Data availability statement
Data are available upon reasonable request.
References
- 1.Karunanantham J, Ali JM, Evans NR, et al. Impact of stroke on outcomes following cardiac surgery: Propensity matched analysis. J Card Surg. 2020;35:3010–6. doi: 10.1111/jocs.14964. [DOI] [PubMed] [Google Scholar]
- 2.Gaudino M, Benesch C, Bakaeen F, et al. Considerations for Reduction of Risk of Perioperative Stroke in Adult Patients Undergoing Cardiac and Thoracic Aortic Operations: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e193–209. doi: 10.1161/CIR.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 3.Riepen B, DeAndrade D, Floyd TF, et al. Postoperative stroke assessment inconsistencies in cardiac surgery: Contributors to higher stroke-related mortality? J Stroke Cerebrovasc Dis. 2023;32:107057. doi: 10.1016/j.jstrokecerebrovasdis.2023.107057. [DOI] [PubMed] [Google Scholar]
- 4.Palmerini T, Savini C, Di Eusanio M. Risks of Stroke After Coronary Artery Bypass Graft - Recent Insights and Perspectives. Interv Cardiol . 2014;9:77–83. doi: 10.15420/icr.2011.9.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borger MA, Ivanov J, Weisel RD, et al. Stroke during coronary bypass surgery: principal role of cerebral macroemboli. Eur J Cardiothorac Surg. 2001;19:627–32. doi: 10.1016/s1010-7940(01)00649-2. [DOI] [PubMed] [Google Scholar]
- 6.Grant SW, Kendall S, Goodwin AT, et al. Trends and outcomes for cardiac surgery in the United Kingdom from 2002 to 2016. JTCVS Open . 2021;7:259–69. doi: 10.1016/j.xjon.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selnes OA, Gottesman RF, Grega MA, et al. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366:250–7. doi: 10.1056/NEJMra1100109. [DOI] [PubMed] [Google Scholar]
- 8.Edmonds HL, Jr, Rodriguez RA, Audenaert SM, et al. The role of neuromonitoring in cardiovascular surgery. J Cardiothorac Vasc Anesth. 1996;10:15–23. doi: 10.1016/S1053-0770(96)80174-1. [DOI] [PubMed] [Google Scholar]
- 9.Florence G, Guerit JM, Gueguen B. Electroencephalography (EEG) and somatosensory evoked potentials (SEP) to prevent cerebral ischaemia in the operating room. Neurophysiol Clin. 2004;34:17–32. doi: 10.1016/j.neucli.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Paras S, Mina A, Crammond DJ, et al. Cardiovascular-related mortality after intraoperative neurophysiologic monitoring changes during carotid endarterectomy. Clin Neurophysiol. 2022;139:43–8. doi: 10.1016/j.clinph.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Nwachuku EL, Balzer JR, Yabes JG, et al. Diagnostic Value of Somatosensory Evoked Potential Changes During Carotid Endarterectomy. JAMA Neurol. 2015;72:73. doi: 10.1001/jamaneurol.2014.3071. [DOI] [PubMed] [Google Scholar]
- 12.Sultan I, Brown JA, Serna-Gallegos D, et al. Intraoperative neurophysiologic monitoring during aortic arch surgery. J Thorac Cardiovasc Surg. 2023;165:1971–81. doi: 10.1016/j.jtcvs.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Harrer M, Waldenberger FR, Weiss G, et al. Aortic arch surgery using bilateral antegrade selective cerebral perfusion in combination with near-infrared spectroscopy. Eur J Cardiothorac Surg. 2010;38:561–7. doi: 10.1016/j.ejcts.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Bozzani A, Arici V, Ticozzelli G, et al. Intraoperative Cerebral Monitoring During Carotid Surgery: A Narrative Review. Ann Vasc Surg. 2022;78:36–44. doi: 10.1016/j.avsg.2021.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Thiagarajan K, Cheng HL, Huang JE, et al. Is Two Really Better Than One? Examining the Superiority of Dual Modality Neurophysiological Monitoring During Carotid Endarterectomy: A Meta-Analysis. World Neurosurg. 2015;84:1941–9. doi: 10.1016/j.wneu.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Zanatta P, Messerotti Benvenuti S, Bosco E, et al. Multimodal brain monitoring reduces major neurologic complications in cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25:1076–85. doi: 10.1053/j.jvca.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Milne B, Gilbey T, Gautel L, et al. Neuromonitoring and Neurocognitive Outcomes in Cardiac Surgery: A Narrative Review. J Cardiothorac Vasc Anesth. 2022;36:2098–113. doi: 10.1053/j.jvca.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Fleseriu CM, Sultan I, Brown JA, et al. Role of Intraoperative Neurophysiological Monitoring in Preventing Stroke After Cardiac Surgery. Ann Thorac Surg. 2023;116:623–9. doi: 10.1016/j.athoracsur.2023.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Klem GH, Lüders HO, Jasper HH, et al. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- 20.McDonagh DL, Berger M, Mathew JP, et al. Neurological complications of cardiac surgery. Lancet Neurol. 2014;13:490–502. doi: 10.1016/S1474-4422(14)70004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogue CW, Jr, Murphy SF, Schechtman KB, et al. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100:642–7. doi: 10.1161/01.cir.100.6.642. [DOI] [PubMed] [Google Scholar]
- 22.Filsoufi F, Rahmanian PB, Castillo JG, et al. Incidence, topography, predictors and long-term survival after stroke in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2008;85:862–70. doi: 10.1016/j.athoracsur.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 23.Head SJ, Börgermann J, Osnabrugge RLJ, et al. Coronary artery bypass grafting: Part 2--optimizing outcomes and future prospects. Eur Heart J. 2013;34:2873–86. doi: 10.1093/eurheartj/eht284. [DOI] [PubMed] [Google Scholar]
- 24.Salazar JD, Wityk RJ, Grega MA, et al. Stroke after cardiac surgery: short- and long-term outcomes. Ann Thorac Surg. 2001;72:1195–201. doi: 10.1016/s0003-4975(01)02929-0. [DOI] [PubMed] [Google Scholar]
- 25.Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg. 1993;106:19–28. [PubMed] [Google Scholar]
- 26.Chen C-H, Peterson MD, Mazer CD, et al. Acute Infarcts on Brain MRI Following Aortic Arch Repair With Circulatory Arrest: Insights From the ACE CardioLink-3 Randomized Trial. Stroke. 2023;54:67–77. doi: 10.1161/STROKEAHA.122.041612. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser HA, Hight D, Avidan MS. A narrative review of electroencephalogram-based monitoring during cardiovascular surgery. Curr Opin Anaesthesiol. 2020;33:92–100. doi: 10.1097/ACO.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 28.Lam AM, Manninen PH, Ferguson GG, et al. Monitoring electrophysiologic function during carotid endarterectomy: a comparison of somatosensory evoked potentials and conventional electroencephalogram. Anesthesiology. 1991;75:15–21. doi: 10.1097/00000542-199107000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Isley MR, Edmonds HL, Stecker M, et al. Guidelines for intraoperative neuromonitoring using raw (analog or digital waveforms) and quantitative electroencephalography: a position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2009;23:369–90. doi: 10.1007/s10877-009-9191-y. [DOI] [PubMed] [Google Scholar]
- 30.Tarakji KG, Sabik JF, Bhudia SK, et al. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA. 2011;305:381–90. doi: 10.1001/jama.2011.37. [DOI] [PubMed] [Google Scholar]
- 31.Thirumala PD, Udesh R, Muralidharan A, et al. Diagnostic Value of Somatosensory-Evoked Potential Monitoring During Cerebral Aneurysm Clipping: A Systematic Review. World Neurosurg. 2016;89:672–80. doi: 10.1016/j.wneu.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Ares WJ, Grandhi RM, Panczykowski DM, et al. Diagnostic Accuracy of Somatosensory Evoked Potential Monitoring in Evaluating Neurological Complications During Endovascular Aneurysm Treatment. Operative Surg . 2018;14:151–7. doi: 10.1093/ons/opx104. [DOI] [PubMed] [Google Scholar]
- 33.Chang R, Reddy RP, Sudadi S, et al. Diagnostic accuracy of various EEG changes during carotid endarterectomy to detect 30-day perioperative stroke: A systematic review. Clin Neurophysiol. 2020;131:1508–16. doi: 10.1016/j.clinph.2020.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.