Abstract
BACKGROUND & AIMS:
There are no contemporary large-scale studies evaluating the burden of Helicobacter pylori in the United States according to detailed demographics. The primary objective was to evaluate H pylori positivity in a large national healthcare system according to individual demographics and geography.
METHODS:
We conducted a nationwide retrospective analysis of adults in the Veterans Health Administration who completed H pylori testing between 1999 and 2018. The primary outcome was H pylori positivity overall, as well as according to zip code–level geography, race, ethnicity, age, sex, and time period.
RESULTS:
Among 913,328 individuals (mean, 58.1 years; 90.2% male) included between 1999 and 2018, H pylori was diagnosed in 25.8%. Positivity was highest in non-Hispanic black (median, 40.2%; 95% confidence interval [CI], 40.0%–40.5%) and Hispanic (36.7%; 95% CI, 36.4%–37.1%) individuals and lowest in non-Hispanic white individuals (20.1%; 95% CI, 20.0%–20.2%). Although H pylori positivity declined in all racial and ethnic groups over the timeframe, the disproportionate burden of H pylori in non-Hispanic black and Hispanic compared with non-Hispanic white individuals persisted. Approximately 4.7% of the variation in H pylori positivity was explained by demographics, with race and ethnicity accounting for the vast majority.
CONCLUSIONS:
The burden of H pylori is substantial in the United States among veterans. These data should (1) motivate research aimed at better understanding why marked demographic differences in H pylori burden persist so that mitigating interventions may be implemented and (2) guide resource allocation to optimize H pylori testing and eradication in high-risk groups.
Keywords: Health Status Disparities, Public Health, Infectious Disease, Gastric Neoplasms, Gastrointestinal Diseases, Disparities/Disparity
An estimated 4.4 billion people are infected with Helicobacter pylori, making it the most common chronic bacterial infection worldwide.1 H pylori, a human carcinogen, accounts for an estimated 80%–90% of gastric cancers globally.2 Successful H pylori eradication is associated with a reduced risk of serious downstream clinical consequences, including gastric cancer and complications of peptic ulcer disease, compared with persistent infection.3 However, the majority of individuals infected with H pylori are asymptomatic or minimally symptomatic and therefore may go undiagnosed unless they are tested.
H pylori positivity varies significantly depending on the population; the reasons underlying this variation are incompletely understood, but demographic, socioeconomic, geographic, topographic, and possibly genetic factors contribute.4–6 One meta-analysis spanning 1970–2016 reported that the highest pooled H pylori positivity rates were in Africa (79.1%; 95% confidence interval [CI], 62.6%–95.6%), whereas the lowest were in North America (37.1%; 95% CI, 32.3%–41.9%), Western Europe (34.3%; 95% CI, 31.3–37.2%), and Oceania(24.4%; 95% CI, 18.5%–30.4%).1 A more recent meta-analysis spanning 1980–2022 reported similar findings, particularly in North America (36.2%; 95% CI, 22.6%–49.9%).7 The quality and depth of data informing these estimates varied widely.1,7 Both studies acknowledged and exposed the lack of robust epidemiologic estimates in many regions of the world, particularly in countries composed of racially and ethnically diverse populations, especially the United States (U.S.). Of the limited U.S. studies included in the meta-analysis by Li et al,7 the largest study was a cross-sectional study of 1,289,641 individuals from a pathology database who had gastric biopsies between 2009 and 2018 and reported an overall H pylori prevalence of 10% based on histology. Apart from this database, the next largest U.S. study evaluated H pylori seroprevalence in 4 Hispanic/Latino communities comprising 16,144 individuals (overall 57% sero-prevalence).8 In the earlier meta-analysis by Hooi et al,1 the largest U.S. study comprised 7465 participants from the National Health and Nutritional Examination Survey (NHANES, 1988–1991) who had banked sera tested for H pylori antibodies. The early time period is relevant because H pylori was not formally discovered until 1982, and recommendations related to H pylori eradication treatment were not included in U.S. guidelines until the mid-late 1990s.9–11 There are no contemporary large-scale nationwide U.S. studies evaluating the descriptive epidemiology of H pylori by detailed demographics, including race and ethnicity, and that evaluate both serologic and non-serologic H pylori testing data over time.12
Closing the gap with high-quality epidemiologic data may improve H pylori–associated outcomes by redirecting resources to disproportionately affected groups. Such data would also provide the foundation for investigations aimed at understanding both fixed and modifiable determinants underlying the differential risk for H pylori susceptibility, treatment outcomes, and complications of chronic H pylori infection. Accordingly, the primary objective of this study was to report the burden of H pylori in the U.S. based on geography, race, ethnicity, age, and sex over the past 2 decades. The secondary aim was to quantify the amount of variation in H pylori positivity that is attributable to these demographic variables. For this analysis, we used nationwide individual-level data from the Veterans Health Administration (VHA), one of the largest integrated healthcare systems in the U.S.
Methods
The local institutional review boards at the Veterans Affairs (VA) Tennessee Valley and San Diego Healthcare Systems, as well as the Research and Development Committees, approved this study with a waiver of informed consent.
Cohort Construction and Data Abstraction
We identified adult U.S. veterans receiving routine care through the VHA, where routine care was defined as at least 1 medical encounter or completed laboratory test between 1999 and 2018. From this cohort, we selected veterans aged ≥18 years who had completed serologic or non-serologic H pylori testing at the VHA. The cohort of tested individuals comprised the primary analytic cohort. Hereafter, we use the term H pylori positivity as opposed to prevalence to describe H pylori burden in this study since we evaluated individuals who completed clinical testing for H pylori (ie, the denominator is not the entire VHA population). A determination of true H pylori population prevalence would necessitate universal population-level testing, which is not clinically recommended in the U.S.13,14
H pylori testing included serologic and non-serologic methods. Non-serologic testing encompassed fecal H pylori antigen test, urea breath test, rapid urease test, Campylobacter-like organism (CLO) test, as well as evaluation of histology obtained via gastric biopsies performed during esophagogastroduodenoscopy (EGD), hereafter referred to as histology.
Each individual was categorized as H pylori positive or negative. Individuals were categorized as positive if they had at least 1 positive test by serology or non-serology (inclusive of histology). Individuals were categorized as negative if they were tested at least once but never satisfied conditions for a positive categorization. To verify the accuracy of H pylori testing and results, we performed iterative cycles of validation using manual review of the electronic health record as the reference standard, with the reviewer (SCS) blinded to the algorithm-generated results. The validation process is detailed in the Supplementary Methods. All methods achieved positive and negative predictive value of 98% or higher (95% CI for lower bound >94%).
We recorded the earliest date of any H pylori positive testing for H pylori positive individuals and the earliest date of negative testing for individuals categorized as H pylori negative. We recorded the respective age, as well as sex, race, ethnicity, and VHA modal station. The modal station was defined as the VHA station most often accessed by the veteran for all healthcare, including medical encounters, laboratory tests, and prescription fills.
Statistical Approach
H pylori positivity was calculated as the number of individuals testing positive for H pylori divided by the total number of individuals in the analytic cohort (either positive or negative). To determine H pylori positivity according to geography, we first categorized VHA stations based on major U.S. Census Regions, followed by individual U.S. state or territory, and then by city and zip code. We geocoded the zip codes associated with each VHA modal station using geonames.org to determine the latitude and longitude for each location.
To characterize the burden of H pylori, we constructed density maps illustrating H pylori positivity across the U.S. based on race, ethnicity, age group (separated at the cohort median age: ≥60 vs <60 years), and sex. Race categories included white, black, and “other”, where “other” comprised Asian, Native Hawaiian or other Pacific Islander, American Indian, or Alaska native (combined because of small counts). Ethnicity categories included Hispanic or non-Hispanic.
We used multivariable logistic regression analysis to estimate the independent association between H pylori positivity and geography, race and ethnicity, age, and sex. In addition, we used an ordinary least squares regression model to estimate the variation in H pylori positivity accounting for these same demographic factors. Variation was estimated by measuring the change in R2 that occurred with the individual removal of each of these variables from the model.
Acknowledging that H pylori positivity rates may have changed over time and to better understand contemporary patterns, we conducted a sensitivity analysis stratifying the analysis by the following time intervals: 1999–2006, 2007–2012, and 2013–2018. To ensure that individuals with more than one H pylori test over time were not duplicated, we considered the date of the first qualifying test for each individual based on the above definitions.
All analyses were conducted using R 4.0.2.
Results
Cohort Characteristics
Of 15,594,932 individuals receiving routine care through the VHA between 1999 and 2018, we identified 914,062 individuals who completed at least 1 H pylori test at the VHA. Of these, 734 were excluded for missing date of birth, sex, VHA station, or for being outside of the prespecified age range (18–110 years). The final analytic cohort comprised 913,328 individuals across the 48 contiguous states, Alaska, Hawaii, Philippines, and Puerto Rico and hereafter encompasses the primary cohort reported below. The mean age was 58.1 years (standard deviation [SD], 14.9), and the majority were male (90.2%) and non-Hispanic white (64.4%) (Table 1). A total of 496,918 individuals underwent serologic testing only (54.4% of the cohort) without evidence of non-serologic testing (Supplementary Figure 1).
Table 1.
Demographics According to H pylori Status for the Full Analytic Cohort (N = 913,328; 1999–2018) and Separated by Time Interval
| 1999–2018 | 1999–2006 | 2007–2012 | 2013–2018 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (no.) | Overall, N = 913,328 | H pylori negative, n = 677,788 | H pylori positive, n = 235,540 | Overall, N = 284,656 | H pylori negative, n = 182,501 | H pylori positive, n = 102,155 | Overall, N = 319,935 | H pylori negative, n = 243,396 | H pylori positive, n = 76,539 | Overall, N = 308,737 | H pylori negative, n = 251,891 | H pylori positive, n = 56,846 |
| Age, y, mean (SD) | 58.06 (14.88) | 57.44 (15.13) | 59.84 (13.96) | 59.30 (14.06) | 58.34 (14.37) | 61.01 (13.32) | 57.69 (15.01) | 57.18 (15.24) | 59.29 (14.13) | 57.30 (15.39) | 57.03 (15.53) | 58.47 (14.68) |
| Male sex, n (%) | 823,879 (90.2) | 606,643 (89.5) | 217,236 (92.2) | 263,494 (92.6) | 167,187 (91.6) | 96,307 (94.3) | 289,196 (90.4) | 218,903 (89.9) | 70,293 (91.8) | 271,189 (87.8) | 220,553 (87.6) | 50,636 (89.1) |
| Race and ethnicity, n (%) | ||||||||||||
| Hispanic | 60,766 (6.7) | 38,442 (5.7) | 22,324 (9.5) | 14,893 (5.2) | 7008 (3.8) | 7885 (7.7) | 21,021 (6.6) | 13,158 (5.4) | 7863 (10.3) | 24,852 (8.0) | 18,276 (7.3) | 6576 (11.6) |
| Non-Hispanic black | 154,083 (16.9) | 92,097 (13.6) | 61,986 (26.3) | 40,430 (14.2) | 19,561 (10.7) | 20,869 (20.4) | 55,203 (17.3) | 32,849 (13.5) | 22,354 (29.2) | 58,450 (18.9) | 39,687 (15.8) | 18,763 (33.0) |
| Non-Hispanic other | 53,649 (5.9) | 38,797 (5.7) | 14,852 (6.3) | 17,571 (6.2) | 11,051 (6.1) | 6520 (6.4) | 18,833 (5.9) | 13,906 (5.7) | 4927 (6.4) | 17,245 (5.6) | 13,840 (5.5) | 3405 (6.0) |
| Non-Hispanic white | 587,848 (64.4) | 469,949 (69.3) | 117,899 (50.1) | 173,856 (61.1) | 121,245 (66.4) | 52,611 (51.5) | 213,570 (66.8) | 174,947 (71.9) | 38,623 (50.5) | 200,422 (64.9) | 173,757 (69.0) | 26,665 (46.9) |
NOTE. Percentages represent column percentages (except H pylori positivity).
SD, standard deviation.
H pylori positivity was 25.8% overall (n = 235,540/913,328) (Table 1). Individuals who were H pylori positive, compared with individuals who were H pylori negative, were more often older (mean, 59.8 [SD, 14.0] vs 57.4 [15.1] years) and male (92.2% vs 89.5%) and less often non-Hispanic white.
H pylori Positivity by Geography
Overall.
H pylori positivity was highest in the South (especially Texas, Alabama, Mississippi, Kentucky, and Georgia) where several regions exceeded 40%. H pylori positivity in California and New York ranged between 20% and 40%, with the highest positivity rates centered in the greater Los Angeles (36%; 95% CI, 35.1%–36.9%) and Bronx (40%; 95% CI, 38.1%–41.9%) regions. In general, much of the West, Midwest, and Northeast, with the exception of some states, demonstrated H pylori positivity of less than 25% (Figure 1, Supplementary Table 1). There were notable differences in H pylori positivity when stratified by race, ethnicity, age group, sex, as detailed below, as well as time period (Supplementary Figure 2).
Figure 1.
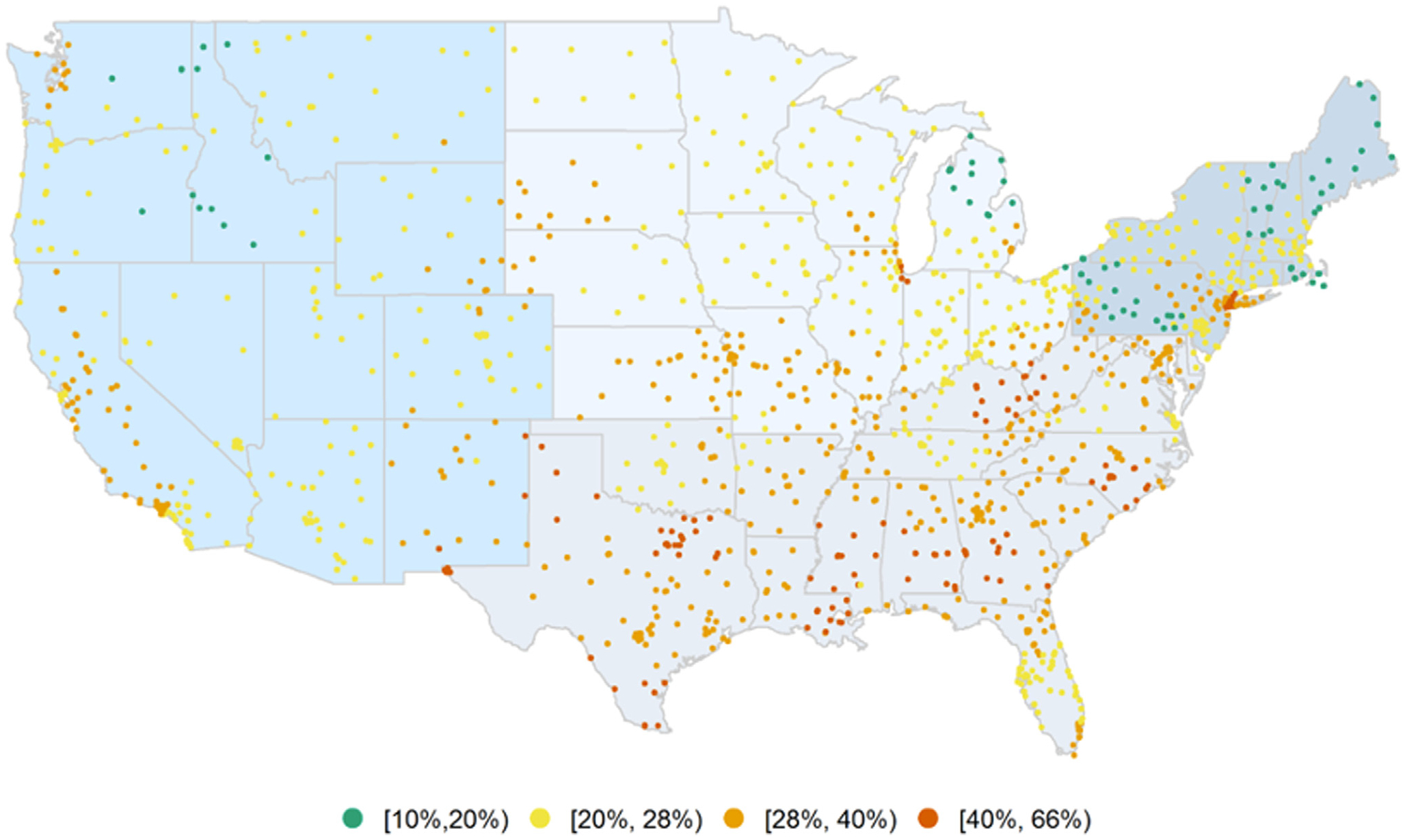
Geographic distribution of H pylori positivity overall in the United States, 1999–2018.
Race and ethnicity.
H pylori positivity differed on the basis of race and ethnicity (Figure 2, Table 2, Supplementary Table 2, Supplementary Figure 3). Irrespective of age, sex, and geography, H pylori positivity was highest in non-Hispanic black (median, 40.2%; 95% CI, 40.0%–40.5%) and Hispanic (36.7%; 95% CI, 36.4%–37.1%) individuals, followed by non-Hispanic other individuals (27.7%; 95% CI, 27.3%–28.1%). Non-Hispanic white individuals had the lowest H pylori positivity (20.1%; 95% CI, 20.0%–20.2%) (Table 2). This same pattern of racial and ethnic variation was seen irrespective of serologic vs non-serologic testing (Table 2), albeit with lower H pylori positivity on non-serologic vs serologic testing across all groups.
Figure 2.
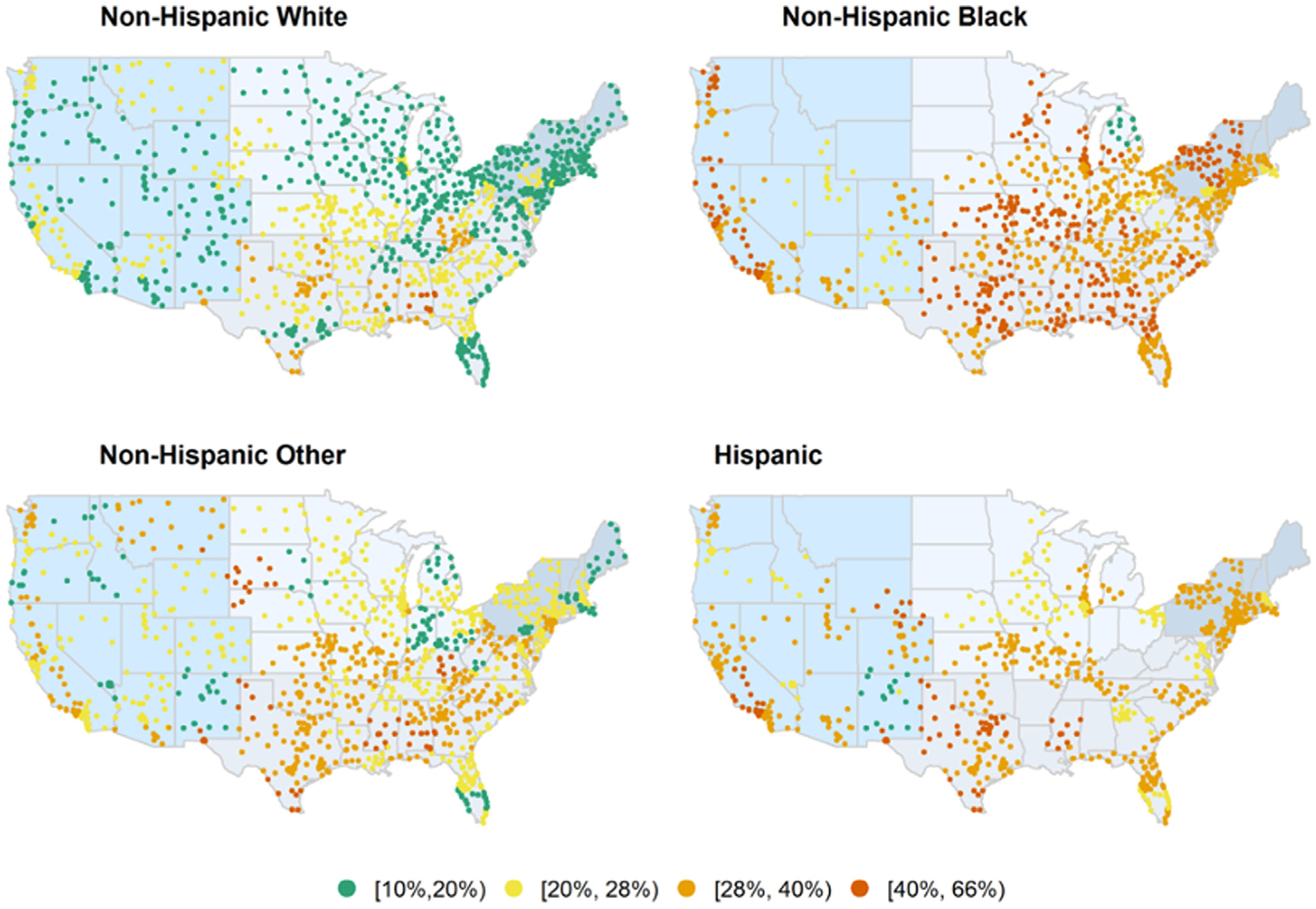
Geographic distribution of H pylori positivity in the United States according to race and ethnicity, 1999–2018.
Table 2.
H pylori Positivity According to Race and Ethnicity for the Full Analytic Cohort (N = 913,328; 1999–2018) and Separated by Time Interval and Testing Type (Serology vs Non-serologya)
| 1999–2018 | 1999–2006 | 2007–2012 | 2013–2018 | |
|---|---|---|---|---|
| Race and ethnicity | Mean (95% CI), % | Mean (95% CI), % | Mean (95% CI), % | Mean (95% CI), % |
| Combined testing | ||||
| Hispanic | 36.7 (36.4–37.1) | 52.9 (52.1–53.7) | 37.4 (36.8–38.1) | 26.5 (25.9–27) |
| Non-Hispanic black | 40.2 (40–40.5) | 51.6 (51.1–52.1) | 40.5 (40.1–40.9) | 32.1 (31.7–32.5) |
| Non-Hispanic other | 27.7 (27.3–28.1) | 37.1 (36.4–37.8) | 26.2 (25.5–26.8) | 19.7 (19.2–20.3) |
| Non-Hispanic white | 20.1 (20–20.2) | 30.3 (30–30.5) | 18.1 (17.9–18.2) | 13.3 (13.2–13.5) |
| Serology | ||||
| Hispanic | 46.9 (46.4–47.4) | 57.5 (56.6–58.4) | 45.2 (44.4–46.1) | 36.8 (35.9–37.7) |
| Non-Hispanic black | 50.9 (50.5–51.2) | 58.7 (58.2–59.3) | 49.1 (48.6–49.7) | 43.2 (42.6–43.8) |
| Non-Hispanic other | 35.3 (34.8–35.8) | 41.9 (41–42.7) | 32.5 (31.7–33.3) | 27.7 (26.8–28.7) |
| Non-Hispanic white | 27.1 (27.0–27.3) | 34.9 (34.6–35.1) | 23.5 (23.2–23.7) | 20.2 (20–20.5) |
| Non-serologya | ||||
| Hispanic | 30.2 (29.7–30.7) | 47.5 (45.9–49.1) | 33.1 (32.2–34.0) | 24.1 (23.5–24.8) |
| Non-Hispanic black | 35.2 (34.8–35.5) | 48.1 (47.2–48.9) | 36.5 (35.9–37.1) | 29.7 (29.2–30.1) |
| Non-Hispanic other | 22.2 (21.7–22.8) | 30.4 (29.0–31.8) | 23.2 (22.3–24.1) | 18.0 (17.3–18.8) |
| Non-Hispanic white | 15.5 (15.3–15.6) | 24.8 (24.4–25.1) | 15.4 (15.2–15.6) | 11.7 (11.5–11.8) |
Non-serology testing includes non-histologic and histologic testing.
H pylori positivity among non-Hispanic white individuals in several parts of the U.S., most notably the Northeast states, Michigan, Minnesota, North Dakota, some Western states, and South Florida, was generally <20%. In contrast, in the majority of states, H pylori positivity among non-Hispanic black individuals exceeded 35%, with the highest rates in Alabama (50.9%; 95% CI, 49.8%–52%) and Mississippi (54%; 95% CI, 52.9%–55.1%). Similarly, irrespective of geography, Hispanic and non-Hispanic other individuals generally had higher H pylori positivity compared with non-Hispanic white individuals (Figure 2, Supplementary Table 2, Supplementary Figure 3).
Age.
Individuals who were ≥60 years old had higher H pylori positivity compared with those who were younger than 60 years (overall, 27.1% vs 24.5%), irrespective of geography (Supplementary Figure 4, Supplementary Table 3). H pylori positivity according to race and ethnicity for those <60 vs ≥60 years old is provided in Supplementary Table 4. Notably, among non-Hispanic black individuals H pylori positivity was slightly higher in those younger vs older than 60 years (40.7% vs 39.5%), whereas among other racial and ethnic groups, positivity rates were lower in younger vs older individuals. In the age group <60 years, non-Hispanic black (40.7%) and Hispanic (34.4%) individuals had 2-fold or higher H pylori positivity compared with non-Hispanic white individuals (17.2%).
Sex.
Men had higher H pylori positivity compared with women, which was generally consistent irrespective of geography, although the magnitude of difference varied depending on the geographic location (Supplementary Figure 5, Supplementary Table 5).
H pylori Positivity Over Time
The demographic breakdown of the cohort according to H pylori status for each of the time intervals (1999–2006, 2007–2012, 2013–2018) is summarized in Table 1. H pylori positivity declined from 35.9% in 1999–2006 to 18.4% in 2013–2018. Similar patterns of geographic differences overall (Supplementary Figures 2) and based on race, ethnicity (Supplemental Figure 3, Table 2), age group (Supplementary Figure 4, Supplementary Table 4), and sex (Supplementary Figure 5) were observed, albeit with a lower magnitude of H pylori positivity across all subgroups over time. H pylori positivity remained significantly higher among non-Hispanic black, Hispanic, and non-Hispanic other compared with non-Hispanic white individuals across all time periods; however, the observed differential between non-Hispanic black (32.1%; 95% CI, 31.7%–32.5%) vs non-Hispanic white individuals (13.3%; 95% CI, 13.2%–13.5%) was greatest (2.4-fold higher) in the most contemporary period (2013–2018). Illustrative maps are provided in the Supplementary Figures.
Variation in H pylori Positivity According to Demographic Factors Over Time
To evaluate the impact of demographic variables on H pylori positivity, we included U.S. Census region, race, ethnicity, age, and sex in a logistic regression model where H pylori positivity was the outcome and evaluated this for the overall study period, 1999–2018, and each time interval (Table 3, Supplementary Figure 6). Approximately 4.7% of the variance in H pylori positivity was explained by these specific demographic factors. Race and ethnicity independently accounted for the majority of this variance (3.5%) (Supplementary Table 6). The strongest effect was seen among non-Hispanic blacks and Hispanics, with these groups having 17%–22% higher odds of H pylori positivity compared with non-Hispanic whites (Table 3). When stratified by time interval, race and ethnicity accounted for a larger percentage of the total variance in H pylori positivity that was explained by the basic demographics model (eg, race and ethnicity independently accounted for 3.05%, 4.53%, and 3.84% of the 4.92%, 5.35%, and 4.23% model variance in 1999–2006 vs 2007–2012 vs 2013–2018, respectively; Supplementary Table 6), findings that are also reflected in the logistic regression model stratified by time interval (Table 3).
Table 3.
Results of Logistic Regression Evaluating the Association Between Demographic Factors and H pylori Positivity, Stratified by Time Interval
| 1999–2018 | 1999–2006 | 2007–2012 | 2013–2018 | |||||
|---|---|---|---|---|---|---|---|---|
| Demographic variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Age, y (ref: <40) | ||||||||
| • 40 to <70 | 1.10 (1.09–1.10) | 1.09 (1.08–1.09) | 1.14 (1.14–1.15) | 1.14 (1.13–1.15) | 1.08 (1.07–1.08) | 1.07 (1.07–1.07) | 1.05 (1.04–1.05) | 1.04 (1.03–1.04) |
| • ≥70 | 1.13 (1.13–1.14) | 1.13 (1.13–1.14) | 1.20 (1.89–1.21) | 1.21 (1.20–1.22) | 1.10 (1.10–1.11) | 1.11 (1.11–1.12) | 1.04 (1.04–1.05) | 1.05 (1.05–1.06) |
| Male sex (ref: female) | 1.06 (1.06–1.06) | 1.06 (1.05–1.06) | 1.09 (1.09–1.10) | 1.07 (1.06–1.08) | 1.04 (1.04–1.05) | 1.04 (1.04–1.05) | 1.02 (1.02–1.03) | 1.03 (1.03–1.04) |
| Race or ethnicity (ref: white) | ||||||||
| • Hispanics | 1.18 (1.18–1.19) | 1.17 (1.17–1.18) | 1.25 (1.24–1.26) | 1.21 (1.20–1.22) | 1.21 (1.21–1.22) | 1.21 (1.20–1.21) | 1.14 (1.13–1.15) | 1.15 (1.14–1.15) |
| • Black | 1.22 (1.22–1.23) | 1.22 (1.22–1.22) | 1.24 (1.23–1.24) | 1.23 (1.23–1.24) | 1.25 (1.25–1.26) | 1.25 (1.24–1.25) | 1.21 (1.20–1.21) | 1.20 (1.20–1.21) |
| • Other | 1.08 (1.08–1.08) | 1.08 (1.08–1.09) | 1.07 (1.06–1.08) | 1.07 (1.06–1.08) | 1.08 (1.08–1.09) | 1.08 (1.08–1.09) | 1.07 (1.06–1.07) | 1.07 (1.07–1.08) |
| US Census Region (ref: Midwest) | ||||||||
| • Northeast | 0.98 (0.97–0.98) | 0.98 (0.97–0.98) | 0.95 (0.94–0.96) | 0.95 (0.95–0.96) | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) | 0.97 (0.97–0.98) | 0.97 (0.96–0.97) |
| • South | 1.07 (1.07–1.08) | 1.05 (1.05–1.05) | 1.10 (1.09–1.10) | 1.08 (1.08–1.09) | 1.08 (1.08–1.09) | 1.05 (1.05–1.06) | 1.03 (1.03–1.04) | 1.01 (1.00–1.01) |
| • West | 1.01 (1.01–1.01) | 1.00 (1.00–1.01) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | 1.04 (1.03–1.04) | 1.03 (1.03–1.04) | 0.98 (0.98–0.99) | 0.98 (0.97–0.98) |
NOTE. Values were rounded to the nearest one-hundredth. Because of high precision of these estimates, some confidence intervals only vary after the second decimal place.
OR, odds ratio.
Discussion
On the basis of a nationwide analysis of 913,328 veterans tested for H pylori, we report a high burden of disease, with more than 25% testing positive between 1999 and 2018. Furthermore, although contemporary data suggest a lower positivity rate (18.4%) among those tested for H pylori in recent years (2013–2018), wide variation based on race and ethnicity persists. Of concern, we found that the gradient of H pylori positivity between non-Hispanic black and non-Hispanic white individuals was widest in the most contemporary time period compared with 1999–2006, suggesting that the disparity gap is worsening. Although there was notable geographic variation in H pylori positivity, our data suggest this might be more so attributed to the geographic variation of race and ethnicity, particularly when considering more recent data.
Our study spanning the 2 decades between 1999 and 2018 extends prior data by reporting that the burden of H pylori remains substantial in the contemporary period, despite clinical guidelines recommending eradication treatment for H pylori when diagnosed. Based on post hoc analyses of H pylori serology in the NHANES III population-based study referenced above (1988–1991), non-Hispanic black and Mexican American individuals had the highest H pylori seropositivity (52.7% and 61.6%, respectively), whereas non-Hispanic white individuals had the lowest (26.2%)15; the H pylori seropositivity rates during the earliest time interval analyzed (1999–2006) in our study parallel these values, especially among non-Hispanic black (58.7%) and Hispanic (57.5%) individuals. In a follow-up NHANES study (1999–2000), the age-standardized H pylori seropositivity in Mexican American (64%) and non-Hispanic black (52%) individuals increased or stayed the same but decreased in non-Hispanic whites (21.2%).16 Importantly, the same disparities were reflected in the burden of active infection with H pylori non-serologic test positivity approaching 30% in non-Hispanic black individuals compared with less than 12% in non-Hispanic white individuals. The reasons underlying the observed racial and ethnic differences, findings that are consistent with prior literature,7,8,17,18 are not fully clear but presumably more so reflect socioeconomic and environmental factors as opposed to genetic predisposition.19,20 Because H pylori colonization most often occurs in childhood or early adolescence, neighborhood socioeconomic characteristics, living situation, water quality, and other environmental exposures early in life appear most relevant, and these social determinants of health correlate with race and ethnicity. One additional consideration unique to the VHA population is environmental exposures related to active-duty service, which may also have certain racial and ethnic selection biases.21,22 In some populations, including individuals of African ancestry residing in the U.S., neighborhood socioeconomic factors alone may not fully explain the increased predilection for H pylori.23 To date, however, genome-wide association studies have not reliably reproduced genetic variants driving H pylori susceptibility.4–6 The observed high H pylori disease burden (both active and prior infection) and possible serious clinical consequences, along with known barriers to successful H pylori eradication, have public health implications and potentiate existing racial and ethnic disparities in healthcare. Indeed, the well-described racial and ethnic disparity in H pylori–associated diseases, especially gastric non-cardia adenocarcinoma, parallels the observations herein.12,24,25
The declining burden of H pylori over time observed in our study population may represent a birth cohort effect as described in H pylori endemic areas,26 as well as time period effects related to H pylori treatment guidelines and, specific to the VA population, related to active-duty exposures (eg, deployment to H pylori endemic areas). Other factors such as improved living conditions, water sanitation, and socioeconomic factors likely also contribute to the observed decline.
Our study has several strengths. This is a U.S. nationwide study that reports H pylori burden according to detailed demographics and that leverages serologic and non-serologic data. We were able to assign geographic locations at the VHA facility zip code level in this nationwide study. This contrasts with prior studies, particularly the NHANES analysis, where data are based on samples of only 15 counties across the U.S. annually. We also quantified the amount of variation in H pylori positivity in the U.S. that can be explained by core demographic variables. There are also important limitations of our study. As a study of the VHA population, the primary limitation is generalizability, particularly for women and certain non-white populations such as Asian Americans. We will note that because of the sheer size of the study population, the absolute number of women represented in this study was still sizeable (n = 89,449), particularly when compared with previous studies. All eligible veterans have equal access to and coverage for medical care; accordingly, it is plausible that the disparities we identified in H pylori burden might demonstrate different patterns among populations not accessing VA care who may experience unequal access to healthcare and coverage. Another limitation is that we were able to evaluate only those individuals tested for H pylori, which is an established limitation of prior studies.1,7,18,27 Although we did not have details regarding the clinical indication for testing, in the U.S., H pylori testing is indicated for symptomatic individuals or for asymptomatic individuals who are considered at high risk for complications of H pylori. We did not include those individuals who had an International Classification of Diseases, revision 9 or 10 code for H pylori (n = 60,472) or who had evidence of H pylori eradication treatment (n = 225,232) without evidence of H pylori testing in the VHA medical record because we would not be able to accurately adjust the denominator. Thus, our data (and, likewise, data from earlier studies) may not accurately reflect the true population prevalence of H pylori. Details regarding immigration history, occupational exposures, deployment history, socioeconomic status, and other potentially relevant risk factors for H pylori acquisition were not available for this study; future analyses specifically investigating these factors may help further explain observed differences in H pylori infection rates, particularly with respect to racial and ethnic differences. The geographic distribution presented in this study may not reflect the geographic location of H pylori acquisition.
In conclusion, the results of this analysis have relevance clinically and for informing public health and the future research agenda. Reporting H pylori burden agnostic of core demographic factors shrouds important differences that are needed to advance clinical and public health interventions. A large percentage of the U.S. population is at risk for benign and malignant consequences of chronic H pylori infection. These data should be used not only to guide resource allocation and interventions to improve H pylori diagnostic and eradication treatment practices, but these data should also motivate research aimed at better understanding the drivers of the persistent marked demographic differences so that mitigating interventions can be optimized and implemented.
Supplementary Material
What You Need to Know.
Background
Manifestations of Helicobacter pylori infection range from asymptomatic to potentially serious consequences, including gastric cancer. H pylori burden varies globally. There are very limited data on H pylori epidemiology in the United States.
Findings
In this nationwide sample of 913,328 people who completed testing for H pylori between 1999 and 2018, H pylori positivity was 25.8% overall. Non-Hispanic blacks and Hispanics had the highest positivity rates. Although H pylori positivity declined in all racial and ethnic groups over the timeframe, the disproportionate burden of H pylori in non-Hispanic black and Hispanic compared with non-Hispanic white individuals persisted and even increased. Nearly 5% of the variation overall was explained by geography, race, ethnicity, age, and sex, with race and ethnicity accounting for the majority (3.5%). Limitations include that this is a retrospective analysis of individuals tested for H pylori and is a predominantly male cohort.
Implications for patient care
One fourth of the U.S. veteran population is at risk for benign and malignant consequences of chronic H pylori infection, with non-white individuals shouldering a disproportionate burden of H pylori infection compared with non-Hispanic white individuals. Research aimed at understanding genetic, environmental, and other drivers of these observed differences is needed. Resources should be allocated to high-risk groups to mitigate downstream consequences of H pylori infection, including non-cardia gastric adenocarcinoma.
Funding
Dr Shah is supported by an American Gastroenterological Association Research Scholar Award (2019), a Veterans Affairs Career Development Award (ICX002027A01), and the San Diego Digestive Diseases Research Center (NIH P30 DK120515).
Abbreviations used in this paper:
- CI
confidence interval
- CLO
Campylobacter-like organism test
- EGD
esophagogastroduodenoscopy
- NHANES
National Health and Nutritional Examination Survey
- SD
standard deviation
- VA
Veterans Affairs
- VHA
Veterans Health Administration
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2023.05.016.
CRediT Authorship Contributions
Shailja C. Shah, MD, MPH (Conceptualization: Lead; Data curation: Equal; Formal analysis: Equal; Methodology: Equal; Resources: Equal; Supervision: Equal; Validation: Equal; Visualization: Equal; Writing – original draft: Lead; Writing – review & editing: Equal; Guarantor of article: Lead)
Alese E. Halvorson, MS (Data curation: Equal; Formal analysis: Equal; Methodology: Equal; Software: Equal; Visualization: Equal; Writing – review & editing: Equal)
David Lee (Formal analysis: Supporting; Visualization: Supporting)
Ranier Bustamante, MS (Validation: Supporting; Writing – review & editing: Supporting)
Brandon McBay, BA, MPH (Visualization: Supporting; Writing – review & editing: Supporting)
Rohan Gupta, BS (Visualization: Supporting; Writing – review & editing: Supporting)
Chad Dorn, MS (Data curation: Supporting)
Otis Wilson, BA (Data curation: Equal; Writing – review & editing: Supporting)
Richard Peek Jr, MD (Writing – review & editing: Supporting)
Samir Gupta, MD, MSCS (Writing – review & editing: Equal)
Lin Liu, PhD (Writing – review & editing: Supporting)
Adriana Hung, MD, MPH (Writing – review & editing: Supporting)
Robert Greevy, PhD (Formal analysis: Equal; Methodology: Equal; Supervision: Equal; Visualization: Equal; Writing – review & editing: Equal)
Christianne L. Roumie, MD, MPH (Formal analysis: Equal; Supervision: Equal; Writing – review & editing: Equal)
Conflicts of interest
This author discloses the following: Shailja C. Shah serves as an ad hoc consultant for Phathom Pharmaceuticals and RedHill Biopharma. The remaining authors disclose no conflicts.
References
- 1.Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 2.Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 2015;136:487–490. [DOI] [PubMed] [Google Scholar]
- 3.Shah S, Hubscher E, Pelletier C, et al. Helicobacter pylori infection treatment in the United States: clinical consequences and costs of eradication treatment failure. Expert Rev Gastroenterol Hepatol 2022;16:341–357. [DOI] [PubMed] [Google Scholar]
- 4.Mayerle J, den Hoed CM, Schurmann C, et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA 2013;309:1912–1920. [DOI] [PubMed] [Google Scholar]
- 5.Lam SY, Mommersteeg MC, Yu B, et al. Toll-like receptor 1 locus re-examined in a genome-wide association study update on anti-Helicobacter pylori IgG titers. Gastroenterology 2022; 162:1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Omar EM. Genetic predisposition for Helicobacter pylori infection: the jury is still out! Gastroenterology 2022; 162:1591–1593. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Choi H, Leung K, et al. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2023. [DOI] [PubMed] [Google Scholar]
- 8.Tsang SH, Avilés-Santa ML, Abnet CC, et al. Seroprevalence and determinants of Helicobacter pylori infection in the Hispanic community health study/study of Latinos. Clin Gastroenterol Hepatol 2022;20:e438–e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection: Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 1998;93:2330–2338. [DOI] [PubMed] [Google Scholar]
- 10.Proceedings of the American Digestive Health Foundation International Update Conference on Helicobacter pylori, McLean, VA, February 13–16, 1997. Gastroenterology 1997;113:S1–S169. [PubMed] [Google Scholar]
- 11.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease: NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA 1994;272:65–69. [PubMed] [Google Scholar]
- 12.Shah SC, McKinley M, Gupta S, et al. Population-based analysis of differences in gastric cancer incidence among races and ethnicities in individuals age 50 years and older. Gastroenterology 2020;159:1705–1714.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212–239. [DOI] [PubMed] [Google Scholar]
- 14.El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018;16:992–1002.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everhart JE, Kruszon-Moran D, Perez-Perez GI, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis 2000; 181:1359–1363. [DOI] [PubMed] [Google Scholar]
- 16.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol 2012; 175:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varga MG, Butt J, Blot WJ, et al. Racial differences in Helicobacter pylori CagA sero-prevalence in a consortium of adult cohorts in the United States. Cancer Epidemiol Biomarkers Prev 2020;29:2084–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnenberg A, Turner KO, Genta RM. Low prevalence of Helicobacter pylori-positive peptic ulcers in private outpatient endoscopy centers in the United States. Am J Gastroenterol 2020;115:244–250. [DOI] [PubMed] [Google Scholar]
- 19.Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine: a time for reckoning with racism. N Engl J Med 2021;384:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddique SM, May FP. Race-based clinical recommendations in gastroenterology. Gastroenterology 2022;162:408–414.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vietnam war deaths, by race, ethnicity and natl origin. Available at: https://www.americanwarlibrary.com/vietnam/vwc10.htm. Accessed September 19, 2022.
- 22.Armey L, Berck P, Lipow J. Racial selection in deployment to Iraq and Afghanistan. SSRN Journal 2018. 10.2139/ssrn.3244866. [DOI] [Google Scholar]
- 23.Epplein M, Cohen SS, Sonderman JS, et al. Neighborhood socioeconomic characteristics, African ancestry, and Helicobacter pylori sero-prevalence. Cancer Causes Control 2012;23:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah SC. Gastric cancer: a neglected threat to racial and ethnic minorities in the USA. Lancet Gastroenterol Hepatol 2021; 6:266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang RJ, Epplein M, Hamashima C, et al. An approach to the primary and secondary prevention of gastric cancer in the United States. Clin Gastroenterol Hepatol 2021;20:2218–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Nishiyama T, Kikuchi S, et al. Changing trends in the prevalence of H. pylori infection in Japan (1908–2003): a systematic review and meta-regression analysis of 170,752 individuals. Sci Rep 2017;7:15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalaly JB, Couturier MR, Burnham C-AD, et al. Multicenter evaluation of Helicobacter pylori IgG antibody seroprevalence among patients seeking clinical care in the US. J Appl Lab Med 2018;2:904–913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


