Abstract
Introduction
High-dose glucocorticoid (GC)-based dual immunosuppressive treatment regimens are still frequently used in active lupus nephritis (LN) despite their known association with dose-dependent toxicities and incomplete efficacy. We hypothesised that the addition of voclosporin to low-dose GCs and mycophenolate mofetil (MMF) would reduce exposure to the toxicities of high-dose GC-based dual immunosuppressive therapy regimens, resulting in an improved safety profile without compromising efficacy.
Methods
Propensity score matching generated two groups of matched participants from the voclosporin arms (in combination with MMF (2 g/day) and low-dose GCs) of the Phase 2 AURA-LV and Phase 3 AURORA 1 studies and the MMF (3 g/day) and intravenous cyclophosphamide (IVC) arms (both in combination with high-dose GCs) of the Aspreva Lupus Management Study (ALMS) induction study. Safety and efficacy outcomes were assessed over 6 months.
Results
There were 179 matched participants identified between the AURA-LV/AURORA 1 studies and ALMS. The overall incidence of adverse events (AEs) was higher in IVC- and MMF-treated participants of ALMS; more voclosporin-treated participants reported AEs by preferred term of glomerular filtration rate decreased, hypertension and anaemia. The incidence of serious AEs was similar across treatments. There were four (2.2%) deaths in IVC- and MMF-treated participants of ALMS compared with seven (3.9%) deaths in voclosporin-treated participants. Significantly more voclosporin-treated participants achieved a ≥25% reduction in urine protein creatinine ratio (UPCR) from baseline at 3 months and ≥50% reduction in UPCR from baseline at 6 months.
Conclusions
Compared with the high-dose GC-based regimens used in ALMS, voclosporin-based triple immunosuppressive therapy resulted in fewer AEs overall and greater and earlier reductions in proteinuria over the first 6 months of treatment. These data reinforce the feasibility of using low doses of GCs and MMF to treat LN when combined with voclosporin as a third agent.
Keywords: Lupus Nephritis; Glucocorticoids; Lupus Erythematosus, Systemic
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
This propensity analysis has allowed for a comparison of a voclosporin-based triple immunosuppressive therapy regimen (voclosporin, MMF, and low-dose GCs) with high-dose GC-based dual immunosuppressive therapy regimens outside of a randomised control trial.
Over the first 6 months of treatment, voclosporin-based triple immunosuppressive therapy was associated with a better overall safety profile and greater and earlier reductions in proteinuria compared with high-dose GC-based, dual immunosuppressive therapy regimens.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These data support treatment guidelines that recommend both minimising patient exposure to GCs and using a voclosporin-based triple-immunosuppressive regimen as an initial therapy in patients with active LN.
Introduction
Lupus nephritis (LN) is the most common manifestation of systemic lupus erythematosus (SLE) and is characterised by proteinuria. The long-term goal of treatment is preservation of kidney function. In the short-term, the goal is to reduce proteinuria, as early reduction in proteinuria has been associated with improved long-term kidney health and overall mortality. Because of this, treatment recommendations include targeting a urine protein creatinine ratio (UPCR) <0.5–0.7 g/g within the first years of treatment.1 2
The Aspreva Lupus Management Study (ALMS, N=370) was an international randomised-controlled trial investigating the effectiveness of mycophenolate mofetil (MMF, 3 g/day) as initial therapy for LN, based on data suggesting MMF to be as effective as monthly pulse intravenous cyclophosphamide (IVC).3 Superiority of MMF over IVC for initial therapy when used in combination with high-dose glucocorticoids (GCs) was not demonstrated. However, improvement in clinical outcomes was observed in both study arms. MMF has since been used in combination with GCs as an alternative to IVC for initial treatment and eventually used for maintenance therapy.4
Despite demonstrated efficacy, the tolerability of IVC and high-dose MMF has been a major drawback to their use. Further, both IVC and MMF are used in combination with high-dose GCs, which are also associated with considerable toxicity.5,8 Recently updated treatment guidelines for LN recommend limiting GC administration to the lowest dose possible (≤5 mg/day) during maintenance.1 2
Voclosporin is a second-generation calcineurin inhibitor (CNI), with a modified side chain yielding an increase in potency and potentially reducing adverse events (AEs) related to calcineurin inhibition.9 10 Voclosporin was studied as part of a triple immunosuppressive regimen in the Phase 2, 48-week AURA-LV and Phase 3, 52-week AURORA 1 studies. These studies, enrolling a total of 534 participants, investigated the addition of voclosporin to low-dose MMF (2 g/day) and low-dose GCs.11,13 Both trials demonstrated significant and earlier reductions in proteinuria with the addition of voclosporin and, the subsequent extension study, AURORA 2, confirmed the acceptable safety profile with durable efficacy up to 36 months.14 15 Based on these results, recent updates to treatment guidelines have included voclosporin-based triple immunosuppressive therapy as an initial option for active LN.1 2
Doses of GCs and MMF used in the voclosporin studies were lower than the doses used in ALMS and many prior LN studies.3 11 12 16 17 Our objective was to compare the safety and efficacy of a voclosporin-based triple immunosuppressive regimen to the high-dose GC-based, IVC and MMF regimens used in ALMS. We hypothesised that triple immunosuppressive therapy with voclosporin, low-dose GCs and MMF as used in recent voclosporin trials would reduce exposure to toxicities associated with the high-dose GC-based dual immunosuppressive therapy regimens used in ALMS, resulting in an improved safety profile and maintained efficacy.
Methods
Study design
The initial/induction phase of ALMS was conducted between July 2005 and March 2007. AURA-LV was conducted between June 2014 and January 2017, and AURORA 1 was conducted between May 2017 and October 2019. Results from the original trials have been published.3 11 12
Participants
The current analysis included participants from the following arms of the parent studies: AURA-LV (n=89): voclosporin 23.7 mg twice daily+MMF 2 g/day+GCs (max 25 mg/day); AURORA 1 (n=178): voclosporin 23.7 mg twice daily+MMF 2 g/day+GCs (max 25 mg/day); and ALMS (n=185): MMF 3 g/day+GCs (max 60 mg/day; n=185); ALMS (n=185): IVC 0.5–1.0 g/m2/month+GCs (max 60 mg/day). The study designs and key differences in exclusion and inclusion criteria are highlighted in online supplemental figure 1 and table 1.
Dosing
The AURA-LV/AURORA 1 participants included in this propensity analysis received voclosporin 23.7 mg twice daily in combination with MMF (2 g/day) and oral GCs. GCs were administered according to a protocol-defined tapering schedule consisting of intravenous methylprednisolone (0.5 g/day; 0.25 g/day if <45 kg) on days 1 and 2, followed by oral prednisone on day 3, starting at 20–25 mg/day and decreasing to ≤2.5 mg/day by week 16 (figure 1). If participants required treatment with intravenous methylprednisolone after day 3, they were discontinued from study treatment and considered a treatment failure. Due to the expected haemodynamic effects of CNIs on estimated glomerular filtration rate (eGFR), the protocols provided guidance on study drug modification for participants experiencing decreases in eGFR or increases in blood pressure.
Figure 1. Protocol-defined GC tapering schedule. In ALMS, oral GCs were initiated at a maximum dose of 60 mg/day and the dose decreased by 10 mg/day every 2 weeks until a dose of 40 mg/day was reached, then by a further 5 mg/day every 2 weeks until 10 mg/day was reached. Reductions below 10 mg/day were allowed after 4 weeks of stable response. In AURA-LV and AURORA 1, intravenous methylprednisolone was administered on days 1 and 2. Oral GCs were initiated on day 3 with 20–25 mg/day prednisone and tapered to a target dose of 2.5 mg/day at week 16. ALMS, Aspreva Lupus Management Study; GC, glucocorticoid.
In ALMS, participants were randomised to MMF (titrated up to 3 g/day by week 3) or IVC (0.5–1.0 g/m2/month × six doses) in combination with oral GCs. GCs were initiated at 0.75–1.0 mg/kg/day (maximum 60 mg/day) and tapered by 10 mg/day every 2 weeks to 40 mg/day, followed by reductions of 5 mg/day every 2 weeks to 10 mg/day (figure 1). Reductions below 10 mg/day were allowed after 4 weeks of stable response. Participants were withdrawn and considered non-responders if they required treatment with intravenous GCs.
Statistical analysis
Propensity score methodology generated two groups of matched participants (179 pairs) from the ALMS (IVC and MMF arms) and AURA-LV/AURORA 1 (voclosporin arms) studies based on the following parameters: age, duration of LN, duration of SLE, albumin, complement (C) 3, C4, serum creatinine, anti-double-stranded deoxyribonucleic acid (dsDNA), eGFR, UPCR, biopsy class, sex and geographical region. The proportions of participants achieving UPCR outcomes at 3 and 6 months were calculated using a logistic regression model with terms for treatment arm, baseline UPCR, biopsy class and region. Change from baseline measures was summarised using least square (LS) means and 95% CIs calculated from a general linear model including covariates for treatment group and baseline value. Median time-to-event measures (95% CI) were calculated using the Kaplan-Meier method; HRs and 95% CIs were derived from the Cox proportional hazards model. Descriptive statistics, including means, SD, counts and percentages, were used to describe baseline demographics, disease characteristics, and the incidence of AEs.
Endpoints
Safety and efficacy were assessed at 3 months (12 weeks) and 6 months (24 weeks). Safety assessments were performed by treatment arm (IVC, MMF and voclosporin), highlighting the safety impact of each therapy, and included AEs occurring on or after the first dose of study drug up to either 3 or 6 months of treatment. AEs were coded by System Organ Class and Preferred Term using MedDRA v9.1 (ALMS), v17.0 (AURA-LV) and v20.0 (AURORA 1). Select laboratory parameters were assessed.
Efficacy outcomes by treatment arm are presented here; efficacy outcomes were also performed by study to preserve the integrity of the propensity matching and are presented in the supplement. Efficacy was analysed based on the following endpoints derived from the 2023 EULAR guidelines: reduction in UPCR from baseline of ≥25% at 3 months, reduction in UPCR from baseline of ≥50% at 6 months and reduction to UPCR <0.5 g/g at 6 months (as the induction phase of the ALMS ended at 6 months). Additionally, time to ≥50% reduction in UPCR from baseline, time to UPCR ≤0.5 g/g and mean UPCR over time were analysed.
We focused on early safety outcomes partly to align with the design and shorter duration of the induction phase of the 24-week ALMS. Also, as the difference between studies in the exposure to GCs was greatest during the first few months of treatment (figure 1), the potential to observe differences in acute tolerability would presumably also be the greatest during this time. We were also interested in efficacy outcomes within the first 6 months given the importance of early reductions in proteinuria on long-term kidney outcomes.18,20
Results
Of 534 participants in the AURA-LV and AURORA 1 studies and 370 participants in ALMS, 179 propensity-matched participants were identified. Of the 179 voclosporin-treated participants, 78 participated in AURA-LV and 101 participated in AURORA 1. From ALMS, 91 IVC-treated and 88 MMF-treated participants were included. As expected, the baseline demographics and disease characteristics were similar between the three treatment arms (table 1; online supplemental table 2).
Table 1. Matched baseline disease characteristics by treatment arm.
| Parameter, n (%) | ALMS | AURA-LV/AURORA 1 | ||
| IVC (n=91) | MMF (n=88) | Total (N=179) | Voclosporin (N=179) | |
| Age, years | ||||
| Median (Min, Max) | 31.0 (12, 52) | 33.0 (14, 64) | 32.0 (12, 64) | 30.0 (18, 66) |
| Sex | ||||
| Male | 11 (12.1) | 8 (9.1) | 19 (10.6) | 19 (10.6) |
| Female | 80 (87.9) | 80 (90.9) | 160 (89.4) | 160 (89.4) |
| Geographical region | ||||
| Asia | 33 (36.3) | 33 (37.5) | 66 (36.9) | 65 (36.3) |
| Europe/South Africa/Australia | 18 (19.8) | 23 (26.1) | 41 (22.9) | 42 (23.5) |
| Latin America | 28 (30.8) | 27 (30.7) | 55 (30.7) | 48 (26.8) |
| USA and Canada | 12 (13.2) | 5 (5.7) | 17 (9.5) | 24 (13.4) |
| Duration of SLE, years | ||||
| Mean (SD) | 4.6 (5.7) | 6.5 (7.0) | 5.5 (6.4) | 5.3 (6.1) |
| Duration of LN, years | ||||
| Mean (SD) | 3.1 (4.6) | 3.3 (4.2) | 3.2 (4.4) | 3.2 (4.5) |
| Biopsy class | ||||
| Class III | 6 (6.6) | 11 (12.5) | 17 (9.5) | 18 (10.1) |
| Class IV | 49 (53.8) | 49 (55.7) | 98 (54.7) | 97 (54.2) |
| Class V | 19 (20.9) | 14 (15.9) | 33 (18.4) | 27 (15.1) |
| Class III and V | 7 (7.7) | 6 (6.8) | 13 (7.3) | 19 (10.6) |
| Class IV and V | 10 (11.0) | 8 (9.1) | 18 (10.1) | 18 (10.1) |
| eGFR, mL/min/1.73 m2 | ||||
| Mean (SD) | 94.6 (32.9) | 85.9 (31.7) | 90.4 (32.5) | 92.8 (30.7) |
| Albumin, g/dL | ||||
| Mean (SD) | 2.8 (0.7) | 3.0 (0.7) | 2.9 (0.7) | 2.9 (0.7) |
| UPCR, g/g | ||||
| Mean (SD) | 4.2 (3.0) | 4.2 (4.6) | 4.2 (3.8) | 4.3 (3.3) |
| Serum creatinine, mg/dL | ||||
| Mean (SD) | 0.8 (0.2) | 0.9 (0.3) | 0.8 (0.3) | 0.8 (0.3) |
| Complement 3, mg/dL | ||||
| Mean (SD) | 69.0 (29.2) | 79.2 (31.0) | 74.0 (30.5) | 76.7 (33.1) |
| Low <90 mg/dL, n (%) | 69 (75.8) | 61 (69.3) | 130 (72.6) | 117 (65.4) |
| Complement 4, mg/dL | ||||
| Mean (SD) | 13.4 (8.9) | 15.3 (12.4) | 14.3 (10.7) | 15 (9.0) |
| Low <10 mg/dL, n (%) | 35 (38.5) | 34 (38.6) | 69 (38.5) | 60 (33.5) |
| Anti-dsDNA, IU/mL | ||||
| Mean (SD) | 122.6 (105.5) | 106.7 (74.3) | 114.8 (91.6) | 114.1 (122.6) |
| High >10 IU/mL, n (%) | 87 (95.6) | 86 (97.7) | 173 (96.6) | 160 (89.4) |
Propensity score methodology was used to generate two groups of matched participants (N=179) from the ALMS and AURA-LV/AURORA 1 (voclosporin) studies based on the following parameters: age, duration of LN, duration of SLE, albumin, complement 3, complement 4, creatinine, anti-dsDNA, eGFR, UPCR, biopsy class, sex, and geographical region. All values are presented as number of participants (percentage) unless otherwise noted.
ALMSAspreva Lupus Management StudyAnti-dsDNA, anti-double strand DNA; eGFR, estimated glomerular filtration rate; LN, lupus nephritis; SLE, systemic lupus erythematosus; UPCR, urine protein creatinine ratio
The majority of participants were female (IVC, 87.9%; MMF, 90.9%; voclosporin, 89.4%), had Class IV disease (IVC, 53.8%; MMF, 55.7%; voclosporin, 54.2%) and a mean duration of LN of 3.1–3.3 years. All three treatment arms had mean (SD) UPCR greater than 4 g/g at baseline (IVC, 4.2 (3.0) g/g; MMF, 4.2 (4.6) g/g; voclosporin, 4.3 (3.3) g/g).
Similar percentages of participants across the three arms were receiving renin–angiotensin–aldosterone system (RAAS) agents at baseline (IVC, 51.6%; MMF, 58.0%; voclosporin, 60.3%), and a greater percentage of participants in the voclosporin group were receiving hydroxychloroquine (IVC, 18.7%; MMF, 15.9%; voclosporin, 45.3%; online supplemental table 2).
Disposition
There were 154 (86.0%; IVC, 79 (86.8%) and MMF, 75 (85.2%)) and 143 (79.9%) participants in the ALMS and AURA-LV/AURORA 1 groups who completed 6 months of treatment.
Dose exposure
Mean (SD) cumulative exposure to intravenous methylprednisolone during days 1 and 2 of AURA-LV/AURORA 1 was 0.76 (0.40) g; the ALMS protocol did not include the use of intravenous GCs. Mean (SD) cumulative exposure to oral GCs was more than twofold higher in the IVC and MMF arms of ALMS than voclosporin-treated participants of AURA-LV/AURORA 1 over both 3 and 6 months (table 2). Mean (SD) daily oral GC doses at 3 months were threefold higher in IVC- and MMF-treated participants compared with voclosporin-treated participants (21.6 (6.1) mg/day vs 21.5 (5.1) mg/day vs 6.6 (5.5) mg/day, respectively); daily doses at 6 months remained nearly twofold greater in the IVC and MMF cohorts compared with voclosporin-treated participants (9.7 (2.8) mg/day vs 10.0 (1.8) mg/day vs 5.2 (11.0) mg/day, respectively). More voclosporin-treated participants achieved an oral GC dose ≤7.5 mg/day at 3 and 6 months (online supplemental table 3).
Table 2. Mean daily doses and cumulative exposure by treatment arm.
| 3 Months | 6 Months | ||||||
| ALMS | AURA-LV/AURORA 1 | ALMS | AURA-LV/AURORA 1 | ||||
| IVC(n=91) | MMF(n=88) | Voclosporin(N=179) | IVC(n=91) | MMF(n=88) | Voclosporin(N=179) | ||
| Oral GC* | n†Daily dose, mean (SD), mg/dayCumulative dose, mean (SD), mg | n=8921.6 (6.1)2964.8 (648.4) | n=8121.5 (5.1)2850.9 (592.3) | n=1696.6 (5.5)1104.0 (336.1) | n=569.7 (2.8)3799.2 (927.5) | n=6110.0 (1.8)3666.9 (854.1) | n=1595.2 (11.0)1485.9 (752.2) |
| MMF | n†Daily dose, mean (SD), g/dayCumulative dose, mean (SD), g | n/a | n=812.7 (0.6)205.1 (48.5) | n=1651.9 (0.5)149.9 (42.1) | n/a | n=692.8 (0.4)397.6 (130.1) | n=1611.9 (0.6)295.1 (94.9) |
Propensity score methodology was used to generate two groups of matched participants (n=179) from the ALMS (IVC and MMF) and AURA-LV/AURORA 1 (voclosporin) studies based on the following parameters: age, duration of lupus nephritis, duration of SLE, albumin, C3, C4, creatinine, anti-dsDNA, eGFR, UPCR, biopsy class, sex, and geographical region. Mean daily dose is the average daily dose of all participants with data available at the specified time point (3 or 6 months) including doses of 0 mg mg. Mean cumulative dose is an average of drug exposure for all participants in each arm regardless of overall treatment duration.
Only includes oral glucocorticoidGCs; doses of intravenous methylprednisolone included at the beginning of AURA-LV and AURORA-1 are not included.
Participants with data available at the respective time point.
ALMSAspreva Lupus Management StudyC, complement; dsDNA, double-stranded DNA; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; IVC, intravenous cyclophosphamide; MMF, mycophenolate mofetil; n/a, not applicable; SLE, systemic lupus erythematosusUPCRurine protein creatinine ratio
Mean (SD) cumulative and daily doses of MMF were higher in the MMF cohort of ALMS than in voclosporin-treated participants in AURA-LV and AURORA 1 (table 2). Mean doses and cumulative exposure to IVC and voclosporin are found in online supplemental table 4.
Safety
The overall incidence of AEs at 3 and 6 months was higher in IVC- and MMF-treated participants of ALMS than in voclosporin-treated participants, although more voclosporin-treated participants reported AEs by preferred term of GFR decreased, hypertension and anaemia (table 3). The incidence of serious AEs was similar across treatments (table 3, online supplemental tables 5 and 6).
Table 3. Adverse event summary table by treatment arm.
| n (%) | 3 Months | 6 Months | ||||||
| ALMS | AURA-LV/ AURORA 1 | ALMS | AURA-LV/ AURORA 1 | |||||
| IVC (n=91) | MMF (n=88) | Total (N=179) | Voclosporin (N=179) | IVC (n=91) | MMF (n=88) | Total (N=179) | Voclosporin (N=179) | |
| Select AEs bysystemorganclass, n (%) | ||||||||
| Gastrointestinal disorders | 54 (59.3) | 46 (52.3) | 100 (55.9) | 62 (34.6) | 60 (65.9) | 54 (61.4) | 114 (63.7) | 69 (38.5) |
| Infections and infestations | 42 (46.2) | 53 (60.2) | 95 (53.1) | 75 (41.9) | 53 (58.2) | 64 (72.7) | 117 (65.4) | 97 (54.2) |
| Skin and subcutaneous tissue disorders | 43 (47.3) | 25 (28.4) | 68 (38.0) | 35 (19.6) | 51 (56.0) | 33 (37.5) | 84 (46.9) | 43 (24.0) |
| Musculoskeletal/ connective tissue disorders | 31 (34.1) | 27 (30.7) | 58 (32.4) | 35 (19.6) | 40 (44.0) | 33 (37.5) | 73 (40.8) | 44 (24.6) |
| Blood and lymphatic system disorders | 19 (20.9) | 13 (14.8) | 32 (17.9) | 21 (11.7) | 37 (40.7) | 21 (23.9) | 58 (32.4) | 33 (18.4) |
| Psychiatric disorders | 12 (13.2) | 15 (17.0) | 27 (15.1) | 4 (2.2) | 14 (15.4) | 15 (17.0) | 29 (16.2) | 6 (3.4) |
| Endocrine disorders | 10 (11.0) | 7 (8.0) | 17 (9.5) | 2 (1.1) | 10 (11.0) | 7 (8.0) | 17 (9.5) | 2 (1.1) |
| Reproductive system and breast disorders | 9 (9.9) | 6 (6.8) | 15 (8.4) | 4 (2.2) | 11 (12.1) | 7 (8.0) | 18 (10.1) | 4 (2.2) |
| Renal and urinary disorders | 6 (6.6) | 4 (4.5) | 10 (5.6) | 13 (7.3) | 8 (8.8) | 8 (9.1) | 16 (8.9) | 19 (10.6) |
| Select AEs bypreferredterm, n (%) | ||||||||
| Alopecia | 33 (36.3) | 9 (10.2) | 42 (23.5) | 6 (3.4) | 37 (40.7) | 10 (11.4) | 47 (26.3) | 11 (6.1) |
| Leucopenia | 12 (13.2) | 1 (1.1) | 13 (7.3) | 2 (1.1) | 23 (25.3) | 6 (6.8) | 29 (16.2) | 4 (2.2) |
| Hypertension | 8 (8.8) | 9 (10.2) | 17 (9.5) | 28 (15.6) | 11 (12.1) | 13 (14.8) | 24 (13.4) | 31 (17.3) |
| Cushingoid | 6 (6.6) | 4 (4.5) | 10 (5.6) | 2 (1.1) | 6 (6.6) | 4 (4.5) | 10 (5.6) | 2 (1.1) |
| Amenorrhea | 6 (6.6) | 1 (1.1) | 7 (3.9) | 0 | 6 (6.6) | 1 (1.1) | 7 (3.9) | 0 |
| Cushing’s syndrome | 3 (3.3) | 3 (3.4) | 6 (3.4) | 0 | 3 (3.3) | 3 (3.4) | 6 (3.4) | 0 |
| Neutropenia | 3 (3.3) | 1 (1.1) | 4 (2.2) | 2 (1.1) | 6 (6.6) | 1 (1.1) | 7 (3.9) | 3 (1.7) |
| Hyperglycaemia | 2 (2.2) | 1 (1.1) | 3 (1.7) | 1 (0.6) | 2 (2.2) | 3 (3.4) | 5 (2.8) | 1 (0.6) |
| Anaemia | 2 (2.2) | 7 (8.0) | 9 (5.0) | 14 (7.8) | 6 (6.6) | 10 (11.4) | 16 (8.9) | 22 (12.3) |
| Dysuria | 2 (2.2) | 0 | 2 (1.1) | 1 (0.6) | 3 (3.3) | 0 | 3 (1.7) | 2 (1.1) |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 1 (1.1) | 0 | 1 (0.6) | 0 |
| GFR decreased | 0 | 0 | 0 | 33 (18.4) | 0 | 0 | 0 | 44 (24.6) |
| Osteoporosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cataract | 1 (1.1) | 1 (1.1) | 2 (1.1) | 2 (1.1) | 2 (2.2) | 1 (1.1) | 3 (1.7) | 4 (2.2) |
| Overallincidence of AEs | ||||||||
| Overall AEs | 83 (91.2) | 83 (94.3) | 166 (92.7) | 149 (83.2) | 87 (95.6) | 84 (95.5) | 171 (95.5) | 161 (89.9) |
| Serious AEs | 11 (12.1) | 20 (22.7) | 31 (17.3) | 26 (14.5) | 14 (15.4) | 22 (25.0) | 36 (20.1) | 35 (19.6) |
| Death | 0 | 4 (4.5) | 4 (2.2) | 6 (3.4) | 0 | 4 (4.5) | 4 (2.2) | 7 (3.9) |
Propensity score methodology was used to generate two groups of matched participants (N=179) from the ALMS (IVC and MMF) and AURA-LV/AURORA 1 (voclosporin) studies based on the following parameters: age, duration of lupus nephritis, duration of SLE, albumin, C3, C4, creatinine, anti-dsDNA, eGFR, UPCR, biopsy class, sex, and geographical region. (AEs) occurred on or after the first dose of study drug up to either 3 or 6 months months of treatment and were coded by System Organ Class and Preferred Term using MedDRA v9.1 (ALMS), v17.0 (AURA-LV) and v20.0 (AURORA 1). AEs were selected for inclusion in this table to evaluate the impact of IVC, MMF, voclosporin, and GCs on these organ systems. Assignation of AEs wereas at the discretion of the study investigator and wereas not based on set definitions or changes from baseline values.
AE, adverse event; ALMSAspreva Lupus Management StudydsDNA, double-stranded DNA; GC, glucocorticoid; GFR, glomerular filtration rate; IVC, intravenous cyclophosphamide; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus; UPCR, urine protein creatinine ratio
Across organ systems, the IVC and MMF arms had greater rates of AEs known to be related to IVC, MMF and GCs (ie, those of the skin, gastrointestinal, endocrine, psychiatric, musculoskeletal and reproductive systems). Over the 6-month period, there were no deaths in the IVC arm; there were 4 (4.5%; one each of interstitial lung disease, gastrointestinal tuberculosis, respiratory tract infection, pneumonia) deaths in the MMF group and 7 (3.9%; 2 each of pneumonia and pulmonary embolism, one each of tuberculous pericarditis, acute respiratory distress syndrome, multiple organ dysfunction syndrome) deaths in the voclosporin group (table 3). All deaths in the voclosporin arm occurred in participants of AURA-LV; the increased incidence of death reported in this study has been explained elsewhere.11
Estimated glomerular filtration rate
Due to the known haemodynamic effects of calcineurin inhibition, there was a decrease in eGFR (mean decrease in eGFR −2.3 mL/min/1.73 m2) within the first 4 weeks of treatment with voclosporin, after which the trajectory of eGFR remained stable, and mean values remained in the normal range (figure 2). Mean corrected eGFR in voclosporin-treated participants was 77.4 mL/min/1.73 m2 at baseline and 78.2 mL/min/1.73 m2 at week 24, compared with 77.4 mL/min/1.73 m2 and 84.2 mL/min/1.73 m2 in the MMF arm, and 78.3 mL/min/1.73 m2 and 82.3 mL/min/1.73 m2 in the IVC arm. Of the 44 (24.6%) AEs of GFR decreased reported in the voclosporin arm, one was considered serious.
Figure 2. Corrected eGFR over 6 months by treatment arm. Propensity score methodology was used to generate two groups of matched participants (n=179) from the ALMS (IVC and MMF) and AURA-LV/AURORA 1 (voclosporin) studies based on the following parameters: age, duration of lupus nephritis, duration of SLE, albumin, C3, C4, creatinine, anti-dsDNA, eGFR, UPCR, biopsy class, sex and geographical region. Only participants with available data at the specified time points are included in the analysis. To account for baseline kidney hyperfiltration known to precede chronic kidney disease, analyses of corrected eGFR (utilising the Modification of Diet in Renal Disease (ALMS) and Chronic Kidney Disease Epidemiology Collaboration (AURA-LV/AURORA 1) equations) constrained all values to a maximum of 90 mL/min/1.73 m2 to mitigate the risk of false negativity. Change from baseline measures were calculated using least square means and 95% CIs calculated from a general linear model including covariates for treatment group and baseline value. ALMS, Aspreva Lupus Management Study; C, complement; dsDNA, double-stranded deoxyribonucleic acid; eGFR, estimated glomerular filtration rate; IVC, intravenous cyclophosphamide; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus; UPCR, urine protein creatinine ratio.
Efficacy
Voclosporin was associated with earlier and greater reductions in proteinuria than IVC or MMF. At 3 months, significantly more participants treated with voclosporin achieved a ≥25% UPCR reduction from baseline (figure 3, table 4, p<0.005). At 6 months, the proportion of participants achieving UPCR ≤0.5 g/g was numerically greater in the voclosporin arm compared to the IVC arm, although the difference did not reach statistical significance; the proportion of participants achieving UPCR ≤0.5 g/g was significantly greater in the voclosporin arm compared to the MMF arm (p<0.005). The proportion of participants achieving a ≥50% UPCR reduction from baseline was significantly greater in voclosporin-treated participants than in either IVC- or MMF-treated participants (figure 3, table 4, p=0.029, p=0.013, respectively).
Figure 3. Efficacy outcomes at 3 and 6 months by treatment arm. Propensity score methodology was used to generate two groups of matched participants (N=179) from the ALMS (IVC and MMF) and AURA-LV/AURORA 1 (voclosporin) studies based on the following parameters: age, duration of lupus nephritis, duration of SLE, albumin, C3, C4, creatinine, anti-dsDNA, eGFR, UPCR, biopsy class, sex and geographical region. The proportion of participants achieving UPCR outcomes at 3 and 6 months was calculated using a logistic regression model with terms for treatment arm, baseline UPCR, biopsy class and region. *p<0.05; **p<0.005; ***p<0.0005. ALMS, Aspreva Lupus Management Study; C, complement; dsDNA, double-stranded deoxyribonucleic acid; eGFR, estimated glomerular filtration rate; IVC, intravenous cyclophosphamide; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus; UPCR, urine protein creatinine ratio.
Table 4. Efficacy outcomes at 3 and 6 months by treatment arm.
| ALMS | AURA-LV/AURORA 1 | ||
| IVC (n=91) | MMF (n=88) | VCS (N=179) | |
| At3 months | |||
| UPCR reduction of ≥25%, n (%) | 56 (61.5) | 68 (77.3) | 164 (91.6) |
| OR (95% CI), p value VCS vs IVC or MMF | 6.88 (3.48,>9.99), p<0.001 | 3.06 (1.47, 6.37), p=0.003 | |
| At6 months | |||
| UPCR ≤0.5 g/g, n (%) | 27 (29.7) | 15 (17.0) | 68 (38.0) |
| OR (95% CI), p value VCS vs IVC or MMF | 1.54 (0.87, 2.73), p=0.141 | 3.24 (1.68, 6.25), p<0.001 | |
| UPCR reduction of ≥50%, n (%) | 52 (57.1) | 50 (56.8) | 127 (70.9) |
| OR (95% CI), p value VCS vs IVC or MMF | 1.83 (1.06, 3.14), p=0.029 | 2.00 (1.16, 3.45), p=0.013 | |
Propensity score methodology was used to generate two groups of matched participants (N=179) from the ALMS (IVC and MMF) and AURA-LV/AURORA 1 (voclosporin) studies based on the following parameters: age, duration of lupus nephritis, duration of SLE, albumin, C3, C4, creatinine, anti-dsDNA, eGFR, UPCR, biopsy class, sex, and geographical region. The proportion of participants achieving UPCR outcomes at 3 and 6 months months wereas calculated using a logistic regression model with terms for treatment arm, baseline UPCR, biopsy class, and region. ORs >unity indicate a benefit of voclosporin treatment over IVC or MMF.
ALMSAspreva Lupus Management StudyC, complement; dsDNA, double-stranded DNA; eGFR, estimated glomerular filtration rate; IVC, intravenous cyclophosphamide; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus; UPCR, urine protein creatinine ratio; VCS, voclosporin
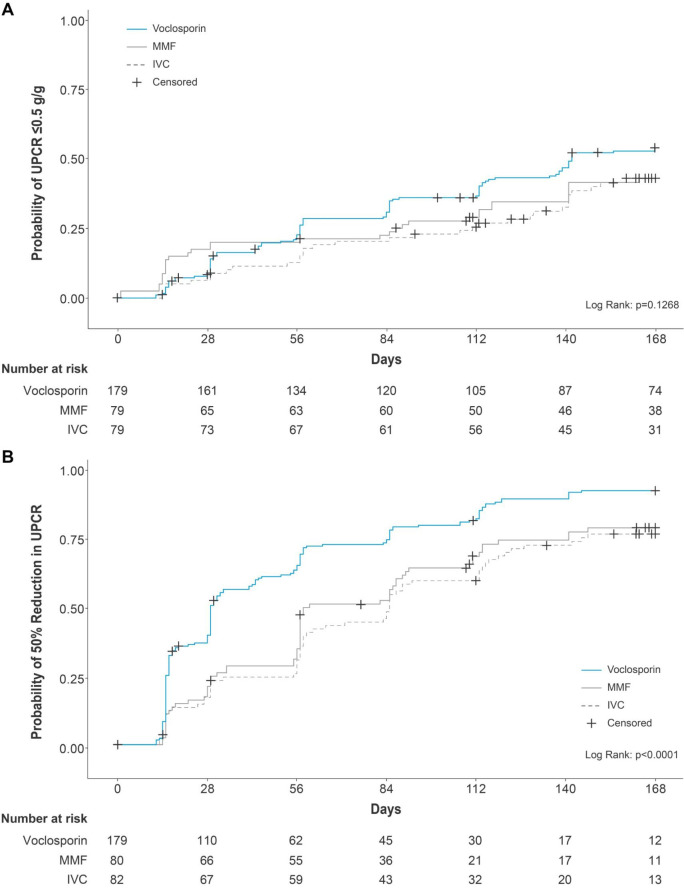
Reduction in UPCR to ≤0.5 g/g at any time during the study period was achieved by 52% of voclosporin-treated participants compared with 40.5% and 41.8% of participants treated with IVC and MMF, respectively; the median time to this endpoint for the voclosporin group was 142 days (95% CI 115.0, not determinable); median times were not determinable for either the IVC or MMF arm as less than 50% of participants achieved the endpoint within the 6-month study period (voclosporin vs IVC: HR 1.43; 95% CI 0.96, 2.13; p=0.083 and voclosporin vs MMF: HR 1.39; 95% CI 0.93, 2.07; p=0.105; figure 4A, online supplemental table 7).
Figure 4. (A) Kaplan-Meier curves for probability of UPCR ≤0.5 g/g by treatment arm. (B) Kaplan-Meier curves for probability of UPCR reduction ≥50% from baseline by treatment arm. Propensity score methodology was used to generate two groups of matched participants (N=179) from the ALMS (IVC and MMF) and AURA-LV/AURORA 1 (voclosporin) studies based on the following parameters: age, duration of lupus nephritis, duration of SLE, albumin, C3, C4, creatinine, anti-dsDNA, eGFR, UPCR, biopsy class, sex and geographical region. Median time to event (95% CI) calculated using Kaplan-Meier methods. Participants who did not achieve the event were censored on the day of their last available UPCR assessment. HRs and 95% CIs were derived from a Cox proportional hazards model with terms for treatment group, baseline UPCR, biopsy class, mycophenolate mofetil use at screening and region. ALMS, Aspreva Lupus Management Study; C, complement; dsDNA, double-stranded deoxyribonucleic acid; eGFR, estimated glomerular filtration rate; IVC, intravenous cyclophosphamide; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus; UPCR, urine protein creatinine ratio.
The proportion of participants achieving ≥50% reduction in UPCR from baseline at any time during the study was 89.9%, 74.4%, and 76.3%, in the voclosporin, MMF, and IVC arms, respectively, with median times to this endpoint of 29, 85, and 58 days (voclosporin vs IVC: HR 2.02; 95% CI 1.50, 2.73; p<0.001; voclosporin vs MMF: HR 1.67; 95% CI 1.24, 2.25; p<0.001; figure 4, online supplemental table 7).
The LS mean change (SE) from baseline in UPCR over the 6-month period was −2.98 (0.15) g/g in voclosporin-treated participants compared with −2.30 (0.22) g/g and −2.75 (0.22) g/g in the IVC and MMF arms, respectively (online supplemental figure 2).
A comparison of outcomes by study (ALMS vs AURA-LV/AURORA 1) also demonstrated a significant benefit with voclosporin-based, triple immunosuppressive therapy across all efficacy endpoints (online supplemental table 8 and figures 3 and 4).
Discussion
This analysis used propensity score methodology to compare early safety and efficacy outcomes associated with the voclosporin-based triple immunosuppressive regimen used in the AURA-LV and AURORA 1 studies with the high-dose GC-based dual immunosuppressive therapy regimens used in ALMS.
We acknowledge that not all dual immunosuppressive therapy regimens require drugs to be given at high doses; indeed, MMF at 2 g/day and low-dose cyclophosphamide dosing based on the Euro-Lupus regimen are also commonly used in clinical practice.21 However, compared with the high-dose GC-based dual immunosuppressive therapy regimens used in ALMS, voclosporin-based triple immunosuppressive therapy resulted in fewer AEs overall and greater and earlier reductions in proteinuria. Taken together with results from the original AURA-LV and AURORA 1 studies and the AURORA 2 continuation study, these data reinforce the feasibility of using low doses of GCs and MMF to treat LN when combined with voclosporin as a third agent.11,13
The safety profile of voclosporin in this study was concordant with that observed in the overall AURA-LV and AURORA-1 trials. Consistent with the known haemodynamic effect of calcineurin inhibition on the kidney vasculature, the voclosporin arm of this analysis saw a small decrease in eGFR within the first 4 weeks of treatment and also reported higher numbers of AEs of GFR decreased and hypertension. However, after the initial reduction in eGFR, mean values remained stable and within the normal range over the 6-month study period (figure 2). In AURORA 2, in which participants were treated with voclosporin for up to 24 months following AURORA 1, long-term renal function was evaluated by calculating the change in eGFR slope during AURORA 2, taking into account the expected acute and early changes in eGFR that occurred in the first year of treatment in AURORA 1. From 12 months onward, the corrected eGFR slope was −0.2 mL/min/1.73 m2 (95% CI −3.0, 2.7) in the voclosporin arm compared with −5.4 mL/min/1.73 m2 (95% CI −8.4, 2.3) in the control arm, suggesting that voclosporin may have a role in kidney preservation with long-term use.15 Additional data from a repeat biopsy substudy of AURORA 2 showed that exposure to voclosporin did not result in CNI-associated nephrotoxicity based on histopathologic evaluation over approximately 18 months of treatment.14
Safety outcomes from the ALMS participants of this analysis were consistent with the known toxicities of pulse IVC and MMF.22,24 The IVC arm had higher rates of AEs associated with the skin, blood and lymphatic, and reproductive systems including alopecia, leucopenia, amenorrhea and neutropenia. As expected, participants in the MMF arm of this analysis had higher rates of AEs of the gastrointestinal tract and infections than participants treated with either IVC or voclosporin.
The incidence of AEs leading to death in this analysis was consistent with that observed in the parent studies. There were fewer deaths in the IVC arm and a similar incidence of deaths in the MMF and voclosporin arms. In the original ALMS, there were 5 (2.8%) deaths in the IVC arm and 9 (4.9%) in the MMF arm, compared with a total of 11 (4.1%) deaths in the voclosporin arms (23.7 mg twice daily) of the AURA-LV/AURORA 1 studies.3 11 12
While the utility of GCs has been recognised for decades, concerns about their safety have brought their use in active LN into question.7 25 26 In SLE, increased end-organ damage and mortality are associated with cumulative GC exposure independent of disease severity or duration.27 In the current analysis, mean exposure to GCs was more than twofold higher in the IVC and MMF arms over the 6-month period than in voclosporin-treated participants of AURA-LV/AURORA 1, and mean daily doses of GCs at 3 and 6 months were threefold and nearly twofold greater, respectively, in ALMS. These data reflect differences in the protocol-specified, GC-tapering regimens used in ALMS compared with the voclosporin trials that were aligned with current standards of care at the time each study was conducted. Consistent with this increased exposure, both arms of ALMS experienced greater rates of AEs associated with GC toxicity, including AEs related to psychiatric disorders, and the endocrine, musculoskeletal and connective tissue systems. Further, participants of ALMS had higher rates of Cushing’s syndrome, cushingoid disease and hyperglycaemia than voclosporin-treated participants. Differences in the safety profiles appeared as early as 3 months and persisted throughout the study.
Recent updates to guidelines on the management of LN suggest tapering GCs to ≤5 mg/day for maintenance dosing depending on the severity of the initial disease.1 2 In this study, and consistent with each study’s respective GC-tapering protocol, 1.1% and 0% of the IVC and MMF arms of ALMS, respectively, achieved a dose of 2.5 mg/day by month 6 compared with 71.5% of the voclosporin group. Yet, even with the lower doses of GCs and MMF administered in AURA-LV and AURORA 1, these participants still achieved greater and earlier reductions in proteinuria than ALMS participants.
Multitargeted therapy has become an increasingly viable and attractive treatment approach in LN, in part due to the ability to address the varied mechanisms underlying the disease.28,30 For example, CNIs, including voclosporin, demonstrate immunosuppressive activity through their effect on T cells and have also been shown to directly protect the podocyte cytoskeleton by inhibiting the calcineurin-mediated dephosphorylation of synaptopodin, stabilising the podocyte and safeguarding against proteinuria.31,33 Further, a multitargeted approach decreases exposure to dose-dependent toxicities associated with the use of individual or dual therapy regimens used at higher doses. In the voclosporin trials, triple therapy with voclosporin and low doses of both GCs and MMF resulted in superior complete response rates compared with low-dose GCs and MMF alone.11,13 It has been argued that the control arms of the voclosporin studies may have been underdosed and comparing voclosporin-based triple immunosuppressive therapy with higher doses of GCs and MMF would yield different results. The question may never be settled in the absence of a prospective, randomised-controlled trial. Yet, we have attempted to address this issue by analysing data from propensity score-matched participants of three of the largest, prospective, randomised-controlled trials in LN.
While propensity score methodology is useful when head-to-head trials are not feasible, there are limitations. Propensity analyses are limited in their ability to adequately represent the incidence of rare safety findings, such as certain AEs and serious AEs, including death. It must be noted that the populations of both the ALMS and pooled AURA-LV/AURORA 1 datasets were substantially reduced in the propensity matching. While the safety data presented here are in line with previous studies, caution should be made when interpreting safety events occurring in small numbers of participants. Additional limitations associated with propensity score matching include the ability to only control for specifically chosen variables. An inherent bias is therefore associated with the exclusion of other variables and of unmatched subgroups of participants, which may change the target population of interest and the outcomes observed.
It should also be noted that there was a 15-year time difference between ALMS and AURA-LV/AURORA 1 during which standards of care changed and certain therapies, including monoclonal antibodies, became more widely available for treating LN. For example, differences in clinical practice may be reflected in the more than twofold higher rate of baseline hydroxychloroquine use in the voclosporin studies compared with ALMS, as more recent data have demonstrated an association between hydroxychloroquine use and reduced risk of renal flares and improved response rates in LN.34,36 Further, ALMS participants could not be exposed to MMF therapy within 12 months prior to randomisation, whereas 48.1% of the voclosporin arm had been receiving MMF at screening. These factors were unable to be controlled through propensity score matching. However, it is possible that AURA-LV/AURORA 1 participants may have received more pretreatment than ALMS participants and therefore been more resistant to therapy or characterised by more refractory disease; despite this, this analysis showed treatment with a voclosporin-based regimen resulted in greater and earlier impacts on proteinuria while improving overall safety outcomes.
Lastly, this analysis only documents safety and efficacy outcomes during the first 6 months of treatment. Yet, it is informative that GC-related toxicities were already apparent in the ALMS participants as early as 3 months. Given the growing body of evidence suggesting that GC-related toxicity is associated both with dose and duration of treatment, the early appearance of toxicities in ALMS is highly suggestive of greater toxicity with continued use. Differences in efficacy were observed across the three treatment arms as early as 3 months as well. This is noteworthy given that early reduction in proteinuria has been shown to be predictive of improved long-term kidney outcomes in LN as well as other proteinuric states.37,40
In conclusion, over the first 6 months of treatment, voclosporin-based triple immunosuppressive therapy (voclosporin, low-dose MMF and GCs) was associated with a better overall safety profile than the high-dose GC-based dual-immunosuppressive therapy regimens used in ALMS, as well as greater and earlier reductions in proteinuria. These data support treatment guidelines that recommend both minimising patient exposure to GCs and using triple immunosuppressive therapy regimens as initial therapy in active LN.
supplementary material
Acknowledgements
The manuscript was drafted by Rachelle Steele (MediComm Partners, London, UK). Aurinia Pharmaceuticals Inc. provided funding for the medical writing support.
Footnotes
Funding: This study was funded by Aurinia Pharmaceuticals Inc.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This is a retrospective analysis of results from three clinical trials involving multiple international study sites—all original study sites obtained local IRB or ethics committee approval as noted in the original publications for each trial. Participants gave informed consent to participate in the study before taking part.
Data availability free text: The aggregated data underlying this article and study protocols will be shared with researchers on reasonable request to the corresponding author. Data will be shared through a secure online platform after signing a data access agreement.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Presented at: The work has been presented previously in abstract form at American College of Rheumatology 2023, LUPUS 2024, National Kidney Foundation 2024, European Renal Association 2024 and European League Against Rheumatism 2024.
Contributor Information
Maria Dall'Era, Email: maria.dallera@ucsf.edu.
Kenneth Kalunian, Email: kkalunian@health.ucsd.edu.
Neil Solomons, Email: neil@solomons.cc.
Matt Truman, Email: mtruman@auriniapharma.com.
Lucy S Hodge, Email: lhodge@auriniapharma.com.
Ernie Yap, Email: eyap@auriniapharma.com.
Anca D Askanase, Email: ada20@cumc.columbia.edu.
Data availability statement
Data are available upon reasonable request.
References
- 1.Fanouriakis A, Kostopoulou M, Andersen J, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. 2023 doi: 10.1136/ard-2024-226636. [DOI] [PubMed] [Google Scholar]
- 2.Rovin BH, Ayoub IM, Chan TM, et al. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the Management of Lupus Nephritis. Kidney Int. 2024;105:31–4. doi: 10.1016/j.kint.2023.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–12. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dooley MA, Jayne D, Ginzler EM, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365:1886–95. doi: 10.1056/NEJMoa1014460. [DOI] [PubMed] [Google Scholar]
- 5.Movahedi M, Beauchamp M-E, Abrahamowicz M, et al. Risk of Incident Diabetes Mellitus Associated With the Dosage and Duration of Oral Glucocorticoid Therapy in Patients With Rheumatoid Arthritis. Arthritis Rheumatol . 2016;68:1089–98. doi: 10.1002/art.39537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice JB, White AG, Scarpati LM, et al. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin Ther. 2017;39:2216–29. doi: 10.1016/j.clinthera.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Einarsdottir MJ, Ekman P, Molin M, et al. High Mortality Rate in Oral Glucocorticoid Users: A Population-Based Matched Cohort Study. Front Endocrinol (Lausanne) 2022;13:918356. doi: 10.3389/fendo.2022.918356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usiskin IM, Kyttaris VC. Estimating glucocorticoid-related morbidity in lupus nephritis using the glucocorticoid toxicity index. Lupus (Los Angel) 2023;32:565–70. doi: 10.1177/09612033231160969. [DOI] [PubMed] [Google Scholar]
- 9.van Gelder T, Huizinga RB, Lisk L, et al. Voclosporin: a novel calcineurin inhibitor with no impact on mycophenolic acid levels in patients with SLE. Nephrol Dial Transplant. 2022;37:917–22. doi: 10.1093/ndt/gfab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Palmisano M, Sun D, et al. Pharmacokinetic Disposition Difference Between Cyclosporine and Voclosporin Drives Their Distinct Efficacy and Safety Profiles in Clinical Studies. Clin Pharmacol. 2020;12:83–96. doi: 10.2147/CPAA.S255789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovin BH, Solomons N, Pendergraft WF, 3rd, et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019;95:219–31. doi: 10.1016/j.kint.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet. 2021;397:2070–80. doi: 10.1016/S0140-6736(21)00578-X. [DOI] [PubMed] [Google Scholar]
- 13.Arriens C, Teng YKO, Ginzler EM, et al. Update on the Efficacy and Safety Profile of Voclosporin: An Integrated Analysis of Clinical Trials in Lupus Nephritis. Arthritis Care & Research . 2023;75:1399–408. doi: 10.1002/acr.25007. [DOI] [PubMed] [Google Scholar]
- 14.Parikh SV, Abner C, Yap E, et al. Repeat Kidney Biopsies from the AURORA 2 Study of Voclosporin in Active Lupus Nephritis. J Am Soc Nephrol. 2023;34:9. doi: 10.1681/ASN.20233411S19a. [DOI] [Google Scholar]
- 15.Saxena A, Ginzler EM, Gibson K, et al. Safety and Efficacy of Long-Term Voclosporin Treatment for Lupus Nephritis in the Phase 3 AURORA 2 Clinical Trial. Arthritis Rheumatol. 2024;76:59–67. doi: 10.1002/art.42657. [DOI] [PubMed] [Google Scholar]
- 16.Furie R, Rovin BH, Houssiau F, et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N Engl J Med. 2020;383:1117–28. doi: 10.1056/NEJMoa2001180. [DOI] [PubMed] [Google Scholar]
- 17.Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219–28. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 18.Ugolini-Lopes MR, Seguro LPC, Castro MXF, et al. Early proteinuria response: a valid real-life situation predictor of long-term lupus renal outcome in an ethnically diverse group with severe biopsy-proven nephritis? Lupus Sci Med. 2017;4:e000213. doi: 10.1136/lupus-2017-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dall’Era M, Stone D, Levesque V, et al. Identification of biomarkers that predict response to treatment of lupus nephritis with mycophenolate mofetil or pulse cyclophosphamide. Arthritis Care Res (Hoboken) 2011;63:351–7. doi: 10.1002/acr.20397. [DOI] [PubMed] [Google Scholar]
- 20.Dall’Era M, Levesque V, Solomons N, et al. Identification of clinical and serological factors during induction treatment of lupus nephritis that are associated with renal outcome. Lupus Sci Med. 2015;2:e000089. doi: 10.1136/lupus-2015-000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121–31. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 22.Chan TM, Li FK, Tang CSO, et al. Efficacy of Mycophenolate Mofetil in Patients with Diffuse Proliferative Lupus Nephritis. N Engl J Med. 2000;343:1156–62. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 23.Goswami RP, Sircar G, Sit H, et al. Cyclophosphamide Versus Mycophenolate Versus Rituximab in Lupus Nephritis Remission Induction. J Clin Rheumatol . 2019;25:28–35. doi: 10.1097/RHU.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 24.Mok CC. Con: Cyclophosphamide for the treatment of lupus nephritis. Nephrol Dial Transplant. 2016;31:1053–7. doi: 10.1093/ndt/gfw068. [DOI] [PubMed] [Google Scholar]
- 25.Mejía-Vilet JM, Ayoub I. The Use of Glucocorticoids in Lupus Nephritis: New Pathways for an Old Drug. Front Med (Lausanne) 2021;8:622225. doi: 10.3389/fmed.2021.622225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeher M, Doria A, Lan J, et al. Efficacy and safety of enteric-coated mycophenolate sodium in combination with two glucocorticoid regimens for the treatment of active lupus nephritis. Lupus (Los Angel) 2011;20:1484–93. doi: 10.1177/0961203311418269. [DOI] [PubMed] [Google Scholar]
- 27.Davidson JE, Fu Q, Rao S, et al. Quantifying the burden of steroid-related damage in SLE in the Hopkins Lupus Cohort. Lupus Sci Med. 2018;5:e000237. doi: 10.1136/lupus-2017-000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Zhang H, Liu Z, et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med. 2015;162:18–26. doi: 10.7326/M14-1030. [DOI] [PubMed] [Google Scholar]
- 29.Rovin BH, Furie R, Teng YKO, et al. A secondary analysis of the Belimumab International Study in Lupus Nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int. 2022;101:403–13. doi: 10.1016/j.kint.2021.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Anders HJ, Saxena R, Zhao MH, et al. Lupus nephritis. Nat Rev Dis Primers. 2020;6:7. doi: 10.1038/s41572-019-0141-9. [DOI] [PubMed] [Google Scholar]
- 31.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–8. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumlin J, Klein J, Viel J. Efficacy of voclosporin in maintaining podocyte function and viability in a rat steptozotocin model of diabetic nephropathy. 14th Biennial International Podocyte Conference. Glomerular Dis. 2023 doi: 10.1159/000530913. [DOI] [Google Scholar]
- 33.Fruman DA, Klee CB, Bierer BE, et al. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci U S A. 1992;89:3686–90. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pons-Estel GJ, Alarcón GS, McGwin G, Jr, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: data from LUMINA, a multiethnic US cohort. Arthritis Rheum. 2009;61:830–9. doi: 10.1002/art.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galindo-Izquierdo M, Rodriguez-Almaraz E, Pego-Reigosa JM, et al. Characterization of Patients With Lupus Nephritis Included in a Large Cohort From the Spanish Society of Rheumatology Registry of Patients With Systemic Lupus Erythematosus (RELESSER) Medicine (Baltimore) 2016;95:e2891. doi: 10.1097/MD.0000000000002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sisó A, Ramos-Casals M, Bové A, et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus (Los Angel) 2008;17:281–8. doi: 10.1177/0961203307086503. [DOI] [PubMed] [Google Scholar]
- 37.Marques F, Reis J, Godinho I, et al. Impact of Early Proteinuria Reduction in Glomerular Disease and Decline of Kidney Function: A Retrospective Cohort. J Clin Med. 2022;11:5968. doi: 10.3390/jcm11195968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anders H-J, Davis JM, Thurau K. Nephron Protection in Diabetic Kidney Disease. N Engl J Med. 2016;375:2096–8. doi: 10.1056/NEJMcibr1608564. [DOI] [PubMed] [Google Scholar]
- 39.de Zeeuw D, Remuzzi G, Parving H-H, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309–20. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 40.Keane WF, Brenner BM, de Zeeuw D, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63:1499–507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.